Abstract
Transforming growth factor β (TGF-β) and structurally related factors use several intracellular signaling pathways in addition to Smad signaling to regulate a wide array of cellular functions. These non-Smad signaling pathways are activated directly by ligand-occupied receptors to reinforce, attenuate, or otherwise modulate downstream cellular responses. This review summarizes the current knowledge of the mechanisms by which non-Smad signaling pathways are directly activated in response to ligand binding, how activation of these pathways impinges on Smads and non-Smad targets, and how final cellular responses are affected in response to these noncanonical signaling modes.
Several non-Smad signaling pathways (e.g., MAPK) can be activated by TGF-β family ligands. Advances in understanding the mechanisms of activation and cellular responses to these pathways have recently been made.
Transforming growth factor β (TGF-β) and structurally related polypeptide growth factors, including bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), activins, nodal, and Müllerian inhibitory substance, have a diverse array of regulatory functions ranging from specifying tissue pattern formation as morphogens during embryonic development to maintaining physiological homeostasis as cytokines in adult organisms (Wu and Hill 2009; Massagué 2012). The functions of the specific factors within the TGF-β family vary and can even be opposing in different cells and at different developmental stages. A frequently noted example of the context-dependent roles is the dichotomy of TGF-β’s roles in tumorigenesis. TGF-β is a tumor suppressor for early-stage tumors and a potent growth inhibitor of cells of epithelial origin, but, in advanced stage of cancers, promotes tumor growth and progression by inducing epithelial-to-mesenchymal transition (EMT) and subsequent tumor invasion and metastasis (Massagué 2012; Katsuno et al. 2013). Understanding the diversity of the signaling activities of the TGF-β family has been a herculean task and a rich ground of important discoveries. Intense studies across different fields over the past three decades have revealed that all TGF-β-related ligands bind a heteromeric complex of type I and type II transmembrane receptors, each equipped with an intracellular kinase domain (Shi and Massagué 2003; Feng and Derynck 2005). In the ligand-bound receptor complex, the type II receptor kinases phosphorylate and thereby activate the type I receptors, which are also known as activin receptor–like kinases (ALKs). Downstream from this focal complex, the main conduit for ligand-initiated signaling events is the Smad family of transcription factors, among which Smad2 and Smad3 are activated by type I receptors for TGF-β, activin, or nodal, for example, ActRIB (ALK-4), TβRI (ALK-5), and ALK-7; whereas Smad1, Smad5, and Smad8 are activated by type I receptors of BMP signaling, for example, BMPRIA (ALK-3) and BMPRIB (ALK-6), or by ALK-1 and ALK-2, two type I receptors that mediate both TGF-β and BMP signaling. These receptor-activated Smads (R-Smads) then form a trimeric complex with Smad4, which acts as a common Smad to all ligand-activated Smad pathways, and accumulate in the nucleus to regulate target gene expression. Inhibitory Smads (I-Smads), that is, Smad6 and Smad7 in vertebrates, play a crucial role in repressing Smad-mediated signaling responses. Against this backdrop of Smad-mediated, canonical TGF-β signaling mechanisms, the activated receptors also signal through other signal transducers, for example, the mitogen-activated protein kinase (MAPK) pathways, including the extracellular signal-regulated kinases (Erks), c-Jun amino terminal kinase (JNK), p38 MAPK, as well as the IκB kinase (IKK), phosphatidylinositol-3 kinase (PI3K) and Akt, and Rho family GTPases, which in the context of TGF-β family signaling are collectively known as non-Smad signaling pathways (Moustakas and Heldin 2005; Zhang 2009). These receptor-activated, non-Smad transducers mediate signaling responses either as stand-alone pathways or in conjunction with Smads, and they converge onto Smads to control Smad activities. This review summarizes our current knowledge of the mechanisms by which the non-Smad signaling pathways are directly activated in response to ligand binding, how activation of these pathways impinges on Smads and non-Smad targets, and the final outcomes of cellular responses to these noncanonical signaling pathways. We aim to provide a comprehensive view that incorporates all data reported. Although we focus the discussion mostly on those pathways activated by TGF-β, the paradigms presented may extend to signaling by other ligands of the TGF-β family.
ACTIVATION OF ERK MAPK BY AND TYROSINE PHOSPHORYLATION OF TGF-β FAMILY RECEPTORS
Already, before the discovery of Smads, Erk MAPKs were thought to play a role in TGF-β signaling because it was shown that TGF-β causes a rapid activation of Ras in normal epithelial as well as colon carcinoma cells (Mulder and Morris 1992; Yan et al. 1994). Activation of Erk1/2 by TGF-β was shown to occur within 5–10 min of ligand treatment, a time frame that is comparable to the kinetics shown by mitogenic growth factors, such as epidermal growth factor (EGF), albeit with a lower intensity (Olsson et al. 2001). Rapid activation of Ras and/or Erk1/2 by BMP was also observed in myoblasts, osteoblasts, stem cells, endothelial cells, and cancer cells (Gallea et al. 2001; Lai and Cheng 2002; Zhou et al. 2007; Le Page et al. 2009). The TGF-β- or BMP-induced activation of Erk1/2 is dependent on the cell type and culture conditions, and caution must be taken in observing Erk activation because Erk activation in response to growth factors in the serum, cell–cell contacts, cell interactions with extracellular matrix, or oncogenic activation in cancer cells could easily obscure the much lower Erk1/2 activation in response to TGF-β or BMP. Interestingly, BMP-induced activation of Erk1/2 in osteoblasts and embryonic stem cells is usually followed by repression of Erk activation (Kua et al. 2012; Li et al. 2012). In some other cells, TGF-β or BMP induces a delayed Erk activation, typically with peak phosphorylation after several hours rather than minutes, implying an indirect mechanism that requires de novo protein synthesis (Simeone et al. 2001). Smad-mediated transcription may play roles in both the delayed activation of Erk1/2 and the inhibition of Erk activity (Javelaud and Mauviel 2005; Li et al. 2012).
Akin to the tyrosine receptor signaling, the TGF-β-induced GTP loading on Ras could lead to recruitment of the proto-oncogene product Raf to the plasma membrane, resulting in activation of Erk1/2 through MEKs. Well known as serine-threonine kinases, both type I and type II TGF-β receptors also phosphorylate on tyrosine and are tyrosine phosphorylated. Three tyrosines of TβRII, Tyr259, Tyr336, and Tyr424, were shown to be autophosphorylated, albeit at a much lower level than the autophosphorylation of TβRII on serines and threonines (Lawler et al. 1997). Tyrosine phosphorylation of TβRII may lead to recruitment of Src homology 2 (SH2) domain–containing (Shc) proteins by analogy to signaling events elicited by receptor tyrosine kinase (RTK) signaling (Schlessinger 2000; McKay and Morrison 2007). In support of this view, Src was shown to phosphorylate TβRII on Tyr284, which serves as a docking site for the recruitment of growth factor receptor-bound protein 2 (Grb2) and Shc, thus linking TβRII to Ras and Erk activation (Galliher and Schiemann 2007). Interestingly, high levels of ectopic TβRII expression preferentially activate Erk1/2 in dermal cells, whereas high levels of ectopic TβRI expression channel the signaling toward the Smad pathway in epidermal cells (Bandyopadhyay et al. 2011), contrasting the difference in substrate preference between type I and type II receptors. Additionally, TβRI was phosphorylated on tyrosine after TGF-β treatment (Lee et al. 2007). Because TβRI is activated by TβRII on ligand binding and forms a tetrameric receptor complex with TβRII, it is not clear whether the tyrosine phosphorylation results from autophosphorylation or phosphorylation in trans by TβRII. Nevertheless, the activated TβRI recruits and phosphorylates ShcA on tyrosine and serine residues, and ShcA tyrosine phosphorylation then promotes the formation of ShcA-Grb2-Sos complexes that lead to activation of Ras at the plasma membrane (Lee et al. 2007). The kinase activities of both TβRI and TβRII are required for ShcA phosphorylation, and overexpressed ShcA mutants lacking either its phosphotyrosine binding (PTB) or its SH2 domain blocked the Erk1/2 activation by TGF-β (Lee et al. 2007). Therefore, recruitment of signaling mediators as a result of receptor tyrosine phosphorylation is one of the mechanisms that enables TGF-β to activate non-Smad signaling (Fig. 1). With this scenario, both TβRI and TβRII should be seen as dual-specific kinase receptors that accommodate the less recognized roles resulting from tyrosine phosphorylation.
