Abstract
A fungus, Colletotrichum fioriniae, was isolated for the first time from fruits of Chinese matrimony vine (Lycium chinense Mill.) in Korea. It was classified as C. fioriniae based on the morphological characteristics and nucleotide sequence of glyceraldehyde-3-phosphate-dehydrogenase and β-tubulin. To the best of our knowledge, this is the first report of C. fioriniae causing anthracnose of Chinese matrimony vine in Korea.
Keywords: Beta-tubulin, Chinese matrimony vine, Colletotrichum fioriniae, Glyceraldehyde-3-phosphate dehydrogenase
Chinese matrimony vine (Lycium chinense Mill.), also known as Chinese boxthorn, wolfberry, or Goji berry, belongs to the Solanaceae family. Originally from Asia and southern Europe, the plant is popular in traditional Chinese medicine. In China, Chinese boxthorn is used as an aphrodisiac (seeds) and antifebrile and antirheumatic tonic (roots). It is also applied as a cure for a wide range of ailments, from skin rashes and eyesight problems to diabetes and lack of energy (as a tonic; berries and leaves) [1]. The fruits are well known in Korea and China for their great nutritional value and medicinal applications [1].
In recent years, utilization of Chinese matrimony vine rapidly increased mainly for its high nutrient value (a high proportion of antioxidants, 12% protein, 68% carbohydrates, 10% fat, and 10% fiber) [2]. The use of Chinese matrimony vine in eastern Asia is becoming more and more prevalent but statistics are not available. In Korea, Chinese matrimony vine was cultivated on approximately 259 ha in 2010 [3]. Several fungi that infect the fruits of Chinese matrimony vine have been identified. These include powdery mildew (Arthrocladiella mougeotii), leaf spot (Cercospora lycii), anthracnose (Colletotrichum acutatum, C. dematium, C. gloeosporioides, and Gloeosporium sp.), brown leaf spot (Phoma sp.), and boxthorn blight (Phytophthora drechsleri and P. nicotianae) [4]. Among these, Colletotrichum spp. have a tremendous effect on Chinese matrimony vine production because of their wide range of infection capabilities. Colletotrichum is an important fungal taxon that causes anthracnose in several economically important plants (crops and grasses) [5].
In October 2015, severe outbreaks of anthracnose on fruits of Chinese matrimony vine occurred, and the pathological signs were observed in the fields of Chungcheongnam-do, South Korea. The infected fruits initially showed small circular brown spots that evolved into sunken zones with concentric rings of orange conidial masses. As the disease progressed, fruits eventually became black, sunken, and blighted (Fig. 1A and 1B). Fresh samples were collected from the infected fruits in the field. The fruits showing the typical disease signs were surface-sterilized with 1% sodium hypochlorite (NaOCl) for 3 min, followed by 3 washes with sterilized distilled water, and were dried on sterilized tissue paper [6]. Then, the fruits were placed in Petri dishes containing blotter paper and incubated at 25 ± 2℃ for 2 days in a 12-hr light and 12-hr dark chamber. After 2 days, spore layers were isolated using autoclaved toothpicks or glass sticks and were mixed with distilled water and streptomycin (300 ppm) in tubes. The mixtures were then spread on a water agar medium containing streptomycin (300 ppm) [7], with incubation for 3 days at 25 ± 2℃. Measurements were taken to ensure that the samples were free of fungal or bacterial contaminants. After 3 days, singlespore isolates of the emerging fungus were transferred to a new potato dextrose agar (PDA; Difco, Sparks, MD, USA) plate to obtain pure culture.
