Abstract
Optimal gene therapy for cancer must (i) deliver DNA to tumor cells with high efficiency, (ii) induce minimal toxicity, and (iii) avoid gene expression in healthy tissues. To this end, we generated a library of >500 degradable, poly(β-amino esters) for potential use as nonviral DNA vectors. Using high-throughput methods, we screened this library in vitro for transfection efficiency and cytotoxicity. We tested the best performing polymer, C32, in mice for toxicity and DNA delivery after intratumor and i.m. injection. C32 delivered DNA intratumorally ≈4-fold better than one of the best commercially available reagents, jetPEI (polyethyleneimine), and 26-fold better than naked DNA. Conversely, the highest transfection levels after i.m. administration were achieved with naked DNA, followed by polyethyleneimine; transfection was rarely observed with C32. Additionally, polyethyleneimine induced significant local toxicity after i.m. injection, whereas C32 demonstrated no toxicity. Finally, we used C32 to deliver a DNA construct encoding the A chain of diphtheria toxin (DT-A) to xenografts derived from LNCaP human prostate cancer cells. This construct regulates toxin expression both at the transcriptional level by the use of a chimeric-modified enhancer/promoter sequence of the human prostate-specific antigen gene and by DNA recombination mediated by Flp recombinase. C32 delivery of the A chain of diphtheria toxin DNA to LNCaP xenografts suppressed tumor growth and even caused 40% of tumors to regress in size. Because C32 transfects tumors locally at high levels, transfects healthy muscle poorly, and displays no toxicity, it may provide a vehicle for the local treatment of cancer.
Keywords: prostate, cationic polymers
Despite technical advances, the main treatments that are used today to treat cancer (surgery, radiation therapy, and chemotherapy) reduce tumor burden but are not curative in about half of cancer patients. The fact that each year more than half a million people in the United States die from cancer underscores the need for more effective treatments.
Gene therapy aimed at targeting the death of cancer cells is an attractive therapeutic option that holds much promise. Although several strategies using so-called suicide genes for the treatment of cancer have been explored, one in particular, a strategy relying on expression of diphtheria toxin (DT) may be especially suited for the treatment of a variety of different kinds of cancer. In contrast to other suicide genes that act by disrupting DNA synthesis, thus targeting rapidly dividing cells, DT is a potent inhibitor of protein synthesis that acts in a cell cycle-independent fashion, killing both quiescent and rapidly dividing tumor cells (1). This property suggests that DT may be an effective therapy for aggressively growing tumors (e.g., melanoma) as well as for other tumor types that grow more slowly (e.g., prostate tumors).
Naturally occurring DT is made by Corynebacterium diphtheriae as a secreted precursor polypeptide composed of two subunits, the A and B chains, linked together by a disulfide bond. The B chain binds to the surface of most eukaryotic cells and then delivers the A chain of DT (DT-A) into the cytoplasm. Once inside the cell, DT-A inhibits protein synthesis by catalyzing ADP-ribosylation of elongation factor 2 (2). A DT gene engineered for use in mammalian cells, DT-A, contains the coding sequence for the DT-A subunit but not for the DT-B subunit (3). The DT-A subunit is retained within the cytoplasm of the cell. In the absence of the B subunit, DT-A released from dead cells is not able to enter other neighboring cells. This property ensures that only targeted cells are killed by the toxin.
DT is extremely potent; a single molecule is sufficient to kill a cell (4). To avoid unwanted deleterious effects to normal, healthy cells, it is therefore important that gene therapy strategies employing DT for the treatment of cancer incorporate mechanisms to tightly regulate and target DT expression to tumor cells. One method of attaining tight, cell-specific regulation of genes encoding toxins is through the use of site-directed recombinases (e.g., Cre and Flp recombinase) (4–6). In these strategies, gene expression is controlled by both transcriptional regulation and regulated DNA recombination mediated by site-directed recombinases. In those cells where a recombinase is expressed, a gene sequence containing the target sequences for the recombinase undergoes recombination, thereby becoming functional. The recombination event takes place only in selected cells because a cell-specific promoter controls the expression of the recombinase. This strategy was previously used, by using replication-defective adenoviruses to deliver DNA, to effectively kill tumor cells in xenografts derived from human prostate cancer cells (5). Infection with two viruses was required to accommodate all of the sequences needed for regulation of DT-A expression, and this requirement proved to be a complicating factor experimentally.