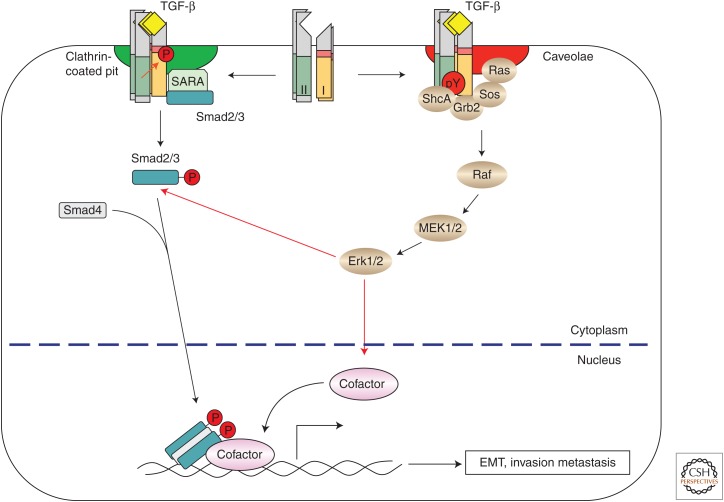
Figure 1.
Transforming growth factor β (TGF-β)-induced activation of the extracellular signal-regulated kinase (Erk) mitogen-activated protein kinase (MAPK) pathway. On binding of TGF-β to its receptor complex, the constitutively active type II receptors phosphorylate the type I receptors at Ser/Thr residues, and induce tyrosine phosphorylation of both the type I and II receptors and ShcA. The phosphorylated tyrosines then recruit Grb2/Sos to activate Erk1/2 MAPK through activation of Ras, Raf, and MEK1/2 in a Smad-independent manner. ShcA directs the TGF-β receptors to caveolin-1-containing lipid raft for Erk MAPK activation. Clathrin-dependent internalization of TGF-β receptors into the Smad anchor for receptor activation (SARA)-containing early endosome is required for Smad activation. Activated Erk MAPK contributes to epithelial-to-mesenchymal transition (EMT) by phosphorylating targeted transcription factors, which in turn control transcription of EMT-related genes without or in cooperation with activated Smad complexes. Erk MAPK also directly phosphorylates R-Smads, thus controlling their activity.
It is not known whether the BMPRII receptor is tyrosine phosphorylated, although c-Src was shown to interact with a carboxy terminal sequence of BMPRII (Wong et al. 2005). The BMPRIA receptor is phosphorylated on tyrosine residues by c-Abl, a non-RTK (Kua et al. 2012). However, c-Abl-mediated tyrosine phosphorylation of BMPRI inhibits BMP-induced Erk activation, and enhances Smad1 and Smad5 activity (Kua et al. 2012). Although the role of tyrosine phosphorylation of BMP receptors remains elusive, Shc is required for BMP-induced Erk activation. BMP-4 stimulation induces rapid dissociation of β3-integrin from BMPRIA and BMPRIB, and association of β3-integrin with focal adhesion kinase (FAK) and Shc, and ultimately, Erk activation (Chang et al. 2009).
A key biological function of TGF-β is the induction of EMT, which normally occurs in embryonic development and is pathologically associated with tumor invasion and dissemination and fibrosis (Moustakas and Heldin 2012; Lamouille et al. 2014). During EMT, cells lose epithelial characteristics and acquire properties of mesenchymal cells, including down-regulation of adherens junctions and associated proteins (e.g., E-cadherin), increased matrix metalloproteinase (MMP) activity, induction of actin stress fibers, and acquisition of motile and invasive properties. In advanced tumors, TGF-β promotes tumorigenesis by inducing EMT through a combination of Smad-dependent and -independent mechanisms. Erk activation was shown to be essential in TGF-β-induced EMT, and is required for disassembly of adherens junctions and cell motility (Zavadil et al. 2001; Xie et al. 2004). Target genes that mediate Erk’s role in TGF-β-induced EMT have been identified (Zavadil et al. 2001), and their functions relate to remodeling integrin-based cell–matrix interactions, and promoting endocytosis, as well as cell motility.
Like TGF-β, BMPs can also induce EMT, cell migration, and invasion in certain cancer cells. Erk activity has been shown to contribute to BMP-7-mediated morphologic conversion in prostate cancer cells (Lim et al. 2011). Blocking Erk activity using chemical inhibitors significantly inhibits BMP-2-induced motility and invasiveness in lung and gastric cancer cells (Hsu et al. 2011; Kang et al. 2011). In addition, Erk activity was shown to be essential for BMP-induced cell differentiation by regulating BMP target genes, such as alkaline phosphatase (ALP), collagen 1, fibronectin, osteopontin, osteocalcin, and Runx2 (Gallea et al. 2001; Lai and Cheng 2002).
One of the mechanisms by which Erk regulates the TGF-β- and BMP-induced biological functions is through phosphorylation of its substrates, such as AP-1 family members, p53, and other transcription factors (Yoon and Seger 2006). Many of these Erk-regulated transcription factors interact and cooperate with Smads to regulate gene expression. In addition, Erk can also regulate the activities of R-Smads, including Smad1, Smad2, and Smad3, through direct phosphorylation (Kretzschmar et al. 1997, 1999; Funaba et al. 2002). In cell culture systems, phosphorylation of Smads by Erk often inhibits the transcription activity of R-Smads, and this regulation has been invoked to explain how oncogenic Ras overrides TGF-β-mediated growth arrest in cancer cells (Kretzschmar et al. 1999). However, others reached different conclusions, in part based on differences in cell type and the response examined (Lehmann et al. 2000; Javelaud and Mauviel 2005). Furthermore, ShcA, although critical for TGF-β-induced Erk activation, can also repress Smad activation by competing with Smad2 or Smad3 for TβRI binding and directing TβRI to caveolin-1-containing lipid raft, a membrane domain that differs from the early endocytic compartment in which Smads are activated (Muthusamy et al. 2015).
ACTIVATION OF TGF-β-ACTIVATED KINASE 1 (TAK1) AND DOWNSTREAM JNK, P38 MAPK, AND IKK
Ligand binding also induces TGF-β receptors to activate the JNK and p38 MAPK signaling pathways (Fig. 2). These two MAPKs are specifically activated by MAP kinase kinases (MKKs) MKK4 or MKK3/6, respectively, in response to cytokines and environmental stress (Weston and Davis 2007). Similarly to cytokine stimulation, TGF-β induces activation of JNK through MKK4 (Frey and Mulder 1997; Engel et al. 1999; Hocevar et al. 1999) and p38 MAPK through MKK3 or MKK6 in various cell lines (Hanafusa et al. 1999; Sano et al. 1999; Bhowmick et al. 2001b; Yu et al. 2002). Depending on cell contexts, the activation of JNK and p38 MAPK by TGF-β or BMP can be either rapid or relatively delayed. The delayed activation usually requires Smad signaling, for example, resulting from Smad-mediated transcription activation of Gadd45β, an upstream activator of MKK4, as seen in pancreatic cancer cells, hepatocytes, and osteoblasts (Takekawa et al. 2002). In contrast, rapid and direct transient activation of JNK and p38 MAPK, independent of Smad activation, is revealed in Smad3−/− cells, Smad4−/− cells, and cells that express a dominant-negative form of Smad3 (Engel et al. 1999; Hocevar et al. 1999). Smad-independent activation of JNK and p38 MAPK is also observed using a mutant TβRI receptor with an altered L45 loop, which renders the receptor defective in Smad binding and phosphorylation, but still retains intact kinase activity (Yu et al. 2002; Itoh et al. 2003).
