Abstract
Prostate Stem Cells (PSCs) are characterized by their intrinsic resistance to Androgen Deprivation Therapy (ADT), possibly due to the lack of Androgen Receptor (AR) expression. PSCs resistance to ADT and PSC expansion in Castration Recurrent Prostate Cancer (CRPC) has sparked great interest in using differentiation therapy as an adjuvant to ADT. Understanding the mechanisms, by which PSCs maintain their undifferentiated phenotype, thus has important implications in differentiation therapy. In the prostate, the ATP Binding Cassette Sub-Family G Member 2 (ABCG2) transporters, which enrich for AR-positive, ADT-resistant PSCs, play an important role in regulating the intracellular androgen levels by effluxing androgens. We hypothesized that the ABCG2-mediated androgen efflux is responsible for maintaining PSCs in an undifferentiated state. Using the HPr-1-AR (non-tumorigenic) and CWR-R1 (tumorigenic) prostate cell lines, it was demonstrated that inhibiting the ABCG2-mediated androgen efflux, with Ko143 (ABCG2 inhibitor), increased the nuclear AR expression due to elevated intracellular androgen levels. Increased nuclear translocation of AR is followed by increased expression of AR regulated genes, a delayed cell growth response, and increased luminal differentiation. Furthermore, Ko143 reduced tumor growth rates in mice implanted with ABCG2-expressing CWR-R1 cells. Additionally, Ko143 treated mice had more differentiated tumors as evidenced by an increased percentage of CK8+/AR+ luminal cells and decreased percentage of ABCG2 expressing cells. Thus, inhibiting ABCG2-mediated androgen efflux forces the PSCs to undergo an AR-modulated differentiation to an ADT-sensitive luminal phenotype.
Implications
This study identifies the mechanism by which the prostate stem cell marker, ABCG2 plays a role in prostate stem cell maintenance and provides a rationale for targeting ABCG2 for differentiation therapy in prostate cancer.
Keywords: ABCG2, Prostate, Androgen Receptor, Androgens, Differentiation
Introduction
The ATP Binding Cassette Sub-Family G Member 2 (ABCG2) transporters belong to the ATP Binding Cassette (ABC) superfamily of transporters and use ATP hydrolysis to catalyze the transport of a wide range of substrates ranging from chemotherapeutic drugs, steroids and xenobiotics from the intracellular to the extracellular milieu. The side population assay is a flow cytometric method that isolates stem cells based on the dye-efflux properties of the ABC transporters and ABCG2 is the molecular determinant of the stem cell enriched side population phenotype in the hematopoietic, retinal, cardiac, limbal and skeletal muscle system [1–5]. The importance of ABCG2 to stem cell maintenance can be adjudged from the observations that ABCG2 expression is lost as stem cells undergo differentiation [1, 2, 6]. In fact, overexpression of ABCG2 has been shown to promote stemness while the loss of ABCG2 expression has been shown to promote lineage commitment in the retinal, hematopoietic and cardiac side population cells [1, 2, 7]. While the importance of ABCG2 in stem cell maintenance has been shown, the precise mechanism by which ABCG2 prevents stem cells from undergoing differentiation is unknown.
An ABCG2-expressing side population is present in normal and cancerous prostate tissues and cell lines [8]. ABCG2 expression is found in ~1% of the cells in the basal compartment [9, 10]. ABCG2-expressing cells are present in biopsies following androgen deprivation therapy [9] and upregulated upon androgen blockade in culture [9, 11]. Although, there are reports of ABCG2 effluxing bicalutamide [12], there have been no studies that show Enzalutamide efflux by ABCG2. Moreover, ABCG2-expressing side population in the prostate demonstrates multipotency and self-renewal properties, thus suggesting an enrichment of stem cells in this population [13]. Transcriptome analysis of ABCG2-expressing prostate cells confirmed the expression of stem cell associated genes such as CD133, Oct4 and nestin and the lack of Androgen Receptor (AR) expression which is typical of Prostate Stem Cells (PSCs) [9, 10, 14]. Although, ABCG2 transporters are suspected to play a role in maintaining the prostate stem cell phenotype, the mechanistic significance of ABCG2 to stem cell maintenance is largely unknown. Elucidating the mechanisms by which PSCs maintain their stemness will provide opportunities to target them in prostate cancer therapy. PSCs are implicated in the etiology of Castration Resistant Prostate Cancer (CRPC) and are resistant to Androgen Deprivation Therapy (ADT) [15]. In fact, ABCG2-expressing prostate cells have been shown to not only survive ADT but are amplified in CRPC [9, 11], thus suggesting a role for the transporters in conferring ADT-resistance to PSCs and in the etiology of CRPC, in addition to stem cell maintenance.
