Abstract
The mammalian Mcm-domain containing 2 (Mcmdc2) gene encodes a protein of unknown function that is homologous to the minichromosome maintenance family of DNA replication licensing and helicase factors. Drosophila melanogaster contains two separate genes, the Mei-MCMs, which appear to have arisen from a single ancestral Mcmdc2 gene. The Mei-MCMs are involved in promoting meiotic crossovers by blocking the anticrossover activity of BLM helicase, a function presumably performed by MSH4 and MSH5 in metazoans. Here, we report that MCMDC2-deficient mice of both sexes are viable but sterile. Males fail to produce spermatozoa, and formation of primordial follicles is disrupted in females. Histology and immunocytological analyses of mutant testes revealed that meiosis is arrested in prophase I, and is characterized by persistent meiotic double-stranded DNA breaks (DSBs), failure of homologous chromosome synapsis and XY body formation, and an absence of crossing over. These phenotypes resembled those of MSH4/5-deficient meiocytes. The data indicate that MCMDC2 is essential for invasion of homologous sequences by RAD51- and DMC1-coated single-stranded DNA filaments, or stabilization of recombination intermediates following strand invasion, both of which are needed to drive stable homolog pairing and DSB repair via recombination in mice.
Keywords: meiosis, recombination, mouse, double strand break repair, synapsis
THE minichromosome maintenance (MCM) family of proteins were discovered based on their crucial functions in DNA replication, and contain conserved MCM and ATPase domains (Tye 1999). However, there are additional MCM family members that function outside of the core MCM2-7 replicative helicase complex. MCM8 and MCM9 function in DNA repair and homologous recombination (Park et al. 2013; Traver et al. 2015). Both are dispensable for DNA replication in mice, but MCM8- and MCM9-deficient cells exhibit defects in homologous recombination repair in response to DNA damage (Hartford et al. 2011; Lutzmann et al. 2012; Nishimura et al. 2012; Park et al. 2013; Lee et al. 2015; Luo and Schimenti 2015). Surprisingly, Mcm9, which is absent from Drosophila, is also required for DNA mismatch repair and this may actually be its primary function (Traver et al. 2015). Mcm8 null mice of both sexes are sterile due to defects in homologous recombination repair during meiotic prophase I (Lutzmann et al. 2012), whereas Mcm9 mutant mice are defective in primordial germ cell proliferation that leads to reduced (males) or absent (females) germ cells (Hartford et al. 2011; Lutzmann et al. 2012).
During meiosis in many organisms, including mice, homologous chromosome pairing and synapsis is driven by recombination, and proper segregation of homologs during the first meiotic division depends upon each chromosome pair undergoing at least one crossover (CO) event. To produce COs, there is an orchestrated process in which abundant programmed double-stranded DNA breaks (DSBs; >200 per cell in mice) are produced, followed by repair of the majority (∼90%) of these breaks by noncrossover (NCO) repair, and the remainder via CO recombination (Handel and Schimenti 2010). The mechanisms regulating CO vs. NCO recombination is an area of intense study. Most of the CO events in both yeast and mice require proteins of the ZMM complex, including MSH4 and MSH5, which stabilize recombination intermediates and facilitate double Holliday junction formation after initial D-loops are formed by invasion of a single-stranded end of a resected DSB (Manhart and Alani 2016). In part, this is done by inhibiting the anti-CO activity of BLM helicase, which otherwise promotes synthesis-dependent strand annealing and NCO repair (Jessop et al. 2006; Oh et al. 2007; Holloway et al. 2010; De Muyt et al. 2012). In Drosophila (which lacks MSH4 and MSH5), a complex consisting of MEI-217, MEI-218, and REC (the latter being an MCM8 ortholog), termed Mei-MCM (meiotic minichromosome maintenance), assumes the role of promoting COs by inhibiting BLM helicase (Kohl et al. 2012).
BLM is a conserved member of the RecQ family of DNA helicases that was identified as a rare recessive genetic disorder in humans (Bloom’s syndrome) characterized by dwarfism, increased cancer susceptibility, and immune deficiency. Mutations in the Drosophila Blm gene result in female sterility, defects in DNA repair, and increased sensitivity to ionizing radiation (Adams et al. 2003; McVey et al. 2007). In mice, Blm deficiency causes embryonic lethality, though heterozygosity or conditional somatic deletion causes increased genomic instability and tumor susceptibility (Chester et al. 1998; Luo et al. 2000; Goss et al. 2002; Chester et al. 2006). Conditional germline deletion of Blm in mice disrupts meiotic prophase I progression; mutant spermatocytes exhibit aberrations in chromosome synapsis and elevated numbers of COs (Holloway et al. 2010).
It was postulated that the Mei-MCM complex in Drosophila evolved to assume the anti-CO activities of MSH4/MSH5. Interestingly, MEI-217 and MEI-218 seem to have arisen from a single ancestral MCM gene, in that the former contains an MCM N-terminal domain and the latter a C-terminal AAA ATPase domain, and these two genes are adjacent in the genome and are expressed as a bicistronic transcript (Liu et al. 2000; Kohl et al. 2012). The mammalian ortholog is a single gene called Mcmdc2 (Mcm-domain containing protein 2) that resembles a typical MCM protein with the aforementioned domain structure. However, unlike other members of the MCM family, Mcmdc2 encodes a much smaller MCM domain than that which is present in other MCM proteins, and the ATPase domains of both MCMDC2 and Mei-218 contain amino acid changes predicted to disrupt actual ATPase activity (Kohl et al. 2012). Mutation of any of the Mei-MCMs (rec, mei-217, or mei-218) in Drosophila causes a severe reduction of COs but not NCO recombination (gene conversion), leading to increased nondisjunction and reduced fertility (Grell 1984; Liu et al. 2000; Blanton et al. 2005; Kohl et al. 2012).
