ABSTRACT
Drought is the most serious problem that impedes crop development and productivity worldwide. Although several studies have documented the root architecture adaption for drought tolerance, little is known about the underlying molecular mechanisms. Our latest study demonstrated that overexpression of the OsERF71 in rice roots under drought conditions modifies root structure including larger aerenchyma and radial root growth, and thereby, protects the rice plants from drought stresses. The OsERF71-mediated root modifications are caused by combinatory overexpression of general stress-inducible, cell wall-associated and lignin biosynthesis genes that contribute to drought tolerance. Here we addressed that the OsERF71-mediated root modifications alter physiological capacity in shoots without evidence of developmental changes for drought tolerance. Thus, the OsERF71-mediated root modifications provide novel molecular insights into drought tolerance mechanisms.
KEYWORDS: AP2, drought, ERF, transcription factor, rice, lignification, radial root growth
Plants have evolved adaptive strategies to cope with the drought stress. Particularly, root architecture modification provides an informative example for plant response to drought stress. Plant roots are capable of detecting soil information such as water contents. Upon drought perception, plants give rise to root structural modification including length, number and radial expansion.1-8 Alternatively or simultaneously, plant roots release uncharacterized drought-inducible signals that move to the aerial parts of the plants and confer drought tolerance to the shoot.9-14 Although several studies have documented the root adaption for drought tolerance, little is known about the underlying molecular mechanisms not only that give rise to root morphological modification but also by which the root morphological adaptation affects plant capacity to drought stresses. Our latest study in Plant Physiology investigated drought-inducible rice OsERF71 transcription factor and explored the molecular mechanisms for drought-related root morphological adaptation that enhance plant capacity against drought stresses.15
OsERF71-mediated root modification for drought tolerance
Expression of OsERF71, a gene for an AP2/ERF transcription factor, is drought-inducible in an ABA independent mechanism. Overexpression of OsERF71 under the control of two different promoters, driving expression either in whole plant body (GOS2 promoter), or specifically in root (RCc3 promoter), results in drought-tolerant phenotypes at the vegetative growth stage. In addition, the transgenic rice plants with root-specific OsERF71 expression show the enhanced grain yield under drought stress in rice paddy field. These data indicate that the OsERF71 overexpression in roots is sufficient to confer drought tolerance. The OsERF71-mediated drought tolerance is connected to a root structure adaptation. The OsERF71 overexpression in roots alters radial root growth including larger aerenchyma and more cell layers between metaxylem cells. The larger aerenchyma are a root modification commonly found in drought tolerant rice plants, such that overexpression of OsNAC5, OsNAC9 and OsNAC10 activates radial root growth that enhances tolerance to drought stress.4,6,7 Maize roots with large cortical aerenchyma also promote drought tolerance since it reduces the metabolic cost of soil exploration under water stress, permitting greater root growth and water acquisition from drying soil.16 In addition to radial root growth, root elongation and high number of roots are associated with root structural adaptation to drought stresses.3,5,8 For example, rice inbred lines (IR20 × MGL-2) with long and thick roots exhibit enhanced drought tolerance.1 What is more, overexpression of TaNAC2 and HRD (HARDY) in A. thaliana or rice promotes primary and lateral root growth, increases root numbers and thereby enhances drought tolerance.3,5 In this way, the drought-inducible OsERF71 that over-produced in rice roots under drought conditions, modifies root structure including larger aerenchyma and radial root growth, and thereby, protects the rice plants from drought stresses.
Molecular mechanisms of the OsERF71-mediated drought tolerance
In our latest study of Plant Physiology, genome-wide analysis was performed to identify numerous downstream genes that are upregulated by OsERF71 transcription factor in the transgenic rice roots. They are divided into three categories: general stress-inducible genes, cell wall-associated genes, and lignin biosynthesis genes. Cell wall-associated proteins such as EXPANSIN, CHITINASE, and PECTINESTERASE are thought to be important for plant adaptation to drought stress by modifying root structure such as larger aerenchyma.17-19 Additionally, OsERF71 controls lignin biosynthesis genes in roots by directly regulating the expression of CINNAMOYL-CoA REDUCTASE 1 (OsCCR1), a key gene in lignin biosynthesis. Since lignin is a key component of cell wall, OsERF71-mediated lignification contributes to cell shape and physiological modification together with other upregulated cell wall-associated genes and thereby, induces radial root growth. Furthermore, root lignification is known to be triggered by drought stress,20-22 suggesting that the hydrophobic lignin property prevents water transpiration from plant tissues under drought conditions. Consequently, the OsERF71-mediated cell wall modifications such as lignification and cell wall loosening provide molecular insights into drought tolerance mechanisms via root structural adaptations.
Can the OsERF71-mediated root modification enhance shoot drought tolerance?
