Abstract
mTOR drives tumor growth but also supports T cell function, rendering the applications of mTOR inhibitors complex especially in T cell malignancies. Here we studied the effects of the mTOR inhibitor rapamycin in mouse EL4 T cell lymphoma. Typical pharmacologic rapamycin (1–8 mg/kg) significantly reduced tumor burden via direct suppression of tumor cell proliferation and improved survival in EL4 challenge independent of anti-tumor immunity. Denileukin diftitox (DD)-mediated depletion of regulatory T cells significantly slowed EL4 growth in vivo in a T cell-dependent fashion. However, typical rapamycin inhibited T cell activation and tumor infiltration in vivo and failed to boost DD treatment effects. Low dose rapamycin (LD, 75 μg/kg) increased potentially beneficial CD44hiCD62L+ CD8+ central memory T cells in EL4 challenge, but without clinical benefit. LD rapamycin significantly enhanced DD treatment efficacy, but DD plus LD rapamycin treatment effects were independent of anti-tumor immunity. Instead, rapamycin up-regulated EL4 IL-2 receptor in vitro and in vivo, facilitating direct DD tumor cell killing. LD rapamycin augmented DD efficacy against B16 melanoma and a human B cell lymphoma, but not against human Jurkat T cell lymphoma or ID8agg ovarian cancer cells. Treatment effects correlated with IL-2R expression, but mechanisms in some tumors were not fully defined. Overall, our data define a distinct, biphasic mechanisms of action of mTOR inhibition at doses that are clinically exploitable, including in T cell lymphomas.
Keywords: lymphoma, carcinoma, rapamycin, temsirolimus, mTOR, immunotherapy, denileukin diftitox
Introduction
The serine/threonine kinase, mammalian target of rapamycin (mTOR), is an important regulator of cell growth, metabolism and stress responses (1) with increased activation in many cancers (2), including B cell and T cell hematologic malignancies (3,4). Treating cancers with rapamycin (sirolimus), a small molecule mTOR inhibitor, or related mTOR inhibitors collectively called rapalogues, is thus a reasonable cancer treatment strategy. Rapalogues are approved to treat certain carcinomas, but with generally modest efficacy (5).
Human B cell (6) and T cell (4,7) malignancies express activated mTOR that can drive their growth. Distinct rapalogues have shown efficacy against them in vitro or in immunocompromised mice (8,9), suggesting direct anti-tumor effects. Rapalogues have some clinical activity in mantle cell lymphoma (9,10), a type of B cell lymphoma, but there are few studies of their clinical effects in T cell lymphomas.
Aside from inhibiting tumor mTOR to slow tumor growth directly, mTOR inhibition also augments conventional (11,12) and γδ T cell (13) functions that can affect anti-cancer immunity (14). We show here that rapamycin has bimodal and novel treatment effects against murine EL4 T cell lymphoma. At typical pharmacologic doses, rapamycin inhibited tumor mTOR and slowed EL4 lymphoma growth in vivo through direct effects on tumor cells, but reduced immune T cell activation. Low dose (LD) rapamycin improved anti-tumor immunity against EL4 when combined with a vaccine plus immune checkpoint blockade (14). However, we found that LD rapamycin did not affect in vivo EL4 growth, minimally inhibited tumor mTOR, but did not blunt immune T cell activation in vivo.
EL4 was refractory to many immunotherapies but responsive to Treg depletion. We tested the IL-2/diphtheria toxin fusion protein, denileukin diftitox (DD) (15,16), as regulatory T cell (Treg) depletion immunotherapy and found that typical rapamycin doses did not improve Treg depletion as immunotherapy, as T cell activation was blunted. By contrast, LD rapamycin improved DD treatment efficacy, but unexpectedly, this LD effect did not require anti-tumor immunity. Instead, rapamycin increased tumor expression of interleukin-2 receptor (IL-2R) that improved killing of tumor cells by DD, which binds this receptor. We used mouse B16 melanoma, ID8agg ovarian carcinoma, human NM001 EBV-transformed B cell lymphoma and human Jurkat CD4+ T cell lymphoma lines in additional rapamycin studies, where we found that rapamycin improved DD efficacy only in IL-2R expressing tumor cells. The mTOR inhibitor, temsirolimus improved DD-mediated cytotoxicity against IL-2R-expressing human B cell lymphoma line NM001, but not against the Jurkat human CD4+ T cell line lacking IL-2R. We thus demonstrate an unexpected mechanism for mTOR inhibitor use in cancer combination immunotherapy and show distinct, biphasic mechanisms of rapamycin action at typical versus low doses of rapamycin, with similar temsirolimus effects.
Materials and Methods
Mice
Wild type (WT), male βδ TCR (T cell receptor) knock out (KO), interferon-γ KO, δ TCR KO, $Rag2 (recombinase activating gene 2) KO, Prf1 (perforin) KO on the C57BL/6J background (BL6) were purchased from Jackson Labs. Male BL6 Foxp3DTR mice were from Alexander Rudensky, University of Washington and used for studies shown. Female WT, βδ TCR KO and Foxp3DTR mice were used as noted. Mice were maintained in specific pathogen-free conditions, provided a normal diet and water ad libitum and used when 8–16-weeks old. All animal studies were approved by our Institutional Animal Care and Use Committee.
Cell lines
Mouse EL4 lymphoma, B16F10 melanoma and human Jurkat CD4+ T cell lymphoma cells were purchased from the ATCC in 2012, 2008 and 1987, respectively. Mouse ID8 was a gift from George Coukos (University of Pennsylvania) in 2004, from which we developed the highly aggressive ID8agg subline (our unpublished data). NM001 is a human EBV-transformed B cell lymphoma line generated as we described (17) in 2015. Cell lines were not independently validated. All murine lines are on the BL6 background and all cells were cultured in medium RPMI-1640 containing 4 mM L-glutamine, 100 Units/ml penicillin and 100 μg/ml streptomycin, 10 mM HEPES, 1 mM sodium pyruvate and 10% fetal bovine serum, in a 5% CO2, humidified atmosphere at 37° C. The pAcGFP1-C1 plasmid was used to generate EL4 cells stably expressing GFP (EL4-GFP) using the Cell Line Nucleofector Kit (Lonza) according to the manufacturer’s instructions. 48 hours after transfection, cells expressing GFP were sorted using a BD FACSAria flow cytometer with FACSDiva software (BD Bioscience), and then maintained in medium containing G418 (1 mg/ml) for selection. ID8agg cells expressing luciferase were generated with the luciferase-encoding plasmid pGL4.51 (Promega) with Attractene transfection reagent and selected with G418 (1 mg/ml). ID8agg-luciferase is derived from a single cell clone and maintained in G418 (0.2 mg/mL) after initial selection.
Tumor challenges and assessments
On day 0, mice were challenged with 40,000 EL4-GFP or 50,000 EL4, or 500,000 B16F10 cells subcutaneously (s.c.) on both shaved flanks. Tumor growth was measured every other day using calipers and volume calculated as (length × width2)/2. ID8agg was given on day 0 as 4 × 106 cells by intraperitoneal (i.p.) injection and growth was assessed by luciferase bioluminescence. Mice were sacrificed when s.c. tumors reached a volume of 1500 mm3, or weight was >30% baseline for ID8agg for survival, or at times indicated for other studies. In bone marrow studies, 1×106 EL4-GFP cells were injected intravenously and mice were sacrificed 5 days later.