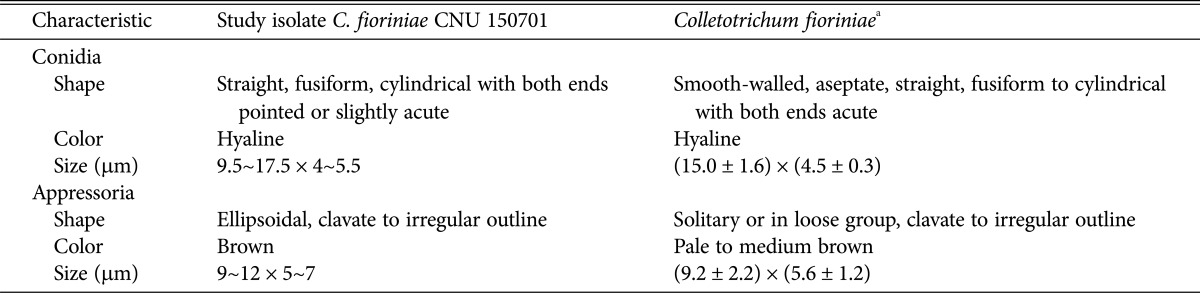
Fig. 1. Anthracnose caused by Colletotrichum fioriniae on Chinese matrimony vine fruits. A, An anthracnose-infected field of matrimony vine; B, Signs of the causal fungus of anthracnose on matrimony vine; C, Signs appearing on inoculated fruits; D, No pathological signs on control fruits; E, Morphological characteristics of conidia (scale bar = 10 µm); F, Appressoria (scale bar = 10 µm); G, H, Fungal colonies on V8 juice agar plates (G, front view; H, back view).
To prove the pathogenicity of the isolated fungus, isolate CNU150701 was inoculated into healthy fruits by wounding and nonwounding methods. Three cultivars (cv. Cheongun, cv. Cheongmyeong, cv. Hwasuu) of matrimony vine were used in the pathogenicity test. A conidial suspension of fungus CNU150701 was prepared from 7-day-old cultures grown on the V8 juice medium at 25℃ in the dark. Uninfected fruits were surface-sterilized with 1% NaOCl for 3min followed by 2 washes with sterilized distilled water. The fruits were dried with sterilized tissue paper and inoculated using the wounding or nonwounding method [8,9]. In the wounding method, the fruits were wounded with a sterile nail to 1-mm depth and inoculated with 10 µL of a conidial suspension (106 conidia/mL) of the pathogen. The nonwounding method involved spraying the conidial suspension (106 conidia/mL) of the pathogen onto the fruits. The fruits sprayed with sterilized distilled water only served as a control. The inoculated fruits were incubated at room temperature in boxes on paper trays. Six fruits were inoculated per cultivar for each method. With the wounding method, all the inoculated fruits showed typical signs (sunken zones with concentric rings of orange conidial masses similar to those observed in the field) after 4 days, whereas no pathological signs were observed in control fruits (Fig. 1C and 1D). With the nonwounding method, the inoculated fruits also showed signs after 7 days, while the control fruits were still symptomless. All 3 cultivars of matrimony vine showed a pathogenicity effect with the wounding method and nonwounding method. The experiment was repeated thrice (each time in triplicate), and the results were similar. Fungus CNU150701 was reisolated from the inoculated fruits and compared with the original pathogen to accomplish Koch's postulates in each test.
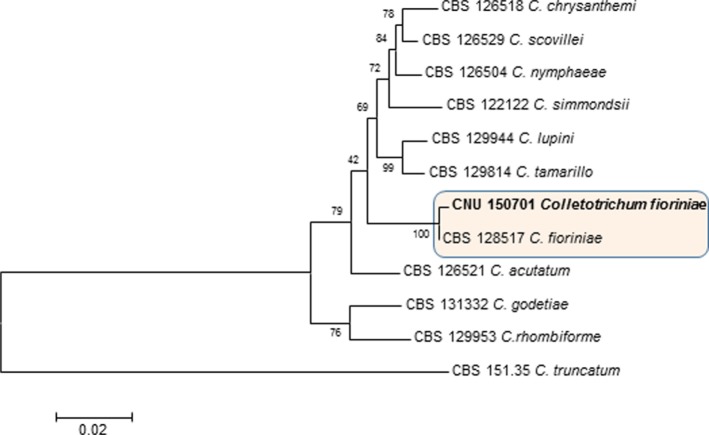
For morphological analyses, a small portion of a mycelium from the fungal culture was removed and mounted in a drop of lacto phenol [10]. Colonies on the PDA medium were pale wine in color with an aerial mycelium containing an orange cluster of conidia. The growth rate was 35~40 mm/wk. Colonies on V8 juice were pale smoke-grey to olivaceous on one side and purplish grey on the reverse side, and the growth rate was 40~45 mm/wk (Fig. 1G and 1H). Setae were not observed. Acervuli were salmon to orange in color. Conidia were hyaline, straight, aseptate, smooth-walled, fusiform to cylindrical with both ends pointed or slightly acute, 9.5~17.5 × 4~5.5 µm (Fig. 1E) in size. Appressoria were solitary or in a loose group having clavate to irregular outline, smooth walled, brown colored, with the entire edge undulating, 9~12 × 5~7 (Fig. 1F). Actually, C. fioriniae is similar to C. acutatum in morphological characteristics. However, appressoria shapes are a little different between these 2 species. Appressoria of C. acutatum were solitary, medium brown, smooth-walled, ellipsoidal to obovate, (7.3 ± 2.0) × (5.4 ± 1.2) µm in size [11]. Morphological characteristics are typical of C. fioriniae [11] as described in Table 1.