Mcmdc2 messenger RNA is present most abundantly in mouse testes (e.g., EMBL-EBI Expression atlas; GEO datasets GSE43717, GSE39970, and GSE44346). Though frequently amplified in various types of cancer (according to TCGA data available through cbioportal.org), this gene was essentially unstudied in mammals until recently (Finsterbusch et al. 2016). To explore the potential role of mammalian Mcmdc2 and whether it performs a role similar to the Drosophila mei-217/218 and/or mammalian Msh4/Msh5, we generated mice bearing a mutant allele. Phenotypic and immunocytological analyses revealed that it is crucial for repair of meiotic DSBs, chromosome synapsis, and fertility, but not other discernable somatic processes. The phenotype is similar to that of MSH4/5, suggesting that it may be involved in aiding their role in stabilizing recombination intermediates and promoting interhomolog recombination and COs.
Materials and Methods
Generation of Mcmdc2Gt animals
All animal use was conducted under a protocol to J.C.S., approved by Cornell’s Institutional Animal Use and Care Committee. Mcmdc2tm4a(EUCOMM)Hmgu (Mcmdc2Gt) embryonic stem (ES) cells were obtained from the European mutant mouse cell repository. Founder animals were genotyped by PCR and crossed for three to four generations into strain B6(Cg)-Tyrc-2J/J. To remove the floxed neomycin resistance (neo) gene from the gene trap allele, females were crossed to a Stra8-Cre male (Sadate-Ngatchou et al. 2008) and the offspring were genotyped for Cre and the gene trap. The neo-deleted animals were then backcrossed two to three generations into C57BL/6J (B6) mice. There were no phenotypic differences between neo-positive vs. neo-negative animals. Genotyping primers are listed in Supplemental Material, Table S1.
Quantitative reverse transcription PCR (RT-qPCR)
Total RNA was isolated from tissue using E.Z.N.A kit (Omega Biotek) according to manufacturer’s instructions. Complementary DNA was made from 500 μg of total RNA using qScript complementary DNA mix (Quanta), and quantitative PCR carried out using iTaq (Bio-Rad, Hercules, CA). All quantitative reverse transcription PCR (RT-qPCR) data were normalized to Gapdh. Primer sequences are listed in Table S1.
Histology and lacZ staining
For hematoxylin and eosin (H&E) staining, testes and ovaries were dissected and fixed in Bouin’s solution for 6–12 hr. Tissues were then washed in 70% ethanol for 2–3 days prior to being paraffin embedded. Sections of 4 μM thickness were stained with Harris H&E. Slides were digitized using a Leica Scanscope CS2 and 20× lens. Images were analyzed using ImageScope and Halo software. For lacZ, tissues were fixed in 4% paraformaldehyde in PBS overnight at 4°. Tissues were washed with PBS and placed in 30% sucrose/PBS overnight at 4°. The fixed tissue was then flash frozen in optimal cutting temperature and 10 μM sections were cut on a cryostat. lacZ staining was carried out as described (Nagy et al. 2007).
Meiotic chromosome surface spreads and immunofluorescence
Isolated testes with the tunica removed were minced into small (2–3 mm) pieces in Dulbecco's Modified Eagle's Medium(DMEM). Meiocytes were hypotonically swollen in in a sucrose solution on slides. Cells were lysed with 0.1% Triton X-100, 0.02% SDS, and 2% paraformaldehyde. Slides were washed and stored at −80° until staining. For staining, slides were brought to room temperature and washed once with PBS and 0.1% Triton X-100. Slides were blocked for 40 min at room temperature with 1× PBS and 0.3% Triton X-100 containing 5% normal goat serum. Primary antibodies were diluted into 1× PBS, 1% BSA, and 0.3% Triton X-100 and incubated overnight at 37° in a humidified chamber. Antibodies and dilutions used include: mouse anti-γH2AX Ser 139 (1:1000; Millipore, Bedford, MA), rabbit anti-Rad51 (1:250; Millipore), rabbit anti-SYCP1 (1:600; Abcam), rabbit anti-SYCP3 (1:600; Abcam), mouse anti-SYCP3 (1:600; Abcam), rabbit anti-MLH1 (1:5;0 Millipore), mouse anti-DMC1 (1:100; Abcam), anti-RPA2 (a gift from P.J. Wang), and guinea pig anti-H1T (1:1000; gift from M.A. Handel). Secondary antibodies were used at 1:1000 in antibody dilution buffer and included: goat anti-rabbit Alexa Fluor 488, goat anti-rabbit Alexa Fluor 594, goat anti-mouse Alexa Fluor 488/594, and goat anti-guinea pig Alexa Fluor 488/594. Images were taken using an Olympus microscope with 63× lens and charged-coupled device camera. Foci were quantified using Fiji (Schindelin et al. 2012) and analyzed in Prism 7 (GraphPad).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Reagents available upon request.
Results and Discussion
To determine the function of mammalian Mcmdc2, we generated knockout mice from embryonic stem (ES) cells. The targeted allele was of a gene trap design, whereby the normal transcript is truncated by virtue of an introduced splice acceptor that created a lacZ fusion. Additionally, a neomycin selectable marker flanked by loxP sites was integrated into an intron (Figure S1A). This allele is referred to as Mcmdc2Gt. Founder animals were genotyped by PCR and the expression pattern of Mcmdc2 was analyzed by RT-qPCR and lacZ activity staining (Figure S1B and Figure S2). These analyses showed that Mcmdc2 is expressed primarily in the gonads and brain, but not the heart, lung, or mouse embryonic fibroblasts. These results are consistent with data from transcriptome datasets as mentioned in the Introduction. Real-time RT-qPCR analysis of multiple Mcmdc2 exons in homozygous mutant testes revealed that extremely low levels of messenger RNA, if any, remained, indicating that the allele is a null or extreme hypomorph (Figure S1C).