Treatments
Days indicated are after tumor challenges. Mice were injected with 100 μg of αB7-H1 (clone 10F.9G2), αPD-1 (clone RMP1-14) or αCTLA-4 (clone 9H10, all from BioXCell) monoclonal antibodies intraperitoneally (i.p.) on days 4, 7 and 12. αCD25 (250 μg, clone PC-61.5.3, BioXCell) was administered i.p. on day 2. Denileukin diftitox (DD, Eisai), a fusion protein of diphtheria toxin and human interleukin-2 (IL-2) (18), that depletes mouse Tregs to treat distinct carcinomas (15,16,19), was given 5 μg i.p. every 4 days for EL4 challenge, and every 5 days for B16 and ID8agg challenge. Foxp3DTR mice engineered to allow Treg-specific depletion using diphtheria toxin (15) were injected i.p. with 1 μg/kg diphtheria toxin every 3 days starting on day 4. Rapamycin (LC Laboratories) or vehicle control (0.25% Tween 80 + 0.25% PEG400) was given i.p. daily for 5 consecutive days/week at indicated doses starting on day 4, for times indicated. Rapamycin at 1–8 mg/kg is defined as typical, and 0.075 mg/kg as low dose (LD).
Flow cytometry
Tumors were isolated and digested with 1.67 Wünsch units/ml Liberase TL (Roche) and 0.2 mg/ml DNase I (Roche) in RPMI-1640 at 37° C for 30 min. Tumors, tumor draining lymph nodes (TDLN) or spleens were stained and analyzed on a BD LSR II flow cytometer using FACSDiva software. For intracellular staining, cells were fixed and permeabilized with Foxp3/transcription factor buffer (eBioscience) per manufacturer’s directions. Anti-mouse antibodies were against CD4 (Tonbo); CD8α (Life Technologies); B7-H1, CD132 (BD Biosciences); Foxp3, Ki-67, Vβ12 TCR, ICOS (eBioscience); and CD3, CD25, CD44, CD62L, CD122, PD-1, Annexin V (Biolegend). Antibodies against human CD25, CD122 and CD132 were from Biolegend. We used Ghost Dye UV 450 viability dye (Tonbo), and Dead Cell Apoptosis Kit with Annexin V Alexa Fluor 488 and propidium iodide (Invitrogen). In the CD3+ gate, EL4 cells were GFP+Vβ12+ and distinguished from resident GFP−Vβ12− T cells. In specific experiments, EL4 cells were stimulated in 96-well round bottom plates using CD3/CD28 beads (Dynabeads Mouse T cell activator, Invitrogen) for 24 hours at 1 bead:5 EL4 cells, before staining for CD25 and CD122.
Immunoblotting
Snap-frozen tumor tissues were homogenized in RIPA buffer (20 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM disodium EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1% Triton-X100) plus 1 mM phenylmethylsulphonyl fluoride and Halt protease and phosphatase inhibitor cocktail (Thermo Scientific), and handled as we described for the bullet blender protocol (20) (5 min at speed 10 with beads; Next Advance). Lysates were centrifuged at 14,000 rpm, 4° C for 10 min, transferred into pre-chilled Eppendorf tubes and protein concentrations were determined by the Bradford method (Thermo). 50 μg protein was separated on 4–15% sodium dodecyl sulfate polyacrylamide gels (BioRad), transferred to PVDF membranes (GE Technologies), blocked in Tris-buffered saline (pH 7.4) plus 0.1% Tween-20 and 5% skim milk, and incubated overnight at 4° C with 1:1000 diluted phospho- and/or total antibodies against rpS6S240/244, 4E-BP1T37/46, P70 S6 KinaseThr 389, and AktS473 (all from Cell Signaling) plus anti-mouse β-Actin or α-Tubulin (Santa Cruz Biotechnology) as a loading control. Membranes were incubated with HRP-conjugated antibodies at ambient temperature for 1 h. Proteins were detected by enhanced chemiluminescence (Pierce). Band quantification was done with Image J software (National Institutes of Health).
In vitro DD sensitization and proliferation assays
Suspension cell (EL4, Jurkat, NM001) proliferation was monitored by the colorimetric water-soluble tetrazolium salt (CCK8) assay using a Cell Counting Kit-8 (Sigma Aldrich) according to the manufacturer’s instructions. Adherent cell proliferation was measured by MTT assay. EL4 (1000–2000 cells/well), B16F10 (500–1000 cells/well), Jurkat or NM001 B cell lymphoma (20,000–40,000 cells/well) cells were seeded in triplicate into 96-well plates and treated with rapamycin alone (0.625–80 nM) or rapamycin (100–200 pg/ml) ± DD (250 ng/ml) and blocking anti-mouse CD25 (10 μg/ml) (BioXCell) and anti-mouse CD122 (10 μg/ml) (BioLegend) antibodies or temsirolimus (200–500 pg/ml) (Sigma Aldrich). After specified incubation times, 5 mg/ml MTT or CCK8 solution was added and optical density was measured at 570 nm for MTT or 450 nm for CCK8 in a microplate reader. Absolute numbers of live cells were counted in triplicate on a hemocytometer using Trypan blue.
Statistics
Statistical analyses were performed using GraphPad Prism 6. For tumor growth, treatments were compared by two-way ANOVA. Survival was determined by the method of Kaplan and Meier and analyzed with the log-rank test. Student’s t test (for two independent groups) or one-way ANOVA (for three or more groups) was used for other single measurements as indicated. P<0.05 was considered significant.
Results
Typical rapamycin treats EL4 T cell lymphoma in vivo
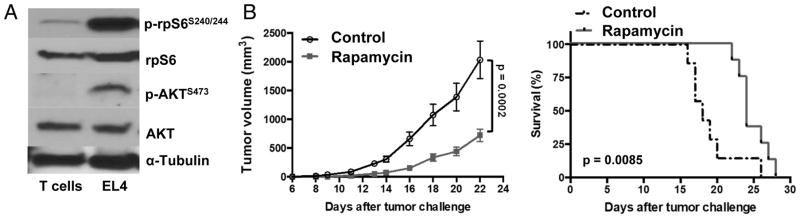
mTOR is comprised of two complexes, mTORC1 and mTORC2, that mediate distinct functions through specific signaling molecules (21). Rapamycin and rapalogues are considered primarily to inhibit mTORC1 (12,22). As activated mTORC1 (increased p-rpS6S240/244) and mTORC2 (increased p-AktS273) was seen in EL4 cells compared with normal spleen T lymphocytes (Fig. 1A), we assessed rapamycin EL4 treatment effects. Typical rapamycin at 8 mg/kg significantly reduced subcutaneous EL4 tumor growth in WT mice, increased survival (Fig. 1B), and protected against intravenous EL4 challenge (Suppl. Fig. 1).
Figure 1. Rapamycin effectively treats EL4 lymphoma in a dose-dependent manner.
(A) Western blot of lysates from CD3+ spleen T cells from naïve mice or in vitro cultured EL4 cells. (B) WT male mice were challenged subcutaneously with 40,000 EL4 cells on day 0. Tumor growth (n=8/group, p value, two-way ANOVA) and survival (p value, log-rank test) of mice treated with rapamycin 8 mg/kg or vehicle control on 5 consecutive days/week for 2 weeks starting on day 4.
