Abstract
Contrary to popular belief, sex hormones act throughout the entire brain of both males and females via both genomic and nongenomic receptors. Many neural and behavioral functions are affected by estrogens, including mood, cognitive function, blood pressure regulation, motor coordination, pain, and opioid sensitivity. Subtle sex differences exist for many of these functions that are developmentally programmed by hormones and by not yet precisely defined genetic factors, including the mitochondrial genome. These sex differences, and responses to sex hormones in brain regions and upon functions not previously regarded as subject to such differences, indicate that we are entering a new era in our ability to understand and appreciate the diversity of gender-related behaviors and brain functions.
Keywords: aging, androgen, depression, epigenetics, estradiol, hippocampus, memory, prefrontal cortex, progesterone, structural plasticity, synapse formation
Abstract
A diferencia de la creencia popular, las hormonas sexuales actúan sobre todo el cerebro tanto de machos como de hembras mediante los receptores genómicos y no genómicos. Muchas funciones neurales y conductuales están influenciadas por los estrógenos, incluyendo el ánimo, la función cognitiva, la regulación de la presión sanguínea, la coordinación motora, el dolor y la sensibilidad a los opioides. Existen sutiles diferencias por sexo para muchas de estas funciones que están programadas a través del desarrollo por hormonas y por factores genéticos no todavía bien definidos, incluyendo el genoma mitocondrial. Estas diferencias de sexo, y las respuestas a las hormonas sexuales en regiones cerebrales y sobre funciones que antes no se consideraban sujetas a tales diferencias, indican que estamos entrando en una nueva era en nuestra capacidad para comprender y apreciar la diversidad de las conductas relacionadas con el género y las funciones cerebrales.
Abstract
Contrairement à la croyance populaire, les hormones sexuelles agissent sur tout le cerveau, masculin et féminin, à travers des récepteurs génomiques et non génomiques. Les estrogènes agissent sur de nombreuses fonctions neuronales et comportementales dont l'humeur, les fonctions cognitives, la régulation de la pression artérielle, la coordination motrice, la douleur et la sensibilité aux opioïdes. Il existe des différences subtiles selon le sexe pour un grand nombre de ces fonctions dont le développement est sous dépendance hormonale et sous l'influence de facteurs génétiques non encore précisément définis, comme le génome mitochondrial. Ces différences selon le sexe et les réponses aux hormones sexuelles dans des régions cérébrales et sur des fonctions considérées auparavant comme non concernées par de telles différences, montrent qu'une nouvelle ère de compréhension et d'appréciation de la variété des comportements et des fonctions cérébrales selon le sexe s'ouvre devant nous.
Introduction
Realization that the brain is a target of sex hormones began with studies of reproductive hormone actions on the hypothalamus, regulating not only gonadotropin secretion and ovulation in females but also sex behavior.1 The work of Geoffrey Harris and subsequent pioneers established the connections between the brain and the endocrine system via the hypothalamus and the portal blood vessels that carry releasing factors from the hypothalamus to the pituitary gland.2,3 After the portal blood supply was shown to carry blood from the hypothalamus to the anterior pituitary,2 heroic efforts using hypothalamus tissue from slaughterhouse animals led to the isolation and structural identification of peptidereleasing factors.4,5 The feedback regulation of hypothalamic and pituitary hormones implied the existence of receptor mechanisms for gonadal, adrenal, and thyroid hormones. Then, the identification of cell nuclear hormone receptors in peripheral tissues6,7 by use of tritiated (3H) steroid and iodinated thyroid hormones led to the demonstration by Don Pfaff, as well as Walter Stumpf, of similar receptor mechanisms in the hypothalamus and pituitary gland.8,9
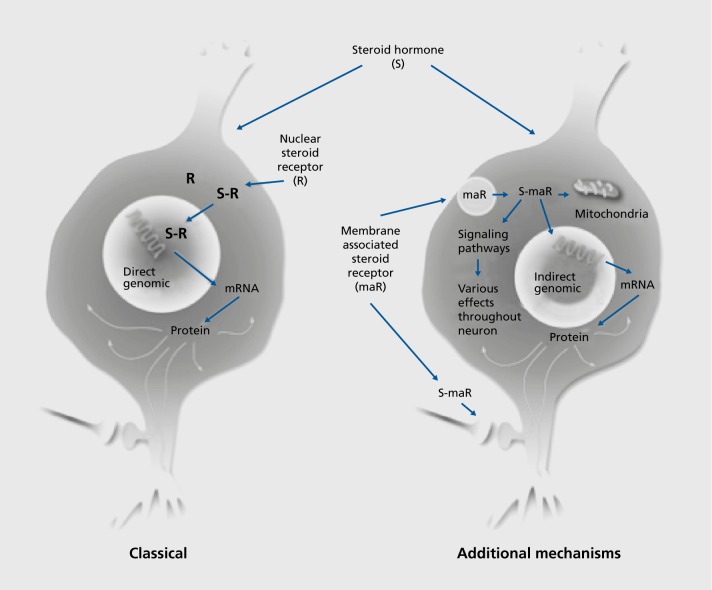
Although the focus at the time was on sexual behavior,1,10 there were other behaviors and neurological states that were known to be influenced by estrogens involving brain regions besides the hypothalamus, including fine motor control, pain mechanisms, seizure activity, mood, cognitive function, and neuroprotection.11 The unexpected discovery of cell nuclear receptor sites for glucocorticoids in the hippocampus, not only of rodents but also monkeys with extension to other species,12 pointed to brain regions other than hypothalamus as targets of circulating hormones. A further serendipitous finding of nuclear estrogen receptors (ERs) in the hippocampus13 also represented a turning point in the realization that not all steroid hormone actions occur via cell nuclear receptors, but rather operate via receptors in other parts of the cell through a variety of signaling pathways (Figure 1).14,15 This is now recognized to be the case for all classes of steroid hormones, including vitamin D, aldosterone, and androgen, as well as estrogens and progestins, to be discussed later in this review.14 First, because structural plasticity of the brain is regulated by hormones, we shall briefly introduce the evolving concepts of plasticity in the adult brain.
