Abstract
RET fusions have been found in lung adenocarcinoma, of which KIF5B-RET is the most prevalent. We established inducible KIF5B-RET transgenic mice and KIF5B-RET-dependent cell lines for preclinical modeling of KIF5B-RET-associated lung adenocarcinoma. Dox-induced CCSP-rtTA/tetO-KIF5B-RET transgenic mice developed invasive lung adenocarcinoma with desmoplastic reaction. Tumors regressed upon suppression of KIF5B-RET expression. By culturing KIF5B-RET-dependent BaF3 (B/KR) cells with increasing concentrations of cabozantinib or vandetanib, we identified cabozantinib-resistant RETV804L mutation and vandetanib-resistant-RETG810A mutation. Among cabozantinib, lenvatinib, ponatinib, and vandetanib, ponatinib was identified as the most potent inhibitor against KIF5B-RET and its drug-resistant mutants. Interestingly, the vandetanib-resistant KIF5B-RETG810A mutant displayed gain-of-sensitivity (GOS) to ponatinib and lenvatinib. Treatment of Dox-induced CCSP-rtTA/tetO-KIF5B-RET bitransgenic mice with ponatinib effectively induced tumor regression. These results indicate that KIF5B-RET-associated lung tumors are addicted to the fusion oncogene and ponatinib is the most effective inhibitor for targeting KIF5B-RET in lung adenocarcinoma. Moreover, this study finds a novel vandetanib-resistant RETG810A mutation and identifies lenvatinib and ponatinib as the secondary drugs to overcome this vandetanib resistance mechanism.
Keywords: KIF5B-RET, fusion oncogene, lung adenocarcinoma, transgenic mice, protein tyrosine kinase inhibitor, drug resistance, mutation, ponatinib
Introduction
Lung adenocarcinoma is a major subtype of non-small cell lung cancer (NSCLC) with increasing incidence in many countries (1). Compared to other histological types of lung cancer, lung adenocarcinoma frequently harbors KRAS or protein tyrosine kinase (PTK) aberrations (2, 3). While mutant KRAS is difficult to target directly at present, PTKs are targetable, allowing precision treatment targeting individual patient’s driver oncogene. Targeting PTK driver oncogenes with small molecule inhibitors in lung adenocarcinoma has shown clinical success in managing NSCLC. This is exemplified by EGFR and ALK inhibitor therapies in EGFR mutation and ALK-fusion lung adenocarcinoma (4, 5). However, acquired resistance to EGFR and ALK kinase inhibitors usually occur within a year after the drug treatment, making it necessary to use secondary drugs to prolong the therapeutic response (6, 7). Moreover, except for EGFR mutations and ALK fusions, other PTK alterations in lung adenocarcinoma occur at <3% rates (8, 9), rendering clinical studies of these PTK oncogenes difficult (10).
Several laboratories have identified recurrent RET fusion genes in 1–2% of lung adenocarcinoma cases (8, 11–14). Among the fusion partners, KIF5B is the most prevalent. RET rearrangements in lung adenocarcinoma are mutually exclusive from other known driver oncogenic mutations, including KRAS, EGFR, ALK, BRAF, and ERBB2 (8). Moreover, ALK, RET, and ROS1 oncofusion-associated lung adenocarcinoma tissues harbor significantly fewer mutations than other lung adenocarcinoma tissues (10). This suggests a strong role of these fusion oncogenes in driving the tumors, implicating them as excellent therapeutic targets.
A number of PTK inhibitors (TKIs) are known to cross-inhibit RET kinase activity (15). These include cabozantinib and vandetanib that have been approved by the United States Food and Drug Administration for the treatment of advanced medullary thyroid cancer, which carries RET point mutations in 30–50% of cases (15). So far, two reports have described the responses of 6 RET-fusion lung adenocarcinoma patients treated with cabozantinib: 4 patients had partial responses and 2 patients had stable disease (16, 17). One patient with RET-fusion lung adenocarcinoma who received vandetanib was reported to have decreased tumor size (18). These are encouraging initial findings but more studies are needed. Several clinical trials of RET kinase inhibitors are ongoing (8, 15). However, the rarity of this molecular subtype of the disease presents a barrier for the current and future clinical studies, as it will take a long time to enroll adequate number of patients into the clinical trials.
To generate a preclinical model of RET fusion lung adenocarcinoma in an immune competent environment, we generated tetO-KIF5B-RET transgenic mice and used CCSP-rtTA/tetO-KIF5B-RET bitransgenic mice to induce KIF5B-RET expression in the lungs by doxycycline (Dox). We found that KIF5B-RET induced invasive lung adenocarcinoma with desmoplastic reaction. After tumor development, continuous expression of the KIF5B-RET fusion gene is required to maintain the lung tumors. Using a parallel in vitro cell-based assay, we identified ponatinib as the most potent inhibitor against KIF5B-RET, its kinase gatekeeper mutations, and a novel vandetanib-resistant RETG810A mutant. Treatment of Dox-induced CCSP-rtTA/tetO-KIF5B-RET transgenic mice with ponatinib resulted in tumor regression.
Materials and Methods
Reagents
Antibodies to phospho-RET(Tyr905) and cleaved-PARP were from Cell Signaling Technology, antibodies to Flag-tag and β-actin were from Sigma, anti-RET antibody was from Santa Cruz Biotech. Anti-TTF1 (ab137061) antibody was from Abcam. Anti-cytokeratin antibody was from Dako (cat. No. Z0622). Ponatinib was from LC Laboratories. Cabozantinib, vandetanib, and lenvatinib were from Selleckchem.
Generation of transgenic mice
The KIF5B-RET (K15;R12, RET51 long form) (12) coding region with a Flag tag coding sequence at the C-terminus was synthesized by GeneArt Gene Synthesis (Life Technologies) and cloned into the L3/L2 loxP-tetO plasmid (19). The 6.6-kb BssHII tetO-KIF5B-RET transgene cassette (Fig. 1A) DNA fragment was used to generate tetO-KIF5B-RET transgenic mice in FVB/N strain as described in Supplementary Information.
Figure 1.