To avoid the limitations and serious side effects associated with viral vectors, as well as toxicity and poor delivery efficiency associated with nonviral systems (6, 7), we report here the synthesis and use of cationic poly(β-amino esters) for DNA delivery to cancer cells. Like other nonviral delivery systems, cationic polymers offer a number of potential advantages, including ease of production, stability, low immunogenicity and toxicity, and capacity to deliver larger DNA payloads (8). Through electrostatic interaction, cationic DNA delivery polymers can spontaneously bind and condense DNA into nanoparticles (6).
We have been particularly interested in poly(β-amino esters) as delivery agents, because they are highly effective in vitro, biodegradable, and easily synthesized by the conjugate addition of a primary amine or bis(secondary amine) monomer to a diacrylate monomer. In previous studies, a large library of >2,500 poly(β-amino esters) was synthesized in a ≈70-mg scale to allow for high-throughput manipulation of reagents (9). It was subsequently discovered that both molecular weight and end-group termination can have order-of-magnitude effects on the transfection potential of poly(β-amino esters) (10).
Using structure/function data from these initial studies, we used preferred monomers and optimized polymerization conditions to generate a new polymer library of >500 poly(β-amino esters), seeking to synthesize polymers with optimal transfection potential and biocompatibility. All members of the polymer library were screened by using an in vitro high-throughput transfection efficiency assay at different polymer/DNA ratios. The top performing DNA-delivery polymers were also screened in vitro for cytotoxicity. From these studies, we identified one polymer, C32, that transfects cells with higher efficiency than all of the other polymers and with no associated cytotoxicity. We tested efficiency of C32 delivery of DNA in mice after intratumoral (i.t.) and i.m. injection and tested for toxicity. C32 delivered DNA i.t. ≈4-fold better than one of the best commercially available reagents, jetPEI (polyethyleneimine, PEI) (Qbiogene, Montreal), and orders of magnitude better than naked DNA. Conversely, the highest transfection levels after i.m. administration were achieved with naked DNA, followed by PEI; transfection was rarely observed with C32. Additionally, PEI induced local toxicity after i.m. injection, whereas C32 demonstrated no toxicity.
Finally, we used C32 to deliver a DNA construct encoding the DT-A to cells in culture and to xenografts derived from LNCaP human prostate cancer cells. We regulated toxin expression both transcriptionally by the use of a chimeric modified enhancer/promoter sequence of the human prostate-specific antigen (PSA) gene (11) and by DNA recombination mediated by the site-directed recombinase, Flp (5). C32 delivery of DT-A DNA arrested protein synthesis in tumor cells in culture and suppressed the growth of human xenografts. In fact, 40% (6 of 15) of treated tumors regressed in size. Direct administration to tumors of C32-delivered DNA encoding targeted DT-A expression may provide a more effective treatment after further clinical trials with fewer toxic side effects and less damage to normal healthy surrounding tissues than those therapies that are currently used to treat localized cancer.
Experimental Procedures
Polymer Synthesis. Monomers were purchased from Aldrich, TCI America (Portland, OR), Pfaltz & Bauer, Matrix Scientific (Columbia, SC), Scientific Polymer (Ontario, NY), and Monomer-Polymer and Dajac Laboratories (Feasterville, PA). Six to 12 versions of each polymer were generated by varying the amine/diacrylate stoichiometric ratio. To synthesize each polymer, 500 mg of amino monomer was weighed into an 8-ml sample vial with a Teflon-lined screw cap. The appropriate amount of diacrylate was added to the vial to yield a stoichiometric ratio ranging from 0.6 to 1.4. A small Teflon-coated stir bar was then put in each vial. Polymers were synthesized on a multiposition magnetic stir-plate residing in an oven at (i) 95°C and solvent free or (ii) 60°C with 2 ml of DMSO added. High-temperature synthesis was performed for ≈12 h, and low temperature synthesis was performed for 2 days. After completion of reaction, all vials were removed from the oven and stored at 4°C. Luciferase transfection assays were performed as described in ref. 9.