While all six genes encoding subunits of the MCM replicative helicase (Mcm2–7) are essential for viability, Mcm8 and Mcm9 are not (Hartford et al. 2011; Lutzmann et al. 2012; Luo and Schimenti 2015). To determine if Mcmdc2 is also essential, Mcmdc2Gt/+ heterozygotes were intercrossed and the offspring genotyped. Consistent with the limited range of expression, all genotypes were obtained in Mendelian ratios, indicating the gene is not required for viability (χ2 = 0.47; Table 1). Homozygotes appeared phenotypically normal, but both male and female animals were found to be sterile when bred to wild-type animals.
Table 1. Mcmdc2Gt/Gt mice are viable.
| +/+ | Mcmdc2Gt/+ | Mcmdc2Gt/Gt | |
|---|---|---|---|
| MALE | 17 | 36 | 21 |
| FEMALE | 14 | 41 | 20 |
| TOTAL | 31 | 77 | 41 |
Numbers were produced from an intercross of heterozygotes.
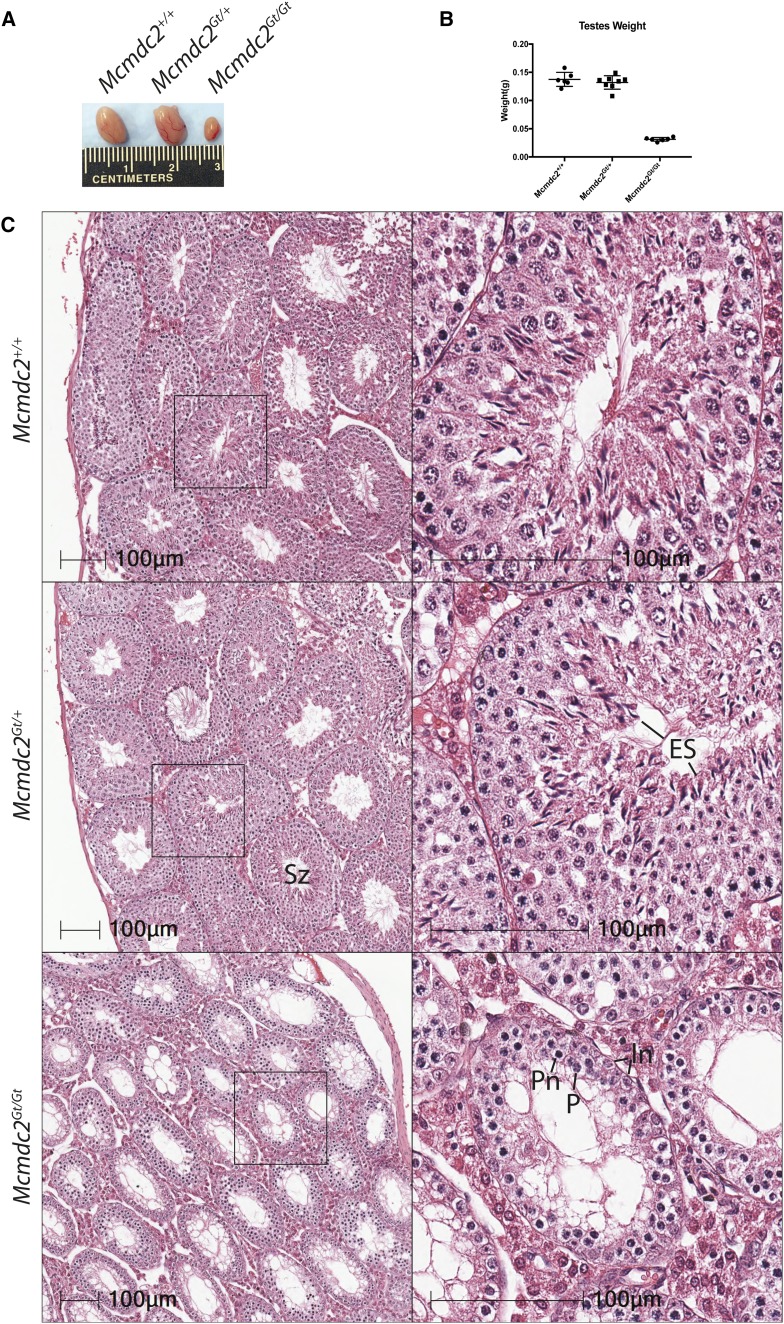
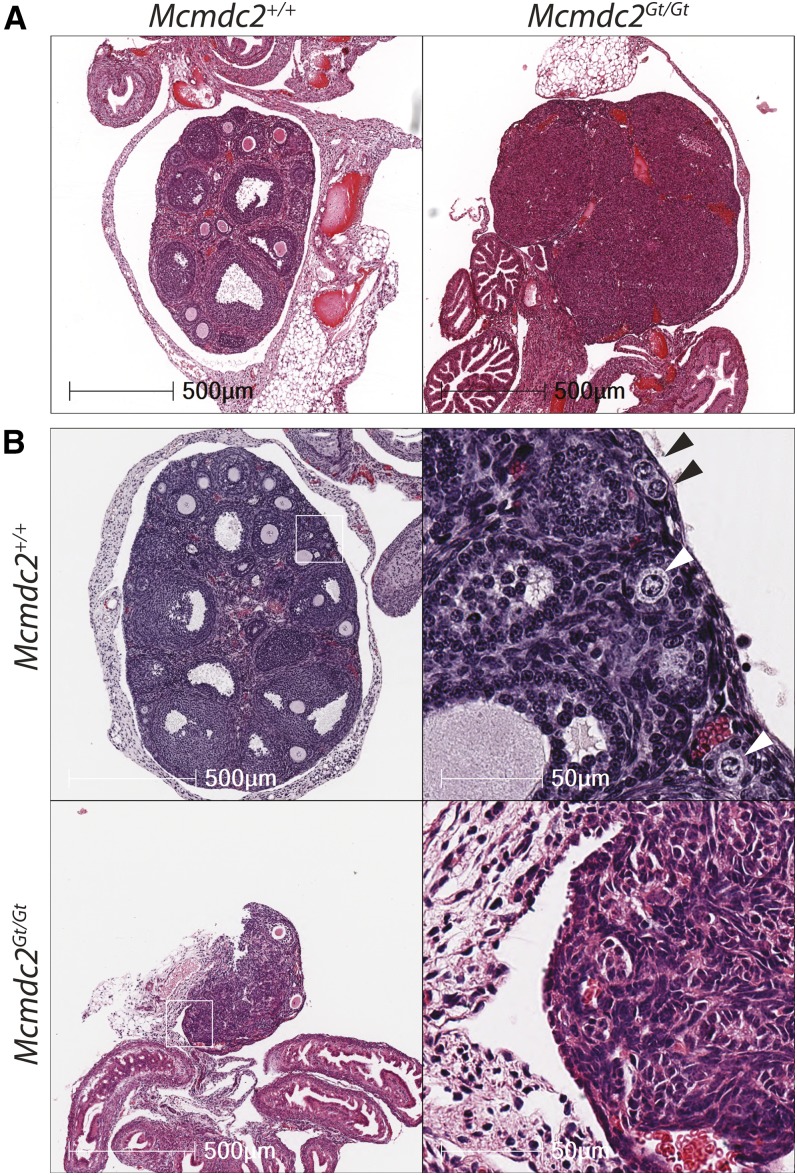
To identify the cause of sterility, we examined gonads of mutant mice. Individual testes isolated from 12-week-old males were much smaller than wild-type or heterozygous littermates of the same age (Figure 1, A and B), weighing ∼4 times less than those of littermates (0.031 ± 0.0013 g vs. 0.13 ± 0.005 g) (Figure 1B). Histological analyses of mutant testes showed a lack of postmeiotic round or elongated spermatids, resulting in seminiferous tubules having luminal areas devoid of normal germ cells compared to heterozygous or wild-type littermates (Figure 1C). Similar to most meiotic mutant mice defective in recombination or chromosome synapsis, the most advanced seminiferous tubules were in a developmental stage (stage IV of the seminiferous epithelium) containing spermatocytes in pachynema (Figure 1C) (Handel and Schimenti 2010). Histology of mutant ovaries revealed an absence of primordial or more advanced follicles in both adult and wean age (24 days postpartum) females (Figure 2, A and B). Therefore, disruption of Mcmdc2 causes catastrophic failure of gametogenesis in both sexes.
Figure 1.