IFN-γ, γδ T cells and adaptive immunity are dispensable for typical rapamycin protection against EL4
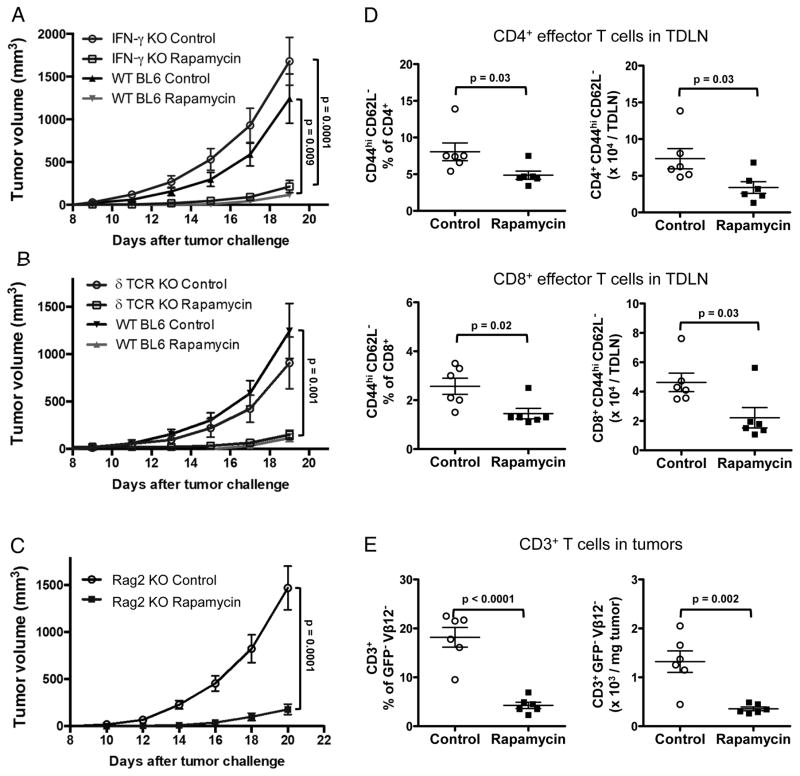
Because rapamycin can improve conventional T cell (12,22–24) and γδ T cell anti-tumor immunity (13), we assessed immune contributions to treatment effects. We challenged syngeneic IFN-γ KO, δ TCR KO (lacking γδ T cells) and Rag2 KO mice (lacking T and B cells) with subcutaneous EL4 and treated with typical rapamycin. Strikingly, in each case typical rapamycin decreased EL4 tumor burden equivalent to WT (Fig. 2A–C), suggesting that its treatment efficacy was independent of tumor-specific immunity. In support, we found that in WT mice, typical rapamycin significantly decreased effector (CD44hiCD62L−) CD4+ and CD8+ T cells in TDLNs (Fig. 2D, Suppl. Fig. 2A) and decreased tumor-infiltrating CD3+ T cells (Fig 2E, Suppl. Fig. 2B).
Figure 2. Rapamycin treats EL4 independent of interferon-γ, γδ T cells or adaptive immunity.
(A–C) IFN-γ KO, δ TCR KO, Rag2 KO and WT male mice (N=8–10/group) were challenged s.c. with 40,000 EL4 cells. Rapamycin 8 mg/kg or vehicle control, starting on day 4, was given on 5 consecutive days/week until the day before sacrifice. P values, two-way ANOVA. WT male mice were treated as above to determine prevalence and numbers of CD44hiCD62L− cells in (D) tumor draining lymph nodes (TDLN) among CD4+ or CD8+ T cells and (E) CD3+ T cells (GFP−Vβ12− cells) within tumors. N= 6/group in D–E. P values, unpaired t test. Error bars, mean ± SEM. KO, knockout.
Typical rapamycin inhibits tumor mTORC1 in vivo and directly inhibits tumor cell proliferation
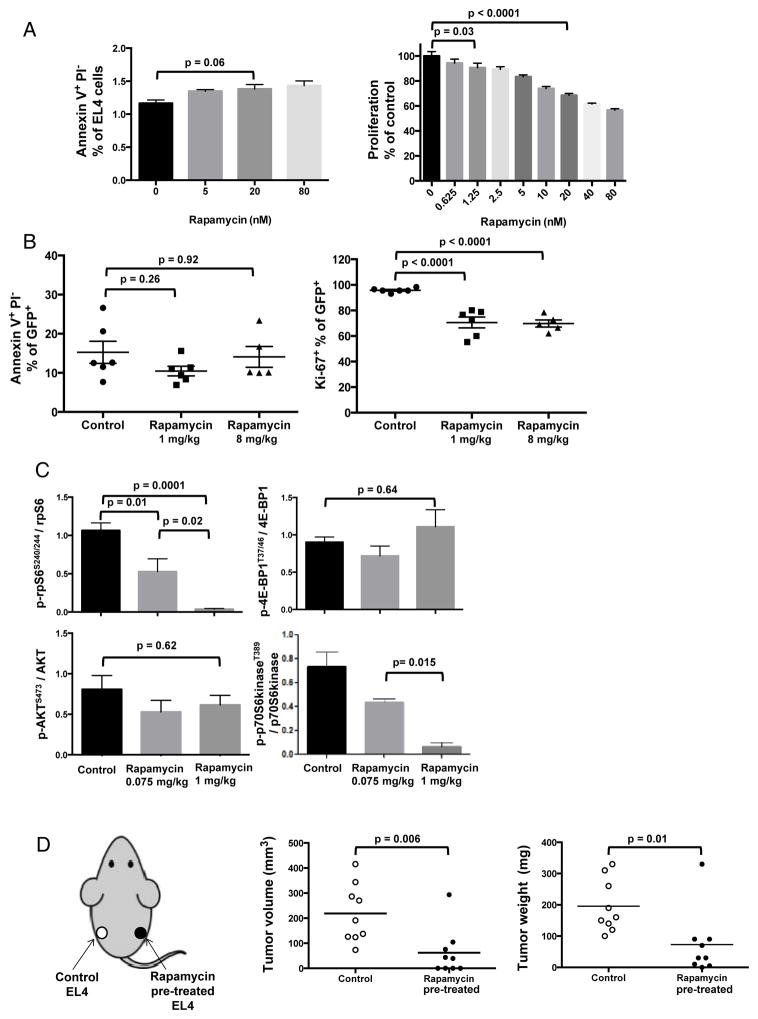
As data suggested direct tumor effects, we tested rapamycin effects in vitro, at concentrations achievable in blood of typical rapamycin-treated mice (25). Rapamycin dose-dependently reduced EL4 tumor cell proliferation without obvious apoptosis induction (<1.5%) in vitro (Fig. 3A). Consistent with in vitro data, typical rapamycin also decreased Ki67+ (proliferating) EL4 tumor cell prevalence without significant apoptosis induction in vivo (Suppl. Fig. 3A, Fig. 3B). Consistent with in vitro results, typical rapamycin (1 mg/kg) significantly reduced tumor mTORC1 (p-rpS6S240/244, p-p70S6kinaseT389) without detectable mTORC2 effect (p-AktS473) in EL4 cells recovered and tested ex vivo. Despite almost complete suppression of some mTORC1 targets, the mTORC1 target p-4E-BP1T37/46 was not affected, suggesting differential effects on specific mTORC1 targets (Fig 3C, Suppl. Fig. 3B). As an additional test of direct tumor effects, we cultured EL4 cells with rapamycin for 2 days in vitro. Treated and untreated tumor cells were challenged into the same mouse on opposite flanks, as depicted on the left in Fig. 3D. Tumors from rapamycin pre-treated cells grew more slowly versus paired control cells (Fig. 3D), confirming that rapamycin anti-T cell lymphoma effects can be directly on tumor cells. Therefore, typical rapamycin suppresses T cell lymphoma mTORC1 signals in vivo and inhibits tumor cell growth in this model through direct tumor cell effects independent of host tumor-specific immunity.