Measurement of Cytotoxicity. COS-7 cells (American Type Culture Collection) were seeded (14,000 cells per well) into clear plates. After 24 h, increasing amounts of polymer, from 10 to 800 μg/ml, in Opti-MEM (Invitrogen) were added to the cells. Cells were incubated with the polymer for 1 h, and then the medium was replaced and metabolic activity was measured by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) Cell Proliferation Assay kit (American Type Culture Collection) after 1 day. Ten microliters of MTT Reagent was added to each well. After 2-h incubation at 37°C, 100 μl of Detergent Reagent was added to each well. The plate was then left in the dark at room temperature for 4 h. Optical absorbance was measured at 570 nm by using a SpectraMax 190 microplate reader (Molecular Devices) and converted to percent viability relative to control (untreated) cells.
Cells. PC3 cells (American Type Culture Collection) were cultured in DMEM supplemented with 10% FBS. LNCaP cells (UroCor, Oklahoma City, OK) were cultured in RPMI medium 1640 supplemented with 10% FBS. All cells were maintained at 37°C in 5% CO2, balance air.
DNA for in Vivo Tests. For details on the construction of three plasmid constructions used in this study, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site. The plasmids are pCAG/luc, containing a firefly luciferase coding sequence regulated by a very strong, ubiquitously expressed promoter/enhancer; RSV/FRT2PSA.FLP/EGFP and pRSV/FRT2PSA.FLP/DT-A, two constructs containing Flp recombinase target sequences (FRT) flanking PSA promoter-driven Flp recombinase; and pRSV/EGFP, used in the construction of pRSV/FRT2PSA.FLP/DT-A.
All restriction enzymes were purchased from Promega. Salmon testes DNA (Sigma-Aldrich) served as a negative control in xenograft experiments.
Muscle and Xenograft Experiments. DNA (either naked or complexed to C32 or PEI) was administered to 8-week old nu/nu male mice (Harlan Bioproducts for Science, Indianapolis) either by i.m. or i.t. injection. To complex plasmid DNA to C32, the polymer was dissolved in DMSO (100 mg/ml). DNA (50 μg) was suspended in 25 μl of 25 mM sodium acetate buffer (pH 5.0) and mixed with C32 polymer (300 or 1,500 μg), also diluted in 25 μl of 25 mM sodium acetate buffer (pH 5.0). After incubation of the polymer/DNA mixture at room temperature for 5 min, 10 μl of 30% glucose in PBS was added to the 50-μl polymer/DNA mixture.
Plasmid DNA was complexed to jetPEI according to the manufacturer's protocol for in vivo administration except that when 50 μg of DNA was complexed, the volume was reduced to 60 μl. Uncomplexed pCAG/luc DNA (50 μg in 100 μl of 5% glucose in 25 mM sodium acetate) was also administered to mice. For i.m. injections, a 28-gauge needle was used to deliver a 100-μl volume to the hind leg muscle. Xenografts were generated by s.c. injection of 5 × 105 PC3 cells and 2 × 106 LNCaP cells in PBS plus 20% Matrigel. A 26-gauge needle was used to deliver a 60-μl volume to tumors. Calipers were used to measure the length and width of some tumors. Mice were killed 2 days after i.t. injections and 20 days after i.m. injections.
Blood Analysis. A cardiac puncture was performed at killing, and serum was sent to an outside laboratory (LabCorp, Research Triangle Park, NC) for analysis of creatinine, total bilirubin, alkaline phosphatase, alanine aminotransferase, γ-glutamyltransferase, lactate dehydrogenase, and creatine kinase.
Optical Imaging. In vivo bioluminescence imaging was performed by using an IVIS imaging system and living image acquisition and analysis software (Xenogen, Alameda, CA). d-luciferin (potassium salt) (Xenogen) was dissolved to 15 mg/ml in PBS and stored at –20°C. Mice were anesthetized with Avertin and subsequently injected i.p. with luciferin (150 μg per g of body weight). Whole-body images were acquired 10–20 min after luciferin administration. An integration time of 1 min (tumors) or 5 min (muscle) was used for luminescent image acquisition.
Histological Analysis. Tumor and muscle samples were fixed in formalin, embedded in paraffin, and sectioned and stained with hematoxylin and eosin according to standard procedures. The samples were thinly sectioned before embedding, and multiple levels were examined for each sample to minimize the possibility of not visualizing the injection site. Microscopic evaluation was performed on a BX41 microscope (Olympus, Melville, NY) equipped with an Olympus Q-Color digital camera for image capture.