Previous studies showed that Mitoxantrone-resistant Rat Progenitor Epithelial (Mx-RPE) cells, which have been selected for based on high ABCG2 expression, had elevated retention of radiolabeled DHT when treated with the ABCG2 inhibitors, Novobiocin and Fumitremorgin C, thus, suggesting that ABCG2 can regulate androgen levels [9]. Several studies have demonstrated that the exogenous introduction of AR in prostate cells that either lack or have very low levels of endogenous AR such as PC3, HPr-1 and PSCs forces cells to undergo differentiation to a luminal phenotype [14, 16, 17]. Thus, we hypothesized that ABCG2 plays a critical role in maintaining PSCs in an undifferentiated state via the efflux of androgens and inhibiting the ABCG2-mediated androgen efflux would force the ABCG2-expressing PSCs to undergo an AR-modulated differentiation to a luminal phenotype. In the present study, we investigate the effects of pharmacological inhibition of ABCG2 on cellular differentiation in the ABCG2-expressing HPr-1-AR (non-tumorigenic prostate epithelial cell line) and the CWR-R1 (tumorigenic CRPC cell line derived from CWR22 xenografts) cells by sphere-formation assays and immunoblots. In vivo differentiation is assessed by treating nude mice implanted with ABCG2-expressing CWR-R1 cells with either placebo or Ko143. Inducing luminal differentiation in PSCs and sensitizing them to ADT is the basis of differentiation therapy. Our findings provide a rationale for targeting ABCG2 for differentiation therapy in prostate cancer.
Materials and Methods
Cell Culture and Treatments
The HPr-1-AR cells were obtained from Dr. Dominic Smiraglia and cultured in Keratinocyte-Serum Free Media (ThermoFisher Scientific) supplemented with 1X Penicillin-Streptomycin. CWR-R1 cells were obtained from Dr. James Mohler and were cultured in Improved MEM supplemented with 2% Fetal Bovine Serum (FBS), 1X Penicillin-Streptomycin, 0.2X Insulin-Transferrin-Selenium (ITS) (Life Technologies), 0.9 mg/L linoleic acid (Sigma-Aldrich) and 100ug/ml EGF (ThermoFisher Scientific). Our preliminary data shows that prolonged charcoal stripping leads to the downregulation of ABCG2 membrane expression in Mitoxantrone-resistant CWR-R1 cells (data not shown). Thus, the ABCG2-expressing CWR-R1 cells were cultured in FBS-supplemented media. All cells were maintained in an atmosphere of 5% CO2 at 37°C. All experiments were done before cells reached passage 65. Treatments were administered in the appropriate media such that DHT (Sigma) was used at a concentration of 0.1nM for the HPr-1-AR cells and 1nM for the CWR-R1 cells. Ko143 (Axon chemicals) and Enzalutamide (Selleck Chemicals) were administered at a concentration of 1μM each.
Magnetic Isolation of ABCG2+ cells
Cells were grown to 75% confluency and the HumanSep FITC Selection Kit (Stem Cell Technologies) was used in conjunction with the Anti-BCRP antibody, clone 5D3, FITC-conjugated (Millipore) to isolate the ABCG2-expressing cells as per the manufacturer’s protocol.
Immunofluorescence Microscopy
Cells were seeded onto coverslips, fixed with 10% formalin and permeabilized with 0.1% Triton X. Cells were blocked with 1% BSA and immunostained with the anti-AR antibody (1:200, Millipore). The Goat anti-Rabbit Antibody, Alexa Fluor 594 conjugate (1:1000, Invitrogen) was used as a secondary antibody. Coverslips were mounted onto slides with Vectashield mounting medium containing DAPI (Vector Laboratories) for analysis under an immunofluorescent microscope.
Thin Layer Chromatography (TLC)
Intracellular androgens from cells treated with [3H]DHT (Perkin Elmer) were extracted using Methy Tert Butyl Ether (Sigma-Aldrich) and reconstituted in a mixture of 9:1 chloroform:acetone. The reconstituted mixture along with steroid standards (Steraloids, Inc) were loaded onto 20cm × 20cm silica coated TLC plates (Millipore). The 9:1 chloroform:acetone mixture was used as the mobile phase to separate the steroids. The standards were visualized on the TLC plate by spraying the plate with p-Anisaldehyde stain. Zones corresponding to the reference steroid standards were scraped into vials containing the BetaMax Scintillation Cocktail (MP Biomedicals) and tritium levels were measured using a liquid scintillation counter.
Western Blotting
RIPA buffer (Invitrogen) was used to prepare cell lysates and protein concentration was measured by the BCA Protein Assay Kit (Thermo Scientific). Cell lysates (20μg) were loaded in the wells of Novex NuPAGE 4–12% Bis-Tris Gels (Thermo Scientific). Electrophoresed proteins from gels were transferred onto PVDF membranes. Membranes were blocked and stained with Anti-AR (1:250, Millipore), Anti-Sox2 (1μg/mL, Millipore), p21 (1:200, C-19) (Santa Cruz), PSA (1:500, Dako), Cytokeratin-8 (1:500, Covance) and Beta-actin (1:10,000, BioVision Inc.) antibodies and counterstained with the appropriate horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Signal from the secondary antibodies was detected using an enhanced chemiluminescence detection system (Pierce).
Sphere-Formation Assay
Cells were plated at a density of 2000 cells/well or 4000 cells/well (after xenograft tumor digestion) in 24-well ultralow attachment plates (Sigma-Aldrich) coated with matrigel (Fisher Scientific). Cells in media were suspended in matrigel at a ratio of 2:3 (cells in media volume: matrigel volume) and this suspension was plated onto the edges of the wells of the plates and allowed to solidify at 37°C for 45 minutes. Upon solidification, treatments were administered and the number and sizes of spheres were quantitated 10–14 days later. Sphere area was measured using the Basic SPOT imaging software. The CWR-R1 spheres were maintained in Improved MEM, original growth media, whereas the HPr-1-AR spheres were cultured in Prostate Epithelial Cell Growth Medium (Lonza).