OsERF71 overexpression in rice roots induces drought tolerance in whole rice plants. To understand whether the OsERF71-induced root modification affects shoot capacity against drought stress, the photochemical efficiency of photosystem II that is determined by Fv/Fm measurements and that is reduced by drought stress, was analyzed with detached leaf discs from transgenic leaves. We treated leaf discs with drought stress for 1 h, because NT (non-transgenic) leaves exhibit a rapid decrease in Fv/Fm values as early as 1 h after the onset of the drought treatment. Under non-drought conditions, the Fv/Fm values were approximately 0.8 in leaf discs from two-week-old transgenic and NT rice plants (Fig. 1); however, under drought conditions, OsERF71 overexpression lines showed 20–35% higher Fv/Fm values than those of the NT controls (Fig. 1). These data suggest that OsERF71 overexpression in rice root is sufficient to enhance drought tolerance in rice shoots.
Figure 1.
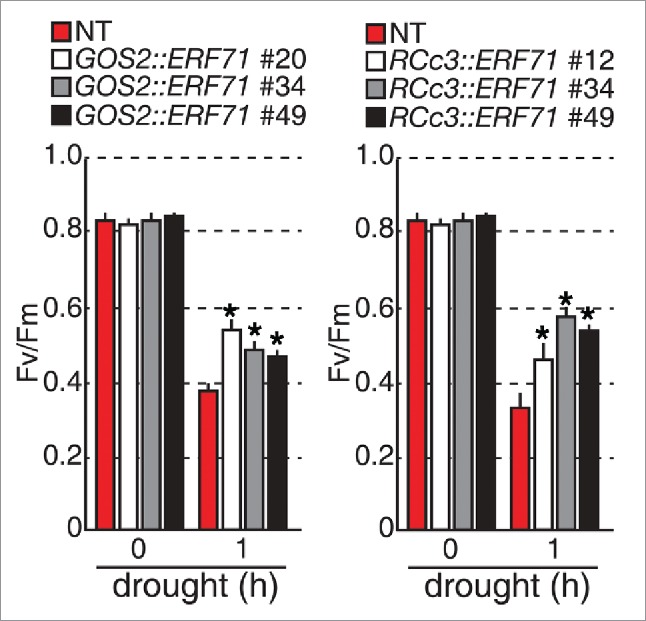
Photochemical efficiency (Fv/Fm) of GOS2::OsERF71 and RCc3::OsERF71 transgenic rice plants. Three independent homozygous GOS2::OsERF71 and RCc3::OsERF71 transgenic lines and NT controls were grown in soil for 2 weeks and detached leaf discs were exposed to a drought stress. Fv/Fm values were measured using a pulse modulation fluorometer. Data are shown as the mean + SD (n = 30). Asterisks indicate significant differences compared with NT control plants (P < 0.05 by Student's t-test).
How does the OsERF71-mediated root modification affect shoot drought capacity against drought stress? The OsERF71-mediated root modification may affect developmental changes of shoots. For example, drought treatment to roots causes leaf growth inhibition and stomatal behavior modification together with leaf cell-wall-hardening via osmotically generated hydraulic signals and ABA.13,23-25 Inhibition of leaf growth is often a primary plant response to moderate water stress.23,26 However, since we found no developmental abnormality in shoots of OsERF71 overexpression rice plants, it is not persuasive. Alternatively, plant shoots get drought tolerant capacity based on physiological modification without developmental changes. The OsERF71 overexpression rice leaves may have high water content due to reduction of water loss in roots by OsERF71-mediated root lignification. For example, transcription levels of two maize lignin biosynthesis CCR1 and CCR2 genes are increased after only 1 hr of drought stress treatment in the root elongation zone.20 Accumulation of lignin in the root is involved in water loss prevention and, therefore can supply water to the aerial part of plants.27 Thus, the OsERF71-mediated root lignification modifies physiological capacity in shoots for drought tolerance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Rural Development Administration under the Next-Generation BioGreen 21 Program (PJ011829012016 to J.-K.K.) and by the Basic Science Research Program through the National Research Foundation of Korea, Ministry of Education (NRF-2014R1A6A3A04053795 to D.-K.L.).