Figure 3. Rapamycin directly inhibits EL4 mTORC1 signals and proliferation.
(A) EL4 cells were treated with rapamycin or vehicle control for 72 h in vitro and stained with propidium iodide (PI) and Annexin V to evaluate apoptosis by flow cytometry. MTT assay was used to assess proliferation. n=6/group. P values, t-test. (B) Apoptosis and Ki67 (proliferation marker) of ex vivo tumor cells from Rag2 KO mice after rapamycin treatment (1 or 8 mg/kg) for 16 days, stained and analyzed by flow cytometry. P values, one-way ANOVA. (C) EL4-challenged WT mice were treated with rapamycin (0.075 or 1 mg/kg) or vehicle control daily from day 4 and sacrificed on day 21 with composite quantification shown from 3 Western blots of tumor lysates. P values, t-test. n=4/group for each blot. Error bars, mean ± SEM. (D) Tumor volumes and weights of EL4 cells pre-treated with rapamycin 100 nM or PBS for 48 h in vitro and then injected (40,000 cells) s.c. into WT mice (1 treated and 1 untreated tumor challenged in one mouse, on opposite flanks, as depicted on the left). N= 10/group. Tumors were measured with calipers and resected for weight one week later. P values, unpaired t test.
Low dose (LD) rapamycin inhibits tumor mTORC1 without clinical benefits in vivo
As typical rapamycin directly inhibited tumor mTORC1 and growth but impaired T cell activation, whereas lower rapamycin doses can improve immunotherapy against EL4 (14), and improves immunity to pathogens (25,26), we assessed if reducing rapamycin could augment clinical efficacy. Rapamycin 2 mg/kg was as clinically effective as 8 mg/kg (Suppl. Fig. 4A). 1 mg/kg rapamycin was less effective but nonetheless significantly decreased EL4 tumor burden (Suppl. Fig. 4B). Rapamycin as low as 0.075 mg/kg (LD) still suppressed tumor mTORC1 (p-rpS6S240/244, p-p70S6kinaseT389) in EL4 cells recovered and tested ex vivo (Fig. 3C), but 0.075 mg/kg or 0.25 mg/kg rapamycin did not delay EL4 growth in vivo (Suppl. Fig. 4B), despite increasing prevalence and numbers of CD8+ T cells with a central memory phenotype (CD44hiCD62L+) in TDLN (Suppl. Fig. 4C).
Regulatory T cell (Treg) depletion with DD treats EL4 lymphoma
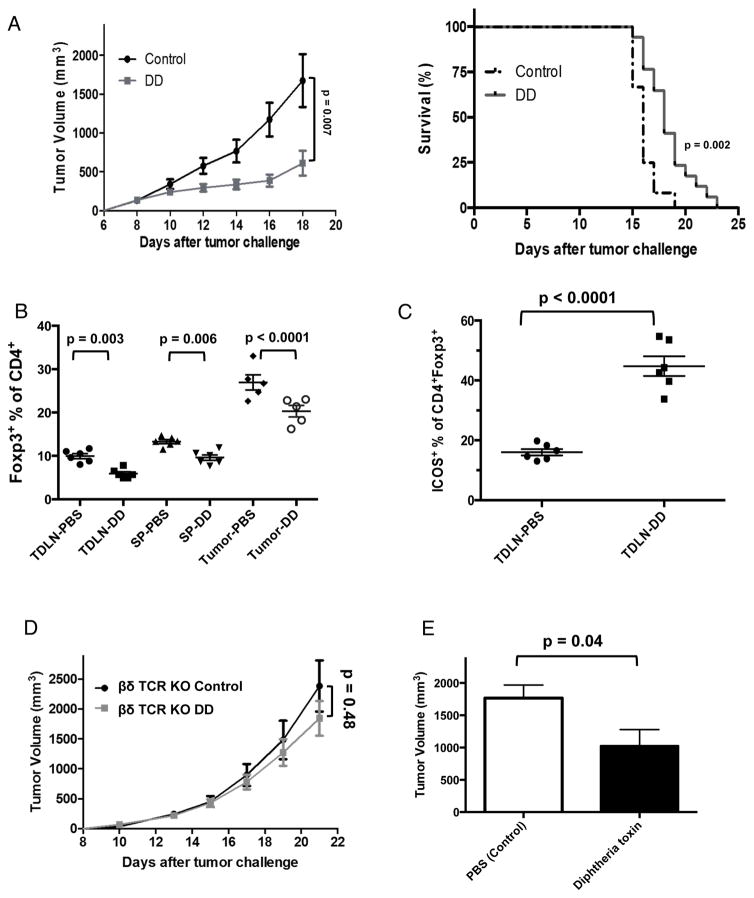
We and others have shown that Treg depletion effectively treats epithelial carcinomas (27,28), including with the fusion immunotoxin denileukin diftitox (DD) (15). DD significantly delayed EL4 tumor growth and increased survival (Fig. 4A). DD reduced CD3+CD4+Foxp3+ Tregs in spleen, TDLNs and tumors as expected (Fig. 4B), but increased Treg ICOS (inducible T cell costimulator) expression (Fig. 4C) associated with increased Treg suppression (29). Adding anti-ICOS or anti-ICOSL antibodies did not improve DD clinical effects (not shown). DD is indicated to treat CD25+ T cell malignancies, thought to be due to direct tumor cytotoxicity (18,30,31), but DD protection was abrogated in βδ TCR KO mice lacking all T cells (Fig. 4D), suggesting that direct tumor cytotoxicity was not an important treatment mechanism, which appeared to be T cell dependent. In support, in EL4-bearing Foxp3DTR mice, in which we can specifically deplete Tregs with diphtheria toxin (15,32), specific Treg depletion significantly reduced tumor growth (Fig. 4E), consistent with Treg depletion as a mechanism for DD treatment effects against EL4.
Figure 4. DD depletes Tregs, slows EL4 growth and improves survival.
(A) Tumor growth and survival of mice treated with DD (5 μg/4 days). P value for tumor growth, two-way ANOVA. Survival data combined from 2 independent experiments, analyzed by log-rank test. N=10–12/group. WT mice were challenged with 50,000 EL4 cells s.c., given 2 doses of DD (5 μg) or PBS on days 4 and 8 and evaluated on day 11 for (B) prevalence of Foxp3+ Tregs from spleen (SP), TDLN and tumors in CD3+ gate after gating out EL4 cells and (C) percentage of ICOS+ Tregs in TDLN. P values, unpaired t test. (D) Tumor growth in βδ TCR KO male mice challenged with 50,000 EL4, treated and analyzed as in (A). (E) Foxp3DTR mice were injected s.c. with 50,000 EL4 cells and treated with diphtheria toxin (1 μg/kg, starting on day 4) every 3 days. Tumor burden on day 15 shown. P value, unpaired t test. Error bars, mean ± SEM. N=10/group.