Derivation and analysis of tetO-KIF5B-RET transgenic mice. A, schematic representation of tetO-KIF5B-RET transgene. B, scheme for production of C/KR bitransgenic mice and induction of the KIF5B-RET transgene expression in the mouse lung type II epithelial cells. C, RT-PCR analysis of KIF5B-RET mRNA expression in various tissues in monotransgenic tetO-KIF5B-RET mouse and in C/KR bitransgenic mouse fed with Dox diet for 1 month. D, after induction with Dox diet for 1 month, cell lysates were prepared from lung tissues of CCSP-rtTA/tetO-KIF5B-RET (C/KR) bitransgenic or wildtype (W) mice. Immunoprecipitation-immunoblotting analysis of KR expression and phosphorylation was performed.
Bitransgenic CCSP-rtTA/tetO-KIF5B-RET (C/KR) mice were produced by crossing tetO-KIF5B-RET mice with CCSP-rtTA mice (also in FVB/N strain) (19–21) (Fig. 1B). To induce KIF5B-RET expression, C/KR mice at 4 weeks old were fed with rodent chow containing 200 mg/kg Dox (Dox diet, Bio-Serv). Both male and female mice were used in the experiments.
Genotyping of CCSP-rtTA transgenic mice was as described previously (19, 21). Genotyping of tetO-KIF5B-RET transgenic mice was performed using GoTaq Hot Start Green Master Mix (Promega) using primer pairs: K5R-3T, 5’-CACGAGAGCTGATGGCACTAACACT and TETO-3B, 5’-CACAGCCCAGCCCAGCCCTCTACT. The 25 µl PCR reaction protocol was: 3 min at 94 °C, 30 cycles of 94 °C for 30 sec, 57 °C for 30 sec and 72 °C for 30 sec with a final extension step of 72 °C for 5 min, which yielded a 309-bp product. Animal experiments were approved by the Institutional Animal Care and Use Committee of University of South Florida.
Cell cultures and analyses
The mouse BaF3 cells were obtained from Dr. H.G. Wang (H. Lee Moffitt Cancer) in 2000 and have been stored in liquid nitrogen. BaF3 cells were cultured in RPMI-1640/10% fetal bovine serum (FBS) supplemented with 2 ng/ml interleukin-3 (IL-3) as described (22). The identity of BaF3 cells were evaluated based on their dependency on mouse IL-3. The authors have not authenticated BaF3 cells using DNA-based method. To generate KIF5B-RET-transformed BaF3 cells (B/KR), BaF3 cells were infected with lentiviruses containing a flag-tagged KIF5B-RET gene. Individual puromycin-resistant cell lines were isolated, screened for the presence of KIF5B-RET by immunoblotting, and evaluated for IL-3-independence.
To identify drug-resistant mutations, established B/KR cells were incubated with increasing concentrations of cabozantinib or vandetanib in RPMI-1640/10%FBS as described in the Results. Individual drug-resistant cell lines were isolated from semi-solid methylcellulose cultures, which contained 4:6 ratio of MethoCult H4100 (Stemcell Technologies) and 20%FBS in RPMI-1640. Genomic DNA of drug-resistant cell lines was isolated and the RET kinase domain coding region was sequenced in both strands of DNA following PCR amplification as described (23).
To determine drug sensitivity, B/KR cells (1,500 cells/well) were incubated in RPMI-1640/10% FBS in the presence of indicated concentrations of drugs in 96-well plates in triplicates. On day 5, viable cells were measured using the CellTiter-Glo reagent (Promega). IC50 measurements were obtained from at least two independent experiments.
Analyses of cell lysates by immunoprecipitation and immunoblotting were as described (19, 21, 23). Immune complex kinase assay using GST-Gab1 as the substrate was performed as described previously (24).
Histological examination
Mice were euthanized by CO2 asphyxiation. Lungs were flushed with 10 ml phosphate-buffered saline and insufflated with 10% buffered formalin. After fixing overnight in 10% buffered formalin, paraffin blocks were prepared. Tissue sections were stained with hematoxylin and eosin (H&E). H&E stain tissue slides were examined by three pathologists and scanned using a ScanScope XT (Aperio). The Genie V1 histology pattern recognition software (Aperio) was used to segment hyperproliferative lesions/tumors from other lung tissue areas and background using the same parameters as described previously (21). Trichrome staining was performed by the Tissue Pathology Core at Stephenson Cancer Center.
Immunohistochemistry (IHC) was performed with a Leica Bond III automated IHC protocol using a Leica Bond-III Polymer Refine Detection reagents (Cat. No.: DS 9800). Epitope retrieval was citrate buffer (pH 6.0) at 100 °C for 20 min. Anti-TTF1 and anti-cytokeratin antibodies were used at 1:400 dilution for 30 min.
Magnetic resonance imaging (MRI) and computerized tomography (CT) protocols
For MRI, mice were anesthetized with 2% isoflurane and transferred to mouse cradle mounted on an insertion device and positioned within the RF coil of the magnet and kept under anesthesia for the duration of the experiment. The mice were physiologically monitored and maintained with a Model 1030 Monitoring and Gating System (SA Instruments, Inc). A respirator sensor pad was placed under each animal to manually control anesthesia mixture whereas a fiber optic rectal thermometer was used for temperature feedback control, which was set to maintain a body temperature of 37 ± 1 °C. Respiration rate was maintained at 50 – 60 breaths per minute and respiratory-gated MR images were acquired during the resting phase after exhalation. MRI was performed using a 7-T horizontal magnet (ASR 310, Agilent Technologies) equipped with nested 205/120/HDS gradient insert in a bore size of 310 mm. Two RF coils, a 35-mm Litzcage coil (Doty Scientific, Inc.) and a 24-mm Litzcage coil (Doty Scientific, Inc), were used depending on the sizes and weights of the mice. Temperature control of the imaging gradients was achieved by means of a water chiller (Neslab Waters) and maintained at 12 °C for all experiments.
Coronal multislice T2-weighted fast spin-echo (FSEMS) respiratory-gated sequences were acquired with TE/TR = 30.05/1497.60 ms, data matrix = 256×128, 20 slices, field of view (FOV) = 90×40 mm2, 16 averages and slice thickness of 1.20 mm over about 10–12 minutes. In T2–weighted images, tumor volumes and dimensions were quantified using manually drawn regions of interest (ROI) within lung on a slice-by-slice basis, where pixels above a given threshold were counted as part of diffuse lesions. Analysis was done with MATLAB™ using a script that employed multi-thresholding segmentation method from the image processing toolbox of MATLAB™. Care was taken to exclude the heart, vessels, and mediastinum.