Testes lacking Mcmdc2 are significantly smaller and lack postmeiotic spermatids. (A) Testes from 12-week-old littermates of the indicated genotypes. (B) Testis weights from Mcmdc2+/+(n = 3), Mcmdc2Gt/+(n = 4), and Mcmdc2Gt/Gt (n = 4) animals. (C) H&E of testes sections from 12-week-old animals of the indicated genotypes. Higher magnification of individual tubules is shown in the insets. The seminiferous tubule enlarged in the lower right panel is representative of the most developmentally advanced observed in mutant testes. As indicated by presence of intermediate spermatogonia and pachytene-like cells (some of which appear necrotic), this appears to be the equivalent of a stage IV tubule (Ahmed and de Rooij 2009). ES, elongated spermatids; In, intermediate spermatogonia; P, pachytene spermatocyte; Pn, likely a necrotic pachytene spermatocyte; Sz, spermatozoa.
Figure 2.
Mcmdc2Gt/Gt ovarian histology reveal lack of oocytes. (A) H&E staining of adult females demonstrates the absence of follicles (and thus, oocytes) in Mcmdc2Gt/Gt ovaries. (B) H&E staining of 24-day-old wild-type or Mcmdc2Gt/Gt females. Wild-type ovaries contain abundant primordial follicles (black arrows) and primary follicles (white arrows), while no primordial follicles are apparent in the Mcmdc2Gt/Gt ovaries. Mcmdc2Gt/Gt ovaries also appear smaller than wild type.
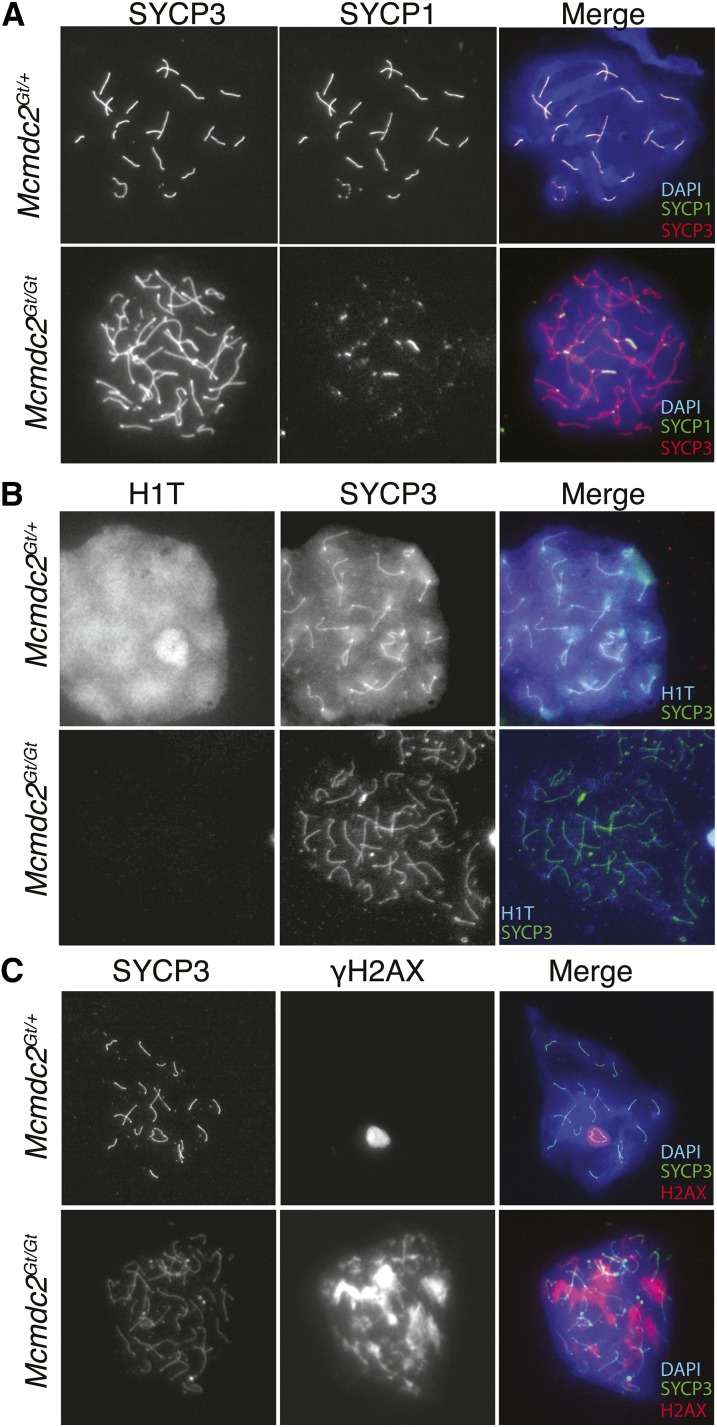
Given the apparent arrest of spermatogenesis in meiotic prophase I, we next evaluated meiotic progression by immunocytological analysis of meiotic chromosomes, using markers of developmental progression, homolog synapsis, and recombination. In wild-type mice aged 19 days postpartum, spermatocytes produced in the first wave of spermatogenesis would normally have progressed through pachynema, the stage at which homologous chromosomes are fully paired and synapsed. Indeed, wild-type testes contained pachytene spermatocytes exhibiting fully synapsed autosomes, as indicated by coincident staining of the synaptonemal complex (SC) axial element protein SYCP3 and SYCP1, an SC central