Abstract
8-Oxo-7, 8-dihydrodeoxyguanosine (8-oxo-dG), one of the representative oxidative DNA lesions, frequently mispairs with the incoming dAMP during mammalian DNA replication. Mispaired dA is removed by post-replicative base excision repair (BER) initiated by adenine DNA glycosylase, MYH, creating an apurinic (AP) site. The subsequent mechanism ensuring a dC:8-oxo-dG pair, a substrate for 8-oxoguanine DNA glycosylase (OGG1), remains to be elucidated. At the nucleotide insertion step, none of the mammalian DNA polymerases examined exclusively inserted dC opposite 8-oxo-dG that was located in a gap. AP endonuclease 1, which possesses 3′→5′ exonuclease activity and potentially serves as a proofreader, did not discriminate dA from dC that was located opposite 8-oxo-dG. However, human DNA ligases I and III joined 3′-dA terminus much more efficiently than 3′-dC terminus when paired to 8-oxo-dG. In reconstituted short-patch BER, repair products contained only dA opposite 8-oxo-dG. These results indicate that human DNA ligases discriminate dC from dA and that MYH-initiated short-patch BER is futile and hence this BER must proceed to long-patch repair, even if it is initiated as short-patch repair, through strand displacement synthesis from the ligation-resistant dC terminus to generate the OGG1 substrate, dC:8-oxo-dG pair.
INTRODUCTION
The integrity of genomic DNA is maintained by accurate DNA replication and elaborate DNA repair in living cells. Among various threats to genetic information, oxidative damage to DNA is most abundant and inevitable as cells produce reactive oxygen species through energy metabolism (1). One of the oxidative DNA lesions, 8-oxo-7, 8-dihydrodeoxyguanosine (8-oxo-dG), is deleterious as it frequently mispairs with the incoming dAMP during DNA replication (2), leading to G:C→T:A transversions (3,4). Adenine paired to 8-oxo-dG is recognized and removed by adenine DNA glycosylase, MYH (MUTYH), a mammalian homolog of bacterial MutY (5,6). Recently, it was reported that inherited defects in the human MYH gene were associated with multiple colorectal tumors and somatic G→T mutations in the adenomatous polyposis coli (APC) gene (7). Furthermore, knockout of the mouse MYH gene resulted in spontaneous cancer (8) and a mutator phenotype in embryonic stem cells (9). These pieces of evidence emphasize the importance of the MYH-initiated base excision repair (BER) in cancer/mutation avoidance.
MYH is an adenine DNA glycosylase and initiates post-replication BER by removing adenine residues from DNA when paired to 8-oxo-dG or dG (5,6). Apurinic (AP) sites generated by MYH glycosylase is cleaved by AP endonuclease (APE), generating 3′-OH and 5′-deoxyribose phosphate (dRP). The 3′-OH residue serves as a primer terminus for a repair synthesis. When short-patch BER proceeds, 1 nt gap is converted to a nick by the actions of gap-filling DNA polymerase (pol) and deoxyribophosphodiesterase (dRPase). When long-patch BER proceeds, a strand displacement synthesis and the excision of a displaced strand by flap-endonuclease 1 (FEN1, DNase IV) are required to produce a ligatable nick (6,10). In both the cases, ligation of the nick follows to complete repair reactions. In BER of 8-oxo-dG:dA to 8-oxo-dG:dC, the mechanism following the removal of adenine base is not understood well. Unlike regular BER, the DNA glycosylase, MYH, removes the undamaged base, adenine, and DNA polymerase inserts a nucleotide opposite the lesion, 8-oxo-dG. The reactions must ensure the formation of 8-oxo-dG:dC pair, which is then repaired to dG:dC by regular BER initiated by 8-oxoguanine DNA glycosylase (OGG1). MYH is located in replication foci (11) and interacts with the proliferating cell nuclear antigen (PCNA), APE1, MSH6 and RPA (12,13). The post-replication repair is coupled with replication (14) and is suggested to follow long-patch BER (12,15,16). However, even if the long-patch BER is the mechanism for the repair, the reason for this choice is not clear. It is plausible that 8-oxo-dG inhibits ligation of a nucleotide inserted opposite this lesion during short-patch BER, hence the repair proceeds to the long-patch repair pathway.
To gain insights into the post-replication repair, we examined the three steps (nucleotide insertion, proofreading and ligation) that are involved in the repair. We also reconstituted short-patch BER of 8-oxo-dG:dA with purified proteins.
MATERIALS AND METHODS
Enzymes
Mouse MYH was purified as described previously (9). Human APE1, PCNA and calf thymus DNA polymerase δ were provided by Carlos de los Santos, Paul Fisher and Holly Miller (SUNY at Stony Brook), respectively. Human DNA polymerases, pol λ, pol η, pol κ, pol β and pol ι, were provided by Luis Blanco (Universidad Autonoma), Fumio Hanaoka (Osaka University), Haruo Ohmori (Kyoto University), Holly Miller (SUNY) and Roger Woodgate (NIH), respectively. Human DNA ligases I and IIIβ were provided by Alan Tomkinson (University of Maryland Medicine).