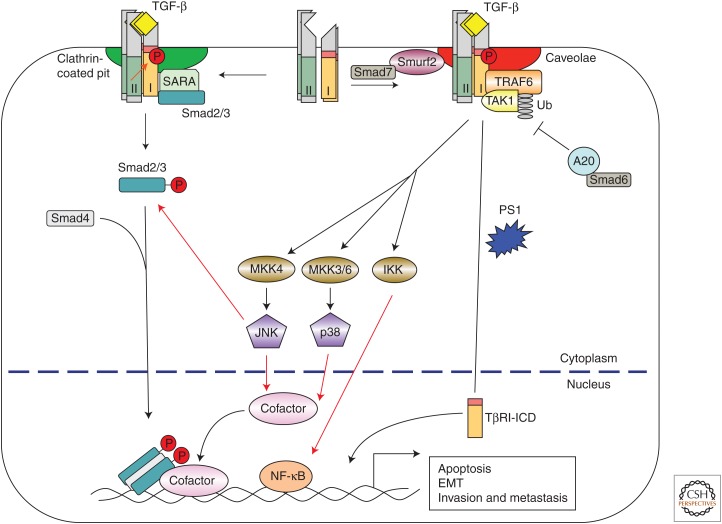
Figure 2.
Transforming growth factor β (TGF-β)-induced activation of c-Jun amino terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) signaling, and the IκB kinase (IKK) pathway. Ligand-bound TGF-β receptors interact with TRAF6 and TGF-β-activated kinase 1 (TAK1), and induce Lys63-linked polyubiquitylation of TRAF6, which then activates TAK1 and downstream kinases, such as JNK, p38 MAPK, and IKK. Smad6 inhibits the TGF-β-induced JNK and p38 MAPK activation by recruiting a deubiquitylase A20 to deubiquitylate TRAF6, whereas Smad7 promotes TGF-β-induced JNK and p38 MAPK activation by directing the TGF-β receptors to caveolin-1-containing lipid rafts through interaction with Smurf2. Activated JNK and p38 MAPK then phosphorylate their targeted transcription factors, and IKK phosphorylates nuclear factor-κB (NF-κB), and these transcription factors cooperate with activated Smads to regulate apoptosis and epithelial-to-mesenchymal transition (EMT). In addition, JNK directly phosphorylates receptor-activated Smad (R-Smad) to regulate Smad activity. TRAF6 can also stimulate proteolytic cleavage of TβRI dependent on presenillin 1 and Lys63 ubiquitylation of TRAF6 to generate the intracellular domain (ICD) of TβRI, which then translocates into the nucleus to regulate cell invasion. SARA, Smad anchor for receptor activation; MKK, MAP kinase kinase; TRAF6, tumor necrosis factor receptor–associated factor 6.
MAP kinase kinase kinases (MAPKKKs) act upstream of and directly activate MKK3/6 and MKK4. Among these, the MAPKKK named TAK1 was identified using a mouse cDNA screen, based on its ability to substitute a latent MAPKKK of Saccharomyces cerevisiae in the yeast-mating pheromone response, and found to be capable of activating TGF-β signaling and to be activated in response to TGF-β (Yamaguchi et al. 1995). In Xenopus, TAK1 is required for BMP-induced mesoderm induction and patterning during embryonic development (Shibuya et al. 1998), whereas in mice, TAK1 is required for the proper development of the vasculature in both embryo and yolk sac, a process that also depends on the type I receptor ALK-1 and the type III receptor endoglin (Shim et al. 2005; Jadrich et al. 2006). The requirement of TAK1 for TGF-β-induced JNK and NF-κB activation was also shown in Tak1-deficient mouse embryonic fibroblasts (MEFs) (Shim et al. 2005).
In contrast to the activation of Smad and Erk pathways by phosphorylation, TGF-β receptors enlist an elaborate mechanism that centers on ubiquitylation by tumor necrosis factor receptor-associated factor 6 (TRAF6), a RING-domain E3 ligase, to activate this branch of non-Smad signaling. Known to control the activation of TAK1 that is induced by the interleukin-1 receptor (IL-1R) and Toll-like receptors (TLRs) (Wu and Arron 2003), TRAF6 was also found to mediate the TGF-β-induced activation of TAK1 and, subsequently, JNK and p38 MAPK (Sorrentino et al. 2008; Yamashita et al. 2008). Mammalian genomes encode six TRAF proteins, identified by a conserved carboxy-terminal TRAF domain, a RING E3 ligase domain, and several Zn fingers in the variable amino terminus (Inoue et al. 2007). Analogous to the mechanism associated with IL-1R or TLR receptor signaling, TRAF6 associates with the activated type II and type I TGF-β receptor complex through its TRAF domain. This binding activates the E3 ligase activity of the RING domain and leads to intramolecular polyubiquitylation of TRAF6 at Lys63 (Yamashita et al. 2008). Unlike ubiquitylation at Lys48, which targets the protein for proteasomal degradation, polyubiquitylation of TRAF6 at Lys63 provides a scaffold for the assembly and activation of protein kinase complexes (Haglund and Dikic 2005). Polyubiquitylated TRAF6 thus recruits TAK1 through association and induces TAK1 activation, allowing TAK1 to activate JNK and p38 MAPK (Fig. 2) (Wang et al. 2001). Recent studies showed that TRAF4 also interacts with TβRI, resulting in Lys63-linked polyubiquitylation of TRAF4 and subsequent activation of TAK1 (Zhang et al. 2013). It will be interesting to determine whether TRAF4 and TRAF6 function in a common complex to activate TAK1, and whether TRAF6 and TRAF4 are required for BMP-mediated TAK1 activation. In addition, other MAPKKKs, such as MEKK1, MEKK4, MLK2, MLK3, and ASK1, were also reported to be involved in TGF-β-mediated activation of JNK or p38 MAPK (Atfi et al. 1997; Brown et al. 1999; Zhang et al. 2003; Kim et al. 2004; Sapkota 2013). It remains to be determined whether they can be recruited to TGF-β receptors in the same manner as the TAK1.
Although independent from R-Smad activation, the activation of the JNK and p38 MAPK through TAK1 by TGF-β receptors is regulated by the inhibitory Smad6 and Smad7. TGF-β and BMP induce expression of Smad6 and Smad7, which in turn inhibit canonical, Smad-dependent TGF-β/BMP signaling (Afrakhte et al. 1998; Ishida et al. 2000; Miyazono 2008). Smad6 and Smad7 also inhibit BMP-induced TAK1 and p38 MAPK activation, possibly through interaction with TAK1 or the TAK1-binding protein, TAB1 (Kimura et al. 2000; Yanagisawa et al. 2001). Smad6 also inhibits the TGF-β-induced activation of TAK1, JNK, and p38 MAPK. This occurs through a different mechanism in which Smad6 recruits the deubiquitylase A20 to TRAF6, thus blocking Lys63-linked polyubiquitylation of TRAF6 (Jung et al. 2013). In contrast, Smad7 was shown to facilitate the TGF-β-induced activation of JNK and p38 MAPK (Mazars et al. 2001; Edlund et al. 2003). The possible function of Smad7 as a scaffold to support the interactions among TAK1, MKK3, and p38 MAPK in TGF-β signaling (Edlund et al. 2003) does not explain how the interaction of Smad7 and TAK1 in BMP signaling inhibits p38 MAPK activation. Additionally, Smad7 may act as an adaptor that bridges TβRI to Smurf2, an HECT-domain E3 ligase, thereby routing TβRI to caveolin-1-containing lipid rafts for turnover by lysosomes (Kavsak et al. 2000; Ebisawa et al. 2001; Di Guglielmo et al. 2003). Endogenous TRAF6 is substantially associated with lipid rafts (Ha et al. 2003a), and sequestration of TRAF6 in the lipid rafts was shown to be required for TRAF6-mediated activation of NF-κB in response to TLR activation or IL-1β signaling (Ha et al. 2003b; Soong et al. 2004; Oakley et al. 2009). It is, therefore, possible that Smad7 enhances TGF-β-induced activation of JNK and p38 MAPK by routing the TGF-β receptors to lipid rafts where TRAF6 is localized. Indeed, TβRI localization in lipid rafts is essential for TGF-β-mediated activation of Erk, JNK, and p38 MAPK (Zuo and Chen 2009; Shapira et al. 2014). The localization of BMP receptors in distinct plasma membrane domains was also shown to have a major impact on BMP signaling specificity (Hartung et al. 2006). In contrast to TGF-β signaling, association of lipid raft with BMP receptors is important for canonical BMP-induced R-Smad phosphorylation but not p38 MAPK activation (Zhou et al. 2010). This could explain why Smad7 enhances TGF-β- but inhibits BMP-induced p38 MAPK activation.