Figure 1. Steroid hormones are now known to act via genomic and nongenomic receptors. In many cases, the same receptor molecule has different functions in the nucleus and non-nuclear sites in the cell. CREB, cyclic AMP-response element binding protein; mRNA, messenger RNA.
Plasticity of the adult brain
Long regarded as a rather static and unchanging organ, except for electrophysiological responsivity, such as long-term potentiation,16 the brain has gradually been recognized as capable of undergoing rewiring after brain damage17 and also able to grow and change, as seen by dendritic branching, angiogenesis, and glial cell proliferation during cumulated experience.18,19 More specific physiological changes in synaptic connectivity were also recognized in relation to hormone action in the spinal cord20 and in environmentally directed plasticity of the adult songbird brain.21 Seasonally varying neurogenesis in restricted areas of the adult songbird brain is recognized as part of this plasticity.22 Indeed, neurogenesis in the adult mammalian brain was initially described23,24 and later rediscovered in the dentate gyrus of the hippocampus.25 Although the existence of adult neurogenesis in the mammalian central nervous system was doubted by some,26 recent evidence clearly proves that the human hippocampus shows significant neurogenesis in adult life.27
Sex hormone actions beyond the hypothalamus
Hippocampus
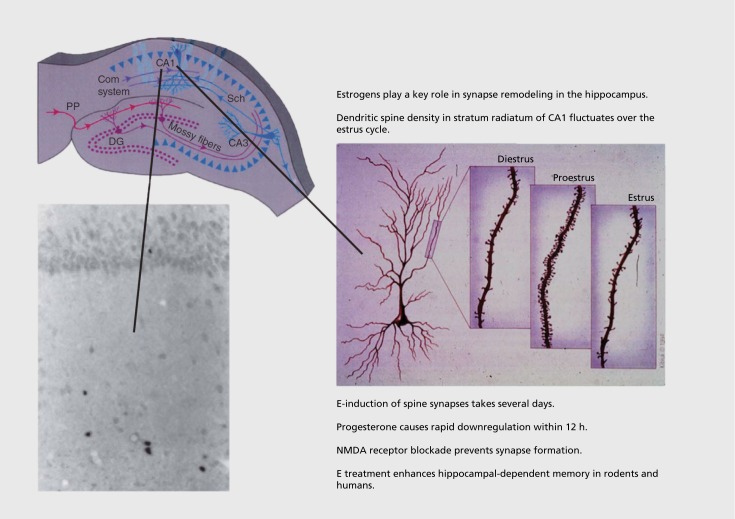
In the original steroid autoradiography studies, a few scattered cells in hippocampus demonstrated strong cell nuclear labeling by 3H estradiol in inhibitory intemeurons.28 Moreover, it was shown that the threshold for eliciting seizure activity in hippocampus was lowest on the day of proestrus when estrogen levels are elevated.29 Moreover, estrogens enhance memory retention of the type involving the hippocampus.30 Using the classical Golgi method, we found cyclic variation in the density of spine synapses on the principal neurons of the CA1 region of the hippocampus, with peak density occurring on the day of proestrus (Figure 2).31 These surprising findings have led to a deeper understanding of mechanisms, as follows32:
Figure 2. The discovery of estrogen actions on synapse formation in hippocampus via both genomic and nongenomic mechanisms has opened the way to understanding actions of estrogens and other steroid hormones throughout the brain, where nuclear steroid hormone receptors are not evident. E, estradiol; NMDA, N-methyl-D-aspartate Adapted from ref 36: McEwen BS, Gray JD, Nasca C. 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol. 2015;226(2):T67-T83. Copyright © Bioscientifica, 2015.