element protein (Figure 3A). Additionally, progression of wild-type spermatocytes into pachynema was confirmed by the presence of diffuse nuclear H1T, a marker of midlate pachynema (Figure 3A) (Meistrich et al. 1985). In contrast, Mcmdc2Gt/Gt spermatocytes lacked H1T staining, indicating a block to developmental progression prior to midpachynema (Figure 3B). Consistent with the block in developmental progress, chromosomes of mutant spermatocytes remained in a zygotene-like state in that they remained unpaired (SYCP3-marked axial elements did not align with other axes of similar length) and unsynapsed, as indicated by lack of colinear SYCP1 staining along the length of chromosome cores (Figure 3A). Interestingly, meiotic spreads from mutant spermatocytes sometimes contained short stretches of SYCP1 staining, although it was uncertain if these patches of SC formation occurred between homologous or nonhomologous chromosomes. Nevertheless, the ability of mutant spermatocytes to form such stretches of SC indicates that MCMDC2 is not required for SC formation per se.
Figure 3.
Mcmdc2Gt/Gt spermatocytes are defective in homologous chromosomes synapsis and fail to progress through pachynema. Immunofluorescent images of meiotic chromosome spreads from Mcmdc2Gt/+ and Mcmdc2Gt/Gt spermatocytes were stained with the indicated antibodies. (A) Mcmdc2Gt/Gt chromosomes fail to pair or synapse along their lengths. Only short patches of SC (marked by SYCP1 labeling) are present in adult spermatocytes. Note: spermatocytes from 19-day-old mice completely lacked such SYCP1 labeling. (B) Mcmdc2Gt/Gt mutants fail to progress to midpachynema as evidenced by a lack of H1T staining and failure to undergo homologous chromosome pairing. The H1T signal exhibits bleed-through in the SYCP3 channel due to cross-reactivity between secondary antibodies. (C) Mcmdc2Gt/Gt mutants have abnormally distributed γH2AX, which marks both asynapsed chromosomes and unrepaired DSBs. Unlike heterozygotes, the mutants lack a discrete XY body that is marked by intense γH2AX.