One of the important consequences of JNK or p38 MAPK activation is apoptosis, which is recognized as an activity of BMP in development and a mechanism of tumor suppression by TGF-β. Consistent with this notion, overexpression of TAK1 causes cells or Xenopus embryos to undergo apoptosis, whereas cells that ectopically express a kinase-inactive mutant TAK1 are protected from TGF-β- or BMP-induced apoptosis (Shibuya et al. 1998; Kimura et al. 2000; Edlund et al. 2003). Moreover, silencing the expression of TRAF6 using siRNAs or treating the cells with a pharmacological inhibitor of p38 MAPK efficiently blocks TGF-β-induced apoptosis (Yu et al. 2002; Sorrentino et al. 2008; Yamashita et al. 2008; Jung et al. 2013). Because Smad3 also plays an essential role in the proapoptotic function of TGF-β (Yamamura et al. 2000; Jang et al. 2002; Valderrama-Carvajal et al. 2002; Yang et al. 2006), therefore, the JNK and p38 MAPK pathway cooperates with the Smad pathway in promoting apoptosis.
Because TAK1 is capable of activating IKKs, which in turn activates NF-κB (Takaesu et al. 2003), TGF-β and BMPs may cross talk with the NF-κB pathway. Indeed, activation of TAK1 by TGF-β has been linked to activation of NF-κB signaling in hepatocytes, fibroblasts, osteoclasts, and hepatocellular carcinomas (Arsura et al. 2003; Gingery et al. 2008). Consistent with this notion, Lys63-linked polyubiquitylation of TAK1 by TRAF6 is required for activation of NF-κB signaling by TGF-β in HepG2 hepatoma cells (Hamidi et al. 2012). Future studies are necessary to determine the cellular context or environmental cues that lead to either the proapoptotic activity of TGF-β or the prosurvival activity of NF-κB by activating TAK1.
In addition to induction of apoptosis, TGF-β-induced JNK and/or p38 MAPK signaling also contributes to TGF-β-induced EMT. Blocking p38 MAPK activity using small molecular inhibitors or by expressing a dominant-negative MKK3 mutant blocks the changes in cell shape and reorganization of actin cytoskeleton that are associated with EMT (Bakin et al. 2002; Yoo et al. 2003). In addition, silencing TRAF6 expression also causes inhibition of TGF-β-induced EMT (Yamashita et al. 2008). The role of TRAF6 in promoting EMT and cancer cell invasion may result from its ability to stimulate proteolytic cleavage of TβRI in a presenillin 1 and Lys63 ubiquitylation-dependent manner to generate an intracellular domain (ICD) of TβRI, which was surprisingly found in the nucleus to promote cell invasion in certain cancer cells (Mu et al. 2011; Gudey et al. 2014). Moreover, TRAF4 was shown to be required for migration, EMT, and metastatic dissemination in response to TGF-β (Zhang et al. 2013). It is likely that non-Smad signaling through TRAF4 or TRAF6 and TAK1 leading to activation of JNK and p38 MAPK signaling is an obligatory step in TGF-β-induced EMT and cancer cell invasion.
THE PI3K-AKT PATHWAY IN TGF-β FAMILY SIGNALING
The serine/threonine kinase protein kinase B, more commonly known as Akt, regulates many biological processes, including cell survival, proliferation, increase in cell size, and metabolism (Carnero et al. 2008; Fruman and Rommel 2014). Growth factors, hormones, and cytokines are known to activate Akt through PI3K. TGF-β and BMP were also shown to activate Akt through PI3K, and Smad-independent or -dependent mechanisms have been reported (Fig. 3) (Bakin et al. 2000; Vinals and Pouysségur 2001; Ghosh-Choudhury et al. 2002; Lamouille and Derynck 2007; Gamell et al. 2008; Boergermann et al. 2010). By immunoprecipitation, p85, the regulatory subunit of PI3K was found constitutively associated with TβRII, but its association with TβRI was shown only in the presence of TGF-β (Yi et al. 2005). Although the interaction between TGF-β receptors and p85 may not be direct, the receptor kinase activities are essential for activation of PI3K, and TβRI kinase inhibitors block the TGF-β-induced activation of Akt by PI3K (Bakin et al. 2000; Lamouille and Derynck 2007). TGF-β-induced PI3K and Akt signaling was also shown to be activated through receptor-mediated Lys63-ubiquitylation of TRAF6, which in this way not only activates TAK1, but also induces ubiquitylation, membrane recruitment, and activation of Akt (Yang et al. 2009). Besides these direct, Smad-independent mechanisms, TGF-β was also shown to activate the PI3K-Akt pathway by inducing the expression of the miR-216a/217 microRNA cluster in kidney glomerular mesangial carcinoma and liver hepatocellular carcinoma cells (Kato et al. 2009; Xia et al. 2013). MiR-216a/217 is capable of inducing hyperactivation of the PI3K-Akt pathway by repressing the expression of Smad7 and PTEN (phosphatase and tensin homolog), an inhibitor of Akt. TGF-β was also reported to activate Akt signaling in mesangial cells by inducing the expression of another microRNA, miR-21, that represses PTEN expression (Dey et al. 2012). Finally, TGF-β can also indirectly activate PI3K-Akt signaling by inducing the expression of TGF-α that activates EGF receptor signaling and, thus, PI3K-Akt signaling (Vinals and Pouysségur 2001). Conversely, the TGF-β-induced, Smad-dependent expression of the lipid phosphatase SHIP (SH2-containing inositol 5′-phosphatase) can down-regulate PI3K-Akt signaling (Valderrama-Carvajal et al. 2002), and this mechanism may account for or contribute to the transient nature of TGF-β-induced Akt phosphorylation.
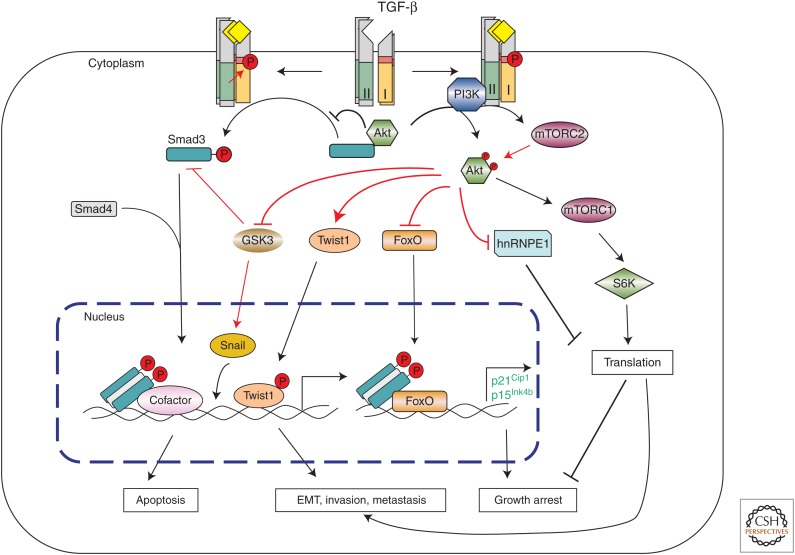
Figure 3.
Transforming growth factor β (TGF-β)-induced activation of the PI3K-Akt pathway. TGF-β can induce PI3K and Akt activation, possibly through interaction of the p85 subunit of PI3K with the TGF-β receptors. Activated Akt then controls translational responses through mTOR1 and S6K, or directly acts on the translational responses by inducing phosphorylation of hnRNPE. TGF-β also induces activation of mTORC2, which contributes to enhanced Akt activation and forms a reinforcing feedback loop in PI3K-Akt activation. These non-Smad-mediated translational responses collaborate with Smad-mediated transcriptional responses during epithelial-to-mesenchymal transition (EMT), but can antagonize Smad-mediated transcription responses during growth arrest or apoptosis. Akt can regulate Smad3 activity by sequestering Smad3 in the cytoplasm or inhibiting GSK3β-mediated Smad3 phosphorylation and degradation. Akt can also regulate apoptosis or EMT by directly phosphorylating FoxO or Twist1 or inducing Snail phosphorylation by GSK3β.
