Abstract
Autism spectrum disorder (ASD) is a severe neurodevelopment disorder. This study tests the hypothesis that children with ASD show atypical intrinsic complexity of brain activity. Electroencephalogram data were collected from boys with ASD and matching normal typically developing children while performing an observation and an imitation task. The multiscale entropy was estimated within the 0.5–30 Hz frequency band over 30 time scales using a coarse-grained procedure. A decreased electroencephalogram complexity was observed in the ASD children both during the observation and during the imitation tasks. On comparing the two tasks, significant differences were observed between groups in the right hemisphere, and also the central cortex for the observation task. Multiscale entropy could provide further evidence of the relationship between ASD and cerebral dysfunction.
Keywords: autism spectrum disorder, complexity, electroencephalogram, multiscale entropy
Introduction
Autism spectrum disorder (ASD) is a severe neurodevelopmental disorder that begins at birth or within the first 2.5 years of life 1. Most autistic children are characterized by deficits in communicative and social skills such as imitation, empathy, and shared attention, as well as restricted interests and repetitive patterns of behaviors 1. The exact cause of autism is still unknown, although many studies have noted differences in the structure and function of brains of individuals with autism 2.
There is a growing consensus to suggest that ASD is associated with dysfunction of the brain, perhaps because of a functional deficit of the frontal mirror neuron system (MNS) 3, and a functional disconnect between brain regions or a functional deficit of the anterior cingulate cortex 4. MNSs are primarily considered to be involved in comprehension and perception of motor actions, but they may also play a critical role in higher order cognitive processes such as the theory of the mind, imitation, empathy, and language 5. Previous studies have found that individuals with ASD may have MNS impairments and dysfunction of the MNS that may lead to impaired self-other representations and imitation 3. In addition, several pieces of evidence have also shown that ASD may be related to weak functional connectivity between remote brain regions, and an overabundance of functional connectivity within local cortical regions 6. Although these recent developments have linked the key symptoms observed in ASD to a function defect of MNS and lack of integration between brain regions, further in-depth research is still required into the complex patterns of brain activity in heterogeneous samples.
The human brain is a complex nonlinear system, and its activity shows complex fluctuations, both in the spatial and in temporal domains. Computational and empirical studies have indicated that variations in functional connectivity may reflect specific cognitive and perceptual states and include the integration of information across detailed regions of the brain. The reciprocity of integration and functional regions can be quantified in particular in terms of neural complexity 7; thus, it has been speculated that disconnections in the brain are associated with an increase in complexity. An unnatural level of complexity in neurophysiologic signals may represent an abnormal neural connectivity pattern in mental disorders 7. Therefore, multiscale signal complexity can thus provide some indication of connectivity 8. Over the past few decades, novel nonlinear approaches on the basis of entropy have been used widely to measure the complexity of physiological signals, including electroencephalogram (EEG) signal 9 and ECG signals 10. Given its fine temporal resolution, low cost, and easy operation, EEG is particularly suitable for investigating inherently complex biological signals arising from brain systems.
Approximate entropy (ApEn) and sample entropy (SampEn) are two traditional methods of measuring complexity, which determine the probability of finding specific patterns or resemblance between time series to examine the irregularity or the predictability of one particular time series 11. However, there is a limitation in the scope of traditional methods as they can only analyze short-range temporal dynamics. To address this limitation, Costa et al. 12,13 proposed multiscale entropy (MSE) as an extension of SampEn, which quantifies the complexity of physiological signals by measuring the entropy across multiple time scales using a coarse-graining procedure. This extension to larger temporal scales can be used to reflect information in long-range temporal dynamics 11. An atypical MSE pattern could indicate disease conditions in the brain and provide useful information on the network controlling mechanisms underlying the physiological dynamics 13. Because of these advantages, the MSE method has been used widely in the study of mental disorders. Mizuno et al. 11 has reported an increase in complexity at larger scale factors (SFs) in Alzheimer’s disease, but the complexity is significantly reduced over smaller scales. An MSE study of drug-naive schizophrenia has found elevated complexity at higher time scales in the centrotemporal region 8. Some researchers have also found an association between autism disease and a significantly decreased MSE complexity 14,15. Therefore, research into this complexity is vital to determine the intrinsically dynamic principles in the human brain.
To characterize the complexity pattern of brain function of ASD and the control samples, we collected the EEG of all participants during the performance of observation and imitation tasks. To test the hypothesis that children with ASD would show atypical intrinsic complexity of brain activity, we calculated and compared the MSE of the EEG activity during the designed tasks.
