Abstract
The integrin heterodimers composed of the alpha 6 subunit with the beta 1 or beta 4 subunit (alpha 6 beta 1 and alpha 6 beta 4) are receptors for laminin and basement membrane components, respectively. The alpha 3 beta 1 integrin recognizes laminin, collagen, fibronectin, or epiligrin. We report the identification of structural variants (A and B) of the alpha 6 and alpha 3 subunits, containing distinct cytoplasmic domains. The expression of one cytoplasmic domain or the other, based probably on alternative exon usage, is cell-type dependent. Most transformed cell lines express both alpha 6A and alpha 6B isoforms, as determined by mRNA amplification or antibody immunoprecipitation. In contrast, embryonic fibroblasts express exclusively alpha 6A, and embryonic stem cells express exclusively alpha 6B. In most normal tissues, both alpha 6 isoforms are detectable, but several tissues express either alpha 6A or alpha 6B. The alpha 3B mRNA was amplified from heart and brain, while all other tissues and cell lines tested contained only alpha 3A mRNA. Alternative cytoplasmic domains may provide a means for varying the cellular responses to the ligands of alpha 6 and alpha 3 integrins according to the cell type.
Full text
PDF
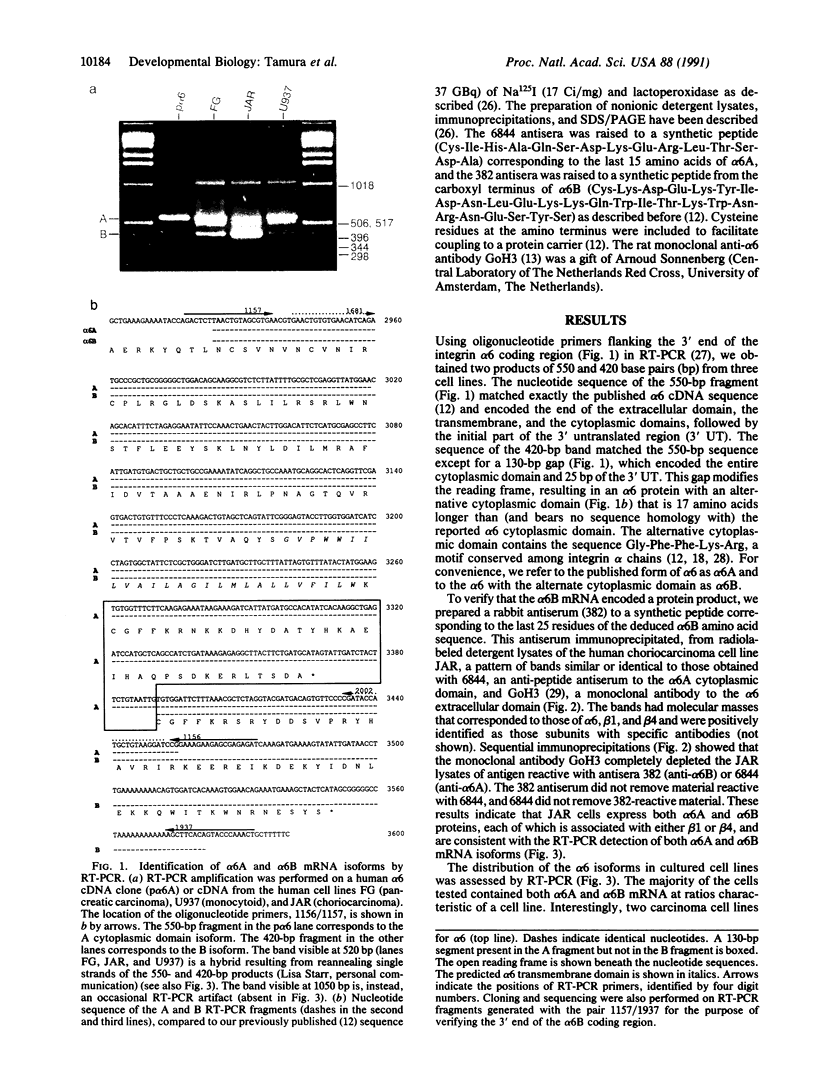

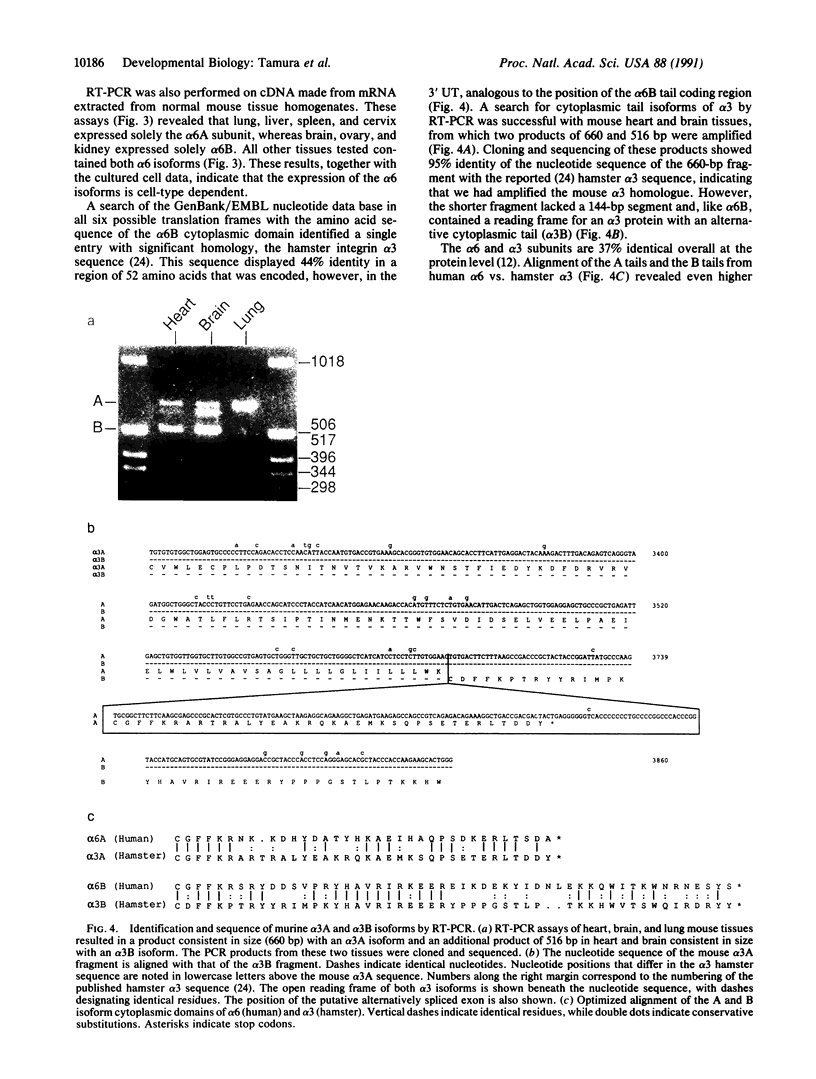

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bray P. F., Leung C. S., Shuman M. A. Human platelets and megakaryocytes contain alternately spliced glycoprotein IIb mRNAs. J Biol Chem. 1990 Jun 15;265(17):9587–9590. [PubMed] [Google Scholar]
- Brown N. H., King D. L., Wilcox M., Kafatos F. C. Developmentally regulated alternative splicing of Drosophila integrin PS2 alpha transcripts. Cell. 1989 Oct 6;59(1):185–195. doi: 10.1016/0092-8674(89)90880-5. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Garcia-Aguilar J., Springer T. A. Genomic structure of an integrin alpha subunit, the leukocyte p150,95 molecule. J Biol Chem. 1990 Feb 15;265(5):2782–2788. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Vestweber D., Kemler R. Cell-matrix interactions and cell adhesion during development. Annu Rev Cell Biol. 1986;2:27–47. doi: 10.1146/annurev.cb.02.110186.000331. [DOI] [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Takada Y., Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988 Jun 5;263(16):7660–7665. [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Large T. H., Houde M., Tawil J. W., Barton A., Esch F., Carbonetto S., Reichardt L. F. Molecular cloning of the rat integrin alpha 1-subunit: a receptor for laminin and collagen. J Cell Biol. 1990 Aug;111(2):709–720. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiji S., Tamura R. N., Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989 Mar;8(3):673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Cheng Y. F., Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990 Sep;111(3):1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandini M. H., Denarier E., Frachet P., Uzan G., Marguerie G. Isolation of the human platelet glycoprotein IIb gene and characterization of the 5' flanking region. Biochem Biophys Res Commun. 1988 Oct 14;156(1):595–601. doi: 10.1016/s0006-291x(88)80884-2. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Jones J. C. The internal affairs of an integrin. Trends Cell Biol. 1991 Jul;1(1):2–4. doi: 10.1016/0962-8924(91)90046-c. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E., Lawrence M., Springer T. A., Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991 Aug;195(2):533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- Shaw L. M., Messier J. M., Mercurio A. M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol. 1990 Jun;110(6):2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Sorokin L., Sonnenberg A., Aumailley M., Timpl R., Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990 Sep;111(3):1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Yamamoto F., Miura Y., Takio K., Titani K., Pawar S., Osawa T., Hakomori S. Characterization through cDNA cloning of galactoprotein b3 (Gap b3), a cell surface membrane glycoprotein showing enhanced expression on oncogenic transformation. Identification of Gap b3 as a member of the integrin superfamily. J Biol Chem. 1990 Apr 25;265(12):7016–7021. [PubMed] [Google Scholar]
- Wacholtz M. C., Patel S. S., Lipsky P. E. Leukocyte function-associated antigen 1 is an activation molecule for human T cells. J Exp Med. 1989 Aug 1;170(2):431–448. doi: 10.1084/jem.170.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I., Quaranta V., Tamura R. N., Reichardt L. F. Laminin receptors in the retina: sequence analysis of the chick integrin alpha 6 subunit. Evidence for transcriptional and posttranslational regulation. J Cell Biol. 1991 Apr;113(2):405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppevelt T. H., Languino L. R., Gailit J. O., Suzuki S., Ruoslahti E. An alternative cytoplasmic domain of the integrin beta 3 subunit. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5415–5418. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]