For µCT, mice were scanned on an Inveon µCT scanner (Inveon micro CT/PET/SPECT, Siemens, Knoxville, TN, USA) using a free-breathing protocol. Animals were anesthetized with a mixture of 2% isoflurane and oxygen. Breathing rate was controlled by a BioVet physiological monitoring system (m2m Imaging Corporation, USA) and kept at average 60 breaths per minute. The 440 projections were taken over 220 degree arc trough 12 minutes over a FOV of 2.7 × 1.5 cm. The X-ray tube scanning parameters were 80 kVp voltage, 480 µA current, 1000ms exposure, we also applied 1.5 mm Al filter.
The acquired data were reconstructed 3D volume with dimensions 1024×576×441 voxels with 27.2669 um effective isotropic voxel size. CCD detector binning was 2×2. Reconstructed data of absorption coefficients were converted to Hounsfield units (HU) in range of −1000 Hu to 1000 HU, where the value of −1000 HU corresponds to the air and 0 HU corresponds to the water (we used 50 ml water phantom for calibration). Image data were evaluated by board certified radiologist using preclinical Inveon Research Workstation 3D Visualization module (Siemens, Knoxville, TN). The lung µCT images were viewed in coronal, sagittal, and axial planes as well as in 3D. The 3D model of lung’s air pathways were rendered from original HU data after applying intensity threshold procedure.
Statistical analysis
Statistical analyses were performed using unpaired t test with Welch’s correction, without assuming equal SDs. The statistical significance was set to 0.05.
Results
Inducible KIF5B-RET transgenic mice
We generated a tetO-KIF5B-RET transgenic mouse line (A1) as described in the supplementary information. These monotransgenic mice had no detectable phenotype and no KIF5B-RET transcript in the tissues that we have examined (Fig. 1C, top panel). CCSP-rtTA/tetO-KIF5B-RET bitransgenic mice (C/KR) were generated by crossing tetO-KIF5B-RET mice with CCSP-rtTA mice (19, 20) and evaluated for Dox-induced expression of KIF5B-RET. As illustrated in Fig. 1C (bottom panel), Dox specifically induced KIF5B-RET mRNA in the lungs but not in the heart, intestine, liver, kidney, or spleen of C/KR mice. Immunoprecipitation of lung tissue lysates with an anti-Flag antibody followed by immunoblotting detected KIF5B-RET protein and phosphorylated KIF5B-RET in the lungs of Dox-induced C/KR mice (Fig. 1D).
In another microinjection experiment performed later, we obtained two other tetO-KIF5B-RET transgenic mouse lines (A2, G6). CCSP-rtTA/tetO-KIF5B-RET bitransgenic mice generated from A2 and G6 lines displayed the same phenotype as that observed in the A1 line (Supplementary Fig. 1). Since the A1 line was generated much earlier, Most of our experiments were conducted using this line.
KIF5B-RET transgenic mice develop lung adenocarcinoma with desmoplastic reaction
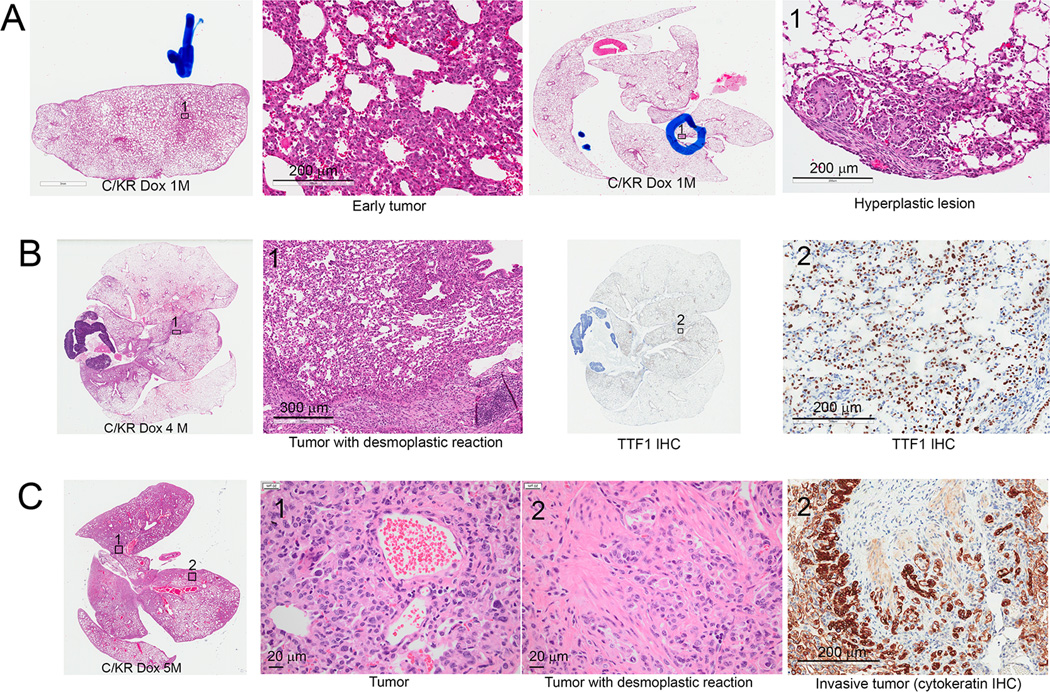
As early as one month after Dox induction, the lungs of C/KR bitransgenic mice developed areas of hyperplastic and early tumor lesions that were often accompanied by thickening of pleural stroma (Fig. 2A). Pleural thickening was observed in human lung adenocarcinoma harboring RET fusion (Supplementary Fig. 2A). By 4–5 months after Dox induction of KIF5B-RET expression, extensive lung tumors were observed in these C/KR mice (Fig. 2B, C). These tumors displayed predominantly lepidic growth pattern with focal solid pattern, visceral pleural involvement, and desmoplastic reaction (Fig. 2B, C). Lymphocytes were often observed near the desmoplastic reaction areas and macrophages were observed in the tumor lesion areas. In total, we examined 12 lungs from C/KR mice induced with Dox for 4–7 months (4 four months, 6 five months, 1 six months, and 1 seven months). Invasive tumors were observed in all 12 lungs, which were confirmed by cytokeratin IHC in every case. We have not observed metastasis of tumors to distal organs. However, we have not ruled out the possibility that distal metastasis may occur when the Dox-induced C/KR mice are kept for a longer period of time.
Figure 2.