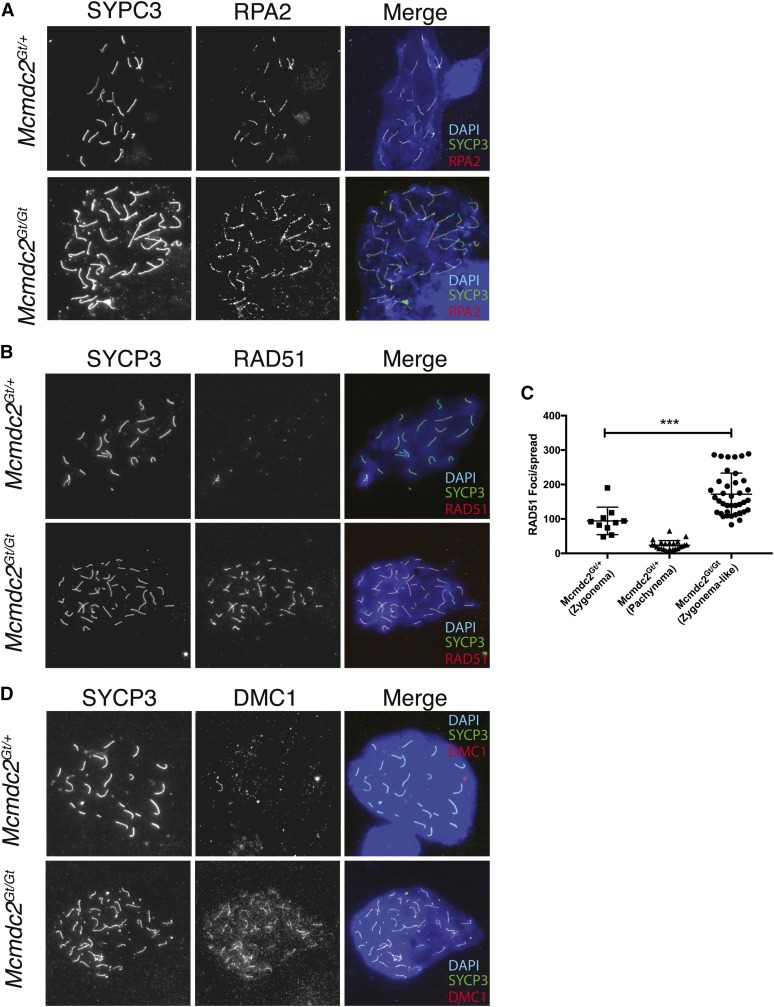
A common cause of failed homolog pairing and synapsis is defective recombination. Recombination is initiated in leptonema by the formation of several hundred DSBs by the SPO11 protein. The induced DSBs are then resected to yield single-stranded 3′ overhangs, which are bound by the single-stranded DNA (ssDNA)-binding trimeric protein complex RPA, which is hypothesized to aid in the processing and/or stabilization of the ssDNA ends and also D-loops of subsequent recombination intermediates (Luo et al. 2013; Ribeiro et al. 2016). Despite the synapsis defects, RPA2 decorated axial elements of zygotene-like mutant spermatocyte chromosomes in a pattern indistinguishable from wild-type, suggesting that meiotic DSBs were normally formed and resected in the absence of MCMDC2 (Figure 4A). Approximately contemporaneous to RPA loading (Ribeiro et al. 2016), the resected ends of DSBs are also bound by recA homologs RAD51 and DMC1, forming cytologically visible foci along SC cores (∼250 per leptotene nucleus) that can be visualized by immunolabeling (Moens et al. 1997). These nucleoprotein filaments drive interchromosomal recombination, which is necessary for homolog pairing and synapsis in mice. The RAD51/DMC1 foci gradually disappear throughout prophase I until DSB repair is complete in midpachynema, at which point autosomes are essentially devoid of foci (Moens et al. 1997). While Mcmdc2Gt/Gt spermatocytes were proficient in loading RAD51 and DMC1 along chromosome axes, they were abnormal in that these foci persisted at high levels (∼200 foci per zygotene-like nucleus), in contrast to heterozygous spermatocytes that exhibited the characteristic disappearance of foci from zygonema to pachynema (Figure 4, B–D).
Figure 4.
Mcmdc2Gt/Gt spermatocytes initiate, but do not complete, meiotic DSB repair. Immunofluorescent images of male meiotic spreads from 19-day-old Mcmdc2Gt/+ and Mcmdc2Gt/Gt animals were labeled with the indicated antibodies. (A) MCMDC2-deficient spermatocytes are proficient for RPA2 loading. The ssDNA-binding protein RPA2 typically localizes to resected DSBs or recombination intermediates on both unsynapsed and synapsed meiotic chromosomes. Shown is a representative control pachytene spermatocyte nucleus on top, and a mutant zygotene-like nucleus on the bottom, in which chromosome axes are decorated with RPA2 foci. (B) Mcmdc2Gt/Gt spermatocytes are defective in DSB repair as indicated by persistent RAD51 foci. The heterozygous nucleus shown in at the pachytene stage, and the mutant is in a zygotene-like stage. (C) Quantification of RAD51 in late zygotene-like Mcmdc2Gt/Gt spread preparations and Mcmdc2Gt/+ zygonema and pachynema spreads. Each data point represents a single meiotic spread. A minimum of three mice per genotype were used. Spreads were staged using SYCP3 for zygonema. Pachytene spreads (heterozygotes) were identified via H1T staining. ***P = 0.0005, unpaired t-test between Mcmdc2Gt/Gt and Mcmdc2Gt/+ zygotene spreads. (D) Mcmdc2Gt/Gt mutants exhibit persistent axial DMC1 foci in the most advanced zygotene-like nuclei. Under normal circumstances, DMC1 foci (like RAD51 foci) disappear from autosomal SCs by midlate pachynema (top). The patterns are representative of 36 Mcmdc2Gt/Gt mutant and 11 heterozygous spreads from three mice per genotype.
In mouse spermatocytes, meiotic prophase I arrest is linked to failure of meiotic sex chromosome inactivation. Normally, the X and Y chromosomes are transcriptionally silenced and sequestered in the XY body, a structure that is marked by concentrated phosphorylated histone H2AX (γH2AX) and DNA damage response proteins (Turner et al. 2004). Disruption of the XY body, which can occur by redirection of silencing machinery to unsynapsed autosomes in a process called meiotic silencing of unsynapsed chromatin (MSUC), allows expression of Y-linked genes that arrest spermatocyte development (Royo et al. 2010). Consistent with the observed lack of DSB repair and asynapsis, Mcmdc2Gt/Gt spermatocytes had abnormal XY bodies and exhibited abundant γH2AX staining over unsynapsed autosomes, which is indicative of both persistent DNA damage and MSUC (Figure 3C) (Turner et al. 2005). These results, along with the histological analysis, indicate MCMDC2 acts early in meiosis to facilitate DNA repair by homologous recombination, and its absence results in meiotic prophase I arrest.
In normal meiocytes, a subset of the meiotic DSBs (∼10%) are repaired and resolved as COs. In pachynema, CO sites are marked by MLH1, and normally there is at least one focus present per chromosome pair. In diplonema, the chromosomes undergo desynapsis and remain attached at CO sites, which are visible as chiasmata. As expected from the gross defect in DSB repair and global synapsis failure, no MLH1 foci were detectible in mutant spermatocytes as are present on heterozygous, synapsed pachytene chromosomes (Figure S3).