(i) Blocking N-methyl-D-aspartate (NMDA) receptors prevents estradiol induction of synapses. Thus, estradiol does not work alone in causing this synapse formation, and the study of underlying mechanisms is revealing some remarkable new aspects not only of hormone action, but also of neuronal plasticity.32
(ii) Estrogen treatment does not induce spine synapses in the adult castrated male hippocampus even though testosterone and dihydrotestosterone do induce spine synapses in the male hippocampus. Paradoxically, male rats treated with aromatase inhibitors at birth, which inhibit defeminization of the brain by endogenous androgen via conversion to estradiol, do respond to estradiol in adulthood with spine synapse induction. Furthermore, androgens are also able to induce spine synapses in the female rat hippocampus (see ref 32 et al).
(iii) The mechanisms implicated in estrogen-induced synapse formation involve interactions among multiple cell types in the hippocampus, as well as multiple signaling pathways. Cholinergic modulation of inhibitory interneurons is involved, and estradiol rapidly and nongenomically stimulates acetylcholine release via ERα on cholinergic terminals in the hippocampus.32 In contrast to the ER story, CA1 pyramidal neurons have ample expression of cell nuclear androgen receptors.33 In the male, these nuclear androgen receptors may play a pivotal role in spine synapse formation, which involves NMDA activity but not cholinergic activity.34 There are also extranuclear androgen receptors with epitopes of the nuclear receptor that are found in the hippocampus in membrane-associated locations in dendrites, spines, and glial cell processes.33 However, the role of nonge nomic forms of androgen receptors in spine synapse formation and other processes is less clear.
(iv) Nongenomic, membrane-associated forms of the classical ER are found in dendrites, synapses, terminals, and glial cell processes (reviewed in ref 32). In the CA1 pyramidal neurons, nongenomic actions of estrogens via phosphatidylinositol 3 (PI3) kinase promote actin polymerization and fllopodia outgrowth to form putative synaptic contacts by dendrites with presynaptic elements. Subsequent PI3 kinase activation via ERs stimulated translation of postsynaptic density protein 95 (PSD-95) in dendrites to provide a postsynaptic scaffold for spine synapse maturation. Signaling pathways implicated in these events include LIM kinase (LIMK) and cofilin phosphorylation and PI3 kinase activation, as well as the Rac/Rho signaling system. It is important to note that the cofilin pathway is implicated in spinogenesis in the ventromedial hypothalamus that contributes to activating lordosis behavior.35
(v) Progesterone treatment after estrogen-induced synapse formation causes rapid (12 h) downregulation of spine synapses; moreover, the progesterone receptor antagonist Ru486 blocked the naturally occurring downregulation of estradiol-induced spines in the estrus cycle. Curiously, the classical progestin receptor is not detectable in cell nuclei within the rat hippocampus, but it is expressed in non-nuclear sites in hippocampal neurons, and virtually all of the detectable progestin receptor is estrogen inducible. The mechanism of progesterone action on synapse downregulation is presently unknown.32,36
Estrogen actions throughout the brain
Besides hippocampus, other brain regions demonstrate estrogen-regulated spine synapse formation and turnover,32,37 including the prefrontal cortex (PFC)38 and primary sensory-motor cortex.39 Moreover, there are sex differences that are developmentally programmed as will now be discussed.
Developmental programming of sex differences
Mechanisms
Developmentally programmed sex differences arise not only from secretion of sex hormones during sensitive periods in development but also through contributions of genes on Y and X chromosomes. In females, there is inactivation of one or the other X chromosome,40; moreover, mitochondria derive from the mother, and mitochondrial genes make important contributions to brain and body functions.
In brain, there are few sexual dimorphisms, ie, complete differences between males and females. The sexually dimorphic nucleus of the preoptic area (SDN-POA) in the rodent brain comes close,41 and Witelson has described an apparent sex dimorphism in the human brain.42 However, the vast majority of sex differences are far more subtle and involve patterns of connectivity and brain regional differences that are controversial.43
Developmental windows and delayed, lasting effects
The contribution of sex hormones to the male and female phenotype occurs primarily during critical windows of early, perinatal development in which there is a considerable delay between testosterone or estradiol exposure and the emergence of sexually dimorphic traits, suggesting an imprinted “memory” of the hormonal exposure.44 For example, sex differences in the stress-induced remodeling of dendrites and synapses in the hippocampus and PFC emerge during peripubertal sexual maturation.45 The search for molecular pathways that sustain sex differences in the brain revealed several sex-biased genes with markedly different expression levels in males and females at diverse stages of development.46
Epigenetic regulation of gene expression
Parsch and Ellegren46 suggested that sex-specific effects on reproduction drive the rapid evolution of sex-biased genes. Males and females adopt different strategies to cope with environmental challenges. Indeed, the response to stress induces sex-specific effects on brain plasticity36,47,48 and activation of neuronal circuitry,49,50 as well as distinct behavioral phenotypes in males and females.51
Transcriptional regulation helps explain the sex-dependent sensitivity to stressful stimuli and the associated risk of neuropsychiatric disorders in males versus females.51 For example, stress-induced transcriptional response of the immediate early gene Fos is higher in females than in males after acute stress.52 Moreover, handling and acute stress induce markedly different immediate early gene expression activation in male versus female mice, with females showing a stronger hippocampal gene activation than males.53 Furthermore, Nugent et al demonstrated that brain feminization is maintained by the active suppression of masculinization through DNA methylation,54 pointing to epigenetic modifications that promote and maintain sex dimorphic features.
