Abstract
The oocyte spindle in most animal species is assembled in the absence of the microtubule-organizing centers called centrosomes. Without the organization provided by centrosomes, acentrosomal meiotic spindle organization may rely heavily on the bundling of microtubules by kinesin motor proteins. Indeed, the minus-end directed kinesin-14 NCD, and the plus-end directed kinesin-6 Subito are known to be required for oocyte spindle organization in Drosophila melanogaster. How multiple microtubule-bundling kinesins interact to produce a functional acentrosomal spindle is not known. In addition, there have been few studies on the meiotic function of one of the most important microtubule-bundlers in mitotic cells, the kinesin-5 KLP61F. We have found that the kinesin-5 KLP61F is required for spindle and centromere symmetry in oocytes. The asymmetry observed in the absence of KLP61F depends on NCD, the kinesin-12 KLP54D, and the microcephaly protein ASP. In contrast, KLP61F and Subito work together in maintaining a bipolar spindle. We propose that the prominent central spindle, stabilized by Subito, provides the framework for the coordination of multiple microtubule-bundling activities. The activities of several proteins, including NCD, KLP54D, and ASP, generate asymmetries within the acentrosomal spindle, while KLP61F and Subito balance these forces, resulting in the capacity to accurately segregate chromosomes.
Keywords: meiosis, kinesin, oocyte, spindle, Drosophila
DURING cell division, chromosomes interact with a bipolar array of microtubules (MTs), called the spindle, to direct their segregation. The organization and stability of the spindle are critical for the proper segregation of chromosomes. In the oocytes of most animal species, the spindle forms in the absence of the MT-organizing centers called centrosomes (Szollosi et al. 1972; Theurkauf and Hawley 1992; Albertson and Thomson 1993). Centrosomes contribute to spindle organization in several ways, including providing cues for bipolarity since there are two centrosomes present during spindle assembly. How oocytes organize a bipolar spindle in the absence of centrosomes remains an important question.
It has been known for many years that two MT-bundling kinesins—the minus-end directed kinesin-14 NCD and the plus-end directed kinesin-6 Subito (SUB)—are required during oocyte spindle assembly in Drosophila melanogaster (Hatsumi and Endow 1992; Giunta et al. 2002). NCD promotes the organization of spindle poles, perhaps through the clustering of MT minus ends (Matthies et al. 1996; Goshima et al. 2005). SUB promotes spindle bipolarity through stabilization of the meiotic central spindle, a structure that consists of overlapping bundles of antiparallel MTs (Jang et al. 2005). Both NCD and SUB play nonessential roles in centrosome-containing cells (Cesario et al. 2006; Goshima et al. 2007). Because these two MT-bundling kinesins have greater impact on acentrosomal oocyte spindle assembly than centrosomal spindle assembly, this led us to hypothesize that MT-bundling activities may substitute in acentrosomal oocytes for the organization typically provided by centrosomes. Therefore, we examined the role of the most prominent MT-bundling kinesin, kinesin-5, in acentrosomal meiosis.
In centrosome-containing cells, kinesin-5—known as KIF11, Eg5, BimC, or KLP61F—is essential for the establishment of spindle bipolarity (Sawin et al. 1992; Heck et al. 1993; Blangy et al. 1995). This occurs through kinesin-5 activity to bundle and slide antiparallel MTs, providing an outward force that keeps centrosomes from collapsing into a monopole (Kapitein et al. 2005). Inhibition of kinesin-5 in mammalian oocytes also results in monopolar spindles despite the lack of centrosomes (Mailhes et al. 2004; Duncan et al. 2012). In mouse oocytes, kinesin-5 acts to fragment and cluster acentriolar MT-organizing centers, resulting in spindle bipolarity (Schuh and Ellenberg 2007; Clift and Schuh 2015). In most organisms, including humans and Drosophila, however, acentriolar MT-organizing centers are not present. This raises the possibility that kinesin-5 acts in additional ways in oocytes to promote bipolarity. In addition, previous studies in intact oocytes have focused on drug inhibition of kinesin-5 activity, which does not prevent MT binding of kinesin-5 (Kapoor and Mitchison 2001), and, therefore, may hamper the observation of possible nonmotor activities.
Importantly, MT bundling kinesins do not act in isolation, but rather in the context of all of the MT-binding proteins associated with the spindle. In centrosome-containing cells, it has been established that kinesin-5 and kinesin-14 work in opposition to generate spindle bipolarity (Mountain et al. 1999; Sharp et al. 2000; Wilson et al. 2004). In addition, the kinesin-12 KIF15 can promote kinesin-5-independent spindle bipolarity (Tanenbaum et al. 2009; Vanneste et al. 2009). Little is known about the coordination of MT-bundling kinesins in oocytes, however. Given the difference in organization of the acentrosomal spindle, it is likely that this coordination operates differently in oocytes.
We report here that loss of the kinesin-5 KLP61F in Drosophila oocytes results in asymmetric bipolar spindles and associated chromosome disorganization, including the failure of homologous chromosomes to biorient. We have also investigated how the MT-bundling activities of kinesin-5, kinesin-6, kinesin-12, and kinesin-14 are coordinated to produce a functional oocyte spindle. Spindle asymmetry depends on the MT-associated protein ASP, the kinesin-14 NCD, and, surprisingly, the kinesin-12 KLP54D. In contrast, the kinesin-6 SUB coordinates with KLP61F to promote spindle symmetry and chromosome organization. These results demonstrate that kinesin-5 and kinesin-6 cooperate, perhaps through the central spindle, to balance forces, which depend on ASP, kinesin-14, and kinesin-12, that generate an asymmetric spindle. We propose that the coordination of several MT-bundling activities compensates for the absence of centrosomes, and is vital to the proper organization of acentrosomal oocyte spindles and chromosome segregation.
Materials and Methods
Fly stocks and genetics
Flies were crossed and maintained on standard media at 25°. Fly stocks were obtained from the Bloomington Stock Center, or the Transgenic RNAi Project at Harvard Medical School (TRiP, Boston, MA, www.flyrnai.org, Ni et al. 2011). Information on genetic loci was obtained from FlyBase (www.flybase.org, Attrill et al. 2015).
To make Klp61F germline clones (Chou and Perrimon 1996), we crossed the P{PZ}Klp61F07012 allele onto a chromosome bearing P{FRT(whs)}2A. Females with this chromosome were crossed in vials to males with a matching FRT chromosome carrying the dominant female sterile mutation ovoD1 and a heat-shock-inducible FLP recombinase. After 3–4 days, parents were transferred to new vials, and progeny were heat-shocked in a 37° water bath for 1 hr. Females carrying both FRT chromosomes and FLPase were selected among the progeny for examination as germline clones.
