Abstract
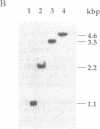
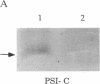
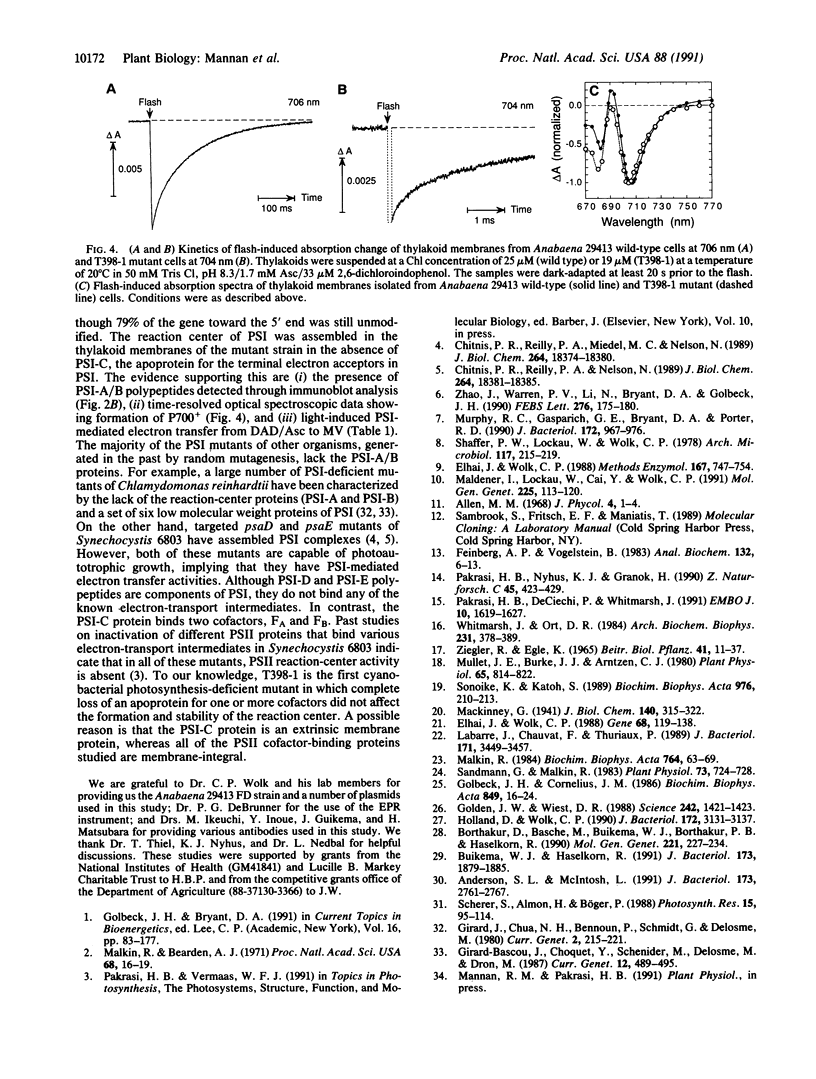
In oxygenic photosynthetic organisms the PSI-C polypeptide, encoded by the psaC gene, provides the ligands for two [4Fe-4S] centers, FA and FB, the terminal electron acceptors in the photosystem I (PSI) complex. An insertion mutation introduced in the psaC locus of the filamentous cyanobacterium Anabaena variabilis ATCC 29413 resulted in the creation of a mutant strain, T398-1, that lacks the PSI-C polypeptide. In medium supplemented with 5 mM fructose, the mutant cells grew well in the dark. However, when grown in the same medium under light, the doubling rate of T398-1 cells was significantly decreased. In intact cells of T398-1, bicarbonate-dependent whole-chain electron transport (PSII and PSI) could not be detected, although partial electron transport reactions involving either one of the two photosystems could be measured at significant rates. The low-temperature EPR signals attributed to the [4Fe-4S] centers FA and FB were absent in the mutant cells. Chemical titration measurements indicated that the ratios of chlorophyll to the primary donor P700 were virtually identical in membranes from the wild-type and mutant cells. Moreover, room-temperature optical spectroscopic analysis of the thylakoid membranes isolated from T398-1 showed flash-induced P700 oxidation followed by dark rereduction, indicating primary photochemistry in PSI. Thus stable assembly of the reaction center of PSI can occur in the absence of the Fe-S cluster cofactors FA and FB. These studies demonstrate that Anabaena 29413 offers a useful genetic system for targeted mutagenesis of the PSI complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. L., McIntosh L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J Bacteriol. 1991 May;173(9):2761–2767. doi: 10.1128/jb.173.9.2761-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur D., Basche M., Buikema W. J., Borthakur P. B., Haselkorn R. Expression, nucleotide sequence and mutational analysis of two open reading frames in the nif gene region of Anabaena sp. strain PCC7120. Mol Gen Genet. 1990 Apr;221(2):227–234. doi: 10.1007/BF00261725. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Mar;173(6):1879–1885. doi: 10.1128/jb.173.6.1879-1885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Miedel M. C., Nelson N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18374–18380. [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Nelson N. Insertional inactivation of the gene encoding subunit II of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18381–18385. [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Girard-Bascou J., Choquet Y., Schneider M., Delosme M., Dron M. Characterization of a chloroplast mutation in the psaA2 gene of Chlamydomonas reinhardtii. Curr Genet. 1987;12(7):489–495. doi: 10.1007/BF00419557. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Holland D., Wolk C. P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990 Jun;172(6):3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarre J., Chauvat F., Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989 Jun;171(6):3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldener I., Lockau W., Cai Y. P., Wolk C. P. Calcium-dependent protease of the cyanobacterium Anabaena: molecular cloning and expression of the gene in Escherichia coli, sequencing and site-directed mutagenesis. Mol Gen Genet. 1991 Jan;225(1):113–120. doi: 10.1007/BF00282649. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C., Gasparich G. E., Bryant D. A., Porter R. D. Nucleotide sequence and further characterization of the Synechococcus sp. strain PCC 7002 recA gene: complementation of a cyanobacterial recA mutation by the Escherichia coli recA gene. J Bacteriol. 1990 Feb;172(2):967–976. doi: 10.1128/jb.172.2.967-976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., De Ciechi P., Whitmarsh J. Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. EMBO J. 1991 Jul;10(7):1619–1627. doi: 10.1002/j.1460-2075.1991.tb07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Nyhus K. J., Granok H. Targeted deletion mutagenesis of the beta subunit of cytochrome b559 protein destabilizes the reaction center of photosystem II. Z Naturforsch C. 1990 May;45(5):423–429. doi: 10.1515/znc-1990-0519. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Malkin R. Iron-sulfur centers and activities of the photosynthetic electron transport chain in iron-deficient cultures of the blue-green alga aphanocapsa. Plant Physiol. 1983 Nov;73(3):724–728. doi: 10.1104/pp.73.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- Zhao J. D., Warren P. V., Li N., Bryant D. A., Golbeck J. H. Reconstitution of electron transport in photosystem I with PsaC and PsaD proteins expressed in Escherichia coli. FEBS Lett. 1990 Dec 10;276(1-2):175–180. doi: 10.1016/0014-5793(90)80536-r. [DOI] [PubMed] [Google Scholar]