Expression and purification of human X-ray repair cross complementing 1 (XRCC1) and DNA ligase IIIα
Plasmids expressing histidine-tagged human XRCC1 (17) and DNA ligase IIIα (18) were provided by Larry Thompson (Lawrence Livermore National Laboratory) and Tomas Lindahl (Cancer Research UK), respectively. Escherichia coli BL21-CodonPlus(DE3)-RIL (Stratagene) was transformed and the protein expression was induced by the addition of isopropyl β-d-1-thiogalactopyranoside at 1 mM. After 2 h incubation at 37°C, bacteria were harvested and suspended in a buffer consisting of 50 mM Tris–HCl (pH 7.5), 10% glycerol, 0.5 M NaCl, 5 mM 2-mercaptoethanol and 1 mM imidazole. Bacteria were sonicated and a crude extract was obtained by ultracentrifugation. The extract was applied to a Ni-NTA agarose column (QIAGEN), and the column was washed successively with 1, 40 and 80 mM imidazole-containing buffers. XRCC1 and DNA ligase IIIα proteins were eluted with a 250 mM imidazole-containing buffer. Identity and purity of proteins were confirmed by SDS–PAGE. Proteins were dialyzed against a buffer containing 50 mM Tris–HCl (pH 7.5), 50% glycerol, 0.1 M NaCl, 1 mM EDTA and 10 mM 2-mercaptoethanol and then stored at −20°C. Protein concentration was determined with Protein Assay reagent (BioRad) using BSA as a standard.
Oligonucleotides
All oligonucleotides were synthesized by the DNA synthesis facility (SUNY, Stony Brook) and purified by electrophoresis in a denaturing 20% polyacrylamide gel. 5′-End labeling was carried out using [γ-32P]ATP (3000 Ci/mmol, Amersham Biosciences) and T4 polynucleotide kinase (New England BioLabs). Labeled oligonucleotides were purified by a MicroSpin G-25 column (Amersham Biosciences). For the preparation of duplex DNA, oligonucleotides were incubated at 80°C for 10 min in a solution (50 μl) containing 0.1× TE and 100 mM NaCl and then slowly cooled to room temperature.
Template oligonucleotides (40mer) were 5′CCAACTTGAAAACGCTCCACXATACCTTACATGCTAGAAC where X = dG or 8-oxo-dG. For nucleotide insertion experiments, 5′-32P-labeled 19mer primer (5′GTTCTAGCATGTAAGGTAT) and 5′-phosphorylated downstream 20mer oligonucleotide (5′GTGGAGCGTTTTCAAGTTGG) were annealed to template 40mer, forming a 1 nt gap in the middle of duplex DNA. For DNA ligation and exonuclease assays, 5′-32P-labeled 20mer oligonucleotides (5′GTTCTAGCATGTAAGGTATX, where X = dA, dC, dG or dT) and 5′-phosphorylated downstream 20mer oligonucleotide (described above) were annealed to template 40mer, generating a substrate with a correctly paired or mispaired 3′ terminus at a nick in the middle of duplex DNA. For MYH-initiated reconstitution experiments, dA:8-oxo-dG mispair-containing 40mer/40mer duplex DNA was employed.
Nucleotide insertion reaction
The reaction mixture (20 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 30 mM KCl, 5 mM DTT, 100 μg/ml BSA, 25 nM 5′-labeled primer/template, 2.5 nM enzyme (pol β, pol η, pol ι, pol κ or pol λ) and dATP or dCTP (concentrations are indicated in Figure 1). For reactions with pol δ, the mixture (20 μl) contained 40 mM bis-Tris (pH 6.7), 6 mM MgCl2, 10 mM DTT, 250 μg/ml BSA, 25 nM 5′-labeled primer/template, 110 nM PCNA (as a trimer), 2.25 U pol δ and dATP or dCTP. For reaction containing both dATP and dCTP, the mixture (20 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 30 mM KCl, 10 mM DTT, 250 μg/ml BSA, 25 nM unlabeled primer/template, 2.5 nM enzyme (pol β, pol η or pol λ), 0.5 μM dATP, 0.5 μM dCTP and 6.6 nM (3000 Ci/mmol) of [α-32P]dATP or [α-32P]dCTP. The reaction was conducted at 37°C for 10 min and terminated by adding 20 μl of a formamide loading buffer (96% formamide, 0.1% bromophenol blue, 0.1% xylene cyanol, 19 mM EDTA) followed by heating at 90°C for 3 min. The products were analyzed by electrophoresis in a denaturing 20% polyacrylamide gel. Radioactive DNA bands were visualized and quantified by a Storm840 PhosphorImager and ImageQuant software (Amersham Biosciences).