RNA isolation and Quantitative Real Time PCR (qRT-PCR)
RNA was isolated using the RNeasy Mini Kit (Qiagen) and cDNA was synthesized using the SuperScript III Reverse Transcriptase Kit (Invitrogen) and oligodT primers. Gene expression was determined using SYBR Green PCR mix (Bio-Rad). Primer sequences are enlisted in supplementary Table 1. qRT- PCR was performed on the Applied Biosystems 7300 machine. The following conditions were used to carry out the RT-PCR reactions: 300 seconds at 95°C; 50 cycles of 10 seconds at 95°C; 20 seconds at 60°C and 20 seconds at 72°C.
In vivo Tumor Xenograft Studies
Twenty-six male nude mice (Harlan Sprague Dawley Inc.) aged 4–5 weeks were castrated and implanted with a testosterone silastic tubing to normalize the serum testosterone levels to 4ng/ml. Mice were subcutaneously injected with 1×106 ABCG2-expressing CWR-R1 cells suspended in matrigel (1:1 ratio). Seven days post inoculation; mice were subdivided into two groups of 13 mice each. The first group of mice received placebo while the second group of mice received 15mg/kg of Ko143 by oral gavage. Treatments were administered three times a week and tumors were measured at the time of drug delivery. Tumor volume was measured by digital calipers and calculated using the formula L1×(L2)2×0.523. Once tumors reached 1500mm3 volume, mice were euthanized and tumors were excised for further analysis.
Statistical Analysis
Three mice from each group with multiple tumors originating from the same injection site or whose tumors failed to reach a volume of 250mm3 on day 45 of cell inoculation were excluded from the study. Linear Mixed Model analysis was used to compute differences in the tumor growth rates between control and Ko143 treated animals. Data obtained from in vitro experiments was analyzed either by One-way or Two-way ANOVA tests. All data analyses were generated using SAS/STAT software, Version 9.4.
Immunohistochemistry
Immunohistochemistry was done on one section of the paraffin embedded tumors. Tissue specimens were immunostained with primary antibodies: anti-Cytokeratin-8 (1:1000, Covance), anti-BCRP antibody, clone BXP-21 (1:500, Millipore) and anti-AR antibody (1:200, Millipore). Biotinylated Horse Anti-Mouse IgG antibody (1:1000, Dako) and biotinylated Goat Anti-Rabbit (1:1000, Abcam) were used as the secondary antibodies as appropriate. Vectastain Elite ABC HRP kit (Vector Laboratories) was used to visualize the immunostained tissue sections. Staining was performed on Dako Omni. Images were scanned using the Aperio scanner.
Results
Inhibiting ABCG2-mediated Androgen Efflux Increases Nuclear AR Expression
Based on previous studies showing the increased retention of radiolabeled DHT in Mx-RPE cells upon incubation with Novobiocin and Fumitremorgin C [9], we propose that inhibiting ABCG2 in the presence of DHT would increase the nuclear AR expression due to increased intracellular retention of androgens. Studies were conducted in the HPr-1-AR and the CWR-R1 cell lines. The HPr-1-AR and CWR-R1 cell lines were chosen due to the presence of functional ABCG2 in a subpopulation and an androgen-responsive phenotype. Additionally, the HPr-1-AR and the CWR-R1 cells offer comparison between non-tumorigenic and tumorigenic cells respectively. Optimal DHT concentrations for treatment were determined by assessing growth curves of the unsorted HPr-1-AR and CWR-R1 cells in response to DHT (Supplementary Figure 1 a,b) and TLC analysis of the intracellular androgens extracted from unsorted HPr-1-AR and CWR-R1 cells treated with increasing concentrations of [3H]DHT (Supplementary Figure 1 c,d). TLC analysis was performed to determine if DHT is metabolized by hydroxysteroid dehydrogenase or aldo-keto reductase expressed in prostate cells. Based on these studies a concentration of 0.1nM DHT was used for the HPr-1-AR cells (Supplementary Figure 1 a,c) and 1nM DHT was used for the CWR-R1 cells (Supplementary Figure 1 b,d) in all further experiments unless otherwise mentioned since these were the lowest concentrations of DHT at which there was both, a retardation in the growth response as well as an observable change in the amount of intracellular androgens. Using DHT concentrations greater than the ones used would overwhelm the ABCG2 transporters and lower concentrations would be undetectable.
To determine the effects of inhibiting the ABCG2-mediated androgen efflux on the expression and cellular localization of AR, ABCG2-expressing HPr-1-AR and CWR-R1 cells were treated with the vehicle control, DHT, Ko143 or a combination of DHT and Ko143 and subjected to immunofluorescent staining. ABCG2 negative cells treated with DHT served as the positive control. In our study, ABCG2 was only pharmacologically inhibited as ~1% of the cell population express ABCG2, thus genetic silencing of ABCG2 expression in such a small population is technically challenging due to low cell yield and genetic silencing of ABCG2 expression in a mixed population of cells would lead to undetectable changes.