Results
Polymer Synthesis. Previous work with poly(β-amino esters) identified several monomers as frequently present in effective gene delivery polymers (9). In general, the most effective polymers were composed of primary amino monomer containing an alcohol, imidazole, or a secondary diamine. Acrylate monomers in effective polymers were almost always hydrophobic (Fig. 1). However, because both molecular weight and end-group termination can have order-of-magnitude effects on the transfection potential of poly(β-amino esters) (10), we sought to further optimize polymer transfection potential.
Fig. 1.
Amino (6–94) and acrylate (B–LL) monomers used to create poly(β-amino ester) library.
Differences in the stoichiometric ratio of amine monomer to acrylate monomer ranging from 0.6 to 1.4 substantially affect the molecular weight of poly(β-amino esters) and their DNA transfection efficiency (10). In particular, it was observed that polymers formed with an excess of amine monomer tend to be more effective. We therefore synthesized some polymers at amine/acrylate ratios of 0.6 and 1.4, and at 10 different ratios between these values (0.6, 0.8, 0.9, 0.95, 0.975, 1.0, 1.025, 1.05, 1.1, 1.2, 1.3, and 1.4). To allow for greater control of monomer stoichiometry and, therefore, better control over polymer molecular weight and chain end groups, polymer synthesis was scaled up to gram amounts. All polymers were synthesized by adding acrylate monomer, resulting in the appropriate stoichiometric ratio, to 500 mg of amine monomer. A total of 70 monomer combinations were used with 6–12 monomer ratios for each combination (Fig. 2). Polymerizations were first performed at 95°C in the absence of solvent to maximize monomer concentration. In some cases, polymers became crosslinked or overly hard at 95°C. Therefore, some polymers were synthesized at 60°C in the presence of 2 ml of DMSO to reduce potential hardening. Residual DMSO was diluted to <0.4% in all cases before addition to cells and had no effect on transfection or cytotoxicity (data not shown).
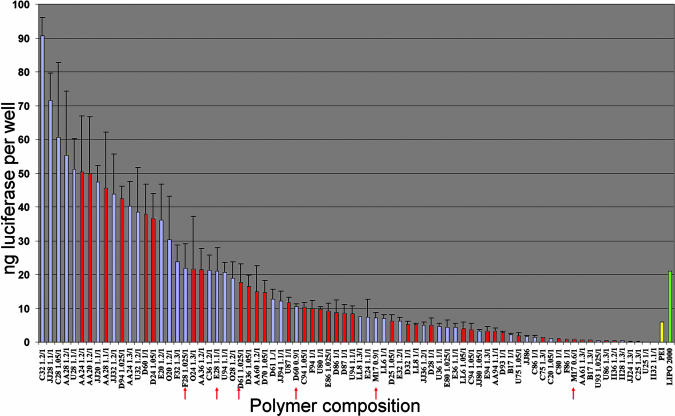
Fig. 2.
In vitro transfection potential of poly(β-amino esters). The transfection potential of polymers synthesized at the optimal amine/acrylate ratio and at the optimal polymer/DNA ratio is shown. Polymers were synthesized at 6 amine/acrylate ratios (1, 1.025, 1.05, 1.1, 1.2, and 1.3) unless marked with a red arrow, in which case they were synthesized at 12 amine/acrylate ratios (0.6, 0.8, 0.9, 0.95, 0.975, 1, 1.025, 1.05, 1.1, 1.2, 1.3, and 1.4). Polymers were synthesized at 95°C in the absence of solvent (blue bars) or at 60°C in the presence of 2 ml of DMSO (red bars). The amine/acrylate ratio of the optimal polymer is listed next to the monomer composition.
Transfection Efficiency. We performed in vitro transfection assays by using all polymers at six different polymer/DNA ratios to determine the transfection efficiency of each polymer. COS-7 cells were transfected with plasmid DNA encoding the firefly luciferase reporter gene (pCMV-Luc). To facilitate performance of the >12,000 transfections (data obtained in quadruplicate), experiments were done in the 96-well plate format. Luciferase expression levels were determined by using a commercially available luciferase assay kit and a 96-well luminescence plate reader.