Epigenetics, brain-derived neurotrophic factor, and mental illness
The incidence of mood disorders is 1.5-to-2-fold higher in women than in men.55 Brain-derived neurotrophic factor (BDNF) has been one of the most studied genes because of its role in neuronal survival and plasticity,56-58 and altered BDNF levels have been associated with altered mental states both in women and in men.59 Estradiol induces BDNF expression, and BDNF mediates some estradiol effects in the hippocampus.32 The discovery of a common single nucleotide polymorphism of the BDNF gene, BDNF Val66Met, led to recognition of subpopulations with differential vulnerability to mood and other disorders and metabolic dysregulation.60 In experimental models with the BDNFMet allele, the estrus cycle critically interacts with the BDNF Val66Met variant to control hippocampal function and the associated behaviors.32
Patterns of gene regulation
A whole-brain transcriptome analysis showed that the gene expression difference between males and females changes over the lifetime and that the greatest expression divergence occurs during the perinatal and peripubertal periods.61 Duclot and Kabbaj62 used RNA sequencing for a genome-wide characterization of sex differences and estrus cycle influence in the rat medial PFC. They found that the transcriptomal difference between females with high and low ovarian hormone levels was greater than the difference between both female conditions and males. Thus, endogenous fluctuation of gonadal hormones may induce alternative gene networks within the same sex. In nucleus accumbens, male and female mice exposed to the same stressors display different transcriptional regulation, and the transcriptional phenotype of the nucleus accumbens predicts the increased behavioral susceptibility to stress in females versus males.63
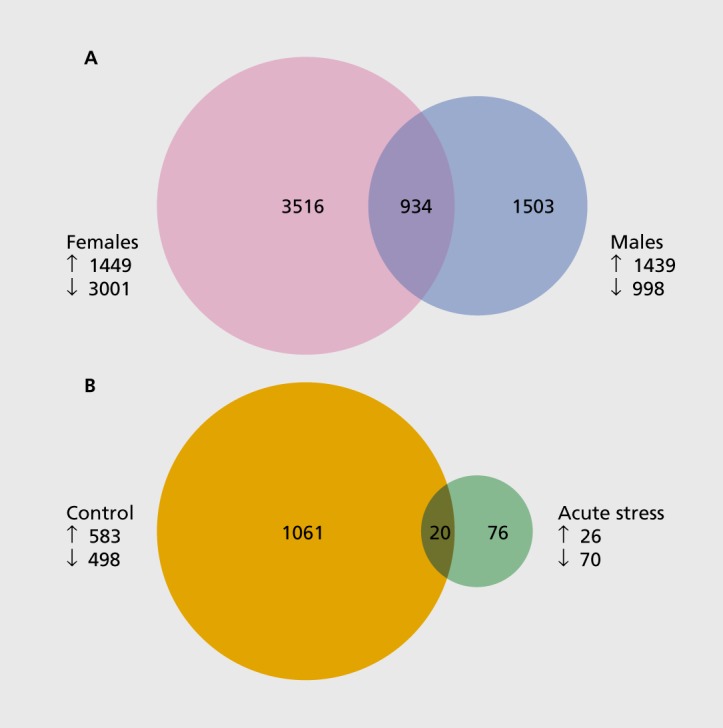
Using the bacterial artificial chromosome transgenic mouse (BAC-TRAP) system,64 the messenger RNA from hippocampal CA3 neurons was extracted and subjected to RNA sequencing. The stress-vulnerable CA3 neurons respond differentially to chronic stress in males and females.36 Acute stress produced markedly different transcriptomic profiles in the CA3 neurons, with females showing a larger number of genes up- or downregulated than with males (Figure 3). Moreover, we found that one episode of acute stress dramatically decreased the gene expression changes observed in unstressed female mice during endogenous fluctuation of estradiol levels (see Figure 3). Thus, targeting discrete brain regions paves the way for novel insights into the molecular underpinnings of hormonal actions and sex differences.
Figure 3. Gene-expression profile generated by RNA sequencing of CA3 pyramidal neurons. (A) Venn diagram depicting the number of genes affected by acute stress in CA3 pyramidal neurons of male and female BAG-TRAP mice. Acute stress induces more genes in females (pink) than in males (blue). The overlap (purple) represents the number of genes commonly changed in males and females. (B) Venn diagram illustrating the number of genes altered during endogenous fluctuation of estradiol levels (high estradiol [proestrus] vs low estradiol [diestrus]) in CA3 pyramidal neurons of female BAG-TRAP mice. Acute stress (green) dramatically decreases the estradiol-biased genes observed in unstressed mice (orange). The overlap (brown) represents the number of genes commonly changed in unstressed and acutely stressed females. BAC-TRAP, bacterial artificial chromosome transgenic mouse; down arrow, downregulated genes; up arrow, upregulated genes.