To generate a mutation in Klp54D, we excised the P{EP}G7530 transposable element, which is inserted 290 bp downstream of the Klp54D coding sequence. We screened excisions for deletions of Klp54D by PCR after crossing to Df(2R)ED3385. We obtained one deletion of ∼2.2 kb upstream of the insertion site, which we designated Klp54D3. A truncated protein of 288 (out of 725) amino acids may be produced from this allele, but half of the kinesin motor domain is missing, suggesting that this protein is likely to be nonfunctional.
To measure X chromosome nondisjunction in ncd-depleted oocytes and Klp54D mutants, females were crossed to males carrying a Y chromosome marked with BS. Mis-segregation of the X chromosome produces XXY females and XO males, which are distinguishable from XX females and XY males because of the BS visible marker. There are also two types of nonviable progeny produced by X chromosome nondisjunction (XXX and OY). To account for these missing progeny, the percentage of X chromosome nondisjunction was calculated by the following formula: (XXY + XO)/[2*(XXY + XO)+(XX + XY)]. P values were calculated using the method described in Zeng et al. (2010).
RNAi in Drosophila oocytes
RNAi constructs generated by TRiP were: Klp61F (HMS00552 and GL00441), Spc105R (GL00392) (Radford et al. 2015), sub (GL00583) (Radford et al. 2015), and asp (GL00108). We designed the ncd shRNA construct using the shRNA predictions from DSIR (http://biodev.extra.cea.fr/DSIR/DSIR.html) and the Public TRC Portal (http://www.broadinstitute.org/rnai/public/seq/search). We obtained the following oligos from IDT:
ctagcagtGCGGCAGTTTCGATAAATAAAtagttatattcaagcataTTTATTTATCGAAACTGCCGCgcg
aattcgcGCGGCAGTTTCGATAAATAAAtatgcttgaatataactaTTTATTTATCGAAACTGCCGCactg
Oligos were annealed and cloned into the NheI and EcoRI sites in pVALIUM22. This construct was inserted into the attP40 site via phiC31 integration and standard germline transformation by Model Systems Genomics (Durham, NC).
To knockdown gene expression by RNAi, the expression of the shRNA is under control of the GAL4/UAS system (Brand and Perrimon 1993). To confine expression to the oocyte, the matα4-GAL-VP16 driver was used, which expresses throughout oogenesis after the initiation of meiosis (Sugimura and Lilly 2006; Radford et al. 2012b). Expression of the Klp61F shRNAs with the nanos-GAL4:VP16 driver, which expresses in the ovary prior to the initiation of meiosis (Rorth 1998), resulted in oogenesis failure, consistent with a role for KLP61F in the premeiotic germline divisions.
To quantify knockdown of gene expression by RNAi, late-stage oocytes were collected from females carrying both driver and shRNA construct by mass disruption of abdomens in a blender filled with phosphate-buffered saline (PBS). Oocytes were filtered through meshes, and allowed to settle in solution to remove body parts and earlier egg chambers. Total RNA was extracted from oocytes using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA), and RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). The reverse transcriptase quantitative PCR (RT-qPCR) was performed in a StepOnePlus real-time PCR system using TaqMan Gene Expression Assays (Thermo Fisher Scientific, Waltham, MA, Dm02151365_g1 for asp, Dm01841845_g1 for Klp61F, Dm02134527_g1 for ncd, and Dm02134593_g1 for Rpll140 as control). Protein was prepared from oocytes by adding SDS gel loading buffer to a final concentration of 1 mg oocytes/8 µl of solution, homogenizing with a pestle, then boiling for 5 min; 4 µl per lane was loaded onto an SDS-PAGE gel (Thermo Fisher Scientific, Waltham, MA). Primary antibodies used were rabbit anti-KLP61F (1:10,000, Brust-Mascher et al. 2009), and rabbit anti-α-tubulin (1:5000, ab15246, Abcam, Cambridge, MA). Secondary antibody used was goat anti-rabbit-HRP (1:5000, Jackson Immunoresearch, West Grove, PA), and signal was detected with Supersignal West Pico (Thermo Fisher Scientific, Waltham, MA).
Immunofluorescence and microscopy
Late-stage oocytes were collected from 2- to 4-day-old Drosophila females aged for 2 days on yeast with males. Oocytes were prepared for immunofluorescence (5% formaldehyde/heptane fixation) and FISH (8% formaldehyde/100 mM cacodylate fixation) as described (Radford et al. 2012a; Radford and McKim 2016). Primary antibodies used for immunofluorescence were mouse anti-α-tubulin conjugated to FITC (1:50 dilution, clone DM1A, Sigma-Aldrich, St. Louis, MO) and rabbit anti-CENP-C (1:5000, Heeger et al. 2005). Cy3-, Cy5-, and AlexaFluor647-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were used. DNA was labeled with Hoechst 33342 (1:1000, Thermo Fisher Scientific, Waltham, MA). FISH probes used were to the AACAC satellite (2nd chromosome), and dodeca satellite (3rd chromosome), as described (Dernburg et al. 1996; Radford et al. 2012a). Samples were mounted in SlowFade Gold (Thermo Fisher Scientific, Waltham, MA). For live imaging, ovaries from females expressing mCherry-Jupiter [gift from Vladimir Gelfand, Northwestern University, Chicago (Lu et al. 2013)], and GFP-CENP-C (gift from Christian Lehner, University of Zurich, Zurich, Switzerland) were dissected in Halocarbon oil 700, then stage 13/14 oocytes were selected, placed on a coverslip, and covered with oil. Images were collected on a Leica TCS SP5 or SP8 microscope with a 63×, 1.4 NA lens using LAS AF software. Images are shown as maximum projections. Image analysis, including fluorescence intensity measurements and centromere foci counting, was performed with Imaris image analysis software (Bitplane, Belfast, United Kingdom).
Statistical analysis
Statistical tests were performed using GraphPad Prism software. Dispersed karyosome, spindle asymmetry, and centromere asymmetry frequencies were compared using Fisher’s exact test.
Data Availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
KLP61F promotes chromosome organization in Drosophila oocytes
We could not generate Klp61F oocytes by making germline clones because it is essential for germline mitosis (see Materials and Methods). Therefore, to test the role of KLP61F in spindle organization and chromosome segregation in oocytes, we used two constructs that express a short hairpin RNA (shRNA) to knock down Klp61F gene expression through RNAi (HMS00552 and GL00441, Ni et al. 2011). Because expression of these constructs is under control of the GAL4/UAS system, we used matα4-GAL-VP16 to confine expression to the female germline after entry into meiosis (Sugimura and Lilly 2006; Radford et al. 2012b). Expression of these constructs resulted in loss of Klp61F mRNA (90% for HMS00552 and 78% for GL00441) and KLP61F protein (82 and 85%, respectively) in late-stage oocytes (Supplemental Material, Figure S1).