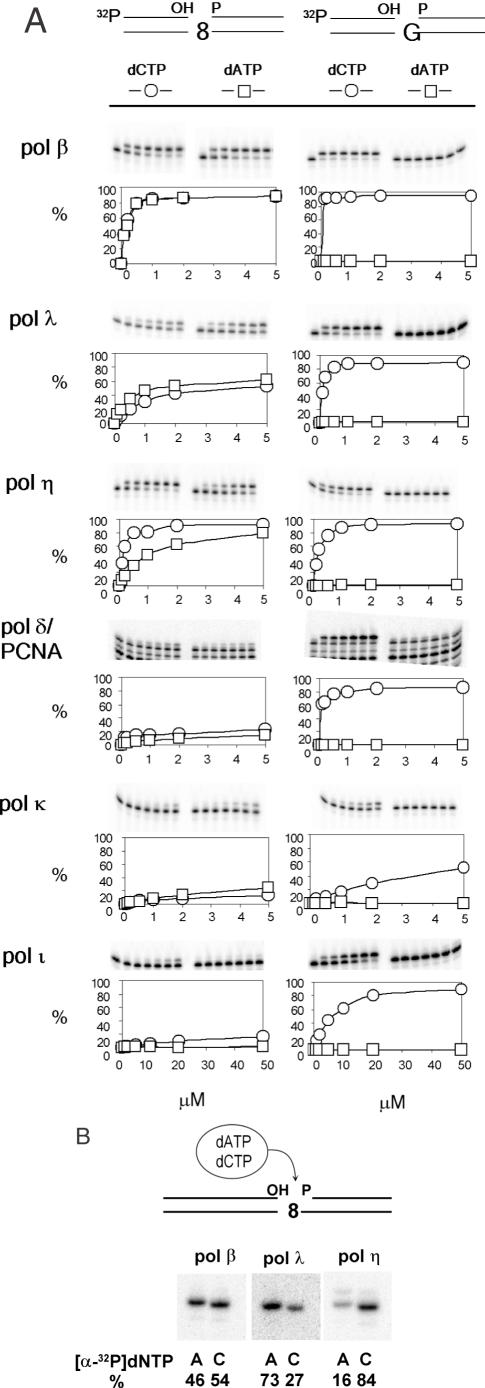
Figure 1.
Nucleotide insertion opposite 8-oxo-dG. (A) Insertion of dAMP or dCMP opposite 8-oxo-dG was determined using 40mer duplex DNA that had a 1 nt gap opposite the lesion. Substrate DNA is shown on the top. Here, ‘8’ stands for 8-oxo-dG. Intensities of radioactive bands were measured and insertion frequency, represented as percent, was plotted as a function of nucleotide concentration (μM). (B) Nucleotide insertion was determined in the presence of both 0.5 μM dATP and 0.5 μM dCTP. Either [α-32P]dATP or [α-32P]dCTP was included in the reaction mixture as a tracer. Relative ratio of insertions of dAMP and dCMP was shown in percentage.
Assay for 3′→5′ exonuclease activity of APE1
The reaction mixture (20 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 30 mM KCl, 5 mM DTT, 50 μg/ml BSA, 10 nM 5′-labeled substrate and 0, 1 or 5 nM APE1. The reaction was performed at 37°C for 30 min and terminated as described above.
DNA ligation reaction
The reaction mixture (20 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 5 mM DTT, 1 mM ATP, 50 μg/ml BSA, 100 mM KCl, 10 nM 5′-labeled substrate and human DNA ligases I (0, 5 and 25 nM), IIIα (0, 0.5 and 2.5 nM) or IIIβ (0, 5 and 25 nM). The ligation mixture was incubated at 37°C for 30 min, after which products were analyzed by gel electrophoresis as described above. In time-course experiments (Figure 4), 50 nM DNA ligase I or 10 nM DNA ligase IIIα/XRCC1 was used in 200 μl of the reaction mixture containing 2 mM ATP (instead of 1 mM) and 0 or 150 mM KCl. Aliquots (10 μl) were removed at various time points during incubation at 37°C, quenched and analyzed for products as described above.
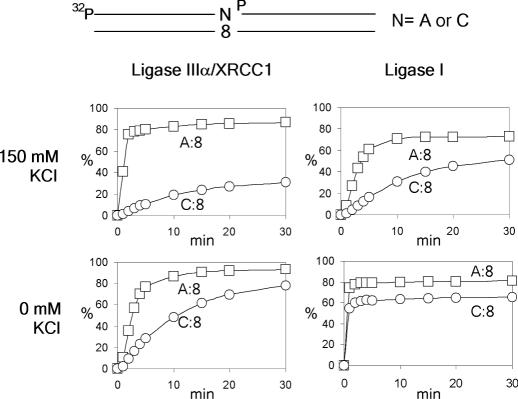
Figure 4.