Dox-induced bitransgenic C/KR mice develop lung tumors. A, H&E stained sections showing C/KR bitransgenic mice developed areas of hyperplastic lesions and early tumors after one month Dox induction. B, lung sections of a C/KR mouse induced with Dox for 4 months. Left, an H&E stained lung section showing lung with desmoplastic tumors. Right, tumor cells were stained positive of nuclear TTF1. C, lung sections of a C/KR mouse induced with Dox for 5 months. Right, H&E stained lung section with extensive tumors and desmoplasia. Right, cytokeratin stain confirmed invasive lung tumor.
Desmoplastic reaction is associated with invasive lung adenocarcinoma in human lung cancer patients (Supplementary Fig. 2B) and was reported in the first case of KIF5B-RET lung adenocarcinoma when KIF5B-RET fusion was discovered (14). In the Cancer Genome Atlas (TCGA) Research Network lung adenocarcinoma study (2), desmoplastic stroma are visible in the tissue sections of RET-fusion lung adenocarcinoma cases (Supplementary Fig. 2A).
Desmoplastic reaction also occurred in the lung tumors in Dox-induced C/KR mice derived from Line A2 and Line G6 of tetO-KIF5B-RET transgenic mice (Supplementary Fig. 1A, B). In comparison, we have not observed desmoplastic reaction in lung adenomas and adenocarcinomas in KrasLA1 mice that express KrasG12D (25) (~ 6 months of age, Supplementary Fig. 1C), Dox-induced CCSP-rtTA/tetO-EGFRL858R transgenic mice (21, 26), or Dox-induced CCSP-rtTA/tetO-SHP2E76K transgenic mice (19) (n >20 lungs examined in each case).
The KIF5B-RET oncogene is required to maintain lung tumors
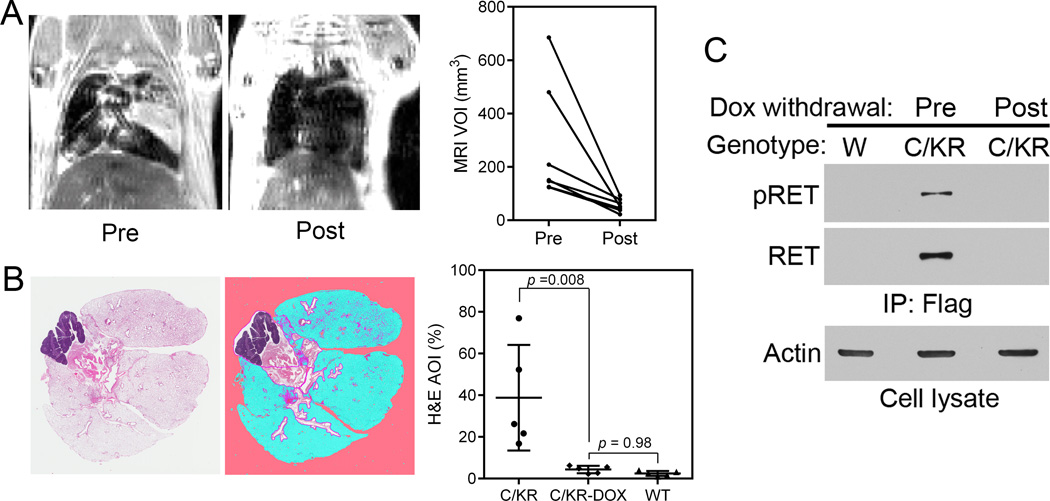
To determine if lung tumors in Dox-induced C/KR mice were addicted to the KIF5B-RET oncogene, we identified 7 Dox-induced C/KR mice (4–5 months) with MRI-detectable lung tumors. These mice were then fed regular chow without Dox. One month after Dox withdrawal, these mice were examined by MRI again. Lung tissue sections were examined by histology and tissue lysates analyzed by immunoprecipitation and immunoblotting for the expression of RET.
As shown in Fig. 3A, Dox withdrawal resulted in regression of MRI-detected tumor lesions in all 7 mice. Lungs from 5 of these mice were examined by histology as illustrated in Fig. 3B. The remaining lesions in the lungs from Dox withdrawn mice were mostly of incompletely resolved fibrotic tissues. Using a histology pattern recognition program (Aperio) as described previously (21), the hyperproliferation/tumor lesion areas were semi-quantified. The area of interest (AOI), which included some of bronchiolar epithelia in addition to hyperproliferation/tumor lesions, in these 5 lungs was 4.33±1.76% (mean ± std). In comparison, the AOI from 5 Dox-induced C/KR mice was 38.80±25.34%, whereas the AOI from the 5 wildtype mice was 2.47%±1.17%. Thus, Dox withdrawal from C/KR mice resulted in a significant reduction (p = 0.008) in the AOI to a level similar to that of normal wildtype mice (p = 0.98) (Fig. 3B).
Figure 3.
KIF5B-RET expression is required to maintain lung tumors. A, C/KR mice developed MRI-detectable lung adenocarcinoma after Dox induction for 5 months. MRI images of representative tumor bearing mouse prior to and one month after Dox withdrawal. The graph shows comparison of MRI-detectable lung tumor burden prior to and post Dox withdrawal. Each line represents an individual mouse (n = 7). VOI, Volume of interest. B, H&E stained tissue section from lung of a mouse after Dox withdrawal (left). Lung tissue image from Genie® v1 histology pattern recognition software (Aperio) analysis (middle). Lung tumor burden was analyzed from H&E stained lung tissues (right) (n = 5 in each group). AOI, areas of interest. White, not lung tissue, area excluded; pink, background; blue, normal; purple: hyperplasia/neoplasia including some areas of normal bronchial epithelia (21). C, comparison of pRET and RET protein in the lungs of C/KR mice induced with Dox (prior to Dox withdrawal) and 1 month post Dox withdrawal. W, wildtype mouse.
Both KIF5B-RET protein and phosphorylated (pY905) KIF5B-RET were detected in Dox-induced C/KR mice (Fig. 3C). They were no longer detected in the lungs one month post-Dox withdrawal. Taken together, these data show that after tumor formation, continuous expression of KIF5B-RET is required to maintain the lung tumors.