Sex differences throughout the brain
Sex differences emerge in many brain regions throughout the life course via both genetic and epigenetic mechanisms because of the widespread distribution of nongenomic, as well as genomic, forms of sex hormone receptors. Below, we present some examples, by no means exhaustive, to illustrate both the widespread nature of sex hormone influences but also the unexpectedly widespread nature of subtle sex differences.
Hippocampus response to stressors
Dendrite remodeling
Twenty-one days of chronic restraint stress (CRS) causes apical dendrites of CA3 neurons to retract; these changes do not occur after CRS in female rats.65
Spatial memory
Female and male rats show opposite effects of chronic stress on hippocampal-dependent memory, with males showing impairment and females showing enhancement or no effect.66
Classical conditioning
Exposure of male and female rats to restraint plus intermittent tail shock yields opposite effects on classical eyeblink conditioning, inhibiting it in females and enhancing it in males; in females, this effect is abolished by ovariectomy and is therefore estrogen dependent.67,68 A morphological correlate of this in the hippocampus: acute stress inhibits estrogen-dependent spine formation in CA1 neurons of the hippocampus, whereas the same acute stressors enhance spine density in male CA1 neurons, possibly by increasing testosterone secretion upon which spine formation in the male CA1 is dependent.68
Neonatal masculinization
Neonatal masculinization of females makes them respond positively, like genetic males, to shock stressor.68 Moreover, in females, depending on reproductive status and previous experience, the negative stress effect is altered, eg, it is absent in mothers and virgin females with experience with infants.69
Opioids
Females have a heightened sensitivity to stress70,71 and can show enhanced cognitive performance after stress,72 which may contribute to their accelerated course of addiction. Ten days of chronic immobilization stress in males essentially “shuts off” the opioid system, whereas chronic stress “primes” the opioid system in all females in a manner that would promote excitation and learning processes after subsequent exposure either to stress or an opiate ligand.70,71
Maturational/developmental component
Sex differences in chronic stress effects on dendrite length and branching appear after puberty. Stress in the pubertal transition causes qualitatively similar responses in males and females in hippocampus, PFC, and amygdala; however, after puberty, distinct sex differences in response to chronic stress become evident.45
Sex differences in prefrontal cortex
CRS for 21 days causes neurons in the medial PFC of the male rat to show dendritic debranching and shrinkage.73 These neurons, which project to cortical areas and not to the amygdala, do not show dendritic changes in females. However, neurons that project to the amygdala from the medial PFC undergo dendritic expansion in females but not in males; this expansion in the female is estrogen-dependent, evidenced by ovariectomized females not showing such changes.50 Estrogens and stress also interact in a regionally specific manner in the PFC in that cortically projecting PFC neurons, which show no dendritic changes after CRS in either intact or ovariectomized animals, display a CRS-induced increase in spine density in ovariectomized animals but not in intact females with circulating estradiol; yet amygdala-projecting PFC neurons show CRS-induced spine density that is enhanced in intact females, accompanying the dendrite expansion.50 Regarding function, as shown by lesion studies, contralateral prefrontal to amygdala projection is key to the ability of acute foot shock stress to impair eyeblink conditioning in female rats.69
Dopaminergic systems
Estradiol stimulates dopamine release independently of nuclear ERs.74 Moreover, there is a sex difference in the ability of estrogen to promote dopamine release where membrane-associated, nongenomic ERs have been demonstrated in dopamine-terminal areas—including the caudate, PFC, and nucleus accumbens—with effects promoting a place memory as opposed to a response memory bias.75 High-dose estrogen treatment used in the initial contraceptive preparations exacerbated symptoms of Parkinson disease in women.76 However, now, with lower doses of estradiol, there is evidence for neuroprotection in Parkinson disease and the involvement of multiple signaling pathways,77 including the G-protein coupled ER (GPER1).75
Sex differences in cerebellum
The cerebellum is responsive to estrogens, generates both estradiol and progesterone during its development, and in humans is implicated in disorders that show sex differences.78,79 Estrogens direct the growth of dendrites in the developing cerebellum and regulate both excitatory and inhibitory balance, affecting not only motor coordination but also memory and mood regulation.79 The cerebellum is involved in associative learning processes of conditioned anticipatory safety from pain and mediates sex differences in the underlying neural processes.80
Sex differences in pain sensitivity and circuitry
Morphine is less potent in alleviating pain in women than in men.80 Gonadal steroids, primarily through ERα and androgen receptors in the periaqueductal gray, are known to exert a sexually dimorphic regulation of spinal antinociception.81,82 For migraine, a pain condition that is more frequent in women than in men, women have been shown to have increased pain sensitivity.83 Moreover, women with migraine have thicker posterior insula and precuneus cortices than male migraineurs, as well as healthy controls of both sexes.83