Table 1. Comparison of morphological characteristics of the isolate under study with those of previously reported Colletotrichum fioriniae.
aSource of description and illustration [11].
Sequence analysis of the type strain is an important method in the research on identification of Colletotrichum [12,13]. Morphological analyses were validated by molecular analysis. For this purpose, genomic DNA was extracted from a representative isolate using the method described by Cenis [14]. For amplification of the internal transcribed spacer (ITS) rDNA regions, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-tubulin-2 (BT2) genes, we used primers ITS1 and ITS4 [15]; GDF and GDR [16], and T1 [17] and BT-2b [18], respectively. Conditions for PCR amplification were described by Prihastuti et al. [7]. The PCR products were purified and directly sequenced with the same primers. The obtained sequence data were compared with all fungal sequence data available in the NCBI-GenBank database using the BLAST search tool [19]. Compared with the sequence of C. fioriniae in GenBank, the amplicons showed 100% homology with the GAPDH sequences (GenBank accession No. KM252196), 99% with the ITS sequence (GenBank accession No. JI121192), and 99% with the BT sequence (GenBank accession No. KT728899). The GAPDH and β-tubulin sequences from a representative isolate (CNU150701) were deposited in GenBank under accession No. KX619446 and No. KX685944. The sequence was aligned manually with those of closely related strains in the ClustalW2 software (http://www.ebi.ac.uk/Tools/phylogeny/clustalw2_phylogeny/). The evolutionary tree was constructed using the distance-based neighbor-joining method of the MEGA 5 software [20]. The reliability of the evolutionary relations in this tree was evaluated by 1,000 bootstrap replications to determine the percentage for each clade (Fig. 2). The sequence of C. truncatum served as the outgroup for both GAPDH and β-tubulin data. Accordingly, the molecular analysis confirmed the morphological identification of the fungal pathogen. Based on the observed pathological signs, pathogenicity, morphology, and molecular characterization, this fungus was identified as C. fioriniae [11].
Fig. 2. The phylogenetic tree generated by a maximum parsimony analysis of a combined dataset of ITS, GAPDH, and BT2 gene sequences of Colletotrichum fioriniae and those of other Colletotrichum spp. obtained from GenBank. The numbers above the branches represent bootstrap values. Colletotrichum truncatum (CBS 151.35) served as the outgroup. The fungal strain identified in this study is shown in boldface. ITS, internal transcribed spacer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; BT2, β-tubulin-2.
C. fioriniae is the only representative of Clade 3 and a phylogenetically distinct species of the C. acutatum complex [11]. The clade has been recognized as a distinct species complex for some years now [21] and was accepted as a separate species [22]. This species commonly causes anthracnose in many plants worldwide and has been reported to be entomopathogenic [11,12,13,14,15,16,17,18,19,20,21,22,23]. Anthracnose on Barbary wolfberry caused by C. fioriniae was reported in China [24]. However, there are no reports of the occurrence of anthracnose caused by C. fioriniae on Chinese matrimony vine in Korea, and to the best of our knowledge, this is the first such study.
ACKNOWLEDGEMENTS
We are greatly thankful to Mr. Lee Boo Hee from Chungnam Agricultural Research and Extension for providing excellent help in many ways with the collection of the Chinese matrimony vine samples. This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (Project No. NRF-2014R1A1A2057824), Republic of Korea.