EL4 is refractory to many other anti-cancer immunotherapies
Given T cell toxicities of typical rapamycin, potentially improved T cell functions with LD rapamycin and reports that LD improves antigen-specific T cell functions (14,25), we asked if LD rapamycin would improve treatment for EL4 in combination with immunotherapies showing promise in epithelial carcinomas (33,34). We thus also assessed EL4 for other candidate tumor immunotherapy targets by flow cytometry and found that EL4 cells expressed low B7-H1 (PD-L1) and high PD-1, but negligible CD25 and CTLA-4 (Suppl. Fig. 5A). Treatments of EL4-bearing WT mice with αPD-L1, αPD-1, αCD25 or αCTLA-4 antibodies at doses that we and others have established to treat epithelial carcinomas (19,35,36) were ineffective in reducing subcutaneous EL4 tumor growth (Suppl. Fig. 5B–C). We therefore focused on Treg depletion as an immunotherapy strategy for further studies.
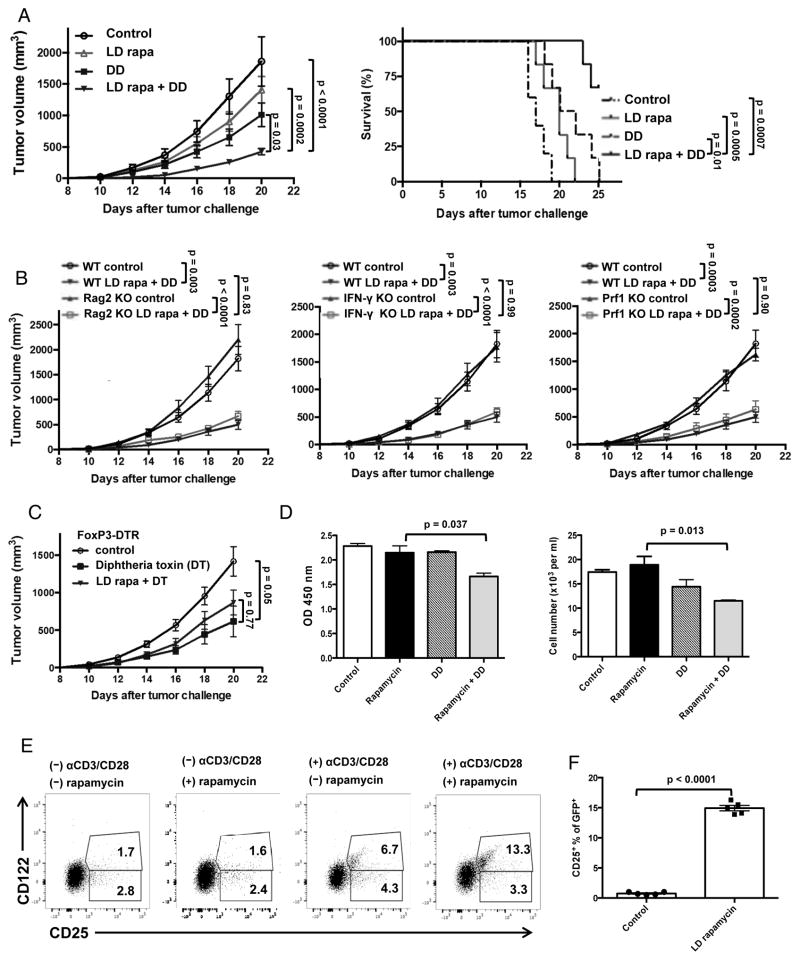
LD rapamycin improves clinical efficacy of DD against EL4 independent of anti-tumor immunity
Given impaired T cell activation and tumor infiltration with typical rapamycin, we expected that it would not improve DD efficacy against EL4, and confirmed that (not shown). However, as Treg depletion improves anti-tumor immunity (15), we hypothesized that LD rapamycin would improve DD treatment effects by improving anti-EL4 immunity, based on LD rapamycin effects in studies of an anti-EL4 vaccine plus anti-CTLA-4 (14). We tested this concept by treating EL4-bearing WT mice with DD, LD rapamycin or both. The DD plus LD rapamycin combination decreased tumor growth and increased survival significantly better than either single agent as predicted (Fig. 5A). To assess expected immune factors contributing to clinical efficacy of this combination, we challenged Rag2 KO (lacking tumor-specific immunity), IFN-γ KO and Prf1 KO mice (lacking perforin, and thus with compromised anti-tumor cytotoxicity) with EL4 and treated with LD rapamycin plus DD. Unexpectedly, the combination of LD rapamycin and DD reduced tumor growth in Rag2 KO, IFN-γ KO and Prf1 KO mice as potently as in syngeneic WT mice (Fig. 5B), suggesting a T cell and anti-tumor immunity independent mechanism. As DD kills IL-2 receptor (IL-2R)+ cells, and not Tregs specifically (15), we attempted to confirm Treg-specific effects by challenging Foxp3DTR mice (genetically engineered so that Tregs are specifically depleted with diphtheria toxin) with EL4. Unexpectedly, though, LD rapamycin did not improve clinical effects of Treg-specific depletion (Fig. 5C), suggesting a Treg-independent effect of the fusion toxin.
Figure 5. LD rapamycin improves DD treatment efficacy independent of anti-EL4 immunity.
Mice challenged with 40,000 EL4 cells on day 0. (A) Tumor growth and survival of WT mice with LD rapamycin (75 μg/kg), DD or both. LD rapamycin administrated daily on days 4–19. DD 5 ug, starting on day 4, given every 4 days until day 16. P values for tumor growth, two-way ANOVA. Survival analyzed by log-rank test. (B) Rag2 KO, IFN-γ KO, and Prf1 KO mice treated and analyzed as in (A). Long bars, comparison between either control. Short bars, comparisons of combo treated groups. (C) Foxp3DTR mice challenged with 40,000 EL4 cells and given diphtheria toxin (DT) 1 μg/kg every 3 days from days 4–16, plus LD rapamycin administrated daily on days 4–19. Tumor growth analyzed by two-way ANOVA. Groups of N=8–10/group in A–C. (D) EL4 cells treated with rapamycin 200 pm, DD 250 ng/ml or both for 5 days. MTT added and OD values measured at 450 nm. Data were confirmed using actual cell counts (Trypan blue). P value, unpaired t test. (E) EL4 cells were incubated with rapamycin 200 pm for 2 days. Cells were then activated with αCD3/CD28 beads ± rapamycin 200 pm for 24 more hours. Representative CD25 and CD122 expression flow cytometry plots of EL4 cells shown. Values in quadrants are percent positive cells in that quadrant. (F) CD25 expression on EL4 tumor cells from LD rapamycin-treated male mice compared with control mice tested ex vivo after 16 days. P value, unpaired t test. Error bars, mean ± SEM.
Rapamycin up-regulates IL-2R expression on EL4 cells in vitro and in vivo
As LD rapamycin unexpectedly did not require anti-tumor immunity to improve DD clinical efficacy, we asked if LD rapamycin could sensitize EL4 to direct DD cytotoxic effects. We cultured EL4 cells in vitro with DD ± rapamycin 200 pg/ml, corresponding to the expected in vivo serum concentration using LD rapamycin (versus ~3–15 ng/ml for typical rapamycin) (22). DD plus rapamycin at 200 pg/ml reduced EL4 cell survival and growth in vitro greater than either DD or rapamycin alone (Fig. 5D), consistent with rapamycin-mediated EL4 sensitization to DD.