Empathy
Assessments of empathy in male and female volunteers, in which both sexes perform equally well on three separate tests, reveal different brain regional patterns of activation as seen by functional magnetic resonance imaging (fMRI).84 Thus, in their daily lives, men and women use different “strategies” in their relationships and approaches to solving problems and yet, on average and with considerable overlap, do most tasks equally well.84,85
Neuroprotection
Estradiol protects neurons from excitotoxic damage due to seizures and stroke, as well as in Alzheimer disease.37,86 The exact role in this process of cell nuclear ERs found on inhibitory interneurons is unclear, but one clue is the ability of estrogens to enhance neuropeptide Y (NPY) expression and release, as NPY has antiexcitatory actions.87,88 Additionally, estradiol translocation via ERβ into mitochondria regulates mitochondrial calcium sequestration, including B-cell lymphoma 2 (Bcl-2) translocation.89
Investigation of the ability of estrogens to protect against stroke damage, as well as Alzheimer and Parkinson disease, has demonstrated that the brain is capable of locally generating estrogens either from androgens or possibly directly from cholesterol.90 Aromatization of androgen precursors produces estrogenic steroids and knock-out of the aromatase enzyme increases ischemic damage even beyond that found after ovariectomy of wild-type mice.86 Moreover, like estrogens, androgens have neuroprotective effects.91 The brain also appears to have the capacity to locally generate the androgen dihydrotestosterone from as yet unknown precursors, independently of the gonads, in an animal model in which mild exercise increases neurogenesis in a manner facilitated by those androgens.92
Estrogen and the aging hippocampus and prefrontal cortex
Estrogen actions have also illuminated the aging process insofar as they have revealed the progressive loss of plasticity and hormone responsiveness, which is not necessarily irreversible.
Hippocampus
In contrast to estrogen modulation of synaptic plasticity in young female rats, estrogen treatment failed to increase synapse density in CA1 of aged ovariectomized rats.37 At the subsynaptic level, whereas estradiol was able to maintain select network properties in the aged hippocampus,37 the loss of structural plasticity in the aged hippocampus was accompanied by half as many synapses containing ERα as in young animals irrespective of estrogen treatment. In addition, though subtle, an estrogen-induced decrease in synaptic ERα levels was observed in both axon terminals and dendritic spines selectively in the young cohort, once again pointing to a loss of responsivity by the neurons in the aged CA1 subfield.93 Moreover, phosphorylated LIMK (pLIMK) located within the CA1 synapses activated by estrogen—which is critically important in regulation of actin dynamics linked to spine formation—showed selective responsivity to estrogen in the young cohort, whereas the aged animals had decreased synaptic pLIMK levels in CA1 that was not reversed by estrogen treatment.94 Age-related losses of responsivity to estrogen with respect to structural plasticity, molecular rearrangement, and biochemical function correlate with an age-related loss of estrogen-induced memory enhancement. In young, but not aged, ovariectomized rats, estrogen rescues the learning deficits associated with experimentally induced cholinergic impairment.37
Prefrontal cortex
The nonhuman primate model has provided the best insights into the aging effects on the PFC, which is important for working memory and self-regulation.37 After ovariectomy, the decrease in spine density in the young monkeys compared with those receiving estradiol-replacement treatment occurred against a background of an adaptive increase in dendritic length in the young vehicle-treated animals, implying that in spite of the higher spine density in the estradiol-treated group, overall there is no difference in the number of spines per neuron in the young cohort. In contrast, there was an estrogen-induced spinogenesis in the aged cohort, which occurred against the background of an age-related reduction in spine density of the aged vehicle- treated group. Thus, the vulnerability to cognitive decline in the aged vehicle-treated group is explained by both an age-induced loss of spines on top of an estrogen deficiency-induced loss of spines.
Because spine size is highly correlated with both synapse size as well as glutamate receptor number,37 it was noteworthy that estrogen shifts the distribution of spine-head diameter toward smaller size in both the young and aged animals, but aging dramatically reduced the representation of spines with small heads and long necks. This age-related selective loss of small spines (and the partial recovery with estrogen) fits in nicely with a developing framework in neurobiology of the essential role that small spines play in learning and memory, with thin spines involved in “learning” and big, mushroom-type spines representing “memory” traces.37 What is therefore most detrimental for the aging animal's cognitive performance is that small, plastic spines are missing in the dorsolateral PFC. When estrogen replacement is provided to the aged animals, their cognitive performance matches the performance of young animals in spite of having an overall smaller spine density than the young estrogen-treated animals. This could indicate that a modest increase in small spines goes a long way in providing neurobiological resilience.