References
- 1.Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 2.Monzón Ballarín S, López-Matas MA, Sáenz Abad D, Pérez-Cinto N, Carnés J. Anaphylaxis associated with the ingestion of Goji berries (Lycium barbarum) J Investig Allergol Clin Immunol. 2011;21:567–570. [PubMed] [Google Scholar]
- 3.Yim DS. Analysis of production and trade of Lycium chinense Mill. in Korea and China. J Korean Soc Int Agric. 2012;24:425–428. [Google Scholar]
- 4.Kim WG, Koo HM, Kim KH, Hyun IH, Hong SK, Cha JS, Lee YK, Kim KH, Choi HS, Kim DG, et al. List of plant disease in Korea. 5th ed. Anyang: Korean Society of Plant Pathology; 2009. pp. 224–225. [Google Scholar]
- 5.Paul NC, Lee HB, Lee JH, Shin KS, Ryu TH, Kwon HR, Kim YK, Youn YN, Yu SH. Endophytic fungi from Lycium chinense Mill and characterization of two new Korean records of Colletotrichum. Int J Mol Sci. 2014;15:15272–15286. doi: 10.3390/ijms150915272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam MH, Park MS, Lee HD, Yu SH. Taxonomic reevaluation of Colletotrichum gloeosporioides isolated from strawberry in Korea. Plant Pathol J. 2013;29:317–322. doi: 10.5423/PPJ.NT.12.2012.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prihastuti H, Cai L, Chen H, McKenzie EH, Hyde KD. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009;39:89–109. [Google Scholar]
- 8.Kanchana-udomkan C, Taylor PW, Mongkolporn O. Development of a bioassay to study anthracnose infection of Capsicum chinense Jacq. fruit caused by Colletotrichum capsici. Thai J Agric Sci. 2004;37:293–297. [Google Scholar]
- 9.Lin Q, Kanchana-udomkan C, Jaunet T, Mongkolporn O. Genetic analysis of resistance to pepper anthracnose caused by Colletotrichum capsici. Thai J Agric Sci. 2002;35:259–264. [Google Scholar]
- 10.Cappuccino JG, Sherman N. Microbiology: a laboratory manual. Tempe (AZ): Benjamin Cummings; 2001. pp. 211–223. [Google Scholar]
- 11.Damm U, Cannon PF, Crous PW. Colletotrichum: complex species or species complexes? Stud Mycol. 2012;73:1–213. doi: 10.3114/sim0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai L, Hyde KD, Taylor PW, Weir BS, Waller JM, Abang MM, Zhang JZ, Yang YL, Phoulivong S, Liu ZY, et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009;39:183–204. [Google Scholar]
- 13.Phoulivong S, Cai L, Chen H, McKenzie EH, Abdelsalam K, Chukeatirote E, Hyde KD. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 2010;44:33–43. [Google Scholar]
- 14.Cenis JL. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acid Res. 1992;20:2380. doi: 10.1093/nar/20.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 16.Templeton MD, Rikkerink EH, Solon SL, Crowhurst RN. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene. 1992;122:225–230. doi: 10.1016/0378-1119(92)90055-t. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 18.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Biotechnology Information. Genebank overview [Internet] Bethesda (MD): National Center for Biomedical Information; 2009. [cited 2009 Nov 20]. Available from: http://www.ncbi.nlm.nih.gov/Genbank. [Google Scholar]
- 20.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talhinhas P, Sreenivasaprasad S, Neves-Martins J, Oliveria H. Molecular and phenotypic analyses reveal association of Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl Environ Microbiol. 2005;71:2987–2998. doi: 10.1128/AEM.71.6.2987-2998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivas RG, Tan YP. A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Divers. 2009;39:111–122. [Google Scholar]
- 23.Marcelino J, Giordano R, Gouli S, Gouli V, Parker BL, Skinner M, TeBeest D, Cesnik R. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia. 2008;100:353–374. doi: 10.3852/07-174r. [DOI] [PubMed] [Google Scholar]
- 24.Liu LP, Yang LY, Liu YN, Yang LN, Lu BH, Yu L, Jin XS, Wang X, Yang C, Li Y, et al. First report of anthracnose disease caused by Colletotrichum fioriniae on barbary wolfberry in China. Plant Dis. 2016;100:2534. [Google Scholar]