Activation of the PI3K-Akt pathway also contributes to TGF-β-induced EMT. Pharmacological inhibitors have implicated PI3K activation in the actin filament reorganization and cell migration during EMT induced by TGF-β. However, caution must be taken when interpreting results with PI3K inhibitors because the dosage routinely applied to block PI3K-Akt-mTOR (mammalian target of rapamycin) signaling also reduces the Smad2 and Smad3 activation (Bakin et al. 2000; Edlund et al. 2004), which may result from interference with the SARA (Smad anchor for receptor activation)-dependent association of Smad2 and/or Smad3 to the TGF-β receptors (Tsukazaki et al. 1998; Hayes et al. 2002).
mTOR, a target of the Akt kinase, plays important roles in the contribution of the PI3K-Akt pathway to TGF-β-induced EMT. In mammary epithelial cells and keratinocytes, TGF-β induces rapid activation of mTOR complex 1 (mTORC1) and S6 kinase (S6K), leading to increased protein synthesis, cell size, motility, and invasion (Lamouille and Derynck 2007). TGF-β also induces activation of mTOR complex 2 (mTORC2), which promotes cytoskeletal reorganization, RhoA activation, and cell migration (Lamouille et al. 2012). In addition, mTORC2 contributes to enhanced Akt activation at a late stage of EMT (Lamouille et al. 2012), thus forming a positive feedback loop in the PI3K-Akt pathway activation. Besides mTOR, Akt also regulates key EMT transcription factors. By phosphorylating glycogen synthase kinase (GSK) 3β, Akt stabilizes NF-κB and the EMT transcription factor Snail, thus enhancing Snail-dependent transcription in EMT (Zhou et al. 2004; Julien et al. 2007). Akt also phosphorylates the EMT transcription factor Twist1, enhancing its activity to induce expression of TGF-β2, which in turn promotes TGF-β receptor signaling, PI3K-Akt pathway activity, and EMT (Xue et al. 2012). Furthermore, TGF-β-activated Akt2 was shown to phosphorylate hnRNP E1 (heterogeneous nuclear ribonucleoprotein E1) and disrupt the hnRNP E1–eEF1A1 (eukaryotic translation elongation factor 1 α1) interaction, thereby releasing translational inhibition of several genes required for EMT (Hussey et al. 2011). Besides its role in EMT, PI3K also affects the TGF-β-induced fibroblast proliferation and morphological transformation (Wilkes et al. 2005).
Akt also directly affects Smad activities in response to TGF-β and, thus, Smad-mediated transcription responses. For example, the association of Akt with unphosphorylated Smad3 restricts TβRI-induced activation and nuclear localization of Smad3 and, thus, attenuates the Smad3-mediated transcription (Conery et al. 2004; Remy et al. 2004). Consistent with this, activation of PI3K or Akt can protect cells from TGF-β-induced apoptosis and growth inhibition (Chen et al. 1998; Shin et al. 2001; Song et al. 2006), whereas TGF-β stimulation inhibits the association of Akt with Smad3, allowing Smad3 to escape Akt-mediated cytoplasmic sequestration (Conery et al. 2004; Remy et al. 2004). Akt can also regulate Smad-mediated transcription by phosphorylating the forkhead transcription factor FoxO, which is required in several Smad-mediated responses important in growth arrest, for example, the induction of p15INK4B and p21CIP1 expressions (Seoane et al. 2004; Gomis et al. 2006). Phosphorylation of FoxO proteins by Akt hampers their nuclear localization, and prevents their participation in Smad-mediated transcription regulation. Akt activation directly promotes TβRI stability by phosphorylating and promoting membrane localization of a deubiquitylating enzyme, USP4 (ubiquitin-specific peptidase 4), which deubiquitylates and thus stabilizes TβRI and promotes non-Smad pathways (Zhang et al. 2012). On the other hand, activation of Akt was shown to enhance TGF-β and BMP-induced transcription responses by stabilizing Smad1 and Smad3. This increased stability results from inactivating GSK3β, because phosphorylation of Smad1 and Smad3 by GSK3β leads to ubiquitylation and degradation (Sapkota et al. 2007; Guo et al. 2008).
ACTIVATION OF RHO-LIKE GTPases IN RESPONSE TO TGF-β PROTEINS
The Rho-like GTPases, RhoA, RhoB, Rac, and Cdc42, control the dynamics of cytoskeletal organization, cell motility, and gene expression through a variety of effectors (Jaffe and Hall 2005). TGF-β and BMP can activate Rho-like GTPases in a cell-type-dependent manner (Fig. 4). For example, in epithelial cells and primary keratinocytes, TGF-β activates RhoA in its GTP-bound state within 5 min, and this activation is followed by a rapid attenuation by 15 min (Bhowmick et al. 2001a; Edlund et al. 2002). In mesenchymal stem cells, BMP induces a rapid activation of RhoA and Rho-associated protein kinase (ROCK) after cell spreading (Wang et al. 2012). Activation of RhoA by TGF-β or BMP is likely independent of Smad2 or Smad3, as suggested by the rapid onset and the inability of a dominant-negative Smad3 mutant or Smad4 deficiency to block RhoA activities (Bhowmick et al. 2001a; Voorneveld et al. 2014). Interestingly, JNK and p38 MAPK can be activated by direct association with these Rho GTPases, as an alternative to the mode of activation by MAPKKKs (Coso et al. 1995; Minden et al. 1995); however, it is not clear whether this mechanism plays a role in TGF-β- or BMP-induced JNK and p38 MAPK activation.
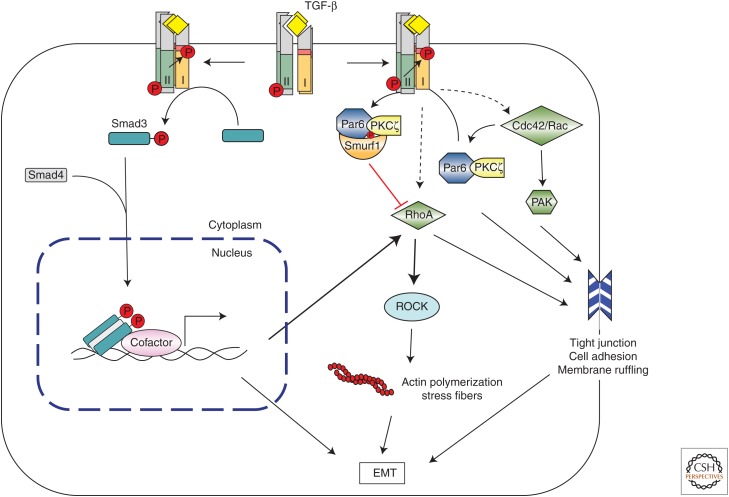
Figure 4.
Transforming growth factor β (TGF-β)-induced regulation of the Rho family of small GTPases. RhoA and Rho-associated protein kinase (ROCK) can be activated by TGF-β by either Smad-dependent or -independent mechanisms to induce actin polymerization and stress fiber formation during epithelial-to-mesenchymal transition (EMT). Additionally, the TβRII receptor can directly phosphorylate Par6 by recruiting Smurf1, thus targeting RhoA for degradation, which leads to tight junction dissociation. TGF-β can also induce tight junction dissociation and cell migration during EMT by recruiting Cdc42 and/or Rac1 to the receptor complex, and activate p21-activated kinase (PAK) signaling. PKC, Protein kinase C.
Paradoxically, TGF-β was found to down-regulate RhoA protein levels through Par6 (Ozdamar et al. 2005), a scaffold protein that binds TβRI at tight junctions and regulates cell polarity in polarized epithelial cells. Engagement with TGF-β causes the receptor complex to accumulate at tight junctions, which then leads to phosphorylation of Par6 by TβRII and, subsequently, recruitment of the ubiquitin E3 ligase Smurf1 to the activated receptor complex. The Par6–Smurf1 complex then mediates localized ubiquitylation and turnover of RhoA in a protein kinase Cζ (PKCζ)-dependent manner, which enables TGF-β-dependent dissolution of tight junctions, a prerequisite for EMT. It is therefore possible that TGF-β regulates RhoA activity in two different phases and in two different cell compartments. Rapid activation of RhoA in the early phase of signaling may be complemented by localized down-regulation of RhoA levels at tight junctions at a later stage. Both phases of this regulation of RhoA in response to TGF-β appear to be essential for the TGF-β-induced EMT.
