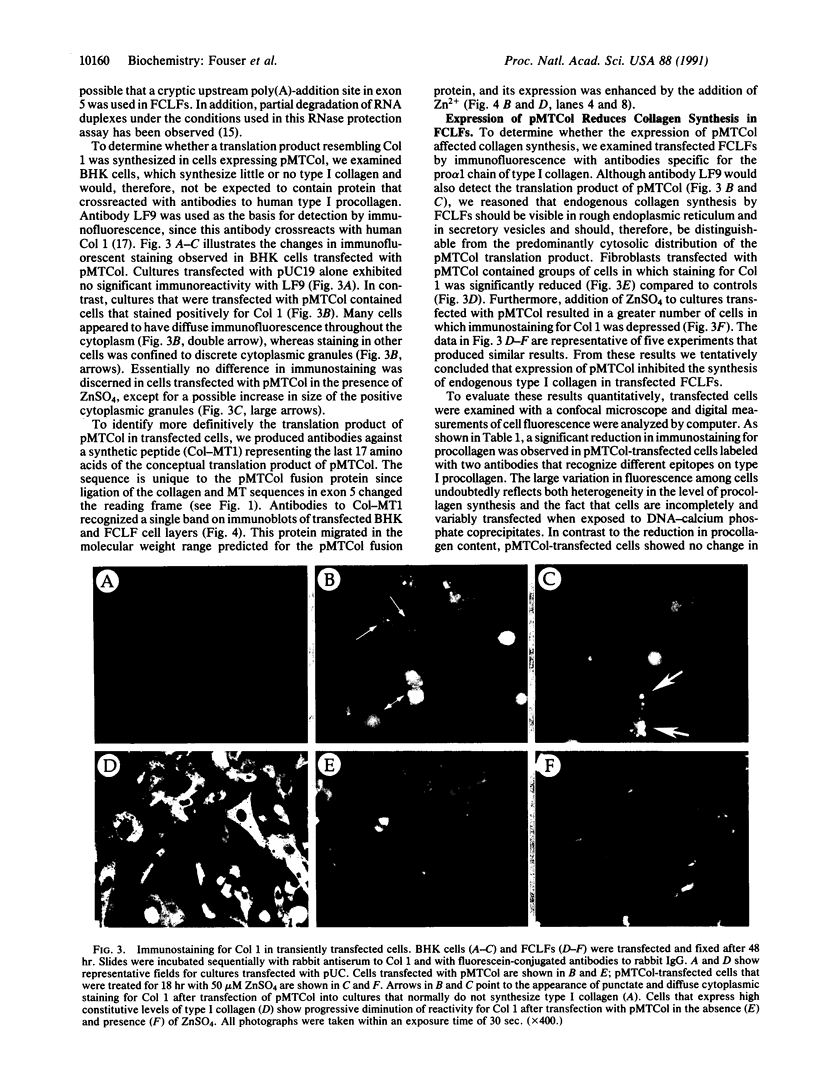
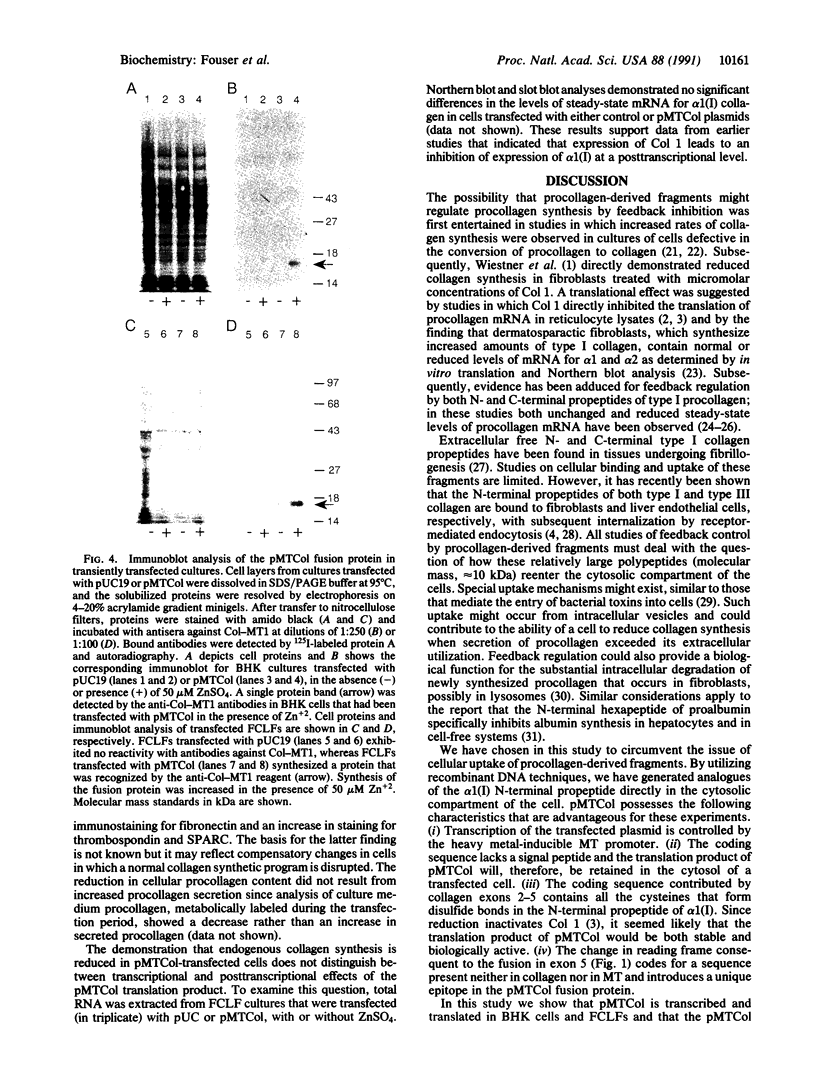
Abstract
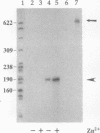
The mechanisms involved in feedback regulation of type I procollagen synthesis by the N-terminal propeptide of the pro alpha 1(I) chain, termed Col 1, are poorly understood. We have constructed a metallothionein-human collagen chimeric minigene (pMTCol) that codes for a Col 1 fusion protein but lacks a signal peptide sequence and, therefore, would be expected to direct the synthesis of the fusion protein to the cytosol. Baby hamster kidney cells and fetal calf ligament cells, transfected with pMTCol, transcribed the gene and synthesized an intracellular antigen that was identified as the fusion protein with a monospecific antibody. Transfected fetal calf ligament fibroblasts showed significantly reduced levels of endogenously produced type I collagen, as determined by imaging and digital quantitation of immunofluorescence by confocal microscopy; synthesis of fibronectin, thrombospondin, and SPARC (secreted protein, acidic and rich in cysteine) was unchanged or increased in these cells. This recombinant approach offers the potential for a systematic analysis of feedback regulation of collagen synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aycock R. S., Raghow R., Stricklin G. P., Seyer J. M., Kang A. H. Post-transcriptional inhibition of collagen and fibronectin synthesis by a synthetic homolog of a portion of the carboxyl-terminal propeptide of human type I collagen. J Biol Chem. 1986 Oct 25;261(30):14355–14360. [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Gelinas R. E. DNA and chromatin structure of the human alpha 1 (I) collagen gene. J Biol Chem. 1984 Dec 10;259(23):14906–14913. [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Devarayalu S., Cook S. C. A highly conserved, 5' untranslated, inverted repeat sequence is ineffective in translational control of the alpha 1(I) collagen gene. Nucleic Acids Res. 1988 Oct 25;16(20):9721–9736. doi: 10.1093/nar/16.20.9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988 Feb 5;263(4):1603–1606. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Robey P. G., Tuross N., Otsuka A. S., Tepen D. A., Esch F. S., Shimasaki S., Termine J. D. The Mr 24,000 phosphoprotein from developing bone is the NH2-terminal propeptide of the alpha 1 chain of type I collagen. J Biol Chem. 1987 Oct 5;262(28):13457–13463. [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Timpl R., Olsen B. R. Procollagen intermediates during tendon fibrillogenesis. J Histochem Cytochem. 1988 Nov;36(11):1425–1432. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- Foster R., Olsson P. E., Gedamu L. Calcium phosphate-mediated transfection alters metallothionein gene expression in response to Cd2+ and Zn2+. Mol Cell Biol. 1989 Sep;9(9):4105–4108. doi: 10.1128/mcb.9.9.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Hörlein D., Fietzek P. P., Wachter E., Lapière C. M., Kühn K. Amino acid sequence of the aminoterminal segment of dermatosparactic calf-skin procollagen type I. Eur J Biochem. 1979 Aug 15;99(1):31–38. doi: 10.1111/j.1432-1033.1979.tb13227.x. [DOI] [PubMed] [Google Scholar]
- Hörlein D., McPherson J., Goh S. H., Bornstein P. Regulation of protein synthesis: translational control by procollagen-derived fragments. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6163–6167. doi: 10.1073/pnas.78.10.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Hasselaar P., Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest. 1991 Feb;64(2):174–186. [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Lozano G., Helle O., Müller P. K. Procollagen alpha 2(I) mRNA in dermatosparactic fibroblasts: evidence for post-transcriptional regulation. EMBO J. 1983;2(8):1223–1227. doi: 10.1002/j.1460-2075.1983.tb01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J. M., Hörlein D., Abbott-Brown D., Bornstein P. Inhibition of protein synthesis in vitro by procollagen-derived fragments is associated with changes in protein phosphorylation. J Biol Chem. 1982 Aug 10;257(15):8557–8560. [PubMed] [Google Scholar]
- Mecham R. P., Lange G., Madaras J., Starcher B. Elastin synthesis by ligamentum nuchae fibroblasts: effects of culture conditions and extracellular matrix on elastin production. J Cell Biol. 1981 Aug;90(2):332–338. doi: 10.1083/jcb.90.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Hudson T. H. Transmembrane transport of diphtheria toxin, related toxins, and colicins. Annu Rev Biochem. 1986;55:195–224. doi: 10.1146/annurev.bi.55.070186.001211. [DOI] [PubMed] [Google Scholar]