Time course of DNA ligation catalyzed by human DNA ligase I and ligase III/XRCC1 in the presence (150 mM) or in the absence of KCl. Substrate DNA was the same as in Figure 3, and ‘N’ stands for A or C. The x-axis and y-axis represent incubation time (min) and generation of ligation products (percent), respectively.
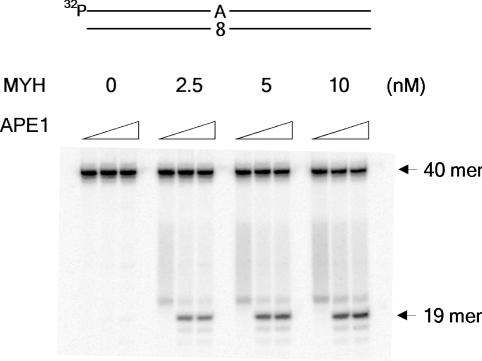
Adenine base excision catalyzed by MYH and APE1
The reaction mixture (20 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 5 mM DTT, 50 μg/ml BSA, 1 nM 5′-labeled 40mer duplex DNA and MYH (0, 2.5, 5 or 10 nM) and was incubated at 37°C for 15 min followed by incubation with APE1 (0, 10 or 50 nM) in the presence of 150 mM KCl at 37°C for 15 min (Figure 5).
Figure 5.
Adenine base excision catalyzed by MYH and APE1. 5′-Labeled duplex 40mer containing dA:8-oxo-dG pair was incubated with MYH at 37°C for 15 min followed by 15 min incubation with APE1 (0, 10 or 50 nM) in the presence of 150 mM KCl.
Reconstitution of MYH-initiated short-patch BER
A starting reaction mixture (100 μl) contained 1 nM unlabeled 40mer duplex DNA and 10 nM MYH and was incubated at 37°C for 30 min. Following the reaction, the mixture was supplemented with 150 mM KCl, 2 mM ATP, 0.5 μM dATP, 0.5 μM dCTP and 33 nM of [α-32P]dATP or [α-32P]dCTP (6000 Ci/mmol), 100 nM APE1, 4 nM pol β and 10 nM of DNA ligase IIIα/XRCC1, and then incubated at 37°C. Aliquots (10 μl) were removed at various time points and the products were analyzed as described above.
RESULTS
Both dAMP and dCMP are inserted opposite 8-oxo-dG by various DNA polymerases
The consecutive action of MYH and APE1 produces a 1 nt gap opposite 8-oxo-dG with 3′-OH group and 5′-dRP moiety, and 8-oxo-dG serves as a template for the repair synthesis. We examined the insertion specificity of several DNA polymerases using a 1 nt gapped substrate (Figure 1A and B). Among DNA polymerases examined, pol β, pol η and pol λ inserted a nucleotide opposite 8-oxo-dG more efficiently than do pol κ, pol ι and pol δ/PCNA. Regarding their specificities, pol η and its close relative, pol ι, preferred dC to dA; pol β and pol δ/PCNA appeared to insert dC and dA at similar frequencies; and pol λ and pol κ preferred dA to dC. All these pols inserted dC opposite dG (Figure 1A) though the activities of pol κ and pol ι were weak when a gapped substrate was used. These results indicate that none of the DNA polymerases exclusively inserts dC opposite 8-oxo-dG.
APE1 exonuclease activity does not discriminate dA from dC pairing to 8-oxo-dG
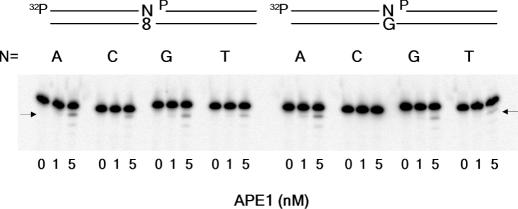
APE1 is reported to have a 3′→5′ exonuclease activity active on 3′-mismatched termini of nicked and gapped DNA molecules and is suggested to compensate for the lack of a proofreading function of pol β (19). This finding raises the possibility that APE1 removes selectively 3′-dA, but not dC, inserted opposite 8-oxo-dG during a gap-filling reaction. To explore this possibility, we used four substrates with different termini at nicks (Figure 2). When dG was a template, all mismatched termini, but not 3′-dC, were subjected to proofreading. When 8-oxo-dG was a template, all four termini were proofread: there was no difference between 3′-dC and 3′-dA, and this result was true under three different KCl concentrations (0, 30 and 150 mM). The exonuclease activity of APE1 was progressively impaired by KCl. We conclude that APE1 does not discriminate 3′-dA from 3′-dC inserted opposite 8-oxo-dG.
Figure 2.
3′→5′ Exonucleolytic proofreading activity of APE1. Nicked DNA substrate is shown above the panel. ‘N’ stands for dA, dC, dG or dT. Arrows indicate proofread products.