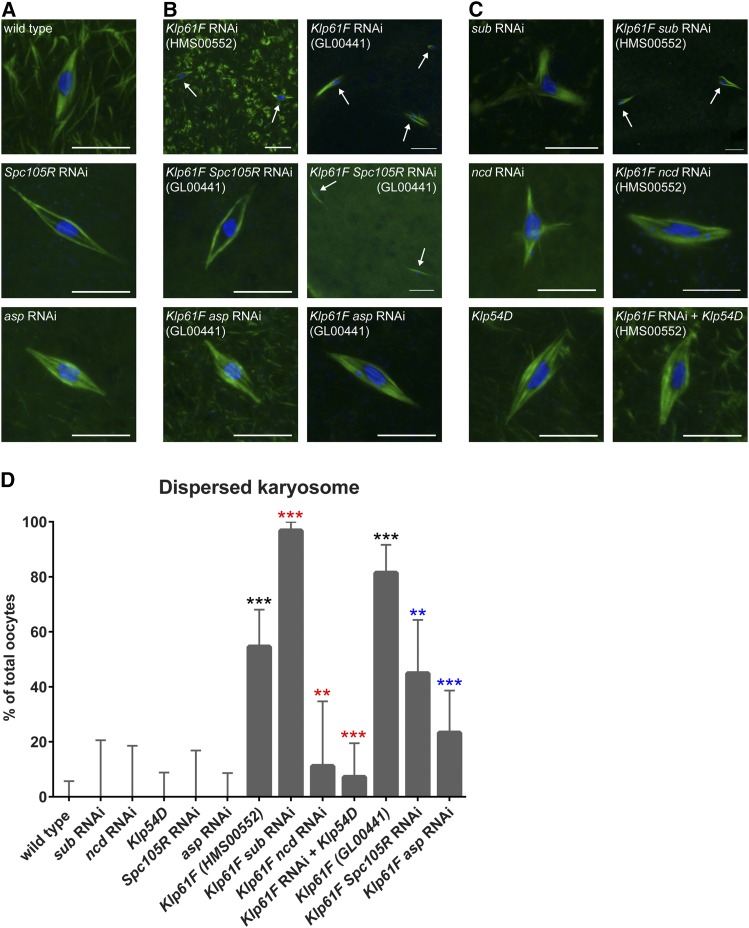
Drosophila oocytes naturally arrest at metaphase of meiosis I, and the cell cycle does not resume until ovulation (King 1970). A collection of late-stage oocytes thus represents stages from early prometaphase through the metaphase I arrest. During this time, the chromosomes are compacted into a karyosome, and traditional chromosome congression does not occur (King 1970). In Klp61F-depleted oocytes, we found that the karyosome was frequently split into multiple masses, and the masses were far apart, forming their own spindles. These “dispersed” karyosomes were found in 55% (n = 55, HMS00552) and 81% (n = 43, GL00441) of Klp61F-depleted oocytes, which is significantly elevated over wild type (0%, n = 63, P < 0.0001, Figure 1A,B,D). Live imaging in Klp61F-depleted oocytes showed that the spindles and centromeres exhibited dramatic movements (File S2) compared to wild type, where stable bipolarity was maintained (File S1). In Klp61F-depleted oocytes, spindles split apart and changed shape around chromosomes that moved apart from each other. This suggests that loss of KLP61F results in an imbalance of MT-based forces on the chromosomes, leading the chromosomes to move away from each other, and become dispersed.
Figure 1.
KLP61F promotes chromosome organization in oocytes. Confocal images of late-stage oocytes. DNA is shown in blue and tubulin is shown in green. Arrows point to parts of a dispersed karyosome. Bar, 10 µm. (A) A wild-type oocyte, and oocytes after knockdown of Spc105R or asp. (B) Oocytes after knockdown of Klp61F, Klp61F and Spc105R, or Klp61F and asp. (C) Oocytes after knockdown of sub, ncd, Klp61F and sub, or Klp61F and ncd. Also shown is a Klp54D mutant oocyte and a Klp54D mutant oocyte after knockdown of Klp61F. (D) Bar graph of the percentage of total oocytes containing a dispersed karyosome. Significance determined by Fisher’s Exact Test: *** P < 0.0001, ** P = 0.002. Comparisons with wild type shown by black asterisks, comparisons with Klp61F (HMS00552) shown by red asterisks, and comparisons with Klp61F (GL00441) shown by blue asterisks. Error bars show 95% confidence intervals.
To test whether the forces causing dispersed karyosomes in the absence of KLP61F depend on kinetochores, we depleted oocytes of Spc105R, which encodes the Drosophila homolog of KNL1. Spc105R-depleted oocytes do not assemble any of the core kinetochore complexes (Radford et al. 2015). In oocytes double-depleted of Klp61F (GL00441) and Spc105R, the karyosome dispersal was significantly reduced compared to Klp61F-depleted oocytes (45%, n = 29, P = 0.002, Figure 1B,D), but remained elevated compared to wild type (P < 0.0001). Therefore, we conclude that KLP61F promotes chromosome organization by opposing both kinetochore- and nonkinetochore-MT-based forces.