Our observations show that mouse MCMDC2 is required for both NCO and CO homologous recombination repair of SPO11-induced meiotic DSBs, and in the absence of recombination, synapsis fails to occur. Because RPA2, DMC1, and RAD51 loaded onto mutant meiotic chromosomes, we conclude that MCMDC2 functions after DSBs are resected (as indicated by binding of RPA) and subsequently recognized by the recA-related recombinases to form DMC1/RAD51 filaments, but that these filaments fail to invade homologous chromosomes in a manner that forms stable recombination intermediates that drive pairing. It is not known if MCMDC2 functions in conjunction with MCM8 (Drosophila rec ortholog), but interestingly, the meiotic phenotypes of Mcm8−/− mutant spermatocytes are quite similar to those of Mcmdc2Gt/Gt (Lutzmann et al. 2012). However, MCM8 has additional functions in S-phase and other tissues; in mitotically growing cells, MCM8 and MCM9 are needed to recruit RAD51 to DSBs and facilitate homologous recombination (Lutzmann et al. 2012; Nishimura et al. 2012; Park et al. 2013). Since MCMDC2 expression is almost exclusive to meiotic cells, any possible functional relationship with MCM8 would be unique to meiosis, and is unlikely related to recruiting RAD51 to SPO11-induced DSBs.
In addition to the similarity with Mcm8 mutants, Mcmdc2Gt/Gt resembles Msh4 and Msh5 knockout mice in that there is defective DSB repair and asynapsis despite loading of RAD51/DMC1 onto DSBs (Kneitz et al. 2000; Her et al. 2001; Reynolds et al. 2013). It is possible that MCMDC2 participates in promoting MSH4/5 function or other members of the ZMM complex that stabilize D-loops formed by invasion of single-stranded ends into homologous sequences (Manhart and Alani 2016). Such a scenario would link to the hypothesis that the Mei-MCM complex in Drosophila adapted to serve the function(s) of MSH4/5 (Kohl et al. 2012). The obvious phenotypic distinction between the mouse Mcm8, Msh4, Msh5, and Mcmdc2 mutants compared to Mei-MCM mutants in Drosophila is that in the latter, oocytes survive and can complete meiosis. This is because meiotic DSBs are repaired in the fly mutants, mainly by NCO recombination. Thus, unlike the situation in mutants deficient for recombinational repair proteins like the RAD51 ortholog, spn-A, activation of the DNA damage checkpoint in Mei-MCM mutants does not occur (Ghabrial and Schupbach 1999; Staeva-Vieira et al. 2003). The failure of mouse meiocytes to repair DSBs in the Mcm8, Msh4, Msh5, and Mcmdc2 mutants may be attributable to an inability to sufficiently stabilize recombination intermediates (following strand invasion) to a degree that would drive pairing and synapsis nucleation. Because interhomolog synapsis occurs independently of meiotic DSBs in flies, the SC may stabilize recombination intermediates sufficiently to permit resolution in the absence of Mei-MCMs; this may also account for why Drosophila does not require MSH4/5 in addition to Mei-MCMs. Alternatively, MCMDC2 may provide the equivalent of the role of fly SC in initial stabilization of recombination intermediates, and MSH4/5 subsequently cements stabilization of intermediates as SC forms in mice.
Currently, there are no proteins known to interact with mammalian MCMDC2 (according to Biogrid.org). The lack of a suitable available antibody precludes studies of its localization in meiocytes or the purification of meiotic complexes in which it may function. Given that MEI-217 interacts with REC (Kohl et al. 2012), an orthologous interaction between MCMDC2 and MCM8 might be expected. Such information will be useful for understanding the biochemical function of MCMDC2 in mice, and the similarities and distinctions compared to Mei-MCMs in Drosophila.
Finally, while this manuscript was under review, another group reported on the phenotype of Mcmdc2 mutant mice conditions (Finsterbusch et al. 2016). Overall, the results are highly congruent, indicating robustness of the phenotype under different breeding conditions and experimental conditions.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.196808/-/DC1.
Acknowledgments
The authors are grateful to R. Munroe and C. Abratte from Cornell’s transgenic facility for ES cell microinjections, and to P.J. Wang for the RPA2 antibody. We also thank J. Sekelsky for editorial and scientific feedback. This work was supported by National Institutes of Health grant GM-45415 to J.C.S. and contract CO29155 from the New York State Stem Cell Program (NYSTEM).