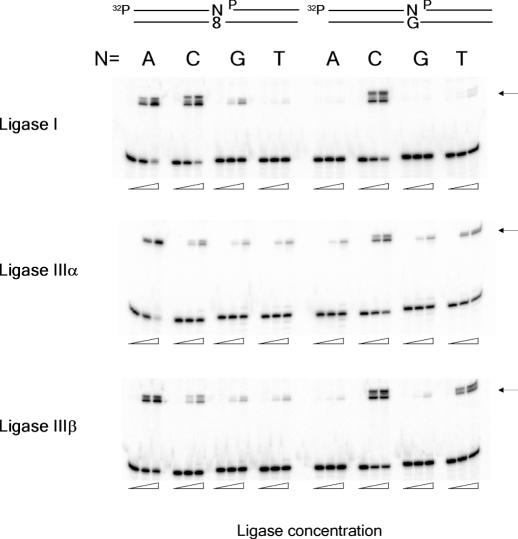
DNA ligases ligate 3′-dA terminus much more efficiently than 3′-dC terminus when pairing to 8-oxo-dG
If a DNA ligase is active on only 3′-dC terminus pairing to 8-oxo-dG, the ligation step will play a critical role in the short-patch BER of dA:8-oxo-dG mispair. We compared the degrees of ligation of four 3′-termini pairing to 8-oxo-dG. Human DNA ligase I, which is believed to be engaged in DNA replication, long-patch BER and nucleotide excision repair appeared to ligate both 3′-dA and 3′-dC termini at similar efficiencies in a qualitative experiment (Figure 3). The other two termini, dG and dT, were poorly ligated by this enzyme. DNA ligases IIIα and IIIβ, which are produced by an alternative splicing (20), appeared to be much more active on 3′-dA than on the other termini. When unmodified DNA was used as a substrate, ligase I exclusively ligated a 3′-dC terminus pairing to dG, whereas ligases IIIα and IIIβ ligated a dT terminus as well as a dC terminus. Since DNA ligase IIIα is believed to be the ligase involved in short-patch BER, we further characterized ligation catalyzed by this enzyme of 3′-dA and 3′-dC termini pairing to 8-oxo-dG (Figure 4). Burst DNA joining occurred on the dA terminus, but not on the dC terminus, in a reaction mixture containing 150 mM KCl, which is considered to represent the physiological salt condition (21). Ligation of the dA terminus was also more efficient than that of the dC terminus in the absence of KCl, but the difference was less drastic. The effects of varying amounts of XRCC1 on the ligation of 3′-dA and -dC termini were also examined, and no significant effects were observed (data not shown). The ligation catalyzed by DNA ligase I was also characterized under the same condition. Both in the presence and in the absence of 150 mM KCl, ligation of a 3′-dA terminus was more efficient than that of a dC terminus pairing to 8-oxo-dG, which was similar to the result with ligase IIIα, but the difference was less marked. We have also tested E.coli and T4 DNA ligases, and the results were similar to those with ligase I (data not shown). From these results, it is concluded that DNA ligases, especially human ligase IIIα, are much more active on a dA terminus than on a dC terminus pairing to 8-oxo-dG.
Figure 3.
Ligation of four 3′-termini pairing with 8-oxo-dG or dG to downstream DNA. Nicked DNA substrate is shown on the top. Ligation products are 40mer indicated by arrows. All oligonucleotides used in this study were purified by gel electrophoresis and showed one band, but ligation products always appeared as two bands. We do not know the reason for this. The concentrations of ligases were 0, 0.5 and 2.5 nM for ligase IIIα and 0, 5 and 25 nM for ligase I and IIIβ.
Adenine base excision and strand incision catalyzed by MYH and APE1
When 5′-32P labeled 40mer duplex bearing a dA:8-oxo-dG pair was incubated with MYH, a faint band migrating above 19mer was detected (Figure 5). This band may be the product of β-elimination reaction of an AP site generated by MYH. When APE1 was added to this reaction mixture in the presence of 150 m KCl, a distinct band was generated. This band corresponds to 19mer with 3′-OH terminus. Thus, the combined action of MYH and APE1 generates a 1 nt gap opposite 8-oxo-dG with 3′-OH and 5′dRP termini.
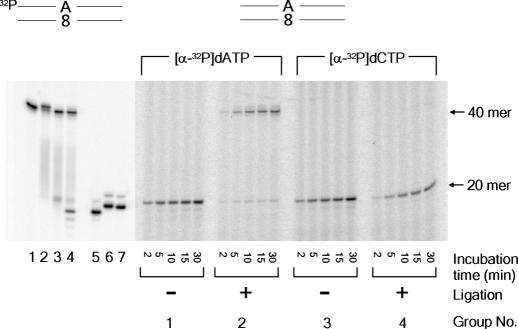
Reconstitution of MYH-initiated short-patch BER of dA:8-oxo-dG mispair
So far, each step of short-patch BER was characterized individually. In this section, short-patch BER was reconstituted using purified proteins. As described in ‘Materials and Methods’, unlabeled duplex 40mer was first incubated with MYH, then the reaction mixture was supplemented with the other protein factors (APE1, pol β and DNA ligase IIIα/XRCC1) and co-factors (KCl, ATP, dATP, dCTP and [α-32P] dATP or [α-32P] dCTP). The dA (group 1) and dC (group 3) were inserted opposite 8-oxo-dG as revealed in reaction mixtures lacking the ligase (Figure 6). Although inserted dA was ligated efficiently (group 2), dC was not (group 4). These results indicate that the short-patch repair is completed only when dAMP is inserted opposite 8-oxo-dG.