KLP61F maintains symmetry in Drosophila oocytes
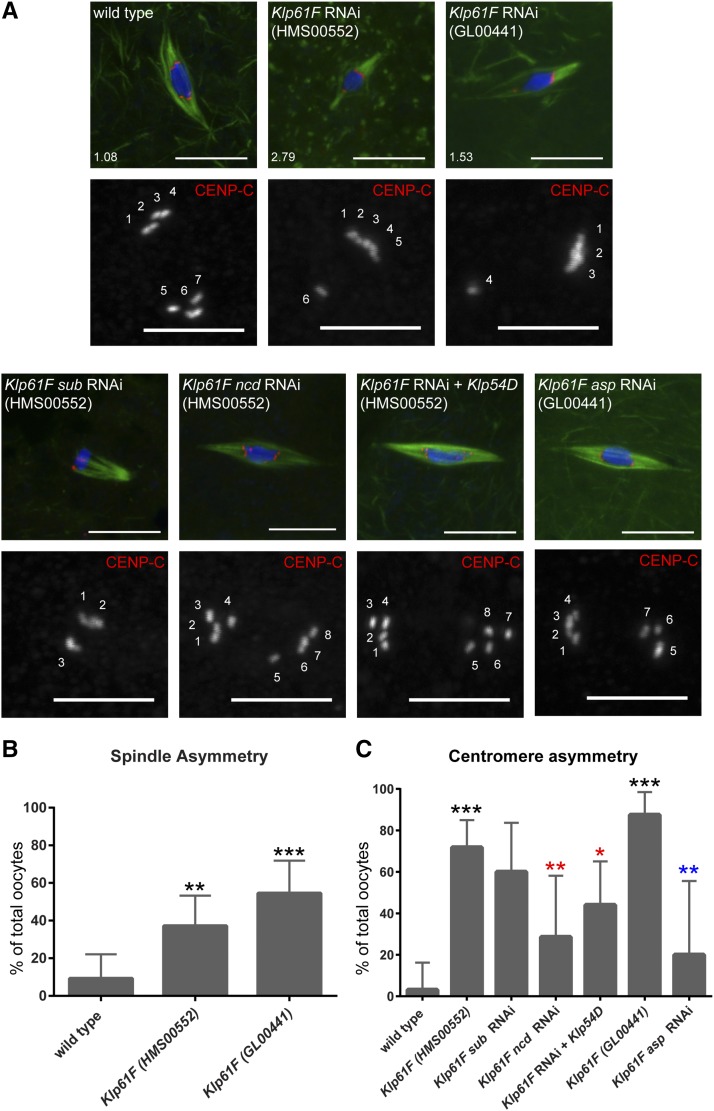
Loss of kinesin-5 in mitotic cells results in the formation of monopolar spindles (Sawin et al. 1992; Heck et al. 1993; Blangy et al. 1995). In contrast, Klp61F-depleted oocytes formed bipolar spindles (Figure 2), and the central spindle formed normally, regardless of whether the karyosome was dispersed (Figure S2). Strikingly, however, these bipolar spindles were frequently asymmetric, such that one half of the spindle appeared to comprise more MTs. To quantify this phenotype, we divided each spindle in half through the midpoint of the karyosome. Then we measured the total fluorescence intensity of the tubulin signal for each spindle half. In oocytes with multiple spindles formed around the dispersed karyosome, each spindle was treated individually. We classified spindles as “asymmetric” if one half of the spindle had >50% more tubulin fluorescence intensity than the other half. In wild type, 9% of spindles were classified as asymmetric (n = 43). This was significantly elevated in Klp61F-depleted oocytes, which showed 37% (HMS00552, n = 43, P = 0.004) and 55% (GL00441, n = 33, P = <0.0001) asymmetric spindles (Figure 2).
Figure 2.
KLP61F promotes spindle and centromere symmetry in oocytes. (A) Confocal images of late-stage oocytes. DNA is shown in blue, tubulin in green, and CENP-C in red in merged images and white in single channel images. Single channel images are zoomed in relative to merged to highlight CENP-C foci. Numbers in lower left corner of merged images represent spindle asymmetry of pictured oocyte. CENP-C foci are numbered in single channel images. Bars, 10 µm in merged, 5 µm in CENP-C single channel images. (B) Bar graph showing the percentage of total oocytes with spindle asymmetry >1.5. Significance determined by Fisher’s Exact Test: *** P < 0.0001, ** P = 0.004. Error bars show 95% confidence intervals. (C) Bar graph showing the percentage of total oocytes with at least 75% of centromeres associated with a single spindle pole. Significance determined by Fisher’s Exact Test: *** P < 0.0001, ** P < 0.01, * P = 0.04. Comparisons with wild type shown by black asterisks, comparisons with Klp61F (HMS00552) shown by red asterisks, and comparisons with Klp61F (GL00441) shown by blue asterisks. Error bars show 95% confidence intervals.
To investigate whether this asymmetry extended to the chromosomes, we determined the location of centromeres with regard to spindle poles. During spindle assembly in Drosophila oocytes, the four pairs of homologous centromeres become bioriented, with four centromeres positioned at each end of the karyosome toward opposite spindle poles (Figure 2). In Klp61F-depleted oocytes, we found that the centromeres were frequently asymmetrically distributed toward each spindle pole (Figure 2). We classified centromeres as “asymmetric” if at least 75% of identified centromeres were associated with one spindle pole. In oocytes with a dispersed karyosome, each set of chromosomes was counted separately. In wild type, 3% of oocytes had asymmetric centromeres (n = 32). In Klp61F-depleted oocytes, on the other hand, centromere asymmetry was significantly elevated to 72% (HMS00552, n = 39, P < 0.0001), and 88% (GL00441, n = 16, P < 0.0001, Figure 2).
If spindle and centromere asymmetry were correlated, we would expect a greater number of cases where the stronger spindle half was associated with more centromeres. Asymmetry was rare in wild-type oocytes: the one example of spindle asymmetry that we observed did not have centromere asymmetry, and vice versa. In Klp61F-depleted oocytes, on the other hand, 69% (HMS00552, n = 13) and 83% (GL00441, n = 6) of the cases where both the spindle and centromeres were classified as “asymmetric,” the stronger spindle half was associated with more centromeres. These results suggest that KLP61F promotes the symmetry of the oocyte spindle, which might be required for the symmetric orientation of centromeres.
The central spindle protein Subito cooperates with KLP61F
The kinesin-6 Subito localizes to, and is required for, the formation of the central spindle, which is a region composed of overlapping MTs in an antiparallel orientation, during prometaphase and metaphase in oocytes (Giunta et al. 2002; Jang et al. 2005). Because both kinesin-5 and kinesin-6 can act on antiparallel MT bundles, we investigated the relationship between them in oocytes. We found that oocytes double-depleted of Klp61F (HMS00552) and sub showed 97% dispersed karyosomes (n = 31, Figure 1C,D). This is significantly elevated over both sub-depleted oocytes (0%, n = 16, P < 0.0001) and Klp61F-depleted oocytes (P < 0.0001), suggesting that these two MT-bundling kinesins are independently required to promote chromosome organization, although we cannot rule out some redundant functions for these two proteins. Combined with the results from depleting Spc105R, we conclude that KLP61F promotes chromosome organization by opposing both kinetochore- and nonkinetochore-MT-based forces.
We found that the frequencies of spindle asymmetry (55%, n = 20, P = 0.3) and centromere asymmetry (60%, n = 15, P = 0.5) in Klp61F sub double-depleted oocytes were not significantly different from Klp61F-depleted oocytes (Figure 2 and Figure S3). However, we found that the magnitude of the spindle asymmetry was amplified in the Klp61F sub-depleted oocytes: whereas 13% (n = 16) of Klp61F-depleted asymmetric spindles had one-half of the spindle 5 times more intense than the other, this was significantly elevated to 55% (n = 11, P = 0.03) in Klp61F sub-depleted asymmetric spindles. These results lead us to conclude that KLP61F and SUB contribute to the symmetry of the oocyte spindle in nonoverlapping roles.
The minus-end-directed kinesin-14 NCD promotes centromere asymmetry and karyosome dispersion in the absence of KLP61F
Building a bipolar spindle with the capacity to accurately segregate chromosomes depends on a balance of MT-based forces. We hypothesized that loss of KLP61F could result in an imbalance of forces, leading to the spindle asymmetry and dispersed karyosomes that we observed. In mitotic cells, the plus-end-directed KLP61F is opposed by the minus-end-directed kinesin-14, known as HSET in mammals and NCD in Drosophila (Mountain et al. 1999; Sharp et al. 2000; Wilson et al. 2004). We tested whether KLP61F and NCD would also oppose one another during acentrosomal spindle assembly in Drosophila oocytes.