The transfection efficiency of polymers synthesized at the optimal monomer ratios and at the optimal polymer/DNA ratio is shown in Fig. 2. Eighteen polymers transfected cells with higher efficiency than did Lipofectamine (21 ng of luciferase per well), and 43 performed better than PEI (polymer/DNA ratio of 1:1 wt/wt, 6 ng of luciferase per well) under the same conditions. The polymers yielding the highest transfection efficiency were C32, JJ28, and C28 (91, 72, and 61 ng of luciferase per well, respectively). Consistent with previous work, these polymers and other top-performing polymers contain amines with alcohol groups and hydrophobic acrylates. It is important to note that the overall transfection levels and the monomer composition of the most effective polymers are both higher than and different from those identified in our preliminary, high-throughput screening library (9). These differences highlight the important influence of molecular weight, chain end-group identity, and polymer/DNA ratio on transfection efficiency.
Cytotoxicity. We tested 13 of the most effective gene delivery poly(β-amino esters) in vitro for cytotoxicity by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (Fig. 7, which is published as supporting information on the PNAS web site). COS-7 cells were incubated with various amounts of polymer (10–800 μg/ml) in Opti-MEM for 1 h and then assayed 24 h later. Although treatment of cells with some polymers (e.g., AA20) resulted in some toxicity, especially at higher polymer concentrations, all of the poly(β-amino esters) were significantly less toxic than PEI. C32 (1.2:1 amine/acrylate ratio), the most efficient transfection polymer, was not toxic over the concentration range tested. Because C32 is both highly effective at gene delivery and demonstrates no toxicity in vitro, we chose to use this polymer for in vivo gene delivery studies.
In Vivo DNA Delivery. To test the utility of C32 for gene delivery in vivo, we examined transfection in a mouse xenograft model. PC3 human prostate tumor cells were mixed with Matrigel and inoculated s.c. into the flanks of nude mice to generate tumors. When tumor volumes were ≈300 mm3, we injected C32-pCAG/luc nanoparticles i.t. (50 μg of DNA per injection, 30:1 polymer/DNA ratio). For comparison, we injected tumors with DNA complexed with in vivo jetPEI, the state-of-the-art commercially available transfection polymer, and with naked DNA. Control tumors were injected with 25 mM sodium acetate buffer at pH 5.0 (n = 5 for each treatment group). Forty-eight hours after injection of DNA, we imaged mice and quantified bioluminescence by using an IVIS Bioluminescence Imaging System (Fig. 3). The average transfection mediated by C32 was 4-fold higher than transfection mediated by PEI and 26-fold higher than transfection by naked DNA.
Fig. 3.
Tumor transfection in vivo. Xenografts of PC3 human prostate tumor cells were injected with C32 (1.2:1 amine/acrylate ratio) complexed to pCAG/luc DNA at a 30:1 polymer/DNA ratio; in vivo jetPEI complexed to pCAG/luc DNA according to the manufacturer's instructions; and naked pCAG/luc DNA. Two days after transfection, mice were imaged and bioluminescence was quantified. (Left) Pseudocolor images representing light emitted from tumors superimposed over grayscale reference image of representative mice from each group of five. (Right) Quantification of the emitted photons from each tumor. Horizontal bars indicate the mean value for each treatment group.
We also measured transfection of healthy muscle by using C32, PEI, and naked DNA (Fig. 4) (n = 5 for each group). We injected complexed DNA or naked DNA into muscle and measured luciferase expression 2, 6, and 20 days after injection. Interestingly, transfection results were completely opposite that of i.t. transfection. Naked DNA resulted in the highest levels of gene expression over the course of the experiment. Transfections with PEI resulted in lower and delayed expression, and those with C32 resulted in even lower transfection.
Fig. 4.
Muscle transfection in vivo. Healthy muscle was injected with C32 (1.2:1 amine/acrylate ratio) complexed to pCAG/luc DNA at a 30:1 polymer/DNA ratio; in vivo jetPEI complexed to pCAG/luc DNA according to the manufacturer's instructions; and naked pCAG/luc DNA. Two, 6, and 20 days after transfection, mice were imaged and bioluminescence was quantified. (Upper) Pseudocolor images representing light emitted from muscle superimposed over grayscale reference image of representative mice from each group of five. (Lower) Quantification of the emitted photons from each injected muscle. Horizontal bars indicate the mean value for each treatment group.