Another finding from the infrahuman primate model is that surgical menopause impairs cognitive function in a manner that is ameliorated by estrogen treatment.95 The presence of abnormal donut-shaped mitochondria that generate excess free radicals was a factor.95,96 Although presynaptic bouton density or size was not significantly different across groups distinguished by age or menses status, cognitive performance accuracy correlated positively with the number of total and straight mitochondria in presynaptic endings of the dorsolateral PFC. In contrast, accuracy correlated inversely with the frequency of boutons containing donut-shaped mitochondria, and those terminals exhibited smaller active zone areas and fewer docked synaptic vesicles than those with straight or curved mitochondria. Estrogen administration ameliorated cognitive performance deficits and reduced the numbers of donut-shaped mitochondria, suggesting that hormone therapy may benefit cognitive aging, in part by promoting mitochondrial and synaptic health in the PFC and possibly in other parts of the brain.95
Conclusion
The origins of sex differences in the brain and behavior depend not only on developmentally programmed secretion of hormones during sensitive periods of early life but also on genes and sex chromosomes, as well as mitochondria from the mother. Moreover, there is continuous interaction between genes and experiences, now referred to as “epigenetics,” which changes the expression of genes over the life course. Knowing that the entire brain is affected by sex hormones with subtle sex differences, we are entering a new era in our ability to understand and appreciate the diversity of genderrelated behaviors and brain functions. This will enhance “personalized medicine” that recognizes sex differences in disorders and their treatment and will improve understanding of how men and women differ, not by ability, but by the “strategies” they use in their daily lives.
Conflict of Interest: The authors declare no conflict of interest.
Contributor Information
Jordan Marrocco, Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, the Rockefeller University, New York, New York, USA.
Bruce S. McEwen, Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, the Rockefeller University, New York, New York, USA.
REFERENCES
- 1.Young WC., Goy RW., Phoenix CH. Hormones and sexual behavior. Science. 1964;143(3603):212–218. doi: 10.1126/science.143.3603.212. [DOI] [PubMed] [Google Scholar]
- 2.Harris GW. Electrical stimulation of the hypothalamus and the mechanism of neural control of the adenohypophysis. J Physiol. 1948;107(4):418–429. doi: 10.1113/jphysiol.1948.sp004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meites J. Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology. 1992;56(1):1–10. doi: 10.1159/000126201. [DOI] [PubMed] [Google Scholar]
- 4.Schally AV., Arimura A., Kastin AJ. Hypothalamic regulatory hormones. Science. 1973;179(71):341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- 5.Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978;202(4366):390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- 6.Jensen E., Greene GL., Closs LE., DeSombre ER., Nadji M. Receptors reconsidered a 20-year perspective. Recent Prog Harm Res. 1982;38:1–40. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- 7.Toft D., Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaff DW., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 9.Stumpf WE., Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogen, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7(11-12):1163–1170. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- 10.Young W. The Hormones and Mating Behavior. In: Young W, ed. Sex and Internal Secretions. 7th ed. Baltimore.MD: Williams and Wilkins . 1961:1173–1239. [Google Scholar]
- 11.McEwen BS., Alves SH. Estrogen actions in the central nervous system. Endocr Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS., DeKloet ER., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 13.Loy R., Gerlach J., McEwen BS. Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Brain Res. 1988;467(2):245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS., Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55(2):343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly MJ., Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12(4):152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 16.Bliss TV., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiology. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parnavelas J., Lynch G., Brecha N., Cotman C., Globus A. Spine loss and regrowth in hippocampus following deafferentation. Nature. 1974;248(5443):71–73. doi: 10.1038/248071a0. [DOI] [PubMed] [Google Scholar]
- 18.Bennett E., Diamond M., Krech D., Rosenzweig M. Chemical and anatomical plasticity of brain. Science. 1964;146(3644):610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 19.Greenough WT., Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40(2):491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- 20.Arnold A., Breedlove S. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 21.DeVoogd T., Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214(4517):202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- 22.Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22(3):624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman J., Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan MS., Bell DH. Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp Brain Res. 1983;52(1):1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- 25.Cameron HA., Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan MS. Environment complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci. 2001;24(10):617–620. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- 27.Spalding KL., Bergmann O., Alkass K., et al Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura NH., Akama KT., Yuen GS., McEwen BS. Thinking outside the pyramidal cell: unexplored contributions of interneurons and neuropeptide Y to estrogen-induced synapse formation in the hippocampus. Rev Neurosci. 2007;18(1):1–13. doi: 10.1515/revneuro.2007.18.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Terasawa E., Timiras P. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83(2):207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 31.Woolley C., Gould E., Frankfurt M., McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS., Akama KT., Spencer-Segal JL., Milner TA., Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabori NE., Stewart LS., Znamensky V., et al Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 34.Romeo RD., Staub D., Jasnow AM., Karatsoreos IN., Thornton JE., McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146(4):2091–2097. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- 35.Christensen A., Dewing P., Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31(48):17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS., Gray JD. Nasca C 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol. 2015;226(2):T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara Y., Waters EM., McEwen BS., Morrison JH. Estrogen Effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95(3):785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao J., Rapp PR., Janssen WG., et al Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104(27):11465–11470 . doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JR., Yan YT., Wang TJ., Chen LJ., Wang YJ., Tseng GF. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex. 2009;19(11):2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy MM., Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorski RA., Gordon JH., Shryne JE., Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 42.Witelson SF., Glezer II., Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;1(5 pt 1):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen B., Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95(1-2):24–39.doi:10.1002/jnr.23809.. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forger NG. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150114. doi: 10.1098/rstb.2015.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eiland L., Ramroop J., Hill MN., Manley J., McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parsch J., Ellegren H. The evolutionary causes and consequences of sex-biased gene expression. Nat Rev Genet. 2013;14(2):83–87. doi: 10.1038/nrg3376. [DOI] [PubMed] [Google Scholar]