Participants and methods
Participants
The study included 20 outpatient boys diagnosed with ASD (age range, 5–8 years; 6.5±1.17 years; right-handed) and 20 age-matched and sex-matched normal control children (age range, 5–10 years; 7.5±1.98 years; right-handed). All participants had full-scale and verbal IQ scores above 70 (WISC-IV). All participants’ parents filled out the Symptoms Autism Diagnostic Observational Scale and the Autism Diagnostic Interview – Revised. An experienced child psychiatrist and a health care psychologist made the diagnosis of age of childhood onset. The criteria for ASD included the following: (i) being within the scale’s diagnostic boundaries; (ii) no history of stimulant medication usage, severe brain injury, or other neuropsychiatric disorders; and (iii) able to cooperate with the EEG data acquisition process. Normal controls were excluded if they were diagnosed with ASD or they had a history of using stimulant medication, a severe brain injury, other neuropsychiatric disorders, cognitive deficits, learning disabilities, or communication problems.
Task description
The experimental procedure used for this research was based on the experimental program of Oberman et al. 16 and included the following: (i) Observation tasks: watching a video of a moving hand. The participants viewed a black-and-white video of an individual opening and closing their right hand with their fingers and thumb held straight. The video was 80 s in length and moved at a rate of 1 Hz, and the distance between the participants and the monitor was 85 cm; (ii) Imitation tasks: moving their own hand. Participants were asked to imitate the video action 80 times at a rate of ~1 Hz and to maintain this action throughout the duration of the task. Their hands were held at eye level and at a comfortable viewing distance. To ensure that participants paid attention to the video stimuli while watching the hand movement video, they were asked to count the number of times the stimulus occurred and report this number at the end of the experiment. All conditions were presented twice to obtain enough clean EEG data for analyses.
Electroencephalogram data acquisition and recording
EEGs were recorded from 18 electrodes at the following scalp locations of the 10–20 system: FP1, FP2, F3, FZ, F4, F7, F8, FT7, FT8, T7, T8, C3, C4, CZ, P3, P4, O1, and O2 (Neuroscan; Compumedics Limited, Charlotte, North Carolina, USA). The electrode impedance was below 5 kΩ. A reference electrode was placed at the left mastoid and a ground electrode was placed between Fpz and Fz. The vertical electrooculogram was recorded from electrodes attached above and below the left eye, and the horizontal electrooculogram was recorded from the outer canthi of both eyes. The EEG was sampled at 24 bits of accuracy and an analogue/digital sampling rate of 1000 Hz. All participants were seated inside an acoustically and electromagnetically shielded room and were asked to minimize all movement during the experiments. Thirty seconds (30 000 samples, continuous data) of artifact-free data (no ocular or muscular artifacts) were selected off-line from the results obtained from each participant. Filter analysis (FIR bandpass filter) was carried out on every EEG data segment, and the MSE was calculated across the 0.5–30 Hz frequency band for each participant.
Multiscale entropy
The MSE method was developed by Costa et al. 12,13 and can be used to quantify the complexity of a signal by calculating the SampEn over multiple temporal scales, which is realized by a coarse-grained procedure. The ApEn was proposed by Pincus 17. SampEn measures the regularity of a time series and is a modified version of ApEn 18.
Given a one-dimensional EEG discrete time series x=[x1,…xN] and SF τ, the time series is calculated for consecutive and nonoverlapping time series yτ using a coarse-grained procedure: 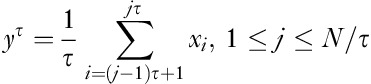 . The first scale is simply the original time series representing the short-range temporal scale and the larger SFs represent longer temporal scales. SampEn is calculated for each series yτ and is defined as the negative value of the logarithmic condition probability that two similar sequences of m consecutive data points will remain similar at the next point (m+1) 14,17: SampEn (m, r, N)=−ln [Cm+1(r)/Cm], where Cm=[number of pairs (i, j) with
. The first scale is simply the original time series representing the short-range temporal scale and the larger SFs represent longer temporal scales. SampEn is calculated for each series yτ and is defined as the negative value of the logarithmic condition probability that two similar sequences of m consecutive data points will remain similar at the next point (m+1) 14,17: SampEn (m, r, N)=−ln [Cm+1(r)/Cm], where Cm=[number of pairs (i, j) with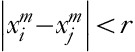 ,
,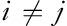 ]/[number of all probable pairs=(N–m+1)/(N–m)], where
]/[number of all probable pairs=(N–m+1)/(N–m)], where 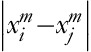 denotes the distance between vectors xim and xjm, m is the dimension of the vectors xim and xjm, r is the tolerable distance between the two vectors, and N represents the length of the time series. When m=1 or m=2 and r is between 0.1 and 0.25 times, the SD of the time series has the best statistical validity 17. In this study, we used the parameters m=2, r=0.15×SD, N=30 000 data points, and we set the SF to 30. After the coarse-grained procedure, there are still 30 000/30=1000 data points, which have been shown to be enough to obtain a reliable estimation of the SampEn value.