- Paglia L., Wilczek J., de Leon L. D., Martin G. R., Hörlein D., Müller P. Inhibition of procollagen cell-free synthesis by amino-terminal extension peptides. Biochemistry. 1979 Oct 30;18(22):5030–5034. doi: 10.1021/bi00589a034. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Mumby S. M., Abbott-Brown D., Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982 Oct;95(1):351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., de Crombrugghe B. Formation of a type I collagen RNA dimer by intermolecular base-pairing of a conserved sequence around the translation initiation site. Nucleic Acids Res. 1987 Nov 11;15(21):8935–8956. doi: 10.1093/nar/15.21.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Schlumberger W., Thie M., Volmer H., Rauterberg J., Robenek H. Binding and uptake of Col 1(I), a peptide capable of inhibiting collagen synthesis in fibroblasts. Eur J Cell Biol. 1988 Jun;46(2):244–252. [PubMed] [Google Scholar]
- Weigand K., Schmid M., Villringer A., Birr C., Heinrich P. C. Hexa- and pentapeptide extension of proalbumin: feedback inhibition of albumin synthesis by its propeptide in isolated hepatocytes and in the cell-free system. Biochemistry. 1982 Nov 23;21(24):6053–6059. doi: 10.1021/bi00267a005. [DOI] [PubMed] [Google Scholar]
- Wiestner M., Krieg T., Hörlein D., Glanville R. W., Fietzek P., Müller P. K. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J Biol Chem. 1979 Aug 10;254(15):7016–7023. [PubMed] [Google Scholar]
- Wiestner M., Rohde H., Helle O., Krieg T., Timpl R., Müller P. K. Low rate of procollagen conversion in dermatosparactic sheep fibroblasts is paralleled by increased synthesis of type I and type III collagens. EMBO J. 1982;1(4):513–516. doi: 10.1002/j.1460-2075.1982.tb01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Donovan C. B., Wu G. Y. Evidence for pretranslational regulation of collagen synthesis by procollagen propeptides. J Biol Chem. 1986 Aug 15;261(23):10482–10484. [PubMed] [Google Scholar]
- Wu C. H., Walton C. M., Wu G. Y. Propeptide-mediated regulation of procollagen synthesis in IMR-90 human lung fibroblast cell cultures. Evidence for transcriptional control. J Biol Chem. 1991 Feb 15;266(5):2983–2987. [PubMed] [Google Scholar]
- Yamada Y., Mudryj M., de Crombrugghe B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J Biol Chem. 1983 Dec 25;258(24):14914–14919. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]