Figure 6.
Reconstitution of MYH-initiated short-patch BER. Substrate DNA (duplex 40mer) is shown above the panel. Lanes 1–4 show base excision reaction catalyzed by MYH, using 5′-32P-labeled substrate. Lane 1, substrate DNA; lanes 2 and 3, incubated with 10 nM MYH for 30 min (lane 2) followed by heating at 90°C for 3 min (lane 3); lane 4, incubated with MYH followed by APE1 treatment for 30 min in the presence of 150 mM KCl; lane 5, 5′-32P-labeled standard 19mer corresponding to MYH/APE1-generated product; lanes 6 and 7, standard 19mer plus 3′-dA (lane 6) or 3′-dC (lane 7). All the other lanes show reconstitution reaction using [α-32P]dATP or [α-32P]dCTP in the presence or in the absence of ligation reaction catalyzed by DNA ligase IIIα/XRCC1.
DISCUSSION
8-Oxo-dG exerts its genotoxity mainly by pairing with dA during DNA replication. In E.coli, the frequency of this misincorporation is ∼30% when measured in mutM−mutY− cells, and more than 90% of these misincorporated dAs are removed by MutY-initiated post-replication repair (22). Although a misincorporation frequency of dA has not yet been determined in human cells, a similar repair process is expected to operate since MYH-defective cells are mutation-prone (9).
In this paper, we conducted biochemical studies using purified proteins to understand the mammalian post-replication repair. We focused on the short-patch BER and examined characteristics of enzymes involved in this repair. None of the mammalian DNA polymerases examined inserted exclusively dC opposite 8-oxo-dG, and mis-inserted dA was not specifically proofread by human APE1. However, at a ligation step, human DNA ligases I and III ligated a 3′-dA terminus pairing to 8-oxo-dG much more efficiently than a 3′-dC terminus. Thus, the ligation step discriminates dA from dC. In reconstitution experiments, DNA was ligated only when dA was inserted opposite 8-oxo-dG, indicating that MYH-initiated short-patch BER is futile and a dC:8-oxo-dG pair must proceed to long-patch BER to complete the repair. Our results support the previous suggestion that the long-patch pathway is responsible for this repair (12,15,16). One of the major questions about the repair of a dA:8-oxo-dG mispair is which DNA polymerase is responsible for dC insertion opposite 8-oxo-dG. In the experiments with cell extracts (15), the gap-filling synthesis step is sensitive to aphidicolin, suggesting that a replicative polymerase, pol δ/pol ε, is critical. However, it is unknown whether the aphidicolin-sensitive polymerase catalyzes both nucleotide insertion opposite 8-oxo-dG and extension. Indeed, there is a suggestion that pol β and pol δ/ε play a role in nucleotide insertion and extension steps, respectively, during a long-patch repair (23,24). Therefore, it is possible that nucleotide insertion opposite 8-oxo-dG and extension from the terminus are catalyzed by different polymerases. Furthermore, there are conflicting reports on the sensitivity of pol λ to aphidicolin (25,26). Since this polymerase possesses dRPase activity (27), it may function in short-patch BER. Therefore, we assessed mammalian DNA polymerases for their abilities to insert a nucleotide opposite 8-oxo-dG, using a gapped substrate. A widely spread idea is that ‘repair’ DNA polymerase preferentially inserts dC opposite 8-oxo-dG. However, none of the DNA polymerases examined exclusively inserted dC. The dRPase activity is required to complete short-patch BER. Pol β (28) and pol λ (27) have dRPase activities while the activity of pol ι is controversial (29,30). Pol β and pol λ, members of X-family polymerases, efficiently inserted both dA and dC into a 1 nt gap opposite 8-oxo-dG (Figure 1). Thus, both enzymes are candidate DNA polymerases involved in this BER. Although pol η preferentially inserted dC, it is reported that pol η, pol κ and pol ι are covalently trapped by a 5′-dRP residue and their repair synthesis activities are greatly reduced (30). In addition, pol ι or pol κ is not very active in inserting a nucleotide opposite 8-oxo-dG. Thus, these polymerases may not be suitable for the repair synthesis. Pol δ also can insert dC and dA opposite the lesion as reported previously (31) and may be responsible for both steps. It is conceivable that a certain factor keeps 8-oxo-dG in the anti conformation and assists DNA polymerase to insert dC opposite 8-oxo-dG (32). However, whichever the polymerase that is responsible for the dC insertion is, short-patch BER cannot complete the post-replication repair since a dC terminus is not ligated efficiently by DNA ligases, especially by ligase III/XRCC1 that is believed to be involved in short-patch BER (6).