denotes the distance between vectors xim and xjm, m is the dimension of the vectors xim and xjm, r is the tolerable distance between the two vectors, and N represents the length of the time series. When m=1 or m=2 and r is between 0.1 and 0.25 times, the SD of the time series has the best statistical validity 17. In this study, we used the parameters m=2, r=0.15×SD, N=30 000 data points, and we set the SF to 30. After the coarse-grained procedure, there are still 30 000/30=1000 data points, which have been shown to be enough to obtain a reliable estimation of the SampEn value.
Statistical analysis
The statistical analysis was carried out using SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA) for Windows. A Kolmogorov–Smirnov normality test was used to confirm the normal distribution of MSE values for every group and every channel. Mauchly’s test of sphericity was used to test whether the data were appropriate for the analysis of variance (ANOVA) or whether a data correction is needed, and then the Greenhouse–Geisser adjustment was applied to each degree of freedom for all of the analyses. The significance value was set as P less than 0.05 for the repeated ANOVA. For the MSE analysis, we applied a four-way repeated-measure ANOVA to test the differences in the two groups (ASD vs. control) in terms of the between-participants factor, the SF (τ=30), the tasks (observation and imitation task) as well as the channels (FP1, FP2, F3, FZ, F4, F7, F8, T7, T8, FT7, FT8, CZ, C3, C4, P3, P4, O1, O2) as well as the within-participants factors. For every task, we then tested the differences in the two groups (ASD vs. control) in terms of the between-participants factor, and SF (τ=30), and the channels (FP1, FP2, F3, FZ, F4, F7, F8, T7, T8, FT7, FT8, CZ, C3, C4, P3, P4, O1, O2) as well as the within-participants factors. For every electrode, ANOVA was then repeatedly measured for the two groups (ASD vs. control) for the between-participant factor and SF (τ=30) as well as the within-participant factor.
Results
The MSE results showed a significant main effect of group difference (F1, 16=8.426, P=0.011) for the four-way ANOVA. The mean SampEn value of the ASD group (mean=1.324, SD=0.044) was significantly lower than that of the normal control group (mean=1.491, SD=0.037).
The results also showed group-by-SF-by-channel interactions. The group-by-SF interaction was further explored using two-way ANOVA for each channel for the two tasks. The results are shown in Table 1 and Fig. 1. For the observation task, a significant group-by-SF interaction was found in C3, C4, CZ, P3, P4, O1, O2, F3, F8, FT8, and T8, where the ASD group was significantly lower than the control group. For the imitation task, a significant group-by-SF interaction was only found in C3, P3, T8, and O1, where the ASD group was significantly lower than the control group.
Table 1.
Group-by-scale factor interaction significance values (autism spectrum disorder vs. control) for each channel in the observation and imitation tasks
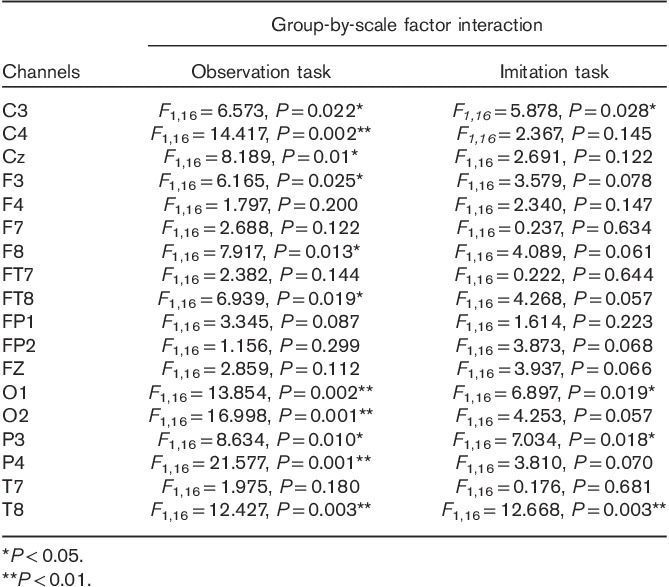
Fig. 1.
