An extensive body of work has demonstrated that atherosclerotic plaques form preferentially at sites of disturbed blood flow encountered at vessel bifurcations, resulting in part from the loss of anti-inflammatory, antithrombotic and antioxidative effects that occur in straight vessel segments due to laminar pulsatile flow1. The production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) is a central mechanism of these protective effects2. Transcriptional and posttranslational regulation of eNOS combine to augment NO production in response to flow-induced mechanical stimulation of the apical endothelial cell surface3. AMP-activated protein kinase (AMPK) and the deacetylase sirtuin 1 (SIRT1) are key mediators of multiple vascular endothelial processes. AMPK mediates metformin-induced protection from oxidative stress4 and regulates coronary hemodynamics5 and arteriogenesis6, while SIRT1 suppresses endothelial senescence produced by disturbed flow7. Laminar flow increases the activity of both AMPK and SIRT1, which then act synergistically on eNOS via phosphorylation by AMPK priming and subsequent deacetylation by SIRT1 to increase NO bioavailability8.
In the current issue of ATVB, Shentu and colleagues now substantially advance our understanding of this important process by demonstrating that AMPK and SIRT1 similarly work together to co-regulate function of the actin-binding protein cortactin, leading to altered peripheral cytoskeletal and membrane structures in vascular endothelial cells that in turn contribute to eNOS activation and decreased atherosclerosis9. Cortactin is a multi-domain cytoskeletal scaffold protein that was first identified more than 20 years ago as a novel F-actin binding protein and substrate of v-Src10. The gene encoding cortactin, EMS1, was initially detected as highly overexpressed in human carcinomas11 but subsequently recognized as ubiquitously expressed in almost all tissues and is especially abundant in the endothelium. Cortactin is an ideal candidate to integrate signal transduction and cytoskeletal structural changes because of its the ability to bind actin, interact with a variety of cytoskeletal effector proteins including Arp 2/3, N-WASP and MLCK, and serve as a target for key regulatory kinases, including Src, ERK and c-Abl12, 13. The peripheral cellular distribution of cortactin and its association with cortical actin were early observations, are implicit in its very name, and further strengthen the idea that cortactin is an important link between extracellular stimuli, cytoskeletal organization and membrane dynamics. Over the last two decades, critical roles for cortactin have been described in cell migration, tumor invasion, host-pathogen interactions, endocytosis, vesicle trafficking, intercellular junctional assembly, leukocyte diapedesis and endothelial barrier function12, 14–16. These processes are essential components of vascular endothelial function and pathology, and it is not surprising that this tissue has served as the backdrop for much of the work highlighting the diverse structural and functional roles of cortactin. As noted above, mechanisms by which blood flow induces atherosclerosis or atheroprotection are intriguing areas of investigation requiring the endothelial cell to translate properties of the local environment, especially flow, into dynamic alterations of the cytoskeleton, cell membrane and biochemical signaling17, 18. The present study identifying cortactin as a key player in atheroprotective eNOS regulation sheds new light on how laminar pulsatile flow mediates atheroprotection and serves to expand the influential role and importance of this protein in endothelial biology.
The authors hypothesized that pulsatile flow leads to concerted regulation of cortactin by AMPK/SIRT1 similar to that observed in eNOS as cortactin contains consensus sequences for both proteins. After confirming specific AMPK phosphorylation of cortactin at Thr-401 in proteomic experiments, they subsequently demonstrate AMPK priming of cortactin for SIRT1 deacetylation in cultured endothelial cells subjected to pulsatile shear stress (PS). Next, employing gene knockdown and both gain and loss of function mutants in an elegant series of experiments, Shentu and colleagues determined that activated cortactin plays a direct role in eNOS activation in response to PS. Mechanistically this occurs via cortactin translocation to the periphery in a close association with F-actin, which subsequently facilitates the transfer of eNOS from lipid to nonlipid raft domains, eNOS phosphorylation, and generation of atheroprotective NO. Finally, in vivo studies in cortactin +/− mice revealed impaired NO production and flow-induced vasodilation associated with reduced caveolae and phosphorylated eNOS at the cellular level. This endothelial dysfunction was associated with an increase in atherosclerotic plaques in ApoE −/− cortactin +/− animals as compared to ApoE −/− cortactin +/+. Taken together these data provide convincing evidence that cytoskeletal and membrane dynamics as regulated by the AMPK/SIRT1/cortactin pathway are key determinants of atheroprotection induced by blood flow patterns.
