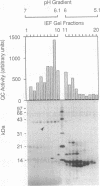
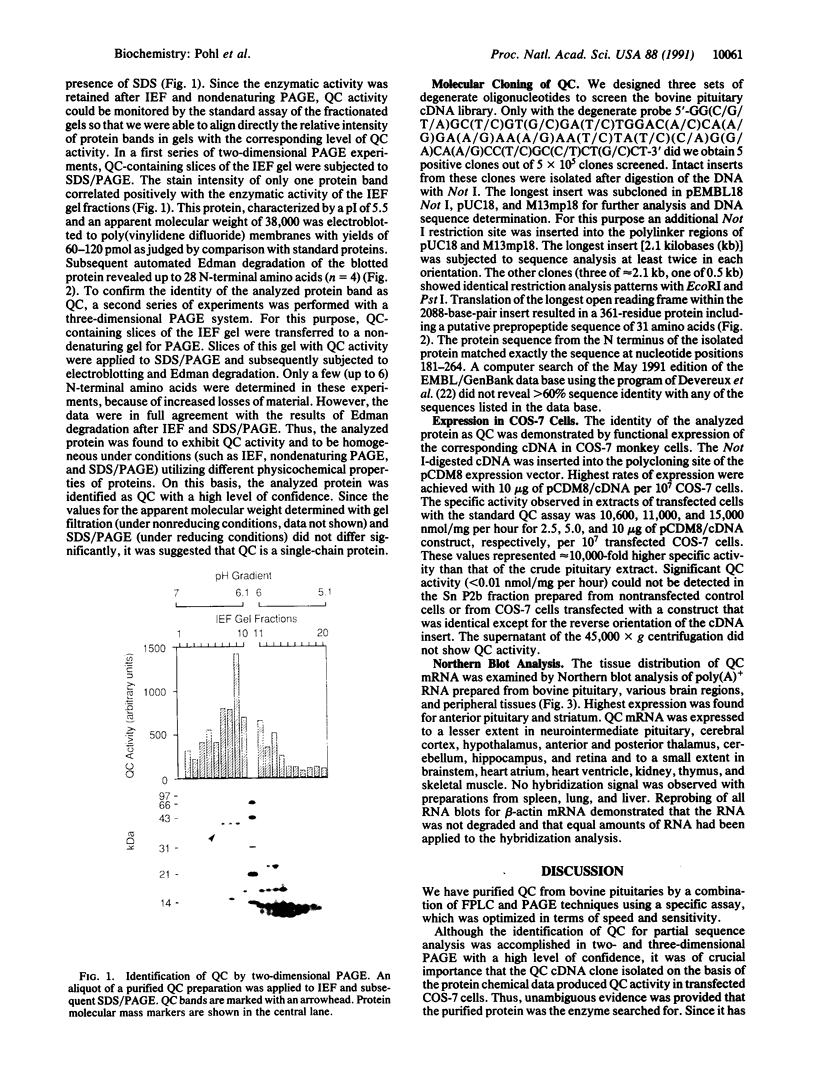
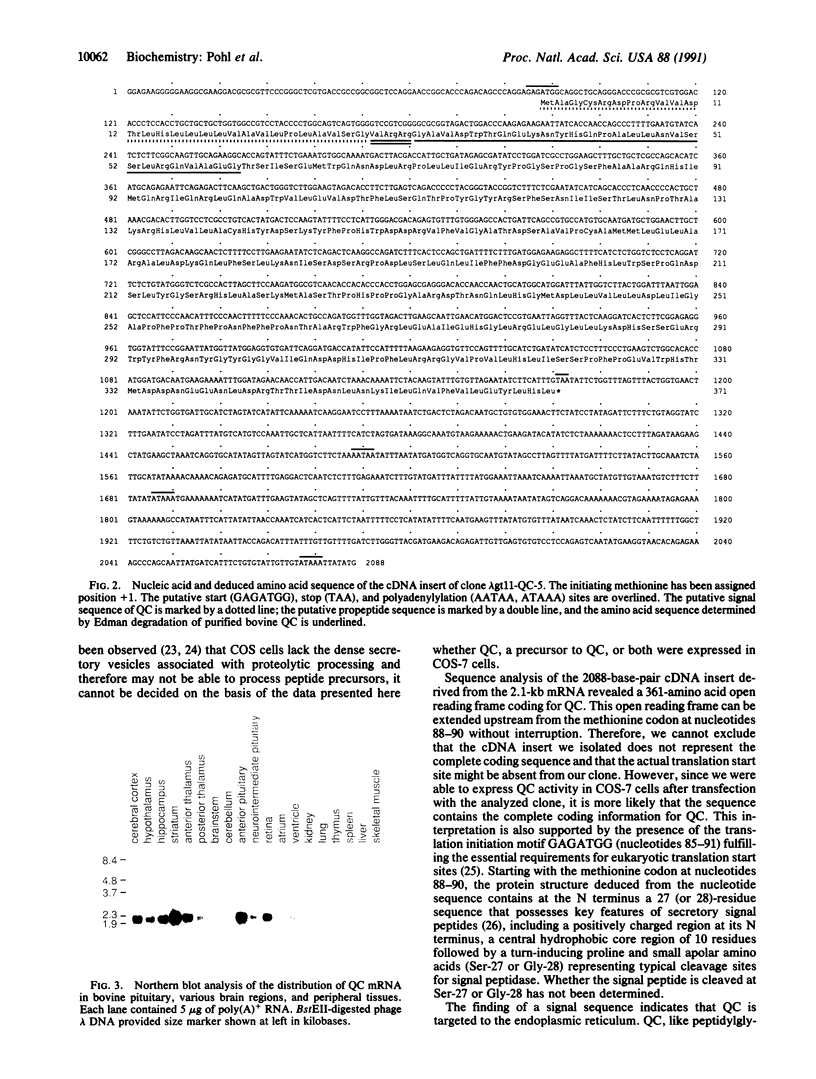
Abstract
A glutaminyl cyclase (QC) that is probably involved in the biosynthesis of pyroglutamyl peptides such as gonadotropin-releasing hormone and thyrotropin-releasing hormone has been purified to homogeneity from bovine anterior pituitary. On the basis of N-terminal sequence analysis, a 2088-base-pair cDNA clone was isolated from a bovine anterior pituitary library. From the nucleotide sequence of this clone, the primary structure of a 330-residue protein and a preceding 31-residue prepropeptide sequence was deduced. By transfection of COS-7 monkey cells with a QC cDNA/pCDM8 vector construct, QC activity was expressed. Hybridization with mRNAs of various bovine tissues revealed expression of QC mainly in brain tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braas K. M., Stoffers D. A., Eipper B. A., May V. Tissue specific expression of rat peptidylglycine alpha-amidating monooxygenase activity and mRNA. Mol Endocrinol. 1989 Sep;3(9):1387–1398. doi: 10.1210/mend-3-9-1387. [DOI] [PubMed] [Google Scholar]
- Busby W. H., Jr, Quackenbush G. E., Humm J., Youngblood W. W., Kizer J. S. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987 Jun 25;262(18):8532–8536. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W. H., Spiess J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3628–3632. doi: 10.1073/pnas.84.11.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koger J. B., Humm J., Kizer J. S. Assay of glutaminylpeptide cyclase. Methods Enzymol. 