Biocompatibility. Histological sections of tumors injected with DNA complexes or with naked DNA revealed a poorly differentiated carcinoma with occasional foci of necrosis and numerous mitotic figures within s.c. tissue; no histologic differences were observed between any of the groups. Histological sections of muscle at the site of injection from all of the PEI/DNA complexes contained foci of calcification associated with myocyte nuclear internalization and atrophy, consistent with myocyte damage (Fig. 5). Similar analysis of muscle injected with C32/DNA complexes and naked DNA demonstrated no pathology. No statistically significant differences were observed between i.m. injection of C32/DNA, PEI/DNA, naked DNA, or buffer in serum levels of markers of renal function (creatinine), liver function (total bilirubin, alkaline phosphatase, alanine aminotransferase, γ-glutamyltransferase, and lactate dehydrogenase), or muscle damage (creatine kinase) (data not shown). The absence of a positive creatine kinase signal for muscle damage with PEI is attributed to the relatively small area of damage at the injection point.
Fig. 5.
Histological analysis of muscle and tumors after transfection. Photomicrographs of hematoxylin and eosin-stained sections of muscle (A and B) and tumor (C and D) taken with a 10× objective. (A) Muscle injected with PEI/DNA shows damaged myocytes with calcifications, indicated with arrows. (B) Muscle injected with C32/DNA shows no pathology. (C) Uninjected tumor control. (D) Tumor injected with C32/DNA shows no histological differences from control tumor.
C32-Delivered DNA Encoding DT-A Arrests Protein Synthesis in Prostate Cancer Cells in Vitro. DT-A catalyzes the transfer of ADP-ribose from NAD to a modified histidine residue on elongation factor 2, thereby inhibiting protein synthesis and resulting in cell death (12). To test the ability of C32-delivered DNA encoding DT-A to inhibit protein synthesis in prostate cancer cells, we transfected LNCaP cells with C32-pCAG/luc and with a second C32 formulation, either C32-pRSV/FRT2PSA.FLP/EGFP or C32-pRSV/FRT2PSA.FLP/DT-A. Control cells were transfected with C32-pRSV/FRT2PSA.FLP/EGFP only. Forty-eight hours later, we prepared protein extracts from cells and assayed luciferase activity. Luciferase activity in cells transfected with the DT-A construct was >10× lower than in cells transfected with the enhanced green fluorescent protein construct (Fig. 8, which is published as supporting information on the PNAS web site). These results demonstrate that after the C32 delivery of a DNA construct in which DT-A expression is regulated both transcriptionally and by DNA recombination, DT-A expression in prostate cancer cells effectively inhibits protein synthesis.
Tumor Regression After C32-Delivered DT-A DNA. Having used a luciferase reporter construct to establish that C32 polymer can effectively transfer DNA to xenografts, we wished to determine whether C32-delivered DNA encoding DT-A would inhibit growth of tumor cells in vivo. LNCaP human prostate cancer cells were mixed with Matrigel and inoculated s.c. into the flanks of nude mice to generate tumors. When tumors attained a volume of ≈100 mm3, we injected either C32-pRSV/FRT2PSA.FLP/DT-A or C32/salmon testes DNA i.t. (50 μg of DNA per injection, 30:1 polymer/DNA ratio). We administered C32/DT-A to tumors five more times every other day, for a total of six injections. We used calipers to measure tumor size before the first injection and on the final day, at which time the mice were killed. The average growth rate of tumors injected with C32-pRSV/FRT2PSA.FLP/DT-A was suppressed 2-fold compared with control tumors (P < 0.0001) (Fig. 6). In fact, 3 of 15 tumors treated with C32/DT-A failed to grow at all and 6 of 15 actually regressed in size. We conclude that expression of C32-delivered DT-A suppressed tumor growth and was capable of achieving tumor regression.
Fig. 6.
Tumor growth after i.t. injection of C32-pRSV/FRT2PSA.FLP/DT-A or C32/salmon sperm DNA nanoparticles. Nanoparticles were injected on day 0 and then every other day for a total of six injections (50 mg of DNA per injection, 30:1 polymer/DNA ratio). Tumor volume was measured with calipers on day 0 and day 11. Fold increase in tumor volume is the ratio of these two measurements. Horizontal bars indicate the mean value for each treatment group.