Cortactin is a rich target for investigation into the coordination of numerous signaling pathways terminating in altered peripheral cytoskeletal and membrane dynamics, qualities vital to endothelial migration, barrier integrity and interaction with the local physical and cellular environment. Interestingly, differential patterns of cortactin phosphorylation lead to markedly different outcomes in actin structure and function. Serine and tyrosine phosphorylation by Src and ERK affect cortactin’s interaction with effectors of actin polymerization and tensile force generation15, 19, 20, with Src tyrosine phosphorylation of cortactin also associated with focal adhesion disassembly and turnover21. While Thr-401 phosphorylation by Akt has been implicated in cancer cell migration and invasion22, the current study adds to this complexity with phosphorylation at this same Thr-401 site by AMPK significantly impacting cortactin-F-actin association and regulation of membrane associated caveolae. The specific alterations in cytoskeletal structure and dynamics responsible for these varied effects remain unknown and are intriguing areas for further study.
The vascular endothelium is a varied and intensely regulated tissue with the ability to adapt its structure and function specifically to the requirements of each organ system and physiologic variable. Exciting work such as the current study by Shentu and colleagues continues to reveal the complex biochemistry underlying such changes at the cellular level. Cortactin represents a prominent cog in this larger wheel. A more thorough understanding of this fascinating protein appears destined to expand the discourse in new and exciting directions which will advance our knowledge of vascular biology and disease, including these newly identified insights into atheroprotective mechanisms mediated by the AMPK/SIRT1 signaling axis.
Figure. Atheroprotective eNOS activation is mediated by AMPK/SIRT1 and cortactin.
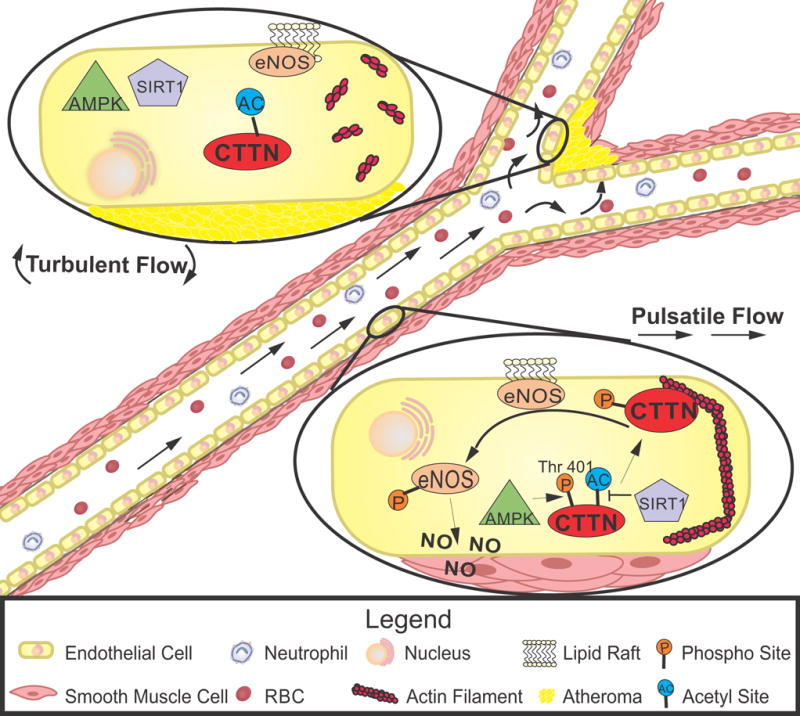
Endothelial cells exposed to laminar pulsatile flow (bottom cell) demonstrate AMPK phosphorylation of cortactin on threonine-401, priming it for subsequent deacetylation by SIRT1. Following these modification, cortactin locates to cortical regions where it associates with F-actin to chaperone eNOS from lipid rafts to the cytoplasm. Subsequent eNOS activation increases nitric oxide to produce vasodilation and reduce atherosclerotic plaque formation. In areas of turbulent flow (top cell), a lack of AMPK/SIRT1 activation and absence of the downstream cytoskeletal rearrangements mediated by cortactin reduce eNOS activation and nitric oxide bioavailability to increase atherosclerotic plaques. AMPK indicates AMP-activated protein kinase; CTTN, cortactin; eNOS, endothelial nitric oxide synthase; SIRT1, sirtuin 1.