References
- 1.Ekanayake IJ, O'Toole JC, Garrity DP, Masajo TM. Inheritance of root characters and their relations to drought resistance in rice. Crop Sci 1985; 25:927-33; http://dx.doi.org/ 10.2135/cropsci1985.0011183X002500060007x [DOI] [Google Scholar]
- 2.Price AH, Tomos AD, Virk DS. Genetic dissection of root growth in rice (Oryza sativa L.). I. A hydrophonic screen. Theor Appl Genet 1997; 95:132-42; http://dx.doi.org/ 10.1007/s001220050541 [DOI] [Google Scholar]
- 3.Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA 2007; 104:15270-5; PMID:17881564; http://dx.doi.org/ 10.1073/pnas.0707294104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Choi YD, Kim M, Reuzeau C, Kim JK. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 2010; 153:185-97; PMID:20335401; http://dx.doi.org/ 10.1104/pp.110.154773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerance in Arabidopsis. J Exp Bot 2012; 63:2933-46; PMID:22330896; http://dx.doi.org/ 10.1093/jxb/err462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redillas MCFR, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, Kim M, Reuzeau C, Kim JK. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol J 2012; 10:792-805; PMID:22551450; http://dx.doi.org/ 10.1111/j.1467-7652.2012.00697.x [DOI] [PubMed] [Google Scholar]
- 7.Jeong JS, Kim YS, Redillas MCFR, Jang G, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, et al.. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increase grain yield in the field. Plant Biotechnol J 2013; 11:101-14; PMID:23094910; http://dx.doi.org/ 10.1111/pbi.12011 [DOI] [PubMed] [Google Scholar]
- 8.Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al.. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genet 2013; 45:1097-102; PMID:23913002; http://dx.doi.org/ 10.1038/ng.2725 [DOI] [PubMed] [Google Scholar]
- 9.Went FW. Effect of the root system on tomato stem growth. Plant Physiol 1943; 18:51-65; PMID:16653830; http://dx.doi.org/ 10.1104/pp.18.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passioura JB. Root signals control leaf expansion in wheat seedling growing in drying soil. Aust J Plant Physiol 1988; 15:687-93; http://dx.doi.org/ 10.1071/PP9880687 [DOI] [Google Scholar]
- 11.Saab IM, Sharp RE. Non-hydraulic signals from maize roots in drying soil: inhibition of leaf elongation but not stomatal conductance. Planta 1989; 179:466-74; PMID:24201770; http://dx.doi.org/ 10.1007/BF00397586 [DOI] [PubMed] [Google Scholar]
- 12.Gowing DJG, Davies WJ, Jones HG. A positive root-sourced signal as an indicator of soil drying in apple, Malus × domestica Borkh. J Exp Bot 1990; 41:1535-40; http://dx.doi.org/ 10.1093/jxb/41.12.1535 [DOI] [Google Scholar]
- 13.Chazen O, Neumann PM. Hydraulic signals from the roots and rapid cell-wall hardening in growing maize (Zea mays L.) leaves are primary response to polyethylene glycol-induced water deficits. Plant Physiol 1994; 104:1385-92; PMID:12232175; http://dx.doi.org/ 10.1104/pp.104.4.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieburth LE, Lee DK. BYPASS1: how a tiny mutant tell a big story about root-to-shoot signaling. J Integr Plant Biol 2010; 52:77-85; PMID:20074142; http://dx.doi.org/ 10.1111/j.1744-7909.2010.00902.x [DOI] [PubMed] [Google Scholar]
- 15.Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Do Choi Y, Kim JK. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol 2016; 172:575-88; PMID:27382137; http://dx.doi.org/ 10.1104/pp.16.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Brown KM, Lynch JP. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 2010; 33:740-9; PMID:20519019; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02099.x [DOI] [PubMed] [Google Scholar]
- 17.Moore JP, Vicre-Gibouin M, Farrant JM, Driouich A. Adaptation of higher plant cell walls to water loss: drought vs desiccation. Physiol Plant 2008; 134:237-45; PMID:18494857; http://dx.doi.org/ 10.1111/j.1399-3054.2008.01134.x [DOI] [PubMed] [Google Scholar]
- 18.Ghosh D, Xu J. Abiotic stress responses in plant roots: a proteomics perspective. Front Plant Sci 2014; 5:6; PMID:24478786; http://dx.doi.org/ 10.3389/fpls.2014.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DK, Ahn JH, Song SK, Choi YD, Lee JS. Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol 2003; 131:985-97; PMID:12644651; http://dx.doi.org/ 10.1104/pp.009902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stellar accumulation of wall phenolics. Plant Physiol 2006; 140:603-12; PMID:16384904; http://dx.doi.org/ 10.1104/pp.105.073130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K. Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 2008; 49:226-41; PMID:18178965; http://dx.doi.org/ 10.1093/pcp/pcm180 [DOI] [PubMed] [Google Scholar]
- 22.Barros J, Serk H, Granlund I, Pesquet E. The cell biology of lignification in higher plants. Ann Bot 2015; 115:1053-74; PMID:25878140; http://dx.doi.org/ 10.1093/aob/mcv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z, Neumann PM. Water stress inhibits hydraulic conductance and leaf growth in rice seedling but not the transport of water via mercury-sensitive water channels in the root. Plant Physiol 1999; 120:143-51; PMID:10318692; http://dx.doi.org/ 10.1104/pp.120.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comstock JP. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J Exp Bot 2002; 53:195-200; PMID:11807122; http://dx.doi.org/ 10.1093/jexbot/53.367.195 [DOI] [PubMed] [Google Scholar]
- 25.Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D, Poni S, Palliotti A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci Rep 2015; 5:12449; PMID:26207993; http://dx.doi.org/ 10.1038/srep12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogoslavsky L, Neumann PM. Rapid regulation by acid pH of cell wall adjustment and leaf growth in maize plants responding to reversal of water stress. Plant Physiol 1998; 118:701-9; PMID:9765556; http://dx.doi.org/ 10.1104/pp.118.2.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C. How tree roots respond to drought. Front Plant Sci 2015; 6:547; PMID:26284083; http://dx.doi.org/ 10.3389/fpls.2015.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]