In addition to RhoA, TGF-β also activates the Cdc42 GTPase. Similarly to activation of RhoA, Cdc42 activation occurs independently of Smads, and blocking both Smad2 and Smad3 phosphorylation does not affect the activation of p21-activated kinase 2 (PAK2), downstream from Rac and Cdc42 activation (Wilkes et al. 2003). Association of Cdc42 with TGF-β receptor complexes at the cell surface has been shown (Barrios-Rodiles et al. 2005), and a cluster of proteins involved in Cdc42 and PAK signaling was found in the TGF-β receptor–associated protein complex. This complex includes the PAK-interacting Cdc42 GTPase, the Rac1 exchange factors α-PIX and β-PIX, PAK1 itself, a PAK1-interacting partner, oxidative stress-responsive kinase-1 (OSR1), and occludin, a tight-junction accessory protein (Barrios-Rodiles et al. 2005). Additionally, LIM kinase 1 (LIMK1), an effector of the PAK network, associates with BMPRII and this interaction synergizes with Cdc42 and activates the catalytic activity of LIMK1, increases the phosphocofilin level, and induces changes in the actin cytoskeleton (Foletta et al. 2003; Lee-Hoeflich et al. 2004). As in the TGF-β pathway, BMP-induced activation of Rho, ROCK, and LIMK is also important for cancer cell dissemination (Voorneveld et al. 2014). It is noteworthy that a delayed peak of RhoA and Cdc42 activation, dependent on new protein synthesis, is observed in certain cells. Induction of this peak of GTPase activity by TGF-β may require expression of NET1, a RhoA-specific guanine exchange factor that activates RhoA through Smad-mediated transcription (Shen et al. 2001).
ACTIVATION OF OTHER NON-Smad SIGNALING PATHWAYS
In some mesenchymal cell lines, but not in epithelial cell lines, TGF-β was shown to activate the c-Abl tyrosine kinase, likely independent of Smad2 or Smad3 activation (Daniels et al. 2004). Moreover, inhibition or loss of c-Abl prevents TGF-β-induced morphological changes, extracellular matrix gene expression, and cell proliferation in fibroblasts (Daniels et al. 2004; Wang et al. 2005). In BMP signaling, c-Abl was found to associate with and phosphorylate BMPRIA, thus skewing BMP signaling toward activation of the Erk1/2 MAPK pathway instead of the Smad pathway, to regulate osteoblast expansion (Kua et al. 2012). It is not clear whether BMP can induce c-Abl activation, nor is the mechanism known that enables TGF-β-induced c-Abl activation. Given the similarity in activation kinetics and promoting TGF-β-mediated morphological changes in the same cell types (Wilkes et al. 2003; Daniels et al. 2004), it is possible that c-Abl functions downstream from the Cdc42-PAK2 pathway. Furthermore, Akt activation in response to TGF-β or BMP may also lead to c-Abl activation.
Activation of Jak-Stat signaling has also been seen in response to TGF-β in several cell types. TGF-β can activate Jak1-Stat3 signaling in hepatic stellate cells, and Jak2 in fibroblasts, and involves Jak-Stat signaling in its ability to promote fibrosis (Dees et al. 2012; Liu et al. 2013). Furthermore, Jak-Stat3 signaling cooperates with TGF-β or BMP pathways in neural progenitor cells and hepatoma cells (Nakashima et al. 1999; Yamamoto et al. 2001). However, conflicting reports suggest that TGF-β inhibits IL-6-induced Stat3 activation in acute myeloid leukemia blast cells and hepatocellular carcinoma and IL-12-induced Jak-Stat signaling in T lymphocytes (Bright and Sriram 1998; Wierenga et al. 2002; Tang et al. 2008). The molecular mechanisms underlying these regulations remain to be determined.
Finally, protein kinase A (PKA) was reported to be activated in response to TGF-β (Wang et al. 1998). Unlike activation of MAPK or GTPase signaling, TGF-β-induced activation of PKA requires the formation of Smad3–Smad4 complexes. However, rather than participating in transcriptional regulation, the Smad3–Smad4 complex interacts with the regulatory subunit of PKA, thereby releasing the catalytic subunit from the PKA holoenzyme and causing its activation (Zhang et al. 2004). Activation of PKA may play an important role in the TGF-β-induced phosphorylation of transcription factor CREB (cAMP-response element-binding protein) and expression of fibronectin (Wang et al. 1998).
CONCLUDING REMARKS
As apparent from this overview, many signaling pathways are controlled by TGF-β and BMP receptors, and the heteromeric receptor complexes that are activated by TGF-β family proteins act as nodal points for multiprotein assemblies that activate different signaling pathways. Future characterization of receptor-associated proteins using proteomics and/or phosphoproteomics analyses may reveal other previously unappreciated non-Smad pathways that are activated in response to TGF-β proteins. The differential activation of non-Smad pathways is often highly context dependent, yet plays important roles in a variety of cellular functions. It is of special importance to determine how TGF-β can direct membrane-bound receptors at the cell surface to recruit and activate multiple effectors, and how the selectivity in downstream signaling is achieved. It is likely that different proteins that associate with the receptor complexes at different activation states or subcellular locales regulate the receptor functions, routing, and pathway activation. A combination of in vivo imaging and manipulation of gene expression through RNA interference, CRISPR/Cas9-mediated genomic editing or traditional gene silencing approaches will be powerful in addressing the roles and mechanisms of TGF-β receptors, their regulators, and their effectors in eliciting the many signaling responses through Smad-dependent and -independent signaling pathways.
As we continue to advance our understanding of TGF-β family signaling networks, the complexity of signaling cross talk with other pathways becomes increasingly apparent, and we start appreciating how subtle perturbations can result in pathological dysregulation. A major challenge is to identify the targets in different context for treatment of different diseases to truly benefit individual patients.