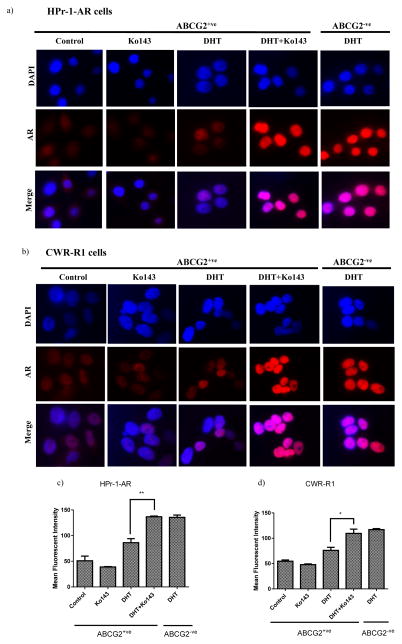
Our results indicate that in both, the HPr-1-AR and the CWR-R1 cells, a combination of DHT and Ko143, significantly increased the nuclear AR expression; the nuclear AR signal was comparable to the ABCG2 negative cells treated with DHT (Figure 1). The vehicle control and Ko143 treated ABCG2-expressing HPr-1-AR cells showed the presence of cytoplasmic AR due to the transfected AR vector (Figure 1a). Upon addition of DHT, cytoplasmic AR translocated to the nucleus, however the nuclear AR signal was very weak (Figure 1 a,c). The control and Ko143 treated ABCG2-expressing CWR-R1 cells showed very weak AR signal, which is in agreement with previous studies that have demonstrated undetectable AR expression in PSCs [10, 14]. The addition of DHT to the ABCG2-expressing CWR-R1 cells slightly increased the nuclear AR expression compared to the control and Ko143 treated cells, however a combination of DHT and Ko143, led to a significant increase in the nuclear AR expression compared to DHT alone (Figure 1 b,d). To confirm that the increased nuclear AR expression upon treatment with a combination of DHT and Ko143 is due to increased intracellular androgen levels, the efflux of androgens by ABCG2 and the consequent increase in the intracellular androgen levels upon inhibiting the ABCG2 transporters was confirmed in the steroidogenic, HPr-1-AR and CWR-R1 cells and the non-steroidogenic Hek293 cells stably transfected with pcDNA3.1ABCG2 vector (Supplementary Figure 2). Thus, our results indicate that inhibiting the ABCG2-mediated androgen efflux increases the nuclear translocation of AR.
Figure 1. Inhibiting the ABCG2-mediated androgen efflux leads to the nuclear translocation of AR.
ABCG2-expressing (ABCG2+ve) (a) HPr-1-AR and (b) CWR-R1 cells obtained by magnetic sorting were treated with either vehicle control, 1μM Ko143, DHT or DHT+ 1μM Ko143 for 24 hours. ABCG2-negative (ABCG2−ve) cells treated with DHT were used as the positive control. Nuclear AR translocation was determined by IF staining. Figures c & d represent the quantitative representation of IF data in the HPr-1-AR and CWR-R1 cells respectively. The mean red intensity in the nucleus of individual cells from 11–15 different fields was computed using Image J. Experiments were done three times and statistical analysis was done using the one-way ANOVA test. (p*<0.05; p**<0.01)
AR nuclear translocation, with ABCG2 inhibition, upregulates expression of AR target genes
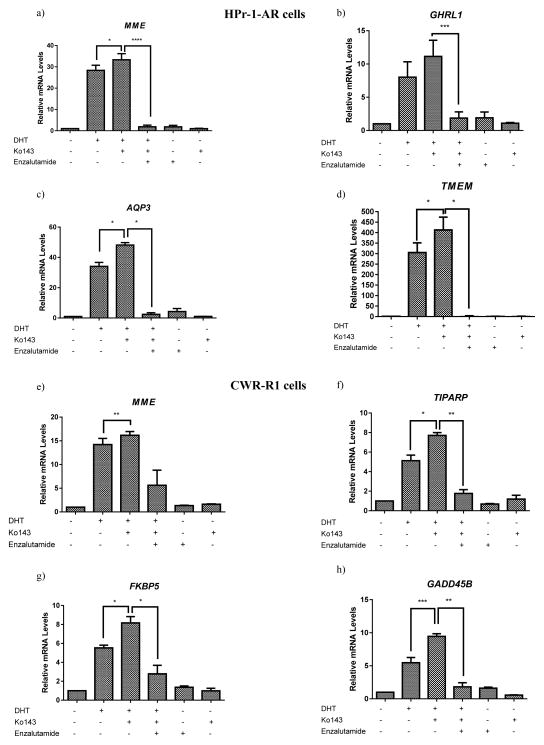
To determine if elevated nuclear AR expression leads to increased AR transcriptional activity, the expression of AR target genes was quantitated by qRT-PCR. To determine the change in the expression of AR target genes upon inhibiting the ABCG2-mediated androgen efflux, ABCG2-expressing HPr-1-AR and CWR-R-1 cells were incubated with either the vehicle control, DHT, Ko143 or DHT in combination with Ko143 for 24 hours. The AR antagonist, Enzalutamide, was added to evaluate the role of AR in the upregulation of AR target genes. Expression of AR target genes was analyzed by qRT-PCR. Different genes were quantitated in the HPr-1-AR (MME, GHRL1, AQP3 and TMEM) and the CWR-R1 cells (TIPARP, MME, FKBP5 and GADD45B) since AR targets different sets of genes in the two cell lines [18, 19]. qRT-PCR analysis revealed that both cell lines treated with a combination of DHT and Ko143 showed a significant increase in the expression of AR target genes compared to cells treated with the vehicle control and DHT (Figure 2) and that Enzalutamide antagonized the ABCG2-mediated upregulation of AR target genes (Figure 2). Although the AR target gene expression showed a maximal increase upon treatment with a combination of DHT and Ko143, we observed an upregulation of AR target genes upon treatment with DHT alone (Figure 2); this could be due to the incomplete efflux of DHT by ABCG2 due to saturation of the transporters with DHT. Additionally, we did not observe any change/downregulation in the AR target gene expression upon treatment with Enzalutamide (Figure 2), possibly due to the low levels of nuclear AR in the ABCG2-expressing cells (Figure 1). Our results confirm that inhibiting the ABCG2-mediated androgen efflux leads to an upregulation of AR target genes and suppression of AR target gene upregulation by Enzalutamide confirms the direct role of AR in upregulating the AR target genes when ABCG2-mediated androgen efflux is inhibited.