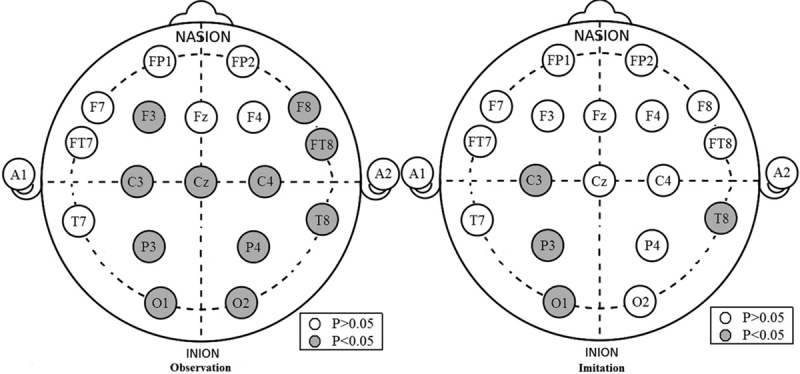
Group-by-scale factor interaction significance values (autism spectrum disorder vs. control) for each channel in the observation and imitation tasks. The gray solid cycle indicates P<0.05.
Discussion
MSE can characterize the complexity of a physiological signal at multiple temporal scales using a coarse-graining procedure 12,13. As such, MSE can measure the inherent complexity of signal dynamics 11. An atypical MSE pattern may reflect a disease condition of the brain and can be used as a reliable index of some mental disorders, such as Alzheimer’s 11 and schizophrenia 8. Similarly, more recent studies have applied MSE to investigate the complexity of electrical brain activity in autism. Bosl et al. 15 was the first to find a pattern of reduced complexity for infants at high risk of autism, particularly in the frontal regions of the brain. Catarino et al. 14 then further reported a reduction of EEG signal complexity within the temporoparietal and occipital regions in an autistic group during a visual matching task. Ghanbari et al. 19 then showed lower complexity in ASD in the frontal regions in the delta band and the occipital–parietal regions in the alpha band. This study also found notably descended EEG SampEn in individuals with autism during both observation tasks and imitation tasks, which was in agreement with other previous complexity studies of ASD. As previous research has shown that changes in local complexity may be associated with atypical neural connectivity 19,20 and that these atypical patterns are related to autism 21,22, EEG complexity can be used as a sensitive method for distinguishing early symptoms of autism as an index for neural processing of information and neural connectivity.
In the current study, a significant group-by-SF-by-electrode interaction suggests that compared with the imitation task, the observation task results in evidently reduced MSE for more regions of the cortex, especially in the right hemisphere and the central brain regions. Although an atypical structural 23 and functional 24 asymmetry phenomenon has been observed in ASD patients, further investigation is needed to determine what this precisely reflects. A previous study discovered that atypical hemispheric asymmetries shift toward the right hemisphere in the ASD group in the visual, auditory, motor, executive, language, and attentional networks, suggesting that atypical asymmetry might be a pervasive feature of functional brain organization in ASD 25. Our results should be considered to be complementary to this previous research.
Moreover, the complexity of the two groups showed significant differences in the central regions during the observation task, but no statistical difference in most of the central regions during the imitation task. These findings were consistent with the study by Oberman et al. 16, who found that the ASD group showed significant μ-suppression during self-performed hand movements, but not while observing hand movements in the central regions. Mirror neurons are a kind of sensory-motor neurons. Its typical feature is that the MNS is activated in the observation and interpretation of movements by others 16. Cook et al. 26 reported that mirror neurons play a key role in understanding the movement of others, which is especially important for social skills. Interestingly, the primary sensorimotor cortices are located in the central brain region. Thus, the present study infers that the lower complexity in the ASD group during the observation task in the central regions is indicative of a possible dysfunction in the MNS in patients with ASD. Furthermore, the lack of a statistical difference in complexity during the imitation task between the two groups suggests normal functioning of other motor systems involved in self-performed actions in the ASD group. The growing consensus indicates that a dysfunction in the MNS early in development could result in a cascade of impairments that are characteristic of ASD 27, such as deficits in imitation, language, theory of mind, empathy, and social communication. Given that ASD is defined by behavioral deficits in many of these areas, the results of this study provide evidence that the altered complexity in central brain regions during observation tasks may contribute toward the behavioral deficits in children with ASD.