ACKNOWLEDGMENTS
Research in Y. E. Zhang’s laboratory is supported by an intramural program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P. 1998. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem Biophys Res Commun 249: 505–511. [DOI] [PubMed] [Google Scholar]
- Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, Factor V, Heuchel R, Mercurio F, Thorgeirsson SS, et al. 2003. Transient activation of NF-κB through a TAK1/IKK kinase pathway by TGF-β1 inhibits AP-1/SMAD signaling and apoptosis: Implications in liver tumor formation. Oncogene 22: 412–425. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. 1997. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem 272: 1429–1432. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. 2002. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115: 3193–3206. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay B, Han A, Dai J, Fan J, Li Y, Chen M, Woodley DT, Li W. 2011. TβRI/Alk5-independent TβRII signaling to ERK1/2 in human skin cells according to distinct levels of TβRII expression. J Cell Sci 124: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, et al. 2005. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307: 1621–1625. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. 2001a. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 12: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. 2001b. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J Biol Chem 276: 46707–46713. [DOI] [PubMed] [Google Scholar]
- Boergermann JH, Kopf J, Yu PB, Knaus P. 2010. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Intern J Biochem Cell Biol 42: 1802–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Sriram S. 1998. TGF-β inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol 161: 1772–1777. [PubMed] [Google Scholar]
- Brown JD, DiChiara MR, Anderson KR, Gimbrone MA Jr, Topper JN. 1999. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J Biol Chem 274: 8797–8805. [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. 2008. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 8: 187–198. [DOI] [PubMed] [Google Scholar]
- Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, Zhou J, Cheng CK, Chang CA, Chiu JJ. 2009. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol Endocrinol 23: 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Su YH, Chuang RL, Chang TY. 1998. Suppression of transforming growth factor-β-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene 17: 1959–1968. [DOI] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, Townsend CM Jr, Ko TC, Luo K. 2004. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-β induced apoptosis. Nat Cell Biol 6: 366–372. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81: 1137–1146. [DOI] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. 2004. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest 114: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees C, Tomcik M, Palumbo-Zerr K, Distler A, Beyer C, Lang V, Horn A, Zerr P, Zwerina J, Gelse K, et al. 2012. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum 64: 3006–3015. [DOI] [PubMed] [Google Scholar]
- Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. 2012. TGFβ-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PloS ONE 7: e42316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. 2003. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5: 410–421. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. 2001. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480. [DOI] [PubMed] [Google Scholar]
- Edlund S, Landström M, Heldin CH, Aspenström P. 2002. Transforming growth factor-β-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell 13: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund S, Bu S, Schuster N, Aspenström P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landström M. 2003. Transforming growth factor-β1 (TGF-β)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-β-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14: 529–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund S, Landström M, Heldin CH, Aspenström P. 2004. Smad7 is required for TGF-β-induced activation of the small GTPase Cdc42. J Cell Sci 117: 1835–1847. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. 1999. Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J Biol Chem 274: 37413–37420. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massagué J, Bernard O. 2003. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, Mulder KM. 1997. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor β in the negative growth control of breast cancer cells. Cancer Res 57: 628–633. [PubMed] [Google Scholar]
- Fruman DA, Rommel C. 2014. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov 13: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaba M, Zimmerman CM, Mathews LS. 2002. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem 277: 41361–41368. [DOI] [PubMed] [Google Scholar]
- Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, et al. 2001. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28: 491–498. [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. 2007. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67: 3752–3758. [DOI] [PubMed] [Google Scholar]
- Gamell C, Osses N, Bartrons R, Ruckle T, Camps M, Rosa JL, Ventura F. 2008. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J Cell Sci 121: 3960–3970. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. 2002. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem 277: 33361–33368. [DOI] [PubMed] [Google Scholar]
- Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ. 2008. TGF-β coordinately activates TAK1/MEK/AKT/NFκB and SMAD pathways to promote osteoclast survival. Exp Cell Res 314: 2725–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massagué J. 2006. A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci 103: 12747–12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudey SK, Sundar R, Mu Y, Wallenius A, Zang G, Bergh A, Heldin CH, Landström M. 2014. TRAF6 stimulates the tumor-promoting effects of TGFβ type I receptor through polyubiquitination and activation of presenilin 1. Sci Signal 7: ra2. [DOI] [PubMed] [Google Scholar]
- Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. 2008. Axin and GSK3-β control Smad3 protein stability and modulate TGF-β signaling. Genes Dev 22: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Kwak HB, Le SW, Kim HH, Lee ZH. 2003a. Lipid rafts are important for the association of RANK and TRAF6. Exp Molec Med 35: 279–284. [DOI] [PubMed] [Google Scholar]
- Ha H, Kwak HB, Lee SK, Na DS, Rudd CE, Lee ZH, Kim HH. 2003b. Membrane rafts play a crucial role in receptor activator of nuclear factor κB signaling and osteoclast function. J Biol Chem 278: 18573–18580. [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I. 2005. Ubiquitylation and cell signaling. EMBO J 24: 3353–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi A, von Bulow V, Hamidi R, Winssinger N, Barluenga S, Heldin CH, Landstrom M. 2012. Polyubiquitination of transforming growth factor β (TGFβ)-associated kinase 1 mediates nuclear factor-κB activation in response to different inflammatory stimuli. J Biol Chem 287: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. 1999. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J Biol Chem 274: 27161–27167. [DOI] [PubMed] [Google Scholar]
- Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. 2006. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol 26: 7791–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. 2002. TGFβ receptor internalization into EEA1-enriched early endosomes: Role in signaling to Smad2. J Cell Biol 158: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. 1999. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY, Kuo PL. 2011. Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem 286: 37335–37346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MC, Merrick WC, Howe PH. 2011. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell 41: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J, Gohda J, Akiyama T. 2007. Characteristics and biological functions of TRAF6. Adv Exp Med Biol 597: 72–79. [DOI] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. 2000. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem 275: 6075–6079. [DOI] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin CH, ten Dijke P. 2003. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J Biol Chem 278: 3751–3761. [DOI] [PubMed] [Google Scholar]
- Jadrich JL, O’Connor MB, Coucouvanis E. 2006. The TGFβ activated kinase TAK1 regulates vascular development in vivo. Development 133: 1529–1541. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. 2005. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269. [DOI] [PubMed] [Google Scholar]
- Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. 2002. TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 4: 51–58. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Mauviel A. 2005. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-β: Implications for carcinogenesis. Oncogene 24: 5742–5750. [DOI] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. 2007. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 26: 7445–7456. [DOI] [PubMed] [Google Scholar]
- Jung SM, Lee JH, Park J, Oh YS, Lee SK, Park JS, Lee YS, Kim JH, Lee JY, Bae YS, et al. 2013. Smad6 inhibits non-canonical TGF-β1 signalling by recruiting the deubiquitinase A20 to TRAF6. Nat Commun 4: 2562. [DOI] [PubMed] [Google Scholar]
- Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, Yoo YA. 2011. Metastatic function of BMP-2 in gastric cancer cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res 317: 1746–1762. [DOI] [PubMed] [Google Scholar]
- Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, et al. 2009. TGF-β activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno Y, Lamouille S, Derynck R. 2013. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 25: 76–84. [DOI] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell 6: 1365–1375. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim BC, Xu Z, Kim SJ. 2004. Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-β-induced apoptosis in hepatoma cells. J Biol Chem 279: 29478–29484. [DOI] [PubMed] [Google Scholar]
- Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. 2000. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem 275: 17647–17652. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. 1997. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 389: 618–622. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massagué J. 1999. A mechanism of repression of TGFβ/ Smad signaling by oncogenic Ras. Genes Dev 13: 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kua HY, Liu H, Leong WF, Li L, Jia D, Ma G, Hu Y, Wang X, Chau JF, Chen YG, et al. 2012. c-Abl promotes osteoblast expansion by differentially regulating canonical and non-canonical BMP pathways and p16INK4a expression. Nat Cell Biol 14: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. 2002. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-β in normal human osteoblastic cells. J Biol Chem 277: 15514–15522. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. 2007. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. 2012. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci 125: 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S, Feng XH, Chen RH, Maruoka EM, Turck CW, Griswold-Prenner I, Derynck R. 1997. The type II transforming growth factor-β receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 272: 14850–14859. [DOI] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. 2007. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. 2004. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J 23: 4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M, Hill CS, Beug H, Downward J. 2000. Raf induces TGFβ production while blocking its apoptotic but not invasive responses: A mechanism leading to increased malignancy in epithelial cells. Genes Dev 14: 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page C, Puiffe ML, Meunier L, Zietarska M, de Ladurantaye M, Tonin PN, Provencher D, Mes-Masson AM. 2009. BMP-2 signaling in ovarian cancer and its association with poor prognosis. J Ovarian Res 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, et al. 2012. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell 10: 171–182. [DOI] [PubMed] [Google Scholar]