Ponatinib is the most potent KIF5B-RET kinase inhibitor
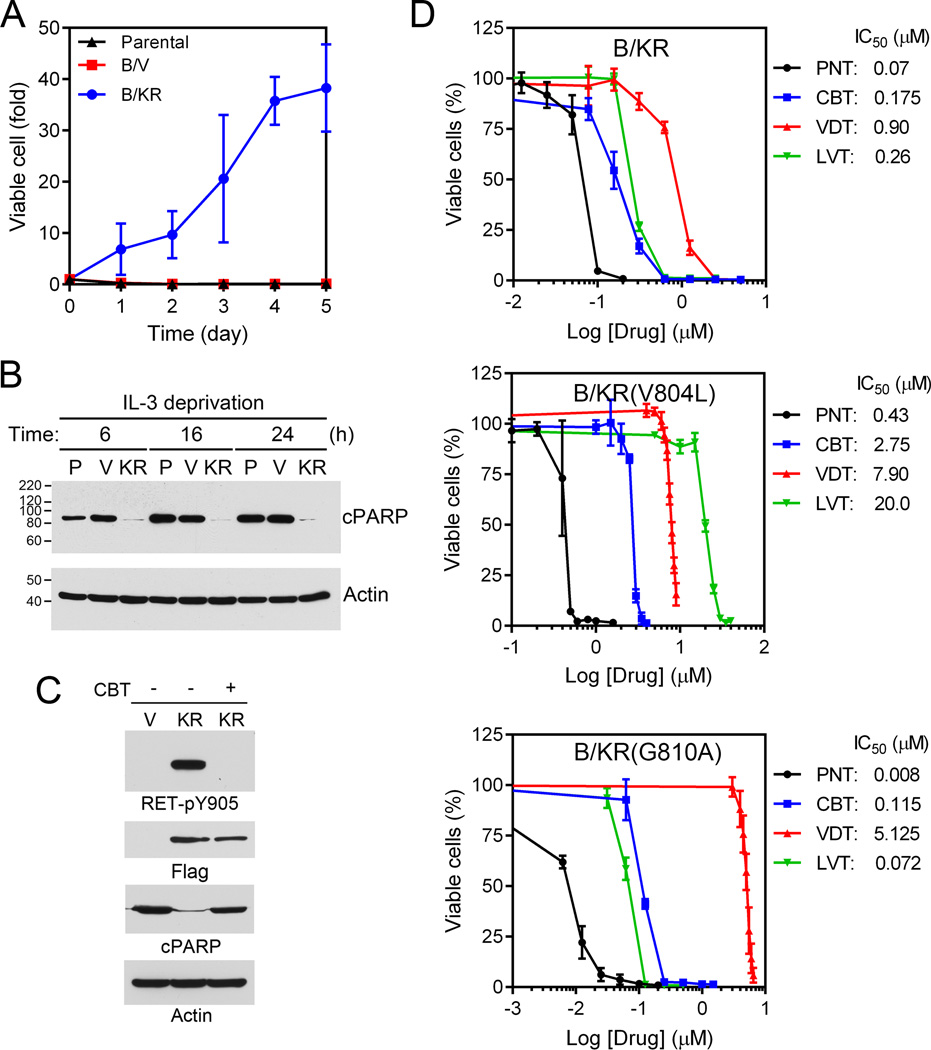
BaF3 cells depend on IL-3 for survival. We established stable BaF3 cells expressing KIF5B-RET (B/KR). When cells were incubated in medium without IL-3, the parental cells and vector-control cells underwent apoptosis in 6 hours as measured by PARP cleavage, whereas B/KR cells continued to grow (Fig. 4A, B). Thus, KIF5B-RET transformed BaF3 cells into cytokine-independence. The KIF5B-RET transformation was inhibited by cabozantinib that suppressed the KIF5B-RET kinase activity (Fig. 4C, D).
Figure 4.
Ponatinib is the most potent KIF5B-RET inhibitors and the vandetanib resistant RETG810A mutation is hypersensitive to ponatinib. A, growth curve of parental, vector control (B/V) and stable BaF3 cells expressing KIF5B-RET (B/KR) without IL-3. B, cells were grown in medium without IL-3 for 6, 16, 24 hours. The cell lysates were immunoblotted with anti-cleaved PARP or anti-β actin. P, parental; V, vector control; KR, KIF5B-RET. C, vector control and KIF5B-RET cells were treated with or without 1 µM cabozantinib (CBT) for 3 hours. The cell lysates were immunoblotted with anti-RET-pY905, anti-Flag, anti-cleaved PARP or anti-beta actin. D, Ba/F3 expressing KIF5B-RET, KIF5B-RETV804L, or KIF5B-RETG810A were seeded on 96-well plates and treated with indicated concentration of ponatinib (PNT), cabozantinib (CBT), vandetanib (VDT) or lenvatinib (LVT) for 5 days. Cell viability was measured using the CellTiter-Glo assay and IC50s were determined. The data were from two triplicate experiments (n = 6).
Cabozantinib and vandetanib inhibited B/KR cells with IC50s of 0.175 µM and 0.90 µM, respectively. To isolate cabozantinib- or vandetanib-resistant KIF5B-RET mutations, we incubated B/KR cells (2×107 cells) with sequentially increasing concentrations of cabozantinib (from 0.175 µM to 0.65 or 0.85 µM in two independent experiments) or vandetanib (from 0.90 µM to 4.5 or 5.5 µM in two independent experiments). Individual drug resistant cells were cloned by methylcellulose culture. After verification of constitutive KIF5B-RET tyrosine kinase activity in these cells in the presence of cabozantinib or vandetanib in the culture medium (Supplementary Fig. 3A), the protein tyrosine kinase domain coding regions of KIF5B-RET from these drug-resistant cell clones were sequenced. The RETV804L gatekeeper mutation was identified in all 10 cabozantinib-resistant cell lines. A novel RETG810A mutation was found in 8 of 10 vandetanib-resistant cell lines. We next immunoprecipitated KIF5B-RET, KIF5B-RETV804L, and KIF5B-RETG810A from these cells and assayed their PTK activity in vitro in the presence of increasing concentrations of cabozantinib or vandetanib. The results confirmed that KIF5B-RETV804L was resistant to cabozantinib and that KIF5B-RETG810A was resistant to vandetanib (Supplementary Fig. 3B).