Figure 2. Inhibiting the ABCG2-mediated androgen efflux increases the expression of AR target genes.
ABCG2-expressing (a–d) HPr-1-AR and (e–h) CWR-R1 cells obtained by magnetic sorting were treated with either vehicle control, DHT, DHT+ 1μM Ko143 or 1μM Ko143 for 24 hours. Enzalutamide at 1μM concentration was added to confirm the role of AR. m-RNA was extracted and reverse transcribed to cDNA. The expression of AR target genes in both the cell lines was evaluated by qRT- PCR. All experiments were done three times in triplicates and statistical analysis was done using the two-way ANOVA test. (p*<0.05; p**<0.01; ***p<0.001; ****p<0.0001).
Inhibiting the ABCG2-mediated androgen efflux leads to a delayed cell growth response and cell differentiation mediated by AR
Exogenous introduction of AR in cells that lack endogenous AR leads to a delayed cell growth response and cell differentiation [14, 16, 17]. Thus, we propose that increased nuclear AR expression in the HPr-1-AR and the CWR-R1 cells, upon inhibiting the ABCG2-mediated androgen efflux, would lead to a delayed cell growth response due to increased luminal differentiation of cells. Cell growth response was assessed in the ABCG2-expressing HPr-1-AR and CWR-R1 cells treated with increasing concentrations of DHT with/without Ko143, by the sphere-forming assay, which serves as a direct indicator of cell growth response of a single sphere-forming cell in a 3D setting. Additionally, the sphere-forming assay can also serve as an indicator of the relative number of stem and differentiated cells in tumor samples. While the proliferative stem cells possess the ability to multiply and form spheres, the differentiated cells are unable to grow as spheres. The sphere forming efficiency was computed by measuring the sphere sizes and assessing sphere number. Sphere number serves as a direct indicator of the proliferative potential of cells capable of forming spheres whereas a change in sphere size indicates the eventual loss/gain of proliferative potential thus leading to smaller/larger spheres respectively. We observed that in both, the HPr-1-AR and the CWR-R1 cells, inhibiting the ABCG2-mediated androgen efflux, significantly impaired the sphere formation efficiency as evidenced by a decrease in sphere size and sphere number (Figure 3 a,b,e,f)). Enzalutamide rescued the impaired sphere formation efficiency of the ABCG2-expressing HPr-1-AR and CWR-R1 cells, thus confirming that the delayed cell growth response upon inhibiting the ABCG2-mediated androgen efflux is modulated by AR (Figure 3 c,d,g,h).
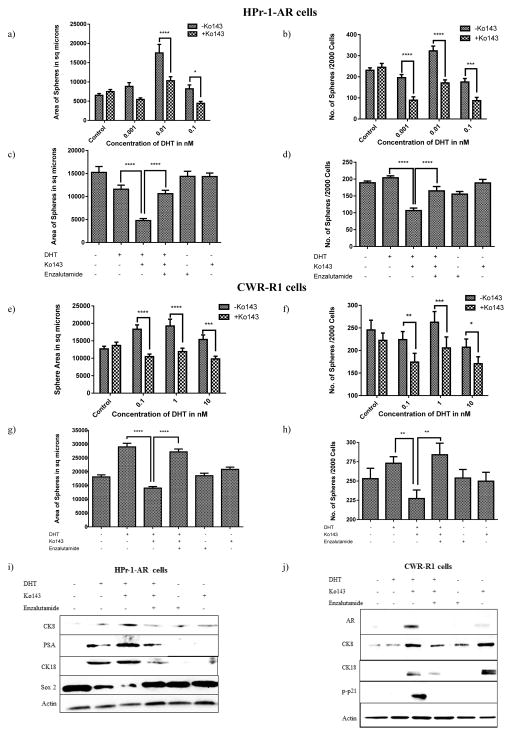
Figure 3. Inhibiting the androgen efflux by ABCG2, leads to an AR-modulated delayed cell growth response and cell differentiation to a luminal phenotype.
ABCG2-expressing (a–d) HPr-1-AR and (e–h) CWR-R1 cells were plated onto 24 well ultralow adhesion plates coated with matrigel at a density of 2000 cells/well for Sphere-Forming Assay. Cells were treated either with different combinations of DHT, Ko143 and Enzalutamide. (a, c, e, g) Sphere sizes were measured and (b, d, f, h) spheres were counted between 10–14 days. Experiments were done three times and spheres from 4–6 wells were counted each time. Statistical analysis was done using the two-way ANOVA test (p**<0.01; ***p<0.001; ****p<0.0001). (i–j) Cell differentiation was assessed by subjecting the cell lysates obtained from DHT, Ko143 and Enzalutamide treated cells to immunoblot analysis and determining the expression levels of luminal markers.