Our results support the previous idea that the long-patch repair is responsible for this post-replicaton repair (12,15,16). This pathway may be selected at the initiation of the repair since proteins necessary for the long-patch repair, such as MYH, PCNA, FEN1 and pol δ, are immediately available in the replication foci (11). Alternatively, it can be the result of the inhibition of short-patch repair as shown here. These two possibilities may be distinguished by identifying a polymerase that catalyzes nucleotide insertion opposite 8-oxo-dG. The possible in vivo roles for pol β and pol λ in this regard are currently under investigation. It is also possible that a short piece of terminal DNA downstream from the 1 nt gap opposite 8-oxo-dG is removed and a replicative, not a gap-filling, synthesis reinitiates opposite 8-oxo-dG.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Luis Blanco, Carlos de los Santos, Paul Fisher, Fumio Hanaoka, Thomas Kunkel, Tomas Lindahl, Holly Miller, Haruo Ohmori, Larry Thompson, Alan Tomkinson and Roger Woodgate for enzymes and expression vectors. We also thank Cecilia Torres for oligonucleotide synthesis. This work was supported by NCI, National Institutes of Health, United States Public Health Service Grants (CA47995 and CA76163), the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15025257), and the Japan Society for the Promotion of Science (15590347 and 16390119).
REFERENCES
- 1.Cooke M.S., Evans,M.D., Dizdaroglu,M. and Lunec,J. (2003) Oxidative DNA damage: mechanism, mutation, and disease. FASEB J., 17, 1195–1214. [DOI] [PubMed] [Google Scholar]
- 2.Shibutani S., Takeshita,M. and Grollman,A.P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature, 349, 431–434. [DOI] [PubMed] [Google Scholar]
- 3.Wood M.L., Dizdaroglu,M., Gajewski,E. and Essigmann,J.M. (1990) Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry, 29, 7024–7032. [DOI] [PubMed] [Google Scholar]
- 4.Moriya M. (1993) Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G·C→T·A transversions in simian kidney cells. Proc. Natl Acad. Sci. USA, 90, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker A.R. and Eshleman,J.R. (2003) Human MutY: gene structure, protein functions and interactions, and role in carcinogenesis. Cell. Mol. Life Sci., 60, 2064–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortini P., Pascucci,B., Parlanti,E., D'Errico,M., Simonelli,V. and Dogliotti,E. (2003) The base excision repair: mechanisms and its relevance for cancer susceptibility. Biochimie, 85, 1053–1071. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tassan N., Chmiel,N.H., Maynard,J., Fleming,N., Livingston,A.L., Williams,G.T., Hodges,A.K., Davies,D.R., David,S.S., Sampson,J.R. and Cheadle,J.P. (2002) Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nature Genet., 30, 227–232. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y., Yang,H., Cunanan,C., Okamoto,K., Shibata,D., Pan,J., Barnes,D.E., Lindahl,T., McIlhatton,M., Fishel,R. and Miller,J.H. (2004) Deficiencies in mouse myh and ogg1 result in tumor predisposition and G to T mutations in codon 12 of the K-Ras oncogene in lung tumors. Cancer Res., 64, 3096–3102. [DOI] [PubMed] [Google Scholar]
- 9.Hirano S., Tominaga,Y., Ichinoe,A., Ushijima,Y., Tsuchimoto,D., Honda-Ohnishi,Y., Ohtsubo,T., Sakumi,K. and Nakabeppu,Y. (2003) Mutator phenotype of MUTYH-null mouse embryonic stem cells. J. Biol. Chem., 278, 38121–38124. [DOI] [PubMed] [Google Scholar]
- 10.Slupphaug G., Kavli,B. and Krokan,H.E. (2003) The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res., 531, 231–251. [DOI] [PubMed] [Google Scholar]
- 11.Boldogh I., Milligan,D., Lee,M.S., Bassett,H., Lloyd,R.S. and McCullough,A.K. (2001) hMYH cell cycle-dependent expression, subcellular localization and association with replication foci: evidence suggesting replication-coupled repair of adenine:8-oxoguanine mispairs. Nucleic Acids Res., 29, 2802–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker A., Gu,Y., Mahoney,W., Lee,S.H., Singh,K.K. and Lu,A-L. (2001) Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair. J. Biol. Chem., 276, 5547–5555. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y., Parker,A., Wilson,T.M., Bai,H., Chang,D.-Y. and Lu,A-L. (2002) Human MutY homolog, a DNA glycosylase involved in base excision repair, physically and functionally interacts with mismatch repair proteins human MutS homolog 2/human MutS homolog 6. J. Biol. Chem., 277, 11135–11142. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H., Tominaga,Y., Hirano,S., McKenna,A.E., Nakabeppu,Y. and Matsumoto,Y. (2002) Replication-associated repair of adenine: 8-oxoguanine mispairs by MYH. Curr. Biol., 12, 335–339. [DOI] [PubMed] [Google Scholar]