Discussion
In this study, we synthesized a library of >500 poly(β-amino esters) and selected C32 polymer for tumor delivery studies based on its high performance in in vitro transfection assays and the absence of cell toxicity and tissue pathology associated with its use. We demonstrate that C32 effectively delivers a DT-A-encoding DNA to human prostate cancer cells, resulting in protein synthesis arrest in cells grown in culture and a significant reduction in tumor growth in mice after i.t. injections. In synthesizing the polymers in this library, we used 70 combinations of amines and acrylates and varied the amine/acrylate ratio for each combination over a range of 0.6 to 1.4. We tested the transfection efficiency of each of the resulting polymers at different polymer/DNA ratios. Consistent with the analysis of a high-throughput screening library, the top-performing polymers, including C32, contain amines with alcohol groups and hydrophobic acrylates (9).
Early in the synthesis and testing of polymers and in previous work (10), we observed that in general, acrylate-terminated polymers transfected cells less effectively than amino-terminated polymers. Therefore, we synthesized a majority of polymers with an excess of the amino monomer. One possible explanation for the observed poor performance of acrylate-terminated polymer is that the reactive ends attach covalently to DNA or cell membranes. Although it is unclear how amino termination improves transfection, the extra positive charge may assist DNA complexation or some step in the transfection process between endocytosis and gene expression.
In further analyses of the polymers in this new library, we have determined that, in addition to the polymer/DNA ratio, polymer molecular weight as well as size and charge of particles after DNA condensation are other properties that affect the efficiency of DNA transfection (unpublished data).
The ability to transfect mammalian cells in vitro could prove useful in the ex vivo modification of cells. However, the ability to successfully mediate in vivo delivery would greatly broaden the scope and utility of these polymers. As a model of local transfection, we examined the ability of our best performing polymer, C32, synthesized at 1.2:1 amine/acrylate ratio, to transfect tumor xenografts and healthy muscle in nude mice. After i.t. injection, C32 transfected tumor cells ≈4-fold better than the state-of-the-art gene-delivery polymer, in vivo jetPEI, and orders of magnitude better than naked DNA (Fig. 3). Conversely, i.m. naked DNA injections result in high levels of gene expression, whereas PEI resulted in a lesser, delayed expression and C32 was ineffective at transfecting muscle (Fig. 4). C32 and naked DNA injection was well tolerated, and no specific pathological changes were observed. However, muscle near the site of injection of PEI/DNA complexes showed signs of myocyte damage (Fig. 5), consistent with previous reports (7). This result is despite the fact that the mass of C32 injected was 30-fold more than that of PEI, further highlighting the toxicity differences between these materials.
Interestingly, although we observed luciferase reporter gene expression after i.t. C32 delivery of DNA to xenografts, no gene expression was observed in muscle after i.m. injection of C32/DNA complexes. This result suggests that clinical use of C32 as a DNA delivery vector may help target DNA expression to tumor cells and avoid unwanted deleterious effects to healthy surrounding tissues. Future studies should explore this possibility further by i.t. injection of C32/DNA to autochthonous prostate tumors in mouse models for prostate cancer, e.g., the TRAMP model (13).
To avoid unwanted, deleterious effects to normal healthy cells, the use of suicide genes in gene therapy strategies for the treatment of cancer requires tight control of gene expression. To address the need for tight control, we used a genetic strategy that is based on both transcriptional regulation and DNA recombination mediated by a site-directed recombinase, Flp recombinase, to regulate the expression of the DT-A gene (5). We used a modified promoter/enhancer of the human PSA gene (11) to target expression of Flp recombinase to prostate cells. A recombination event required for the expression of DT-A provides a second level of control, helping to ensure that DT is expressed only in cells in which the PSA promoter/enhancer is expressed above basal levels.