Previous studies have shown that the exogenous introduction of AR in PSCs drive differentiation to a luminal phenotype [16]. To investigate whether inhibiting the ABCG2-mediated androgen efflux leads to an AR-modulated cell differentiation, ABCG2-expressing cells, treated with different combinations of DHT, Ko143 and Enzalutamide for 5 days were analyzed for the expression of differentiation and stem cell markers. We observed that ABCG2-expressing cells treated with the vehicle control lacked the expression of prostate luminal markers. However, DHT in combination with Ko143, increased the expression levels of the prostate luminal markers, CK8 and CK18 in both the cell lines (Figure 3 i,j). In the ABCG2-expressing HPr-1-AR cells, treatment with a combination of DHT and Ko143 led to an upregulation of PSA and the downregulation of Sox2 (stem cell marker) (Figure 3i), whereas in the ABCG2-expressing CWR-R1 cells, treatment with a combination of DHT and Ko143 led to an increase in AR and phosphorylated p21 (cell cycle arrest marker) expression (Figure 3j). Different markers were analyzed in both the cell lines since a) we could not detect phosphorylated p21 in the ABCG2-expressing HPr-1-AR cells and Sox2 in the ABCG2-expressing CWR-R1 cells (Data not shown) b) In the CWR-R1 cells, PSA expression is not altered by DHT [19] and c) HPr-1-AR cells have a stably transfected AR vector, such that AR is cytosolic in the absence of androgens and nuclear upon treatment with androgens; Since the analysis was done on whole cell lysates, the intracellular AR expression in the ABCG2-expressing HPr-1AR cells would remain unchanged. Of note, the CWR-R1 cells are known to have both, mutated AR as well as AR variants (Supplementary Figure 3b) [20]. However, we did not observe AR variants in the ABCG2-expressing cells upon treatment with DHT and Ko143 (Figure 3j). The reason for this observation could be that AR variants are generated in response to ADT; however, PSCs are intrinsically resistant to ADT [21, 22].
Interestingly, high levels of CK8 and CK18 were also observed in the Ko143 treated ABCG2-expressing CWR-R1 cells (Figure 3j). However, the Ko143 treated CWR-R1 cells showed the absence of phosphorylated p21 expression (Figure 3j). The ABCG2-expressing CWR-R1 cells were cultured in FBS-supplemented media because growth in charcoal stripped FBS-supplemented media leads to the downregulation of ABCG2-expressing CWR-R1 cells. The HPr-1-AR cells are routinely cultured in a serum-free media, thus obviating the need for charcoal stripped serum. Serum contains a wide range of steroids such as testosterone, progesterone and cholesterol. Furthermore, prostate cells can synthesize DHT from the steroids through the intact, cholesterol and the backdoor pathway [23]. Pump inhibition with Ko143 could have trapped the steroidal androgens from the serum in the cells thus eliciting a weak AR response. Thus, inhibiting ABCG2, albeit in the absence of exogenous DHT, was sufficient to induce a very weak AR expression and consequently upregulate CK8 and CK18 but not p-p21 (Figure 3j). The immunoblot results suggest that steroidal androgens from serum can partially induce the expression of some luminal differentiation markers but not induce cell growth arrest. These results are in accordance with the observations from the sphere-forming assay where Ko143 treated ABCG2-expressing CWR-R1 cells showed no change in the sphere formation efficiency compared to cells treated with the vehicle control (Figure 3 e,f). The role of AR in the forced differentiation of ABCG2-expressing cells was confirmed by Enzalutamide (Figure 3 i,j). Thus, our results suggest that inhibiting the ABCG2-mediated androgen efflux forces prostate cells to undergo AR regulated luminal differentiation.
Inhibiting ABCG2 leads to delayed tumor progression and increased overall survival in vivo
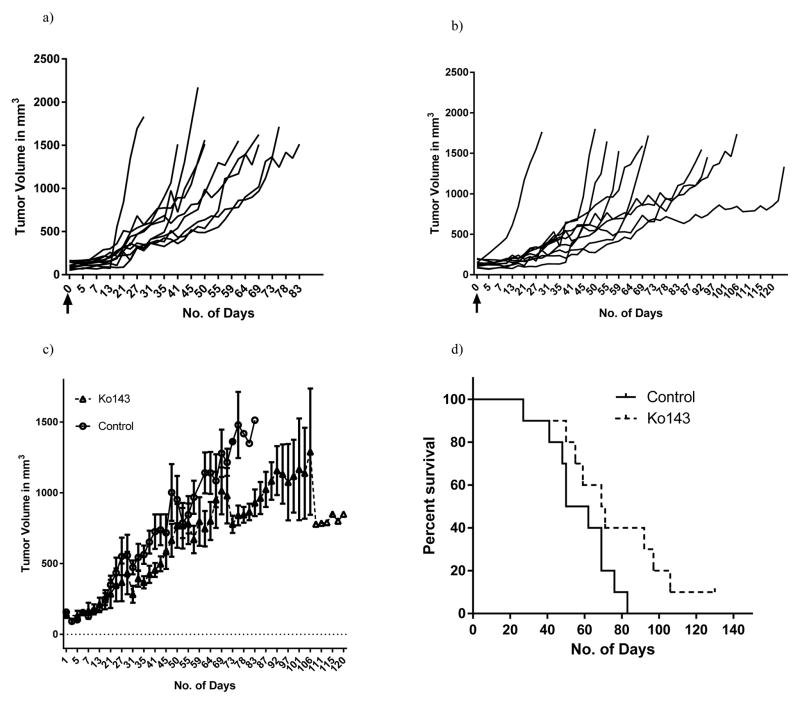
Based on our in vitro observations, we proposed that inhibiting ABCG2 in vivo, would slow tumor progression and increase the overall survival in mice. To determine whether Ko143 slows tumor progression, tumor volume was monitored in testosterone-supplemented nude mice subcutaneously implanted with ABCG2-expressing CWR-R1 cells treated with either placebo or 15mg/Kg Ko143. Survival was computed by the time it took for tumors to reach a volume of 1500mm3. Our results show that mice treated with Ko143 showed significantly slower tumor growth rates. Linear Mixed Model analysis that took the individual tumor volume trajectories into account revealed statistically significant differences between the two cohorts (p<0.01) (Figure 4 a,b,c).