- Lim M, Chuong CM, Roy-Burman P. 2011. PI3K, Erk signaling in BMP7-induced epithelial-mesenchymal transition (EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional cultures. Horm Cancer 2: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Meyer C, Li J, Nadalin S, Konigsrainer A, Weng H, Dooley S, ten Dijke P. 2013. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem 288: 30708–30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars A, Lallemand F, Prunier C, Marais J, Ferrand N, Pessah M, Cherqui G, Atfi A. 2001. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem 276: 36797–36803. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. 2007. Integrating signals from RTKs to ERK/MAPK. Oncogene 26: 3113–3121. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81: 1147–1157. [DOI] [PubMed] [Google Scholar]
- Miyazono K. 2008. Regulation of TGF-β family signaling by inhibitory Smads. In The TGF-β family (ed. Derynck R, Miyazono K), pp. 363–387. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2005. Non-Smad TGF-β signals. J Cell Sci 118: 3573–3584. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. 2012. Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin Cancer Biol 22: 446–454. [DOI] [PubMed] [Google Scholar]
- Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, et al. 2011. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun 2: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder KM, Morris SL. 1992. Activation of p21ras by transforming growth factor β in epithelial cells. J Biol Chem 267: 5029–5031. [PubMed] [Google Scholar]
- Muthusamy BP, Budi EH, Katsuno Y, Lee MK, Smith SM, Mirza AM, Akhurst RJ, Derynck R. 2015. ShcA protects against epithelial-mesenchymal transition through compartmentalized inhibition of TGF-β-induced Smad activation. PLoS Biol 13: e1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. 1999. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284: 479–482. [DOI] [PubMed] [Google Scholar]
- Oakley FD, Smith RL, Engelhardt JF. 2009. Lipid rafts and caveolin-1 coordinate interleukin-1β (IL-1β)-dependent activation of NFκB by controlling endocytosis of Nox2 and IL-1β receptor 1 from the plasma membrane. J Biol Chem 284: 33255–33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson N, Piek E, Sundstrom M, ten Dijke P, Nilsson G. 2001. Transforming growth factor-β-mediated mast cell migration depends on mitogen-activated protein kinase activity. Cell Signal 13: 483–490. [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. 2005. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307: 1603–1609. [DOI] [PubMed] [Google Scholar]
- Remy I, Montmarquette A, Michnick SW. 2004. PKB/Akt modulates TGF-β signalling through a direct interaction with Smad3. Nat Cell Biol 6: 358–365. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. 1999. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J Biol Chem 274: 8949–8957. [DOI] [PubMed] [Google Scholar]
- Sapkota GP. 2013. The TGFβ-induced phosphorylation and activation of p38 mitogen-activated protein kinase is mediated by MAP3K4 and MAP3K10 but not TAK1. Open Biol 3: 130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massagué J. 2007. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25: 441–454. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103: 211–225. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massagué J. 2004. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117: 211–223. [DOI] [PubMed] [Google Scholar]
- Shapira KE, Hirschhorn T, Barzilay L, Smorodinsky NI, Henis YI, Ehrlich M. 2014. Dab2 inhibits the cholesterol-dependent activation of JNK by TGF-β. Mol Biol Cell 25: 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Li J, Hu PP, Waddell D, Zhang J, Wang XF. 2001. The activity of guanine exchange factor NET1 is essential for transforming growth factor-β-mediated stress fiber formation. J Biol Chem 276: 15362–15368. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. 1998. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J 17: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19: 2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I, Bakin AV, Rodeck U, Brunet A, Arteaga CL. 2001. Transforming growth factor β enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol Biol Cell 12: 3328–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone DM, Zhang L, Graziano K, Nicke B, Pham T, Schaefer C, Logsdon CD. 2001. Smad4 mediates activation of mitogen-activated protein kinases by TGF-β in pancreatic acinar cells. Am J Physiol Cell Physiol 281: C311–C319. [DOI] [PubMed] [Google Scholar]
- Song K, Wang H, Krebs TL, Danielpour D. 2006. Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J 25: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong G, Reddy B, Sokol S, Adamo R, Prince A. 2004. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest 113: 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landström M. 2008. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 10: 1199–1207. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. 2003. TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J Mol Biol 326: 105–115. [DOI] [PubMed] [Google Scholar]
- Takekawa M, Tatebayashi K, Itoh F, Adachi M, Imai K, Saito H. 2002. Smad-dependent GADD45β expression mediates delayed activation of p38 MAP kinase by TGF-β. EMBO J 21: 6473–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, et al. 2008. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-β and IL-6 signaling. Proc Natl Acad Sci 105: 2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. 1998. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell 95: 779–791. [DOI] [PubMed] [Google Scholar]
- Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S, Lebrun JJ. 2002. Activin/TGF-β induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol 4: 963–969. [DOI] [PubMed] [Google Scholar]
- Vinals F, Pouysségur J. 2001. Transforming growth factor β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signaling. Mol Cell Biol 21: 7218–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, Hoogenboom JP, Biemond I, Verspaget HW, Hommes DW, de Rooij K, et al. 2014. Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 147: 196–208. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu Y, Sharma K. 1998. Transforming growth factor-β1 stimulates protein kinase A in mesangial cells. J Biol Chem 273: 8522–8527. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351. [DOI] [PubMed] [Google Scholar]
- Wang S, Wilkes MC, Leof EB, Hirschberg R. 2005. Imatinib mesylate blocks a non-Smad TGF-β pathway and reduces renal fibrogenesis in vivo. FASEB J 19: 1–11. [DOI] [PubMed] [Google Scholar]
- Wang YK, Yu X, Cohen DM, Wozniak MA, Yang MT, Gao L, Eyckmans J, Chen CS. 2012. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev 21: 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. 2007. The JNK signal transduction pathway. Curr Opin Cell Biol 19: 142–149. [DOI] [PubMed] [Google Scholar]
- Wierenga AT, Schuringa JJ, Eggen BJ, Kruijer W, Vellenga E. 2002. Downregulation of IL-6-induced STAT3 tyrosine phosphorylation by TGF-β1 is mediated by caspase-dependent and -independent processes. Leukemia 16: 675–682. [DOI] [PubMed] [Google Scholar]
- Wilkes MC, Murphy SJ, Garamszegi N, Leof EB. 2003. Cell-type-specific activation of PAK2 by transforming growth factor β independent of Smad2 and Smad3. Mol Cell Biol 23: 8878–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes MC, Mitchell H, Penheiter SG, Dore JJ, Suzuki K, Edens M, Sharma DK, Pagano RE, Leof EB. 2005. Transforming growth factor-β activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21–activated kinase-2. Cancer Res 65: 10431–10440. [DOI] [PubMed] [Google Scholar]
- Wong WK, Knowles JA, Morse JH. 2005. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: Implication for a role in pulmonary arterial hypertension. Am J Respir Cell Molec Biol 33: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Arron JR. 2003. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays 25: 1096–1105. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. 2009. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell 16: 329–343. [DOI] [PubMed] [Google Scholar]
- Xia H, Ooi LL, Hui KM. 2013. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology 58: 629–641. [DOI] [PubMed] [Google Scholar]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. 2004. Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia 6: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, Ruegg C, Hemmings BA. 2012. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer Discov 2: 248–259. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270: 2008–2011. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuda T, Muraguchi A, Miyazono K, Kawabata M. 2001. Cross-talk between IL-6 and TGF-β signaling in hepatoma cells. FEBS Lett 492: 247–253. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Hua X, Bergelson S, Lodish HF. 2000. Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J Biol Chem 275: 36295–36302. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. 2008. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol Cell 31: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Winawer S, Friedman E. 1994. Two different signal transduction pathways can be activated by transforming growth factor β1 in epithelial cells. J Biol Chem 269: 13231–13237. [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takeda K, Ochiai W, Takizawa T, Ueno M, Takizawa M, Shibuya H, Taga T. 2001. Inhibition of BMP2-induced, TAK1 kinase-mediated neurite outgrowth by Smad6 and Smad7. Genes Cells 6: 1091–1099. [DOI] [PubMed] [Google Scholar]
- Yang YA, Zhang GM, Feigenbaum L, Zhang YE. 2006. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell 9: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, et al. 2009. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325: 1134–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JY, Shin I, Arteaga CL. 2005. Type I transforming growth factor β receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem 280: 10870–10876. [DOI] [PubMed] [Google Scholar]
- Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ Jr, Liebermann DA, Böttinger EP, Roberts AB. 2003. Transforming growth factor-β-induced apoptosis is mediated by Smad-dependent expression of GADD45β through p38 activation. J Biol Chem 278: 43001–43007. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R. 2006. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 24: 21–44. [DOI] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. 2002. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J 21: 3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. 2001. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc Natl Acad Sci 98: 6686–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. 2009. Non-Smad pathways in TGF-β signaling. Cell Res 19: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang W, Hayashi Y, Jester JV, Birk DE, Gao M, Liu CY, Kao WW, Karin M, Xia Y. 2003. A role for MEK kinase 1 in TGF-β/activin-induced epithelium movement and embryonic eyelid closure. EMBO J 22: 4443–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Duan CJ, Binkley C, Li G, Uhler MD, Logsdon CD, Simeone DM. 2004. A transforming growth factor β-induced Smad3/Smad4 complex directly activates protein kinase A. Mol Cell Biol 24: 2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, et al. 2012. USP4 is regulated by Akt phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol 14: 717–726. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Garcia de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A, et al. 2013. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell 51: 559–572. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. 2004. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931–940. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, Patterson C, Moser M. 2007. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res 76: 390–399. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xie J, Lee D, Liu Y, Jung J, Zhou L, Xiong S, Mei L, Xiong WC. 2010. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev Cell 19: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Chen YG. 2009. Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell 20: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]