To confirm that the novel RETG810A mutation renders vandetanib resistance and to evaluate if drug resistance may be attributed to overexpression of KIF5B-RET protein, we recreated a BaF3/KIF5B-RETG810A cell line (B/KRG810A-REC) using lentivirus encoding the KIF5B-RETG810A. We then compared expression of KIF5B-RET and KIF5B-RETG810A protein in these cells and vandetanib resistance between B/KRG810A and B/KRG810A-REC. As shown in Supplementary Fig. 3C, KIF5B-RET and KIF5B-RETG810A protein levels were similar between B/KR and the B/KR-derived B/KRG810A cells. The independently generated B/KRG810A-REC cells had a 20% higher level of KIF5B-RETG180A protein. The vandetanib IC50s were 5.310 µM and 5.675 µM for B/KRG810A and B/KRG810A-REC cells (sFig. 3D). These data confirm that the RETG810A mutant is resistant to vandetanib. While we do not rule out the possibility that increased KIF5B-RET expression may confer some extent of drug tolerance, our results indicate that vandetanib resistance of B/KRG810A cells is mainly due to the G810A mutation.
Cabozantinib, vandetanib, ponatinib and lenvatinib are multi-kinase TKIs that have been reported in on-going clinical trials of RET-fusion NSCLC (15). We compared these four drugs in the KIF5B-RET-dependent growth of B/KR cells. As shown in Fig. 4D, ponatinib was the most potent inhibitor with an IC50 of 0.07 µM for B/KR cells. B/KRV804L gatekeeper mutation cells were pan-resistant to all four TKIs, resulting in 6.2, 8.7, 15.7, and 76-fold increase in IC50s for ponatinib, vandetanib, cabozantinib, and lenvatinib, respectively (Fig. 4D). However, ponatinib had the least fold increase in IC50 and remained the most potent inhibitor for B/KRV804L cells with a sub-micromolar IC50.
Vandetanib IC50 for B/KRG810A cells was increased 5.7-fold to 5.1 µM compared to B/KR cells. Interestingly, while cabozantinib had similar IC50s in B/KRG810A cells and B/KR cells, ponatinib and lenvatinib had lower IC50s in B/KRG810A cells by 8.7- and 3.6-folds, respectively. Thus, while the RETG810A mutation causes vandetanib resistance, it becomes hypersensitive to ponatinib and lenvatinib. We hereby term this type of mutation as a gain-of-sensitivity (GOS) mutation.
To further compare the potencies of these four drugs for RET and RETV804L and also to determine the potencies of these drugs for another known RET gatekeeper mutant RETV804M, IC50s for inhibition of recombinant RET, RETV804L, and RETV804M in an in vitro kinase assay were measured in parallel via a commercial service (Reaction Biology, Malvern, PA). As shown in Supplementary Fig. 4, ponatinib again displayed the most potent inhibitory activities against RET, RETV804L, and RETV804M among these four compounds.
Ponatinib induces lung tumor regression in Dox-induced C/KR bitransgenic mice
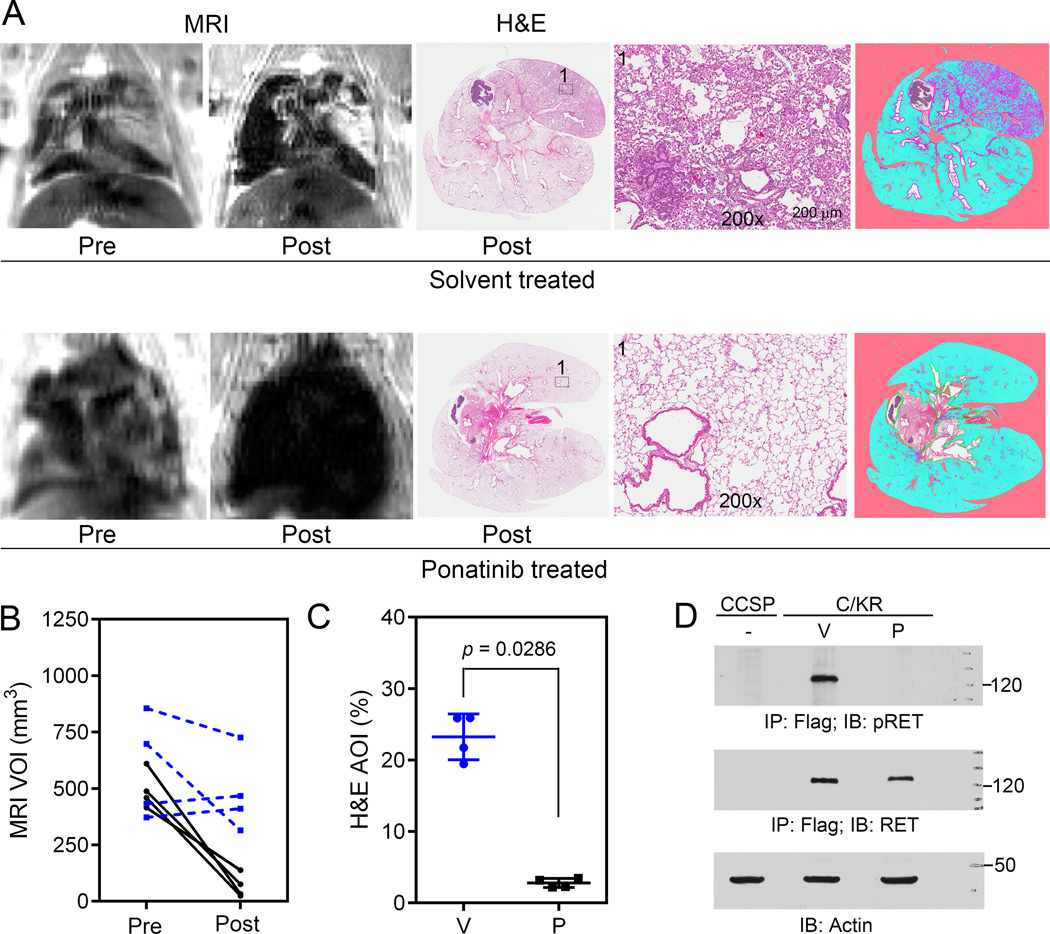
Since ponatinib was the most potent TKI for KIF5B-RET and its gatekeeper mutations, we chose it for the in vivo study in our KIF5B-RET transgenic animal model of NSCLC. Dox-induced C/KR mice (4–5 months) were examined by MRI and micro-CT. Eight mice with MRI-detected lung tumor lesions were divided into two groups and treated with ponatinib or vehicle (4 mice/group) by oral gavage (30 mg/kg/day, 5 days/week) (see Supplementary Fig. 5 for treatment schedule and body weight monitoring data). After 4 weeks of drug treatment, the lungs of these mice were examined again by MRI and µCT, and lung tissue sections were analyzed by histology.