- 15.Parlanti E., Fortini,P., Macpherson,P., Laval,J. and Dogliotti,E. (2002) Base excision repair of adenine/8-oxoguanine mispairs by an aphidicolin-sensitive DNA polymerase in human cell extracts. Oncogene, 21, 5204–5212. [DOI] [PubMed] [Google Scholar]
- 16.Fortini P., Pascucci,B., Parlanti,E., D'Errico,M., Simonelli,V. and Dogliotti,E. (2003) 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat. Res., 531, 127–139. [DOI] [PubMed] [Google Scholar]
- 17.Caldecott K.W., Tucker,J.D., Stanker,L.H. and Thompson,L.H. (1995) Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res., 23, 4836–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash R.A., Caldecott,K.W., Barnes,D.E. and Lindahl,T. (1997) XRCC1 protein interacts with one of two distinct forms of DNA ligase III. Biochemistry, 36, 5207–5211. [DOI] [PubMed] [Google Scholar]
- 19.Chou K.M. and Cheng,Y.C. (2002) An exonucleolytic activity of human apurinic/apyrimidinic endonuclease on 3′ mispaired DNA. Nature, 415, 655–659. [DOI] [PubMed] [Google Scholar]
- 20.Mackey Z.B., Ramos,W., Levin,D.S., Walter,C.A., McCarrey,J.R. and Tomkinson,A.E. (1997) An alternative splicing event which occurs in mouse pachytene spermatocytes generates a form of DNA ligase III with distinct biochemical properties that may function in meiotic recombination. Mol. Cell. Biol., 17, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhagwat A.S., Sanderson,R.J. and Lindahl,T. (1999) Delayed DNA joining at 3′ mismatches by human DNA ligases. Nucleic Acids Res., 27, 4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriya M. and Grollman,A.P. (1993) Mutations in the MutY gene of Escherichia coli enhance the frequency of targeted G:C→T:A transversions induced by a single 8-oxoguanine residue in single-stranded DNA. Mol. Gen. Genet., 239, 72–76. [DOI] [PubMed] [Google Scholar]
- 23.Podlutsky A.J., Dianova,I.I., Podust,V.N., Bohr,V.A. and Dianov,G.L. (2001) Human DNA polymerase β initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J., 20, 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlanti E., Pascucci,B., Terrados,G., Blanco,L. and Dogliotti,E. (2004) Aphidicolin-resistant and -sensitive base excision repair in wild-type and DNA polymerase β-defective mouse cells. DNA Repair, 3, 703–710. [DOI] [PubMed] [Google Scholar]
- 25.Ramadan K., Shevelev,I.V., Maga,G. and Hubscher,U. (2002) DNA polymerase λ from calf thymus preferentially replicates damaged DNA. J. Biol. Chem., 277, 18454–18458. [DOI] [PubMed] [Google Scholar]
- 26.Shimazaki N., Yoshida,K., Kobayashi,T., Toji,S., Tamai,K. and Koiwai,O. (2002) Over-expression of human DNA polymerase lambda in E.coli and characterization of the recombinant enzyme. Genes Cells, 7, 639–651. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Diaz M., Bebenek,K., Kunkel,T.A. and Blanco,L. (2001) Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase λ. J. Biol. Chem., 276, 34659–34663. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto Y. and Kim,K. (1995) Excision of deoxyribose phosphate residues by DNA polymerase β during DNA repair. Science, 269, 699–702. [DOI] [PubMed] [Google Scholar]
- 29.Bebenek K., Tissier,A., Frank,E.G., McDonald,J.P., Prasad,R., Wilson,S.H., Woodgate,R. and Kunkel,T.A. (2001) 5′-Deoxyribose phosphate lyase activity of human DNA polymerase ι in vitro. Science, 291, 2156–2159. [DOI] [PubMed] [Google Scholar]
- 30.Haracska L., Prakash,L. and Prakash,S. (2003) A mechanism for the exclusion of low-fidelity human Y-family DNA polymerases from base excision repair. Genes Dev., 17, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Einolf H.J. and Guengerich,F.P. (2001) Fidelity of nucleotide insertion at 8-oxo-7, 8-dihydroguanine by mammalian DNA polymerase δ. Steady-state and pre-steady-state kinetic analysis. J. Biol. Chem., 276, 3764–3771. [DOI] [PubMed] [Google Scholar]
- 32.Tominaga Y., Ushijima,Y., Tsuchimoto,D., Mishima,M., Shirakawa,M., Hirano,S., Sakumi,K. and Nakabeppu,Y. (2004) MUTYH prevents OGG1 or APEX1 from inappropriately processing its substrate or reaction product with its C-terminal domain. Nucleic Acids Res., 32, 3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]