In this study, we explored the MSE method to analyze the EEG signals of children with and without ASD during an observation task and an imitation task. The results of this study showed an altered complexity pattern for ASD children compared with normal controls for both tasks. In addition, compared with the imitation task, the complexity of the ASD group in the right hemisphere and the central cortex regions showed a significant decline during the observation task. These findings, which were consistent with previous ASD studies, provide further evidence of the relationship between ASD and cerebral dysfunction.
Acknowledgements
The authors would like to thank the following funding organizations for support: the National Natural Science Foundation of China (Grant 61503295), the China Postdoctoral Science Foundation (Grant 2015M570840), the Natural Science Foundation of Shaanxi Province (Grant 2015JQ8318), and the Science and Technology Project of Guangdong Province (Grant 2014A020215032).
Tian Liu: conceived and designed the experiments, carried out the data analysis, and wrote the manuscript; Yanni Chen: conducted the experiments; Desheng Chen: contributed materials and analysis tools; Chenxi Li: analyzed the data; Yusheng Qiu: revised the manuscript; Jue Wang: approved the final version.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-5, 5th ed Washington, DC, USA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol 2015; 14:1121–1134. [DOI] [PubMed] [Google Scholar]
- 3.Schunke O, Schöttle D, Vettorazzi E, Brandt V, Kahl U, Bäumer T, et al. Mirror me: imitative responses in adults with autism. Autism 2016; 20:134–144. [DOI] [PubMed] [Google Scholar]
- 4.McPartland JC, Jeste SS. Connectivity in context: emphasizing neurodevelopment in autism spectrum disorder. Biol Psychiatry 2015; 77:772–774. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher S, Varga S. Conceptual issues in autism spectrum disorders. Curr Opin Psychiatry 2015; 28:127–132. [DOI] [PubMed] [Google Scholar]
- 6.Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: evidence from an fMRI study of an embedded figures task. Autism Res 2010; 3:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T. Complexity of spontaneous brain activity in mental disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 2013; 45:258–266. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Cho RY, Mizuno T, Kikuchi M, Murata T, Takahashi K, Wada Y. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 2010; 51:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández A, Gómez C, Hornero R, López-Ibor JJ. Complexity and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 2013; 45:267–276. [DOI] [PubMed] [Google Scholar]
- 10.Šliupaitė A, Navickas Z, Vainoras A. Evaluation of complexity of ECG parameters using sample entropy and Hankel matrix. Elektronika ir Elektrotechnika 2015; 92:107–110. [Google Scholar]
- 11.Mizuno T, Takahashi T, Cho RY, Kikuchi M, Murata T, Takahashi K, Wada Y. Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol 2015; 121:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E 2005; 71:021906. [DOI] [PubMed] [Google Scholar]
- 13.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 2002; 89:068102. [DOI] [PubMed] [Google Scholar]
- 14.Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol 2011; 122:2375–2383. [DOI] [PubMed] [Google Scholar]
- 15.Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med 2011; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res Cogn Brain Res 2005; 24:190–198. [DOI] [PubMed] [Google Scholar]
- 17.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci 1991; 88:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lake DE. Improved entropy rate estimation in physiological data. In: Proceedings of the 33rd annual international conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September; 2011. [DOI] [PubMed]
- 19.Ghanbari Y, Bloy L, Christopher EJ, Blaskey L, Verma R, Roberts TP. Joint analysis of band-specific functional connectivity and signal complexity in autism. J Autism Dev Disord 2015; 45:444–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nastase SA, Iacovella V, Davis B, Hasson U. Connectivity in the human brain dissociates entropy and complexity of auditory inputs. NeuroImage 2015; 31:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 2015; 49:254–263. [DOI] [PubMed] [Google Scholar]
- 22.Wass S. Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn 2011; 75:18–28. [DOI] [PubMed] [Google Scholar]
- 23.Dougherty CC, Evans DW, Katuwal GJ, Michael AM. Asymmetry of fusiform structure in autism spectrum disorder: trajectory and association with symptom severity. Mol Autism 2016; 24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 2012; 135:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardinale RC, Shih P, Fishman I, Ford LM, Müller RA. Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry 2013; 70:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behav Brain Sci 2014; 37:177–192. [DOI] [PubMed] [Google Scholar]
- 27.Neta MG, Varanda C. The role of mirror neurons in autism impairment. Eur Psychiatry 2016; 31:S374–S375. [Google Scholar]