Acknowledgments
None
Sources of Funding: None
Footnotes
Disclosure: None
References
- 1.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. The Journal of clinical investigation. 2016;126:821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying no: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circulation research. 2016;119:375–396. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 3.Balligand JL, Feron O, Dessy C. Enos activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiological reviews. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 4.Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:830–836. doi: 10.1161/ATVBAHA.113.301938. [DOI] [PubMed] [Google Scholar]
- 5.Enkhjargal B, Godo S, Sawada A, Suvd N, Saito H, Noda K, Satoh K, Shimokawa H. Endothelial amp-activated protein kinase regulates blood pressure and coronary flow responses through hyperpolarization mechanism in mice. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1505–1513. doi: 10.1161/ATVBAHA.114.303735. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Zhang M, Liu Z, Xing J, Moriasi C, Dai X, Zou MH. Amp-activated protein kinase alpha1 in macrophages promotes collateral remodeling and arteriogenesis in mice in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:1868–1878. doi: 10.1161/ATVBAHA.116.307743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warboys CM, de Luca A, Amini N, et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:985–995. doi: 10.1161/ATVBAHA.114.303415. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, sirt1, and vascular homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shentu T-P, He M, Sun X, Zhang J, Zhang F, Gongol B, Marin TL, Zhang J, Wen L, Wang Y, Johnson DA, Shyy JY. Ampk and sirt1 coregulation of cortactin contributes to endothelial function. Arteriosclerosis, thrombosis, and vascular biology. 2016 doi: 10.1161/ATVBAHA.116.307871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. The Journal of cell biology. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, u21b31/prad1 and ems1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- 12.Cosen-Binker LI, Kapus A. Cortactin: The gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo AN, Aman J, van Nieuw Amerongen GP, Dudek SM. Targeting abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1071–1079. doi: 10.1161/ATVBAHA.115.305085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XM, Huang BQ, Splinter PL, Cao H, Zhu G, McNiven MA, LaRusso NF. Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-src. Gastroenterology. 2003;125:216–228. doi: 10.1016/s0016-5085(03)00662-0. [DOI] [PubMed] [Google Scholar]
- 15.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: Roles for cortactin and myosin light chain kinase. The Journal of biological chemistry. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 16.MacGrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. Journal of cell science. 2012;125:1621–1626. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe J, Berk BC. Novel mechanisms of endothelial mechanotransduction. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2378–2386. doi: 10.1161/ATVBAHA.114.303428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate n-wasp. Molecular and cellular biology. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruchten AE, Krueger EW, Wang Y, McNiven MA. Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. American journal of physiology. Cell physiology. 2008;295:C1113–1122. doi: 10.1152/ajpcell.00238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Renuse S, Sahasrabuddhe NA, et al. Activation of diverse signalling pathways by oncogenic pik3ca mutations. Nature communications. 2014;5:4961. doi: 10.1038/ncomms5961. [DOI] [PMC free article] [PubMed] [Google Scholar]