The IL-2R consists of CD25 (IL-2Rα chain), CD122 (IL-2Rβ chain) and CD132 (common γ chain). DD acts primarily by binding CD25, but CD122 (and CD132 to a lesser extent) can participate (37). EL4 cells cultured in vitro with rapamycin alone at 200 pg/ml did not significantly alter expression of the IL-2R components CD25 or CD122 (baseline ~4% CD25+ of which about 1/3 co-express CD122). αCD3/αCD28 bead stimulation alone increased CD25+ cells to ~11% (of which about half co-expressed CD122), whereas upon stimulation with αCD3/αCD28 beads, rapamycin increased CD25 expression significantly (11% versus 16.6%, the further increase solely in CD122 co-expressing cells, Fig. 5E). In vivo, LD rapamycin strongly increased CD25 expression on EL4 tumor cells recovered and tested ex vivo (Fig. 5F). However, rapamycin effects on DD-mediated cytotoxicity were not abrogated in vivo by αCD25 antibody blockade alone (Suppl. Fig. 6).
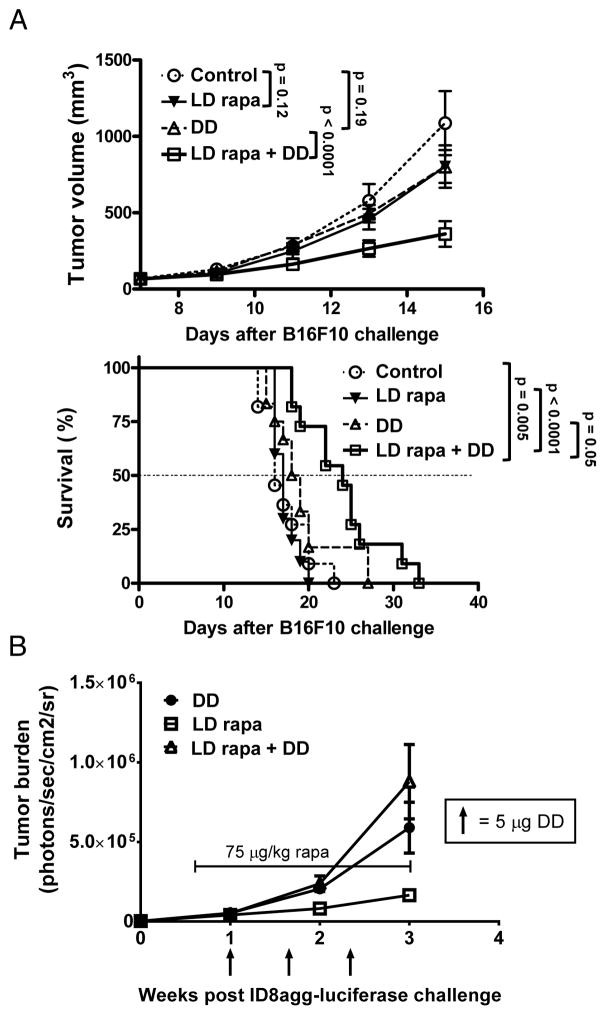
LD rapamycin boosts DD cytotoxicity against B16F10 without augmenting IL-2R expression
To understand if effects were specific to EL4, or hematologic malignancies, we challenged WT mice with B16F10 melanoma cells and found that DD or LD rapamycin alone were not effective, but the combination was significantly superior to either agent alone (Fig. 6A, Suppl. Fig. 7). In vitro, rapamycin also boosted DD-mediated cytotoxicity in a CD25/CD122-dependent fashion in EL4 and B16F10 (Suppl. Fig. 8) consistent with altered CD25 and CD122 expression by flow cytometry in EL-4 (Fig. 5E). B16F10 expression of CD25 or CD122 was minimal and did not increase significantly after in vitro culture in rapamycin and in fact CD25 mean fluorescence intensity was slightly reduced (Suppl. Fig. 9). Because we did not detect significant rapamycin-mediated increase in EL4 IL-2R without activation through the T cell receptor (Fig 5E), we tested in vivo rapamycin effects on IL-2R in B16, as there is no known protocol to activate B16 cells in vitro. Surprisingly, we did not find a significant increase in IL-2R+ B16 prevalence and saw a slight decrease in mean fluorescence intensity (Suppl. Fig. 10) as was seen in vitro (Suppl. Fig. 9A). ID8agg did not express basal or rapamycin-induced IL-2R (Suppl. Fig. 9B) and did not respond in vivo to DD + LD rapamycin (Fig. 6B). Thus, carcinomas can respond to this strategy, although not always with a detectable rapamycin-mediated increase in IL-2R in vivo.
Figure 6. LD rapamycin improves DD treatment efficacy of B16F10 but not ID8agg tumors.
Mice challenged with 500,000 B16F10 cells on both flanks or with 4×106 ID8agg cells i.p. on day 0. (A) Top: B16 tumor growth, and bottom: survival of WT mice with control, LD rapamycin (75 μg/kg), DD or both. LD rapamycin administrated daily on days 7–15. DD (5 ug/mouse), starting on day 7, given every 5 days. P values for tumor growth, two-way ANOVA. Survival was analyzed by log-rank test. N= 10/group. (B) WT mice challenged with ID8agg-luciferase cells plus LD rapamycin (75 μg/kg), DD or both. LD rapamycin administrated daily on days 4–21. DD 5 μg, starting on day 7, given every 5 days until day 17. N=5/group. Arrows indicate DD administrations. Bracketed line encompasses rapamycin treatment dates. Bioluminescence detected following Luciferin injection IV.
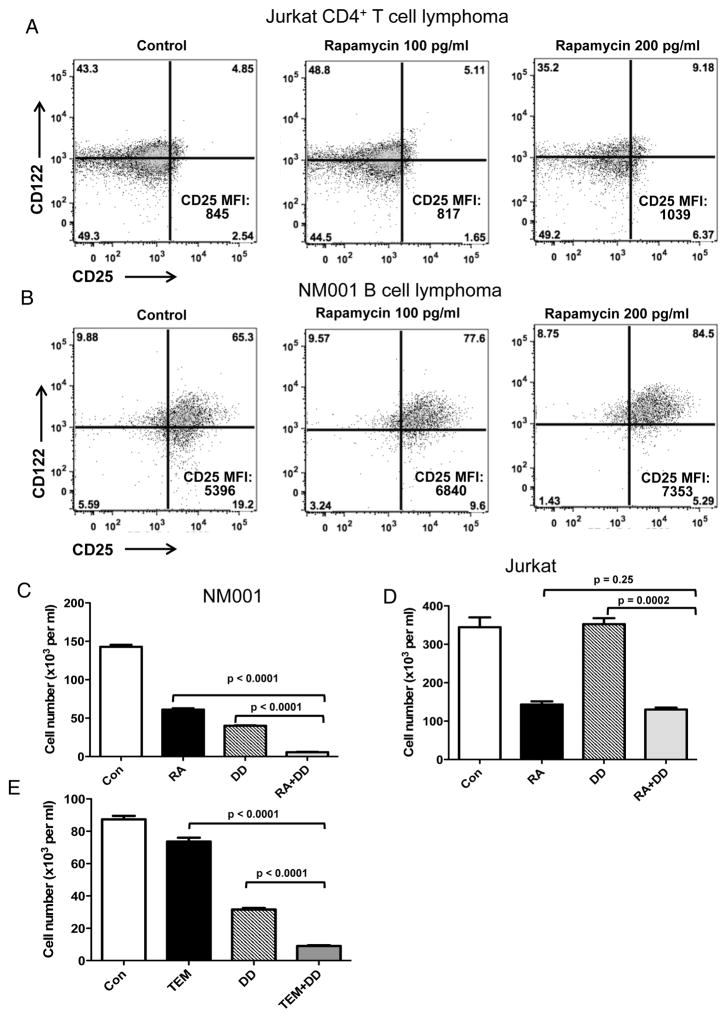
To examine human relevance to hematologic malignancies we found that the human NM001 B cell lymphoma line expressed basal and rapamycin-inducible CD25 and CD122 while the human Jurkat CD4+ T cell lymphoma cell line expressed basal CD122 but minimal CD25 that was significantly inducible in vitro with sufficient rapamycin (Fig. 7A, B). Nonetheless, NM001 B lymphoma was susceptible to rapamycin-augmented DD-mediated cytotoxicity in vitro (Fig. 7C) whereas Jurkat was not (Fig. 7D).