Oocytes depleted of ncd showed 98% reduction in ncd mRNA and had several defects, including elevated X chromosome nondisjunction (48%, n = 216) and broken and/or frayed spindles (100%, n = 18), consistent with previously reported phenotypes of ncd mutants (Davis 1969; Kimble and Church 1983; Hatsumi and Endow 1992; Matthies et al. 1996). Oocytes depleted of both Klp61F (HMS00552) and ncd showed significant rescue of the abnormal spindles observed in ncd-depleted oocytes (67%, n = 18, P = 0.02, Figure 1C and Figure 2) and the dispersed karyosomes observed in Klp61F-depleted oocytes (11%, n = 18, P = 0.002, Figure 1D). Spindle asymmetry was not significantly different (24%, n = 21, P = 0.4, Figure S3). Because the size and shape of Drosophila oocyte spindles is variable, it is only possible to definitively identify extreme changes in spindle symmetry (such as the difference between wild type and Klp61F-depleted oocytes). The centromere asymmetry in Klp61F-depleted oocytes, on the other hand, was significantly rescued in Klp61F ncd-depleted oocytes (29%, n = 14, P = 0.009, Figure 2). These results suggest that NCD contributes to the asymmetry of centromeres and/or disorganized chromosomes, which are prevented by KLP61F in acentrosomal oocytes. Although the two phenotypes are correlated, we do not know if the disorganized chromosomes are a result of asymmetry.
The kinesin-12 KLP54D promotes centromere asymmetry and karyosome dispersion in the absence of KLP61F
Spindle bipolarity in human cells can be maintained in the absence of kinesin-5 (Kapoor et al. 2000), dependent on the kinesin-12 motor protein, known as KIF15 or Hklp2 (Tanenbaum et al. 2009; Vanneste et al. 2009). In C. elegans, oocyte spindle organization is dependent on the kinesin-12 KLP-18 (Segbert et al. 2003), but kinesin-5 is not essential. To determine the relationship between kinesin-5 and kinesin-12 in Drosophila oocytes, we generated a mutation in the gene encoding Drosophila kinesin-12, Klp54D. This mutation removes half of the kinesin motor domain, but mutants were homozygous and hemizygous viable and fertile, with no elevation in X chromosome segregation errors in oocytes (0.7%, n = 2276 vs. 0.4%, n = 1455 in wild type, P = 0.4). Because these Klp54D mutants do not have a non wild-type phenotype, it is not possible to determine genetically if they are null alleles.
To test whether there is an interaction between KLP61F and KLP54D, we depleted Klp61F (HMS00552) in Klp54D hemizygous mutants. Loss of KLP54D in the absence of KLP61F resulted in spindle asymmetry that was not significantly different from that of Klp61F-depleted oocytes (35%, n = 26, P = 1.0, Figure 3). As with oocytes depleted of both Klp61F and ncd, the size and shape of Drosophila oocyte spindles is variable, making it possible to observe only extreme changes in spindle symmetry. The centromere asymmetry, on the other hand, was significantly improved (44%, n = 25, P = 0.04, Figure 2), and the dispersed karyosome phenotype was significantly rescued by the loss of KLP54D (7%, n = 42, P < 0.0001, Figure 1C,D). These results demonstrate that KLP54D contributes to the asymmetry of centromeres and chromosome organization defects in Klp61F-depleted oocytes.
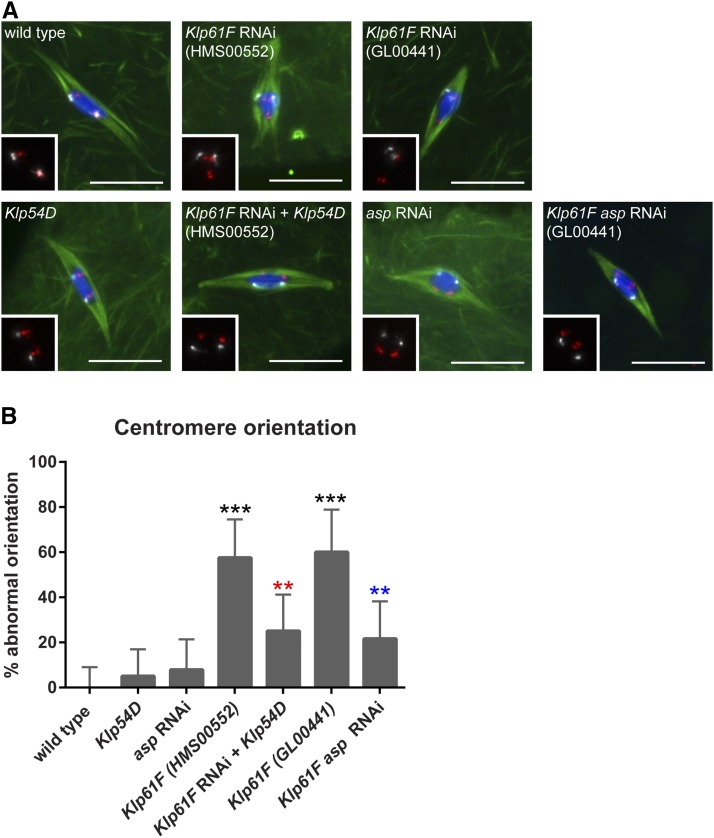
Figure 3.
KLP61F is required for homologous centromere biorientation in oocytes. (A) Confocal images of late-stage oocytes. DNA is shown in blue, tubulin is shown in green, FISH probe marking the 2nd chromosome is shown in red, the 3rd chromosome is shown in white. Inset shows FISH probes only. Bars, 10 µm. (B) Bar graph showing the percentage of centromeres that were abnormally oriented. Significance determined by Fisher’s Exact Test: *** P < 0.0001, ** P < 0.01. Comparisons with wild type shown by black asterisks, comparisons with Klp61F (HMS00552) shown by red asterisks, and comparisons with Klp61F (GL00441) shown by blue asterisks. Error bars show 95% confidence intervals.