Figure 4. Inhibiting the ABCG2 transporters by Ko143 slows tumor progression and increases the overall survival in vivo.
Tumor trajectories of individual mice in the (a) control (n=10) and (b) Ko143 treated group (n=10) respectively. The black arrows represent day 0, when the treatments were started. Figure (c) depicts the average tumor growth rate of mice in the control and Ko143 treated group. Statistical analysis was done using the Linear mixed modelling. Figure (d) represents the overall survival of mice in both the groups as computed by the time taken for the tumors to reach a volume of 1500mm3. Difference in the slopes of placebo and Ko143 treated animals was computed using the Log-rank Test.
In addition to slowing down tumor progression, mice treated with Ko143 demonstrated a trend for increased survival. The median survival in the control group was 56 days as opposed to 70 days in the treated cohort. We observed a strong tendency of Ko143 to increase the overall survival; however the log-rank analysis revealed that the differences were not statistically significant (Figure 4d).
Ko143 disrupts the sphere forming ability of cells isolated from tumor xenografts
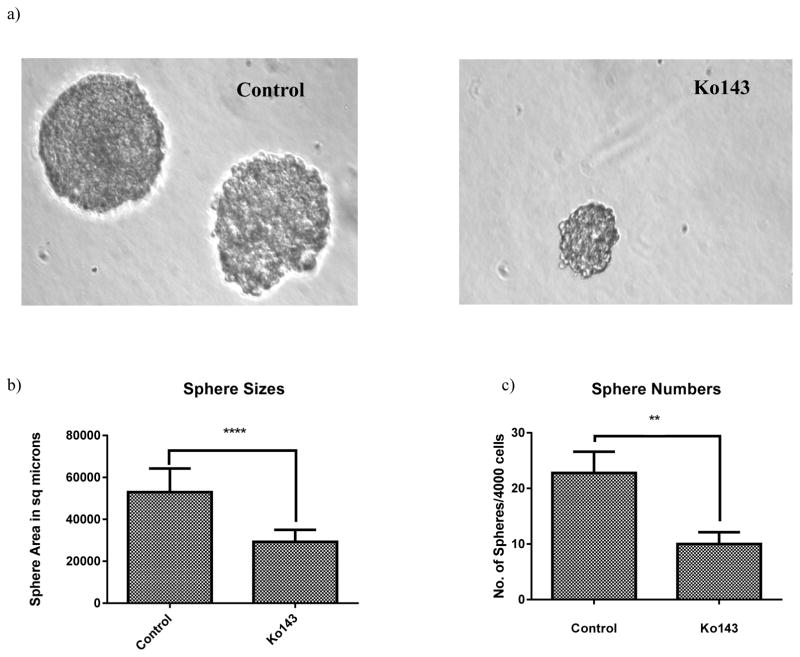
Results from our in vivo data show that Ko143 slows the tumor growth rate. Slower tumor growth rates are a direct indicator of impaired cellular proliferation. To confirm the results of our in vivo observation that Ko143 leads to a delayed cell response, we isolated cells from the tumors of mice by protease digestion and subjected the isolated cells to sphere-formation assay. Sphere-formation assay revealed that cells isolated from tumors of Ko143 treated group formed significantly smaller and fewer spheres (Figure 5). Based on our sphere formation data, we can infer that the tumors of mice treated with Ko143 had a significantly higher percentage of differentiated cells, which are unable to grow into spheres whereas tumors of mice in the placebo group had comparatively greater number of stem cells.
Figure 5. Cells isolated from tumors of mice in the treated group showed impaired sphere formation.
Cells were isolated from tumors of mice in the control (n=3) and Ko143 group (n=3) by protease digestion and plated in 24 well ultralow attachment plates coated with matrigel at a density of 4000 cells/well. On day 14, spheres were (a) imaged under 20X magnification, (b) sphere sizes were measured by the basic SPOT software and (c) spheres were counted. The student’s t test was used for statistical analysis (p**<0.01; ****p<0.0001).