Figure 7. Rapamycin and DD synergize in reducing NM001 B cell lymphoma but not Jurkat CD4+ T cell lymphoma cell numbers in vitro.
Jurkat CD4+ T cell lymphoma (A) and NM001 B lymphoma (B) cell lines were treated in vitro with 0, 100 or 200 pg/ml rapamycin for 48 hrs. Representative flow cytometry dot plots of CD25 and CD122 expression. CD25 mean fluorescence intensity (MFI) of the total live gate indicated in the lower right. NM001 B lymphoma (C) and Jurkat cell lines (D) were cultured for 5 days in vitro in control medium (Con), 100 pg/ml rapamycin (RA), 250 ng/ml (DD) or both and cell numbers counted. (E) NM001 B lymphoma cells were cultured for 5 days in vitro in control medium (Con), 500 pg/ml temsirolimus (TEM), 250 ng/ml (DD) or both. Viable cells were counted. P value, unpaired t test. Error bars, mean ± SEM.
Temsirolimus reproduces some rapamycin effects on CD25 expression and cytotoxicity enhancement in human cancer cells
Rapamycin is FDA-approved to prevent organ allograft rejection but not for cancer treatment (13,38). Temsirolimus is a rapalogue that is FDA-approved to treat renal cell carcinoma (39). To test comparability of temsirolimus, we cultured human B cell lymphoma NM001 cells or Jurkat T lymphoma cells with temsirolimus, DD or both. Temsirolimus did not increase CD25 and CD122 expression on NM001 or Jurkat (Suppl. Fig. 11) but it did improve DD-mediated cytotoxicity against NM001 (Fig. 7E). Antibodies blocking human IL-2 from binding human IL-2R are not reported and thus, IL-2R-related mechanisms could not be specifically assessed in human cells.
Discussion
Rapamycin was originally approved to prevent renal allograft rejection by reducing T cell activation, and thus has been considered immunosuppressive (12,40). However, much recent work now shows that mTOR inhibition can improve T cell functions (12,13,26,40–42), such as enhancing virus- or tumor-specific T cell immunity in mice (25,43). The rapalogue everolimus improved antibody responses to influenza vaccine in elderly humans (40). Rapamycin was recently shown to boost anti-CTLA-4 plus tumor vaccination in this mouse EL4 T cell lymphoma by improving anti-tumor immunity (14).
There is therefore much interest in mTOR inhibition in cancer therapy given successes of immunotherapy and the potential for mTOR inhibition to improve tumor-specific immunity. We undertook these studies to fill the information gap on rapalogue treatment effects against T cell lymphomas, expecting that rapalogues could further augment immunotherapy for them. We also made important insights into rapamycin effects on B cell lymphomas and carcinomas.
We used mouse EL4 as a relevant T cell lymphoma model as it overexpresses mTOR. Over a range of pharmacologically achievable doses, rapamycin suppressed EL4 mTOR and directly inhibited its growth in vitro and in vivo. Nonetheless, these typical rapamycin doses also suppressed T cell activation, which could be detrimental in combination with immunotherapies.
EL4 is highly aggressive, a concept that we extend by showing its refractoriness to many approaches to immune checkpoint blockade as immunotherapy. However, DD was approved to treat T cell lymphomas, and we have shown that it also depletes Tregs in mice (15,16,19) and humans (44), which could useful in immunotherapy. To capitalize on these DD features, we treated EL4-bearing mice with DD and found significant clinical treatment effects that appeared to be T cell dependent. We further showed that typical rapamycin doses did not improve DD treatment effects, which we considered likely due to rapamycin-mediated T cell inhibition. We thus used the LD rapamycin dose shown to augment anti-EL4 immunity in a vaccine plus αCTLA-4 model (14). When we found that LD rapamycin plus DD was better than either individual agent, we expected to show that efficacy was from improved anti-tumor immunity.
Surprisingly, LD rapamycin plus DD effects against EL4 did not require tumor-specific immunity, or interferon-γ or perforin, other important mediators of anti-tumor immunity. We thus considered that rapamycin could sensitize EL4 cells to DD-mediated cytotoxicity. We then showed that rapamycin increased T cell capacity to augment EL4 expression of IL-2R components that bind DD, and confirmed this as a mechanism for augmented EL4 cytotoxicity in vitro. Anti-CD25 antibody alone failed to reverse LD rapamycin + DD effects on EL4 challenge in vivo, which could be due to the need for simultaneous CD122 blockade or effects of anti-CD25 on anti-tumor immune cells, such as Tregs (28), among other considerations.
We further found that LD rapamycin augmented DD treatment of the mouse B16 melanoma line B16F10 but not the ID8agg ovarian carcinoma line in vivo. Mechanistic studies showed that rapamycin improved IL-2R-dependent DD-mediated cytotoxicity against EL4 and B16F10 in vitro, tumors in which we found basal IL-2R expression and in vivo efficacy of the combination. By contrast, ID8agg expressed negligible IL-2R that was not rapamycin-inducible, and rapamycin did not boost DD-mediated cytotoxicity in vitro, or augment in vivo combinatorial clinical efficacy. Nonetheless, IL-2R expression on B16F10 was minimal, and did not increase in vivo. It is possible that the mechanism for B16 treatment effects in vivo could also include altered DD affinity, cellular survival mechanisms or anti-tumor immunity (14). Although rapamycin did not increase IL-2R on unstimulated EL4 cells in vitro, it did in vivo, and after in vitro EL4 T cell receptor activation. Thus, additional in vivo factors could contribute to augmenting IL-2R expression, DD interactions or rapamycin signals that facilitate DD-mediated tumor cytotoxicity in some tumors, an area that requires additional study.
To test human relevance, we assessed human T cell (Jurkat) and B cell (NM001) lymphoma lines. NM001 was basal IL-2R+ and underwent rapamycin-mediated augmentation of IL-2R and DD-mediated cytotoxicity in vitro. Jurkat was IL-2R low, but sufficient rapamycin augmented its IL-2R expression. Nonetheless, rapamycin did not boost DD-mediated cytotoxicity. The common feature for lymphoma or carcinoma cells that responded to the combination of LD rapamycin plus DD was IL-2R expression, not whether rapamycin further augmented IL-2R expression. These data suggest that tumor IL-2R expression is necessary but not sufficient for rapamycin to enhance DD cytotoxicity. Other factors, such as the differing mutational landscapes of distinct tumors (45–47) could also alter treatment effects in ways to be defined.