Because centromere asymmetry was rescued in Klp54D mutant oocytes depleted of Klp61F, this raised the question of whether homologous chromosomes were being accurately bioriented. To test this, we examined homologous centromeres using fluorescent in situ hybridization (FISH) of probes to the heterochromatic repeats present at Drosophila centromeres. In Drosophila oocytes, centromeres are clustered prior to spindle assembly (Dernburg et al. 1996). As the spindle assembles, centromeres separate and homologous centromeres orient toward opposite spindle poles, termed biorientation. We found that homologous centromeres were often misoriented in Klp61F-depleted oocytes (HMS00552, 58%, n = 33; GL00441, 60%, n = 25; Figure 3), consistent with a failure to establish biorientation. We found that misorientation was significantly reduced in Klp54D hemizygous mutants depleted of Klp61F compared to Klp61F-depleted oocytes (HMS00552, 25%, n = 40, P = 0.008, Figure 3). This demonstrates that the centromere asymmetry is suppressed because the homologous chromosomes orient correctly in Klp61F-depleted oocytes that are mutant for Klp54D.
Loss of the MT-associated protein ASP prevents centromere asymmetry and karyosome dispersion in the absence of KLP61F
Our results thus far show that KLP61F plays an important role in promoting spindle and chromosome symmetry in the oocyte. The oocyte divisions are highly asymmetric, with two rounds of division producing a single large egg that retains most of the cytoplasm. To test whether KLP61F opposes proteins that promote asymmetric cell division, we investigated ASP, the Drosophila homolog of the microcephaly protein ASPM. ASP is required for the asymmetric neuroblast cell divisions (Rujano et al. 2013). Females depleted of asp in their germline showed 65% reduction in asp mRNA, and were sterile. In asp-depleted oocytes, chromosome and spindle organization were normal (Figure 1B, Figure 3, and Figure S3), consistent with previous observations using asp mutants (Riparbelli et al. 2002). In oocytes double-depleted of Klp61F (GL00441) and asp, we observed a significant rescue of the dispersed karyosome phenotype compared to Klp61F-depleted oocytes (23%, n = 43, P = <0.0001, Figure 1D). Spindle asymmetry was not significantly different (32%, n = 31, P = 0.08, Figure S3), although the trend was toward more symmetry than Klp61F-depleted oocytes. Centromere asymmetry, on the other hand, was significantly reduced in double-depleted oocytes (20%, n = 10, P = 0.001, Figure 2). FISH experiments also showed that centromere misorientation was significantly rescued in double-depleted oocytes (22%, n = 37, P = 0.003, Figure 3). These results demonstrate that the centromere asymmetry observed in the absence of KLP61F is dependent on ASP.
Discussion
We undertook a study of kinesin-5 in oocytes for two reasons. First, MT-bundling kinesins appear to have an increased importance in oocytes. Second, kinesin-5 promotes spindle bipolarity in mitotic cells by keeping centrosomes separated, but oocytes lack centrosomes. Thus, we expected that the Drosophila kinesin-5 KLP61F would have a role in organizing the oocyte spindle due to its MT-bundling activity, but whether KLP61F would promote spindle bipolarity in the absence of centrosomes was not clear.
Instead of monopolar spindles, loss of KLP61F in Drosophila oocytes resulted in bipolar spindles but with two striking defects. First, the chromosomes were frequently disorganized, resulting in the karyosome breaking into multiple masses. Second, the bipolar spindles formed in the absence of KLP61F were highly asymmetric. We do not know if the two phenotypes are due to the same defect or if the karyosome dispersal is a consequence of the asymmetry. However, because the karyosome dispersal and the centromere asymmetry phenotypes depend on the same genes, they may result from the same defect. Our results also show that KLP61F cooperates with the kinesin-6 SUB to promote karyosome integrity. In contrast, the kinesin-12 KLP54D, the kinesin-14 NCD, and the spindle pole protein ASP cooperate to promote dispersal of the chromosomes. These conclusions are based on patterns of suppression or enhancement of the Klp61F depletion phenotype, so although not all RNAi or mutants used are proven null, the following interpretations of our results do not depend on complete loss of function.
Mechanisms and forces that generate asymmetric spindles in oocytes
Spindle asymmetry in Klp61F-depleted oocytes depends on the spindle pole protein ASP, and the motors NCD and KLP54D (Figure 4, B and D). ASP has previously been linked to spindle positioning and asymmetric cell division in Drosophila (Rujano et al. 2013) and mammals (Fish et al. 2006; Gai et al. 2016), but this represents a novel function for NCD and KLP54D. However, the observation that loss of kinesin-5 is rescued by loss of dynein at the poles (Miyamoto et al. 2004; Mitchison et al. 2005), may be mechanistically similar to the rescue we see by NCD and ASP. The relationship between kinesin-5 and kinesin-12 may appear as antagonism or cooperation, depending on the situation, which may reflect different effects on parallel and antiparallel MT bundles (Tanenbaum et al. 2009; Vanneste et al. 2009; Sturgill and Ohi 2013; Drechsler and McAinsh 2016). The mammalian kinesin-12 KIF15 typically localizes to kinetochore-MTs (Sturgill and Ohi 2013), and a property it shares with kinesin-14 is bundling parallel MTs (McDonald et al. 1990; Fink et al. 2009). Similarly, ASP is known to focus minus-ends of MTs with a MT cross-linking activity (Ito and Goshima 2015), which could enhance the activity of NCD and KLP54D. We suggest that, in the absence of KLP61F, the bundling activity of parallel MTs by NCD and KLP54D results in a structural difference between the two halves of the spindle (Figure 4D). This would increase the number of available kinetochore-MTs in one half of the spindle, and possibly lead to an asymmetric distribution of centromeres (Figure 4E).
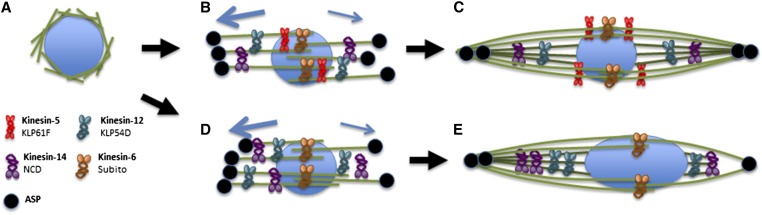
Figure 4.
Model for asymmetric spindle organization in oocytes. A pathway for assembly and the early establishment of symmetry in the Drosophila oocyte spindle. The MT-bundling kinesins are shown interacting with their preferred MT substrates: SUB (brown) at the central spindle, NCD (purple), and KLP54D (blue) on parallel MTs and KLP61F (red) on all MTs. ASP (black), which also bundles MTs, is shown where it is most abundant, at the poles. (A) Spindle assembly begins with the accumulation of MTs around the chromosomes (Theurkauf and Hawley 1992). (B) The MTs are sorted in two directions. We propose that the proteins promoting the bundling of parallel MTs, ASP, KLP54D and NCD, respond to activators (arrows) that are more abundant or stronger toward one pole. KLP61F balances this activity by specifically bundling and sliding pairs of antiparallel MTs in each direction. SUB also bundles antiparallel MTs but has less ability to discriminate an even number of MTs. (C) Eventually, two types of MTs are present (Radford et al. 2015): antiparallel MTs whose plus-ends overlap (light green) and parallel MTs whose plus-ends interact with kinetochores (dark green). We propose that the antiparallel MTs promote symmetry while parallel MTs promote asymmetry. (D and E) In the absence of KLP61F, antiparallel MTs, and thus a central spindle and a bipolar spindle, are maintained by SUB. However, the stronger bundling of parallel MTs at one of the poles leads to an asymmetric spindle.