In contrast to our studies using an adenoviral vector to deliver DT-A-encoding DNA to cells (5), in this study we use nonviral polymeric nanoparticles as the DNA vector. Previously, two DNA constructs were necessary, each delivered by a virus, to introduce DNA encoding DT-A and the necessary regulatory sequences to cells. Infection of cells with two viruses necessitated high viral loads and proved technically challenging. With an eye to making our strategy more practical for clinical use, we generated the RSV/FRT2PSA.FLP/DT-A construct used in this study. We inserted this construct, containing DT-A and all of the required regulatory elements and designed for economy of sequence length (≈7 kb), into a standard adenoviral shuttle vector. To our surprise, we discovered that it was impossible to produce a virus containing this sequence because the PSA regulatory sequence is active in 293 packaging cells. As a result, the cells expressed Flp recombinase, and viruses contained sequences that had undergone recombination resulting in excision of the FRT-flanked PSA.FLP cassette. By necessity, then, we turned to nonviral polymeric nanoparticles to deliver the RSV/FRT2PSA.FLP/DT-A sequence. In addition to providing a means for circumventing the problems we encountered with the production of viruses, use of polymeric nanoparticles allows for multiple administrations and avoids the risk associated with using adenoviral vectors for therapeutic treatments.
We have generated a library of degradable polymer-based vectors that surpasses the best commercially available nonviral vectors for in vitro and in vivo gene transfer. In particular, C32 displays many of the characteristics of an effective, local tumor delivery system: (i) an ability to induce high levels of local transfection in tumors, (ii) an inability to transfect healthy muscle, and (iii) an absence of toxicity. Furthermore, we have shown here that direct administration to tumors of a poly(β-amino ester) complexed to DNA encoding the toxin DT-A effectively inhibits tumor growth. By judicious selection of other cell and/or tumor-specific promoter sequences to regulate DT-A expression transcriptionally, this strategy can be adapted to the treatment of tumors in other tissues. Modifying polymers for efficient delivery of DNA to specific cells after systemic administration should extend application of this gene therapy to the treatment of metastatic disease.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (through the Massachusetts Institute of Technology Biotechnology Process and Engineering Center) (R.L.), National Institutes of Health Grants EB00244 (to R.L.) and CA90841 (to J.A.S.), and the John S. Sharpe Foundation of Bryn Mawr Hospital (J.A.S.).
Abbreviations: DT, diphtheria toxin; DT-A, A chain of DT; FRT, Flp recombinase target sequences; i.t., intratumoral; PEI, polyethyleneimine; PSA, prostate-specific antigen.
References
- 1.Rodriguez, R., Lim, H.-Y., Bartkowski, L. M. & Simons, J. W. (1998) Prostate 34, 259–269. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield, L., Bjorn, M. J., Horn, G., Fong, D., Buck, G. A., Collier, R. J. & Kaplan, D. A. (1983) Proc. Natl. Acad. Sci. USA 80, 6853–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxwell, I. H., Maxwell, F. & Glode, L. M. (1986) Cancer Res. 46, 4660–4664. [PubMed] [Google Scholar]
- 4.Yamaizumi, M., Mekada, E., Uchida, T. & Okada, Y. (1978) Cell 15, 245–250. [DOI] [PubMed] [Google Scholar]
- 5.Peng, W., Verbitsky, A., Bao, Y. & Sawicki, J. A. (2002) Mol. Ther. 6, 537–545. [DOI] [PubMed] [Google Scholar]
- 6.Luo, D. & Saltzman, W. M. (2000) Nat. Biotechnol. 18, 33–37. [DOI] [PubMed] [Google Scholar]
- 7.Wang, J., Zhang, P. C., Mao, H. Q. & Leong, K. W. (2002) Gene Ther. 9, 1254–1261. [DOI] [PubMed] [Google Scholar]
- 8.Ledley, F. D. (1995) Human Gene Ther. 6, 1129–1144. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, D. G., Lynn, D. M. & Langer, R. (2003) Angew. Chem. Int. Ed. 42, 3153–3158. [DOI] [PubMed] [Google Scholar]
- 10.Akinc, A. B., Anderson, D. G., Lynn, D. M. & Langer, R. (2003) Bioconjugate Chem. 14, 979–988. [DOI] [PubMed] [Google Scholar]
- 11.Wu, L., Matherly, J., Smallwood, A., Adams, J. Y., Billick, E., Belldegrun, A. & Carey, M. (2001) Gene Ther. 8, 1416–1426. [DOI] [PubMed] [Google Scholar]
- 12.Collier, R. J. (1975) Bacteriol. Rev. 39, 54–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg, N. M., DeMayo, F., Finegold, M. J., Medina, D., Tilley, W. D., Aspinall, J. O., Cunha, G. R., Donjacour, A. A., Matusik, R. J. & Rosen, J. M. (1995) Proc. Natl. Acad. Sci. USA 92, 3439–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.