Since rapamycin is not FDA-approved for cancer, we also tested temsirolimus, which is FDA-approved for renal cell cancer (39). Temsirolimus augmented DD-mediated cytotoxicity against NM001. Nonetheless, neither rapamycin nor temsirolimus significantly augmented NM001 IL-2R at concentrations that significantly augmented DD-mediated cytotoxicity. This discordance could be due to altered signaling by DD or rapalogues in combination, or rapalogue-mediated increased DD affinity or alterations in tumor metabolism or survival mechanisms that increase susceptibility to cytotoxicity, in addition to effects on anti-tumor immunity, areas of current investigations.
In summary, we define novel rapalogue effects that could be useful to treat T cell hematologic malignancies, our original study goal, that also appear to be useful in selected B cell malignancies and carcinomas. Our proof-of-concept work shows novel rapalogue effects including augmentation of DD-mediated tumor killing and clinical efficacy. These effects are manifest at rapamycin doses significantly lower than those currently used clinically, suggesting that improved clinical outcomes with reduced toxicities are possible. Agents are FDA-approved and can be easily tested clinically. Assessments of whether LD rapamycin sensitizes against other cytotoxic agents, and whether it can augment specific immunotherapies as we suggest, and assessment of mTOR inhibitors with distinct mechanisms of action all merit additional attention.
Supplementary Material
Acknowledgments
Financial support: Yang Liu (Xiangya School of Medicine); Vinh Dao (AG038048, TL1TR001119, CA180377); Tyler Curiel (CA170491, CA54174, The Holly Beach Public Library, The Owens Foundation, The Barker Foundation, The Skinner endowment).
Thanks to Wanjao Chen for technical help. Studies were funded by The Xiangya School of Medicine, The Holly Beach Public Library Association, 5P30CA54174, AG038048, TL1TR001119, CA180377, Texas STARS, the Owens Foundation, the Barker Foundation and the Skinner Endowment.
Footnotes
The authors have no relevant financial conflicts of interest to declare.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Mark A, Hajdu M, Varadi Z, Sticz TB, Nagy N, Csomor J, et al. Characteristic mTOR activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease--a combined tissue microarray, in vitro and in vivo study. BMC cancer. 2013;13:250. doi: 10.1186/1471-2407-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witzig TE, Reeder C, Han JJ, LaPlant B, Stenson M, Tun HW, et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood. 2015;126:328–35. doi: 10.1182/blood-2015-02-629543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–80. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 6.Majchrzak A, Witkowska M, Smolewski P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B-cell lymphoma: current knowledge and clinical significance. Molecules. 2014;19:14304–15. doi: 10.3390/molecules190914304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia. 2010;24:13–21. doi: 10.1038/leu.2009.223. [DOI] [PubMed] [Google Scholar]
- 8.Evangelisti C, Ricci F, Tazzari P, Tabellini G, Battistelli M, Falcieri E, et al. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia. 2011;25:781–91. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Karim IA, Giles FJ. Mammalian target of rapamycin as a target in hematological malignancies. Curr Probl Cancer. 2008;32:161–77. doi: 10.1016/j.currproblcancer.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3822–9. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 11.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dao V, Liu Y, Pandeswara S, Svatek RS, Gelfond JA, Liu A, et al. Immune stimulatory effects of rapamycin are mediated by stimulation of antitumor γδ T cells. Cancer Res. doi: 10.1158/0008-5472.CAN-16-0091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedicord VA, Cross JR, Montalvo-Ortiz W, Miller ML, Allison JP. Friends not foes: CTLA-4 blockade and mTOR inhibition cooperate during CD8+ T cell priming to promote memory formation and metabolic readiness. J Immunol. 2015;194:2089–98. doi: 10.4049/jimmunol.1402390. [DOI] [PubMed] [Google Scholar]
- 15.Hurez V, Daniel BJ, Sun L, Liu AJ, Ludwig SM, Kious MJ, et al. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res. 2012;72:2089–99. doi: 10.1158/0008-5472.CAN-11-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purner MB, Berens RL, Krug EC, Curiel TJ. Epstein-Barr virus-transformed B cells, a potentially convenient source of autologous antigen-presenting cells for the propagation of certain human cytotoxic T lymphocytes. Clin Diagn Lab Immunol. 1994;1:696–700. doi: 10.1128/cdli.1.6.696-700.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 19.Lin PY, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, et al. B7-H1-Dependent Sex-Related Differences in Tumor Immunity and Immunotherapy Responses. J Immunol. 2010;185:2747–53. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10519–24. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dao V, Pandeswara S, Liu Y, Hurez V, Dodds S, Callaway D, et al. Prevention of carcinogen and inflammation-induced dermal cancer by oral rapamycin includes reducing genetic damage. Cancer Prev Res (Phila) 2015;8:400–9. doi: 10.1158/1940-6207.CAPR-14-0313-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–52. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14:1266–76. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menetrier-Caux C, Curiel T, Faget J, Manuel M, Caux C, Zou W. Targeting regulatory T cells. Target Oncol. 2012;7:15–28. doi: 10.1007/s11523-012-0208-y. [DOI] [PubMed] [Google Scholar]
- 28.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–6. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foss FM. DAB(389)IL-2 (denileukin diftitox, ONTAK): a new fusion protein technology. Clin Lymphoma. 2000;1(Suppl 1):S27–31. [PubMed] [Google Scholar]
- 31.Kaminetzky D, Hymes KB. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics: targets & therapy. 2008;2:717–24. doi: 10.2147/btt.s3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Hurez VJ, Thibodeaux SR, Kious MJ, Liu A, Lin P, et al. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012;11:509–19. doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 33.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drerup JM, Liu Y, Padron AS, Murthy K, Hurez V, Zhang B, et al. Immunotherapy for ovarian cancer. Curr Treat Options Oncol. 2015;16:317. doi: 10.1007/s11864-014-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, et al. Denileukin diftitox reduces EL4 lymphoma growth by depleting regulatory T cells, which can be improved by αICOS. Journal of immunology. 2015;194(1 Supplement):213.7. [Google Scholar]
- 36.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 37.Foss F. Clinical experience with denileukin diftitox (ONTAK) Semin Oncol. 2006;33(1 Suppl 3):S11–6. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–56. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortolani S, Ciccarese C, Cingarlini S, Tortora G, Massari F. Suppression of mTOR pathway in solid tumors: lessons learned from clinical experience in renal cell carcinoma and neuroendocrine tumors and new perspectives. Future Oncol. 2015;11:1809–28. doi: 10.2217/fon.15.81. [DOI] [PubMed] [Google Scholar]
- 40.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 41.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–90. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. The Journal of clinical investigation. 2015;125:2090–108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diken M, Kreiter S, Vascotto F, Selmi A, Attig S, Diekmann J, et al. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer immunology research. 2013;1(6):386–92. doi: 10.1158/2326-6066.CIR-13-0046. [DOI] [PubMed] [Google Scholar]
- 44.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–77. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 45.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2009;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.