We do not know the source of the spindle asymmetry observed in Klp61F-depleted oocytes (Figure 4, B and D). One possibility is that spindle asymmetry could result from stochastic forces within the spindle. For example, in the absence of KLP61F, homologous centromeres misorient, and the asymmetric spindle may result from a greater number of centromeres facing one of the poles. Alternatively, spindle-independent forces could establish the asymmetry. In this case, homolog misorientation is not a cause, but a consequence, of the asymmetric spindle in Klp61F-depleted oocytes. We favor this model, because it can explain how suppression by several factors occurs, including KLP54D and ASP, that also restore biorientation. The meiotic divisions are asymmetric in order to generate one oocyte and smaller polar bodies. The mechanism that establishes this asymmetry is poorly understood, but in different systems has been proposed to involve the tubulin or actin cytoskeleton (Fabritius et al. 2011). Whether the asymmetric oocyte division is preceded by an asymmetric metaphase spindle is not known. In maize meiosis, there is evidence that the spindle is asymmetric, and this depends on the position of the spindle within the cell (Nannas et al. 2016). We suggest the mechanism enforcing the asymmetric division can influence the activities of proteins like NCD, KLP54D, and ASP, and result in an asymmetric spindle, while the activity of KLP61F counteracts these forces. We cannot rule out the possibility, however, that NCD, KLP54D, and ASP activity can result in an imbalance of forces within the spindle, and this is what KLP61F counteracts.
KLP61F activity prevents asymmetric spindles in oocytes
KLP61F and the kinesin-6 Subito are synergistic. Therefore, we suggest the function of KLP61F is to promote spindle symmetry by sorting parallel and antiparallel MTs (Figure 4B). Furthermore, we propose that the central spindle, stabilized by SUB, is the dominant spindle feature required for bipolarity in Drosophila oocytes. SUB stabilizes the central spindle, which promotes spindle bipolarity, while KLP61F provides MT forces that promote spindle symmetry and chromosome organization, which is partially dependent on a stable central spindle. A strong central spindle stabilized by SUB may explain why loss of KLP61F in oocytes did not result in monopolar spindles, although it remains possible the reduction in KLP61F levels after RNAi, while drastic, was not sufficient to elicit monopolar spindles (Kwok et al. 2004; Brust-Mascher et al. 2009).
A similar mechanism may be operating in mouse oocytes. HURP is recruited to the central spindle in mouse oocytes by kinesin-5 activity (Breuer et al. 2010). HURP and Eg5 are proposed to sort MTs toward each pole. We suggest this sorting is able to distribute an approximately equal number of MTs to each half-spindle, over-riding pole-derived forces that promote bundling of more parallel MTs on one side of the spindle (Figure 4C). If the asymmetric forces are particularly strong, a defect in this process could result in all MTs on one side, resulting in the monopolar spindles observed in mammalian oocytes depleted of kinesin-5 (Mailhes et al. 2004; Duncan et al. 2012).
An interesting consequence of this work is that centromeres may take advantage of spindle asymmetry, resulting in cases of biased chromosome inheritance, often termed “meiotic drive” (Malik and Bayes 2006; Ross and Malik 2014). Biased chromosome segregation requires the ability to discriminate which spindle pole will direct chromosomes into the oocyte, as opposed to the polar bodies. An attractive model is that there is an inherent asymmetry in the oocyte spindle itself (Figure 4B); however, there has previously been very little evidence that the spindle itself is asymmetric (Hewitt 1976). We believe our results constitute some of that evidence. Further study is necessary to determine if oocyte spindle asymmetry contributes to biased chromosome inheritance and meiotic drive.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.194647/-/DC1.
Acknowledgments
We thank Le Nguyen for technical assistance; Vladimir Gelfand, Christian Lehner, and Jonathan Scholey for reagents; and Mercedes Gyuricza and Lin-Ing Wang for helpful comments on the manuscript. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks and plasmid vectors used in this study.
Author contributions: S.J.R. and K.S.M. conceived and designed the experiments. S.J.R. and A.M.M.G. performed the experiments. S.J.R. and K.S.M. analyzed the data and wrote the article.
Footnotes
Communicating editor: M. P. Colaiacovo
Literature Cited
- Albertson D. G., Thomson J. N., 1993. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res. 1: 15–26. [DOI] [PubMed] [Google Scholar]
- Attrill H., Falls K., Goodman J. L., Millburn G. H., Antonazzo G., et al. , 2015. FlyBase: establishing a gene group resource for Drosophila melanogaster. Nucleic Acids Res. 44: D786–D792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A., Lane H. A., d’Hérin P., Harper M., Kress M., et al. , 1995. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83: 1159–1169. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Breuer M., Kolano A., Kwon M., Li C. C., Tsai T. F., et al. , 2010. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J. Cell Biol. 191: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust-Mascher I., Sommi P., Cheerambathur D. K., Scholey J. M., 2009. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol. Biol. Cell 20: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario J. M., Jang J. K., Redding B., Shah N., Rahman T., et al. , 2006. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J. Cell Sci. 119: 4770–4780. [DOI] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N., 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D., Schuh M., 2015. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat. Commun. 6: 7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. G., 1969. Chromosome behavior under the influence of claret- nondisjunctional in Drosophila melanogaster. Genetics 61: 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A. F., Sedat J. W., Hawley R. S., 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Drechsler H., McAinsh A. D., 2016. Kinesin-12 motors cooperate to suppress microtubule catastrophes and drive the formation of parallel microtubule bundles. Proc. Natl. Acad. Sci. USA 113: E1635–E1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F. E., Hornick J. E., Woodruff T. K., 2012. Bipolar-to-monopolar spindle collapse in human eggs. Mol. Reprod. Dev. 79: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritius A. S., Ellefson M. L., McNally F. J., 2011. Nuclear and spindle positioning during oocyte meiosis. Curr. Opin. Cell Biol. 23: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G., Hajdo L., Skowronek K. J., Reuther C., Kasprzak A. A., et al. , 2009. The mitotic kinesin-14 Ncd drives directional microtubule-microtubule sliding. Nat. Cell Biol. 11: 717–723. [DOI] [PubMed] [Google Scholar]
- Fish J. L., Kosodo Y., Enard W., Paabo S., Huttner W. B., 2006. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 103: 10438–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai M., Bianchi F. T., Vagnoni C., Verni F., Bonaccorsi S., et al. , 2016. ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules. EMBO Rep. 17: 1396–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta K. L., Jang J. K., Manheim E. A., Subramanian G., McKim K. S., 2002. subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160: 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Nedelec F., Vale R. D., 2005. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 171: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S. S., Zhang N., Scholey J. M., et al. , 2007. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi M., Endow S. A., 1992. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J. Cell Sci. 101: 547–559. [DOI] [PubMed] [Google Scholar]