1989;168:358–365. doi: 10.1016/0076-6879(89)68027-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lapps W., Eng J., Stern A. S., Gubler U. Expression of porcine cholecystokinin cDNA in a murine neuroendocrine cell line. Proteolytic processing, sulfation, and regulated secretion of cholecystokinin peptides. J Biol Chem. 1988 Sep 15;263(26):13456–13462. [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lechan R. M., Wu P., Jackson I. M., Wolf H., Cooperman S., Mandel G., Goodman R. H. Thyrotropin-releasing hormone precursor: characterization in rat brain. Science. 1986 Jan 10;231(4734):159–161. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- MESSER M., OTTESEN M. ISOLATION AND PROPERTIES OF GLUTAMINE CYCLOTRANSFERASE OF DRIED PAPAYA LATEX. Biochim Biophys Acta. 1964 Nov 22;92:409–411. doi: 10.1016/0926-6569(64)90204-4. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Snyder S. H., Ross C. A. Carboxypeptidase E (enkephalin convertase): mRNA distribution in rat brain by in situ hybridization. J Neurosci. 1990 Aug;10(8):2850–2860. doi: 10.1523/JNEUROSCI.10-08-02850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Richter K., Kawashima E., Egger R., Kreil G. Biosynthesis of thyrotropin releasing hormone in the skin of Xenopus laevis: partial sequence of the precursor deduced from cloned cDNA. EMBO J. 1984 Mar;3(3):617–621. doi: 10.1002/j.1460-2075.1984.tb01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Adelman J. P. Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature. 1984 Oct 18;311(5987):666–668. doi: 10.1038/311666a0. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Hruby D. E. Gene transfer techniques to study neuropeptide processing. Annu Rev Physiol. 1988;50:323–332. doi: 10.1146/annurev.ph.50.030188.001543. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]