Lung tumor lesions in the vehicle-treated mice were similar or worse based on MRI and µCT examination (Fig 5A, B, Supplementary Fig. 6, 7, Supplementary Information 2, sMovie). In contrast, the lung lesions were greatly reduced in ponatinib-treated mice. Semi-quantitative measurement of tumor lesions by MRI showed that the mean VOI was significantly (p = 0.0286) decreased from 494±83 mm3 (prior to treatment) to 68±51 mm3 (post treatment) in the ponatinib-treated cohort (Fig. 5B). In comparison, the mean MRI VOI was not significantly changed (p = 0.6571) in the vehicle-control cohort before (580±227 mm3) and after (480±176 mm3) treatment. Analysis of H&E stained lung tissue sections (Supplementary Fig. 6) by the histology pattern recognition program show that vehicle-treated cohort had a mean 23.25±3.20% of AOI with hyperplasia or neoplasia, whereas ponatinib-treated mice had significantly (p = 0.0286) less AOI (mean = 2.79±0.63%) similar to that of the wildtype mice (2.47%±1.17%, Fig. 3B, 5C). In another experiment, lung tissue lysates from Dox-induced C/KR mice treated with vehicle or ponatinib for 1 month were analyzed for tyrosine phosphorylation of KIF5B-RET. As shown in Fig. 5D, KIF5B-RET protein was decreased and its tyrosine phosphorylation was suppressed in the lungs of ponatinib-treated animals. Thus, ponatinib is effective in inhibiting KIF5B-RET kinase activity and reducing the lung tumors of C/KR mice.
Figure 5.
Ponatinib treatment results in tumor regression. A, C/KR mice with MRI-detectable lung tumors were treated with vehicle (upper panel) or ponatinib (30 mg/kg/day, 5 days/week, p.o., lower panel) for 1 month and then analyzed by MRI again (n = 4 in each group). Lung tissue sections at the end point were stained with H&E. Representative images of MRI, H&E sections, and image from Genie® v1 histology pattern recognition software analysis (right) are shown. B, comparison of MRI-detectable lung tumor burden prior to and post vehicle or ponatinib treatment. Each line represents an individual mouse. Blue, vehicle-treated; Black, ponatinib-treated. C, lung tumor burden analyzed from samples of H&E stained lung tissue sections. V, vehicle-treated; P, ponatinib-treated. D, comparison of pRET, RET and β-actin proteins in the lungs of CCSP and C/KR mice treated with vehicle or ponatinib. CCSP, negative control mouse.
Discussion
RET oncogene fusions occur in 1–2% of lung adenocarcinoma. The rarity of the molecular lesion presents a barrier for clinical studies of RET fusion-associated lung adenocarcinoma (10). To facilitate in vivo studies of RET-associated lung adenocarcinoma, we have established an inducible transgenic mouse model of KIF5B-RET-associated lung adenocarcinoma.
KIF5B-RET displayed a constitutively active PTK activity as measured by pRET when it was expressed in the lungs of Dox-induced C/KR transgenic mice. Hyper-proliferative lesions and early tumors in the lungs were observed as early as one month after Dox induction of the C/KR mice, and progressed to invasive lung tumors in 4–5 months after Dox induction of KIF5B-RET expression in the C/KR mice. Lung tumors formed in this Dox-induced C/KR model resemble human invasive lung adenocarcinoma and are associated with desmoplastic reaction and visceral pleural involvement. Desmoplastic stroma was also evident in the RET fusion-positive lung adenocarcinoma cases in TCGA. Various histological patterns of RET fusion-positive lung adenocarcinoma have been observed, including the prevalence of poorly differentiated, solid-predominant histologic features in RET fusion cases of lung adenocarcinoma (17, 27–29). Lung tumors in our C/KR mouse model recapitulate the invasive desmoplastic feature of RET fusion-associated tumors.
Similar to the absence of desmoplastic stroma in the lung tumors of KrasLA1 mice that we observed in this study, a previous study using the adenovirus-Cre recombinase/LSL-RasG12D mouse model also found that lung adenoma and adenocarcinoma developed in that mouse model lack stromal reaction (30). Knockout of tgfbr2 in the LSL-KrasG12D model allows the KrasG12D-driven lung tumors to progress to invasive lung adenocarcinoma with desmoplastic reaction (30). Human NSCLC often can cause desmoplasia when they invade into stroma. Among 230 cases of human lung adenocarcinoma characterized in TCGA (2), there are 1 case with TGFBR2 deletion and 1 case with TGFBR2 point mutation (E257D), both of them occur in KRASG12C tumors (www.cbioportal.org). While many other lung adenocarcinoma cases in the TCGA cohort show the desmoplastic histology, such as RET-fusion cases, they are not associated with TGFBR2 deficiency. Thus, although TGFBR2 deficiency could promote desmoplastic stroma deposition, other factors are also likely to contribute to the stromal response to the invasive tumors.
Genomic analysis has suggested the exclusive dependency of lung adenocarcinoma on ALK, RET, or ROS fusion oncogenes (10). Consistently, data from our Dox withdrawal experiment indicate that lung tumors developed in Dox-induced C/KR mice require the continuing expression of the KIF5B-RET fusion oncogene to maintain the malignancy. This property suggests that KIF5B-RET is an effective target for drug treatment to eliminate KIF5B-RET-associated lung adenocarcinoma.
By culturing KIF5B-RET-dependent B/KR cells with RET kinase inhibitors, we identified cabozantinib-resistant RETV804L gatekeeper mutation and a novel vandetanib-resistant RETG810A mutation. Interestingly, while the RETG810A mutant remained sensitive to cabozantinib, it acquired GOS to ponatinib and lenvatinib. Recent clinical experience in EGFR and ALK inhibitor therapy of lung adenocarcinoma has shown that a major mechanism of developing drug resistance is secondary mutations in the targeted PTK kinase domain. Mutation-sensitive secondary TKIs can be developed and used to prolong the therapeutic response (31–33). Our finding here suggests that ponatinib or lenvatinib are the secondary drugs to use when RETG810A mutation occurs in vandetanib-treated RET-associated cancer, including lung adenocarcinoma and metastatic thyroid cancer.
Among cabozantinib, lenvatinib, ponatinib, and vandetanib, our data show that ponatinib is the most potent inhibitor of RET PTK. Treatment of lung tumors induced by KIF5B-RET in the transgenic animals resulted in tumor regression. Thus, ponatinib is an effective drug for treating KIF5B-RET-associated lung adenocarcinoma in this genetically engineered mouse model and is predicted to show anti-tumor efficacy in RET fusion lung adenocarcinoma patients. While the gatekeeper RETV804L and RETV804M mutations are less sensitive to ponatinib than the wildtype RET, ponatinib remains the most potent inhibitor (Fig. 4 and Supplementary Fig. 4). Thus, ponatinib appears to be the drug of choice for treating RET fusion-associated lung adenocarcinoma.