- 47.Leuner B., Mendolia-loffredo S., Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56(12):964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galea LA., Wainwright SR., Roes MM., Duarte-Guterman P., Chow C., Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25(11):1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 49.Bangasser DA., Curtis A., Reyes BA., et al Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shansky RM., Hamo C., Hof PR., Lou W., McEwen BS., Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20(11):2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarthy MM. Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150106. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babb JA., Masini CV., Day HE., Campeau S. Stressor-specific effects of sex on HPA axis hormones and activation of stress-related neurocircuitry. Stress. 2013;16(6):664–677. doi: 10.3109/10253890.2013.840282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohacek J., Manuella F., Roszkowski M., Mansuy IM. Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology. 2015;52:1–12. doi: 10.1016/j.psyneuen.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 54.Nugent BM., Wright CL., Shetty AC., et al Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy MM., Arnold AP., Ball GF., Blaustein JD., De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duman RS., Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Magarinos AM., Li CJ., Gal Toth J., et al Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21(3):253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bath KG., Schilit A., Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 59.Sen S., Duman R., Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Notaras M., Hill R., van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20(8):916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- 61.Shi L., Zhang Z., Su B. Sex biased gene expression profiling of human brains at major developmental stages. Sci Rep. 2016;6:21181. doi: 10.1038/srep21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duclot F., Kabbaj M. The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biol. 2015;16:256. doi: 10.1186/s13059-015-0815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodes GE., Pfau ML., Purushothaman I., et al Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35(50):16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heiman M., Schaefer A., Gong S., et al A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135(4):738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galea LA., McEwen BS., Tanapat P., Deak T., Spencer RL., Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 66.Bowman RE., Beck KD., Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43(1):48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 67.Wood GE., Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95(7):4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21(16):6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shors TJ. A trip down memory lane about sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150124. doi: 10.1098/rstb.2015.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker JB., Monteggia LM., Perrot-Sinal TS., et al Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27(44):11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milner TA., Burstein SR., Marrone GF., et al Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67(11):757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luine VN., Beck KD., Bowman RE., Frankfurt M., MacLusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19(10):743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 73.McEwen BS., Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mermelstein PG., Becker JB., Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almey A., Milner TA., Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bedard PJ., Langelier P., Villeneuve A. Oestrogens and the extrapyramidal system. Lancet. 1977;2(8052-8053):1367–1368. doi: 10.1016/s0140-6736(77)90429-9. [DOI] [PubMed] [Google Scholar]
- 77.Bourque M., Dluzen DE., Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson's disease. Front Neuroendocrinol. 2012;33(2):169–178. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Dean SL., McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7(1):38–47. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedges VL., Ebner TJ., Meisel RL., Mermelstein PG. The cerebellum as a target for estrogen action. Front Neuroendocrinol. 2012;33(4):403–411. doi: 10.1016/j.yfrne.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Labrenz F., Icenhour A., Thurling M., et al Sex differences in cerebellar mechanisms involved in pain-related safety learning. Neurobiol Learn Mem. 2015;123:92–99. doi: 10.1016/j.nlm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Loyd DR., Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57–63. doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 83.Maleki N., Linnman C., Brawn J., Burstein R., Becerra L., Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012;135( pt 8):2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Derntl B., Finkelmeyer A., Eickhoff S., et al Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 85.McEwen BS., Lasley EN. The End Of Sex As We Know It. Cerebrum: The Dana Forum on Brain Science; vol 7. Washington, DC: Dana Press. 2005 [Google Scholar]
- 86.McCullough LD., Blizzard K., Simpson ER., Oz OK., Hum PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23(25):8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura NH., Resell DR., Akama KT., McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80(5):308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- 88.Ledoux VA., Smejkalova T., May RM., Cooke BM., Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29(5):1457–1468. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nilsen J., Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3(4):297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 90.Hojo Y., Hattori TA., Enami T., et al Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101(3):865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pike CJ., Nguyen TV., Ramsden M., Yao M., Murphy MP., Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53(5):693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okamoto M., Hojo Y., Inoue K., et al Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci U S A. 2012;109(32):13100–13105. doi: 10.1073/pnas.1210023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adams MM., Fink SE., Shah RA., et al Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22(9):3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yildirim M., Janssen WG., Tabori NE., et al Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152(2):360–370. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hara Y., Yuk F., Puri R., Janssen WG., Rapp PR., Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111(1):486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Picard M., McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A. 2014;111(1):7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]