- Heck M. M., Pereira A., Pesavento P., Yannoni Y., Spradling A. C., et al. , 1993. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 123: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger S., Leismann O., Schittenhelm R., Schraidt O., Heidmann S., et al. , 2005. Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev. 19: 2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt G. M., 1976. Meiotic drive for B-chromosomes in the primary oocytes of Myrmeleotettix maculatus (Orthopera: Acrididae). Chromosoma 56: 381–391. [DOI] [PubMed] [Google Scholar]
- Ito A., Goshima G., 2015. Microcephaly protein Asp focuses the minus ends of spindle microtubules at the pole and within the spindle. J. Cell Biol. 211: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J. K., Rahman T., McKim K. S., 2005. The kinesinlike protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol. Biol. Cell 16: 4684–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein L. C., Peterman E. J., Kwok B. H., Kim J. H., Kapoor T. M., et al. , 2005. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435: 114–118. [DOI] [PubMed] [Google Scholar]
- Kapoor T. M., Mitchison T. J., 2001. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. J. Cell Biol. 154: 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor T. M., Mayer T. U., Coughlin M. L., Mitchison T. J., 2000. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150: 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble M., Church K., 1983. Meiosis and early cleavage in Drosophila melanogaster eggs: effects on the claret-non-disjunctional mutation. J. Cell Sci. 62: 301–318. [DOI] [PubMed] [Google Scholar]
- King R. C., 1970. Ovarian Development in Drosophila melanogaster. Academic Press Inc., New York. [Google Scholar]
- Kwok B. H., Yang J. G., Kapoor T. M., 2004. The rate of bipolar spindle assembly depends on the microtubule-gliding velocity of the mitotic kinesin Eg5. Curr. Biol. 14: 1783–1788. [DOI] [PubMed] [Google Scholar]
- Lu W., Fox P., Lakonishok M., Davidson M. W., Gelfand V. I., 2013. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr. Biol. 23: 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhes J. B., Mastromatteo C., Fuseler J. W., 2004. Transient exposure to the Eg5 kinesin inhibitor monastrol leads to syntelic orientation of chromosomes and aneuploidy in mouse oocytes. Mutat. Res. 559: 153–167. [DOI] [PubMed] [Google Scholar]
- Malik H. S., Bayes J. J., 2006. Genetic conflicts during meiosis and the evolutionary origins of centromere complexity. Biochem. Soc. Trans. 34: 569–573. [DOI] [PubMed] [Google Scholar]
- Matthies H. J., McDonald H. B., Goldstein L. S., Theurkauf W. E., 1996. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 134: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. B., Stewart R. J., Goldstein L. S., 1990. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell 63: 1159–1165. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Maddox P., Gaetz J., Groen A., Shirasu M., et al. , 2005. Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol. Biol. Cell 16: 3064–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto D. T., Perlman Z. E., Burbank K. S., Groen A. C., Mitchison T. J., 2004. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 167: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain V., Simerly C., Howard L., Ando A., Schatten G., et al. , 1999. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 147: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannas N. J., Higgins D. M., Dawe R. K., 2016. Anaphase asymmetry and dynamic repositioning of the division plane during maize meiosis. J. Cell Sci. 129: 4014–4024. [DOI] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., et al. , 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. J., McKim K. S., 2016. Techniques for imaging prometaphase and metaphase of meiosis I in fixed Drosophila oocytes. J. Vis. Exp. 116: e54666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. J., Harrison A. M., McKim K. S., 2012a Microtubule-depolymerizing kinesin KLP10A restricts the length of the acentrosomal meiotic spindle in Drosophila females. Genetics 192: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. J., Jang J. K., McKim K. S., 2012b The chromosomal passenger complex is required for meiotic acentrosomal spindle assembly and chromosome bi-orientation. Genetics 192: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. J., Hoang T. L., Głuszek A. A., Ohkura H., McKim K. S., 2015. Lateral and end-on kinetochore attachments are coordinated to achieve bi-orientation in Drosophila oocytes. PLoS Genet. 11: e1005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M. G., Callaini G., Glover D. M., Avides M. C., 2002. A requirement for the abnormal spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J. Cell Sci. 115: 913–922. [DOI] [PubMed] [Google Scholar]
- Rorth P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Malik H. S., 2014. Genetic conflicts: stronger centromeres win tug-of-war in female meiosis. Curr. Biol. 24: R966–R968. [DOI] [PubMed] [Google Scholar]
- Rujano M. A., Sanchez-Pulido L., Pennetier C., le Dez G., Basto R., 2013. The microcephaly protein Asp regulates neuroepithelium morphogenesis by controlling the spatial distribution of myosin II. Nat. Cell Biol. 15: 1294–1306. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., LeGuellec K., Philippe M., Mitchison T. J., 1992. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359: 540–543. [DOI] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J., 2007. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130: 484–498. [DOI] [PubMed] [Google Scholar]
- Segbert C., Barkus R., Powers J., Strome S., Saxton W. M., et al. , 2003. KLP-18, a Klp2 kinesin, is required for assembly of acentrosomal meiotic spindles in Caenorhabditis elegans. Mol. Biol. Cell 14: 4458–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. J., Brown H. M., Kwon M., Rogers G. C., Holland G., et al. , 2000. Functional coordination of three mitotic motors in Drosophila embryos. Mol. Biol. Cell 11: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill E. G., Ohi R., 2013. Kinesin-12 differentially affects spindle assembly depending on its microtubule substrate. Curr. Biol. 23: 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura I., Lilly M. A., 2006. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell 10: 127–135. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P., 1972. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 11: 521–541. [DOI] [PubMed] [Google Scholar]
- Tanenbaum M. E., Macurek L., Janssen A., Geers E. F., Alvarez-Fernandez M., et al. , 2009. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr. Biol. 19: 1703–1711. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Hawley R. S., 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste D., Takagi M., Imamoto N., Vernos I., 2009. The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr. Biol. 19: 1712–1717. [DOI] [PubMed] [Google Scholar]
- Wilson P. G., Simmons R., Shigali S., 2004. Novel nuclear defects in KLP61F-deficient mutants in Drosophila are partially suppressed by loss of Ncd function. J. Cell Sci. 117: 4921–4933. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Li H., Schweppe N. M., Hawley R. S., Gilliland W. D., 2010. Statistical analysis of nondisjunction assays in Drosophila. Genetics 186: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.