It is envisioned that more drugs will be developed, re-purposed, and evaluated for therapy of RET fusion-associated lung adenocarcinoma, either as novel single agent or in combination treatment. The immune competent C/KR mouse model that we developed here provides a resource allowing the direct side-by-side comparison of two drugs in preclinical studies or co-clinical trials (34, 35) of RET fusion-associated lung adenocarcinoma.
Supplementary Material
Acknowledgments
Financial information
This study was supported by National Institutes of Health grants R21CA175603 (to J. Wu), R01CA178456 (to J. Wu), P30CA076292 (to T. Sellers), P50CA119997 (to E. B. Haura), and P20GM103639 (to K.M. Fung).
We thank H. Lee Moffitt Cancer Center Core facility staff, including Animal, DNA sequencing, Histology, Microscopy Core facilities, and Stephenson Cancer Center/University of Oklahoma Health Sciences Center Histology Core facility staff for assistance. We also thank J.A. Whitsett for the CCSP-rtTA transgenic mice, D.C. Radisky and A.P. Fields for advice and assistance, and K. Politi and G. Felsenfeld for reagents.
Abbreviations list
- AOI
area of interest
- B/KR
BaF3/KIF5B-RET
- CCSP
Clara cell secretory protein
- C/KR
CCSP-rtTA/tetO-KIF5B-RET
- CT
computerized tomography
- Dox
doxycycline
- GOS
gain-of-sensitivity
- IHC
immunohistochemistry
- IL-3
interleukin-3
- MRI
magnetic resonance imaging
- NSCLC
non-small cell lung cancer
- PTK
protein tyrosine kinase
- TCGA
the Cancer Genome Atlas
- TKI
protein tyrosine kinase inhibitor
Footnotes
Disclosure of Potential Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surgery today. 2014;44:1004–1012. doi: 10.1007/s00595-013-0636-z. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Disc. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riely GJ, Yu HA. EGFR: The Paradigm of an Oncogene-Driven Lung Cancer. Cli Cancer Res. 2015;21:2221–2226. doi: 10.1158/1078-0432.CCR-14-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Cli Cancer Res. 2015;21:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–164. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. The Oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito M, Shimada Y, Shiraishi K, Sakamoto H, Tsuta K, Totsuka H, et al. Development of lung adenocarcinomas with exclusive dependence on oncogene fusions. Cancer Res. 2015;75:2264–2271. doi: 10.1158/0008-5472.CAN-14-3282. [DOI] [PubMed] [Google Scholar]
- 11.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 13.Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju YS, Lee WC, Shin JY, Lee S, Bleazard T, Won JK, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno T, Tsuta K, Tsuchihara K, Nakaoku T, Yoh K, Goto K. RET fusion gene: Translation to personalized lung cancer therapy. Cancer Sci. 2013;104:1396–1400. doi: 10.1111/cas.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Disc. 2013;3:630–635. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Pennell NA, Ali SM, Ross JS, Ma PC, Velcheti V. RET-rearranged lung adenocarcinomas with lymphangitic spread, psammoma bodies, and clinical responses to cabozantinib. J Thoracic Oncology. 2014;9:1714–1719. doi: 10.1097/JTO.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 18.Gautschi O, Zander T, Keller FA, Strobel K, Hirschmann A, Aebi S, et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thoracic Oncology. 2013;8:e43–e44. doi: 10.1097/JTO.0b013e31828a4d07. [DOI] [PubMed] [Google Scholar]
- 19.Schneeberger VE, Luetteke N, Ren Y, Berns H, Chen L, Foroutan P, et al. SHP2E76K mutant promotes lung tumorigenesis in transgenic mice. Carcinogenesis. 2014;35:1717–1725. doi: 10.1093/carcin/bgu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 21.Schneeberger VE, Ren Y, Luetteke N, Huang Q, Chen L, Lawrence HR, et al. Inhibition of Shp2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget. 2015;6:6191–6202. doi: 10.18632/oncotarget.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorsey JF, Cunnick JM, Lanehart R, Huang M, Kraker AJ, Bhalla KN, et al. Interleukin-3 protects Bcr-Abl-transformed hematopoietic progenitor cells from apoptosis induced by Bcr-Abl tyrosine kinase inhibitors. Leukemia. 2002;16:1589–1595. doi: 10.1038/sj.leu.2402678. [DOI] [PubMed] [Google Scholar]
- 23.Ren Y, Zhang Y, Liu RZ, Fenstermacher DA, Wright KL, Teer JK, et al. JAK1 truncating mutations in gynecologic cancer define new role of cancer-associated protein tyrosine kinase aberrations. Sci Rep. 2013;3:3042. doi: 10.1038/srep03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J Biol Chem. 2004;279:8497–8505. doi: 10.1074/jbc.M312575200. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 26.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai TH, Wu SG, Hsieh MS, Yu CJ, Yang JC, Shih JY. Clinical and prognostic implications of RET rearrangements in metastatic lung adenocarcinoma patients with malignant pleural effusion. Lung Cancer. 2015;88:208–214. doi: 10.1016/j.lungcan.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. British J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y, Zhang Y, Li Y, Hu H, Wang L, Li H, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121–126. doi: 10.1016/j.lungcan.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Borczuk AC, Sole M, Lu P, Chen J, Wilgus ML, Friedman RA, et al. Progression of human bronchioloalveolar carcinoma to invasive adenocarcinoma is modeled in a transgenic mouse model of K-ras-induced lung cancer by loss of the TGF-beta type II receptor. Cancer Res. 2011;71:6665–6675. doi: 10.1158/0008-5472.CAN-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Disc. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. New England J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 33.Katayama R, Friboulet L, Koike S, Lockerman EL, Khan TM, Gainor JF, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20:5686–5696. doi: 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Akbay E, Mikse O, Tupper T, Cheng K, Wang Y, et al. Co-clinical trials demonstrate superiority of crizotinib to chemotherapy in ALK-rearranged non-small cell lung cancer and predict strategies to overcome resistance. Clin Cancer Res. 2014;20:1204–1211. doi: 10.1158/1078-0432.CCR-13-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.