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Adams M. D., McVey M., Sekelsky J. J., 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. [DOI] [PubMed] [Google Scholar]
- Ahmed E. A., de Rooij D. G., 2009. Staging of mouse seminiferous tubule cross-sections. Methods Mol. Biol. 558: 263–277. [DOI] [PubMed] [Google Scholar]
- Blanton H. L., Radford S. J., McMahan S., Kearney H. M., Ibrahim J. G., et al. , 2005. REC, Drosophila MCM8, drives formation of meiotic crossovers. PLoS Genet. 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester N., Kuo F., Kozak C., O’Hara C. D., Leder P., 1998. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom’s syndrome gene. Genes Dev. 12: 3382–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester N., Babbe H., Pinkas J., Manning C., Leder P., 2006. Mutation of the murine Bloom’s syndrome gene produces global genome destabilization. Mol. Cell. Biol. 26: 6713–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muyt A., Jessop L., Kolar E., Sourirajan A., Chen J., et al. , 2012. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol. Cell 46: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterbusch F., Ravindranathan R., Dereli I., Stanzione M., Trankner D., et al. , 2016. Alignment of homologous chromosomes and effective repair of programmed DNA double-strand breaks during mouse meiosis require the minichromosome maintenance domain containing 2 (MCMDC2) protein. PLoS Genet. 12: e1006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial A., Schupbach T., 1999. Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nat. Cell Biol. 1: 354–357. [DOI] [PubMed] [Google Scholar]
- Goss K. H., Risinger M. A., Kordich J. J., Sanz M. M., Straughen J. E., et al. , 2002. Enhanced tumor formation in mice heterozygous for Blm mutation. Science 297: 2051–2053. [DOI] [PubMed] [Google Scholar]
- Grell R. F., 1984. Time of recombination in the DROSOPHILA MELANOGASTER Oocyte. III. Selection and characterization of temperature-sensitive and -insensitive, recombination-deficient alleles in Drosophila. Genetics 108: 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M. A., Schimenti J. C., 2010. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet. 11: 124–136. [DOI] [PubMed] [Google Scholar]
- Hartford S. A., Luo Y., Southard T. L., Min I. M., Lis J. T., et al. , 2011. Minichromosome maintenance helicase paralog MCM9 is dispensible for DNA replication but functions in germ-line stem cells and tumor suppression. Proc. Natl. Acad. Sci. USA 108: 17702–17707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her C., Wu X., Bailey S. M., Doggett N. A., 2001. Mouse MutS homolog 4 is predominantly expressed in testis and interacts with MutS homolog 5. Mamm. Genome 12: 73–76. [DOI] [PubMed] [Google Scholar]
- Holloway J. K., Morelli M. A., Borst P. L., Cohen P. E., 2010. Mammalian BLM helicase is critical for integrating multiple pathways of meiotic recombination. J. Cell Biol. 188: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop L., Rockmill B., Roeder G. S., Lichten M., 2006. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of sgs1. PLoS Genet. 2: e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitz B., Cohen P. E., Avdievich E., Zhu L., Kane M. F., et al. , 2000. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 14: 1085–1097. [PMC free article] [PubMed] [Google Scholar]
- Kohl K. P., Jones C. D., Sekelsky J., 2012. Evolution of an MCM complex in flies that promotes meiotic crossovers by blocking BLM helicase. Science 338: 1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Im J. S., Shibata E., Park J., Handa N., et al. , 2015. MCM8–9 complex promotes resection of double-strand break ends by MRE11–RAD50–NBS1 complex. Nat. Commun. 6: 7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Jang J. K., Graham J., Nycz K., McKim K. S., 2000. Two genes required for meiotic recombination in Drosophila are expressed from a dicistronic message. Genetics 154: 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Santoro I. M., McDaniel L. D., Nishijima I., Mills M., et al. , 2000. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 26: 424–429. [DOI] [PubMed] [Google Scholar]
- Luo M., Yang F., Leu N. A., Landaiche J., Handel M. A., et al. , 2013. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nat. Commun. 4: 2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Schimenti J. C., 2015. MCM9 deficiency delays primordial germ cell proliferation independent of the ATM pathway. Genesis 53: 678–684. [DOI] [PubMed] [Google Scholar]
- Lutzmann M., Grey C., Traver S., Ganier O., Maya-Mendoza A., et al. , 2012. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol. Cell 47: 523–534. [DOI] [PubMed] [Google Scholar]
- Manhart C. M., Alani E., 2016. Roles for mismatch repair family proteins in promoting meiotic crossing over. DNA Repair (Amst.) 38: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M., Andersen S. L., Broze Y., Sekelsky J., 2007. Multiple functions of Drosophila BLM helicase in maintenance of genome stability. Genetics 176: 1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich M. L., Bucci L. R., Trostle-Weige P. K., Brock W. A., 1985. Histone variants in rat spermatogonia and primary spermatocytes. Dev. Biol. 112: 230–240. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Chen D. J., Shen Z. Y., Kolas N., Tarsounas M., et al. , 1997. Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma 106: 207–215. [DOI] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vintersten K., Behringer R., 2007. Staining frozen mouse embryo sections for {beta}-galactosidase (lacZ), activity. CSH Protoc. 2007: pdb.prot4726. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Ishiai M., Horikawa K., Fukagawa T., Takata M., et al. , 2012. Mcm8 and Mcm9 form a complex that functions in homologous recombination repair induced by DNA interstrand crosslinks. Mol. Cell 47: 511–522. [DOI] [PubMed] [Google Scholar]
- Oh S. D., Lao J. P., Hwang P. Y., Taylor A. F., Smith G. R., et al. , 2007. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Long D. T., Lee K. Y., Abbas T., Shibata E., et al. , 2013. The MCM8–MCM9 complex promotes RAD51 recruitment at DNA damage sites to facilitate homologous recombination. Mol. Cell. Biol. 33: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A., Qiao H., Yang Y., Chen J. K., Jackson N., et al. , 2013. RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat. Genet. 45: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J., Abby E., Livera G., Martini E., 2016. RPA homologs and ssDNA processing during meiotic recombination. Chromosoma 125: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H., Polikiewicz G., Mahadevaiah S. K., Prosser H., Mitchell M., et al. , 2010. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr. Biol. 20: 2117–2123. [DOI] [PubMed] [Google Scholar]
- Sadate-Ngatchou P. I., Payne C. J., Dearth A. T., Braun R. E., 2008. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46: 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., et al. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva-Vieira E., Yoo S., Lehmann R., 2003. An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. EMBO J. 22: 5863–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver S., Coulombe P., Peiffer I., Hutchins J. R., Kitzmann M., et al. , 2015. MCM9 is required for mammalian DNA mismatch repair. Mol. Cell 59: 831–839. [DOI] [PubMed] [Google Scholar]
- Turner J. M., Aprelikova O., Xu X., Wang R., Kim S., et al. , 2004. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14: 2135–2142. [DOI] [PubMed] [Google Scholar]
- Turner J. M., Mahadevaiah S. K., Fernandez-Capetillo O., Nussenzweig A., Xu X., et al. , 2005. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37: 41–47. [DOI] [PubMed] [Google Scholar]
- Tye B. K., 1999. MCM proteins in DNA replication. Annu. Rev. Biochem. 68: 649–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Reagents available upon request.