Mice treated with Ko143 have a higher percentage of luminal cells and a lower percentage of ABCG2-expressing cells in tumors
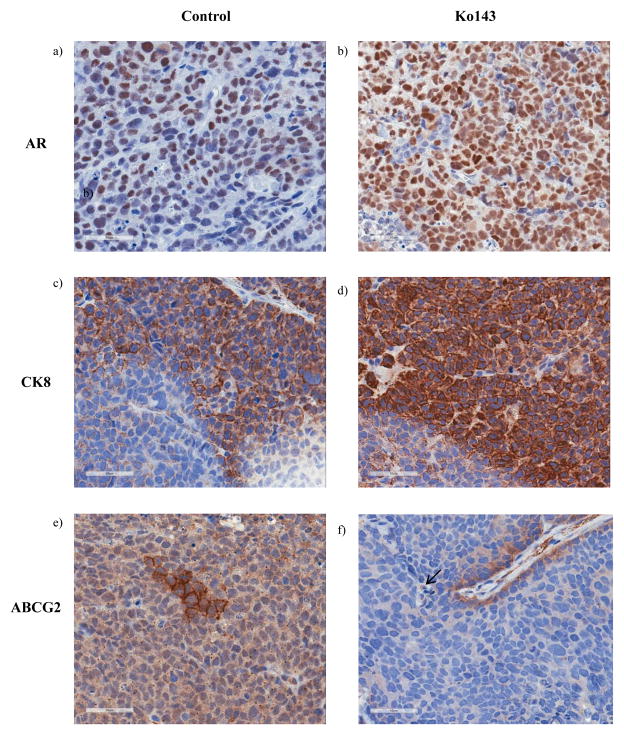
Based on our observations from in vitro experiments, showing that the inhibition of the ABCG2-mediated androgen efflux forces prostate stem cells to differentiate to a luminal phenotype, we hypothesized that Ko143 treated mice would have more differentiated tumors compared to mice from the control group. Luminal differentiation was assessed by immunostaining the paraffin embedded tumor sections for CK8 and AR. We observed that tumors from the Ko143 treated group had a higher percentage of CK8 expressing cells (~86.4% in the Ko143 group vs ~63.75% in the control group) and AR expressing cells (~74% in the Ko143 group vs ~57% in the control group) compared to the control group (Figure 6 a,b,c,d). Also, we observed a general overlap between CK8 and AR expressing cells. Additionally, tumor sections from the Ko143 cohort showed a 2.5 fold reduction in the number of ABCG2expressing cells compared to tumors from the control group. In the control group, ~87.5% of the tumors contained at least one foci (areas with ≥1 ABCG2 expressing cell) of ABCG2 expressing cells as opposed to only 55.5% of the tumors from the treated cohort (Figure 6 e,f). Our results thus suggest that the Ko143 treated group had more differentiated tumors compared to the control group as evidenced by an increased percentage of luminal and decreased percentage of ABCG2 expressing cells. The reduction in tumor growth rate and increased survival in the treated group could be attributed to increased differentiation.
Figure 6. IHC analyses of the paraffin embedded tumor sections.
Tumors from control (n=8) and Ko143 (n=9) treated mice were dissected upon autopsy and a part of the tumors were subjected to paraffin embedding. The paraffin embedded sections were immunostained with (ab) AR, (c–d) CK8 and (e–f) ABCG2 and imaged under 40X magnification. Analysis was done in one section of the tumor from each animal.
Discussion
Elucidating the mechanisms by which stem cells maintain their undifferentiated phenotype is important to improve the efficacy of cancer therapy due to their role in the etiology of cancer and their intrinsic resistance to conventional therapies. Although, ABCG2 is suspected to play an important role in stem cell maintenance, the precise mechanism is unknown. Our study dissects the mechanism by which ABCG2 protects PSCs from undergoing differentiation. Using the HPr-1-AR and CWR-R1 cell lines, we have shown that ABCG2 maintains PSCs in an undifferentiated state by effluxing androgens. Inhibiting the ABCG2-mediated androgen efflux leads to an AR-modulated luminal differentiation and a delayed cell growth response (Figure 7). Our observations are consistent with previous reports, which show that inducing AR expression in PSCs leads to a delayed cell growth response and luminal differentiation rather than cell proliferation [14, 16]. In accordance with our in vitro observations, we observed that Ko143 treatment reduced the tumor growth rate, increased survival and led to more differentiated tumors in mice transplanted with ABCG2-expressing CWR-R1 cells. Most of the studies on ABCG2 and its relevance to stem cells have shown that ABCG2 maintains the stem cell phenotype either by effluxing cytotoxic substrates or by acting as a downstream effector of stem cell specific pathways such as the Wnt, Notch and Hedgehog pathways [2, 24, 25]. This is the first study to show that ABCG2 plays a role in stem cell maintenance by effluxing differentiation-inducing substrates. Additionally, our study provides a plausible reason for the lack of AR expression in PSCs. Previous studies show that PSCs have low AR levels due to the methylation of the AR promoter CpG islands [14]. However, we detected AR transcripts in ABCG2-expressing HPr-1-AR and CWR-R1 cells (data not shown). Previous reports have confirmed the presence of AR transcripts and shown that AR is unstable and degraded in the absence of androgens in PSCs [26, 27]. Our study suggests that androgen efflux by ABCG2 may facilitate AR degradation whereas inhibiting the ABCG2-mediated androgen efflux may lead to stabilization and the nuclear translocation of AR in the PSCs.
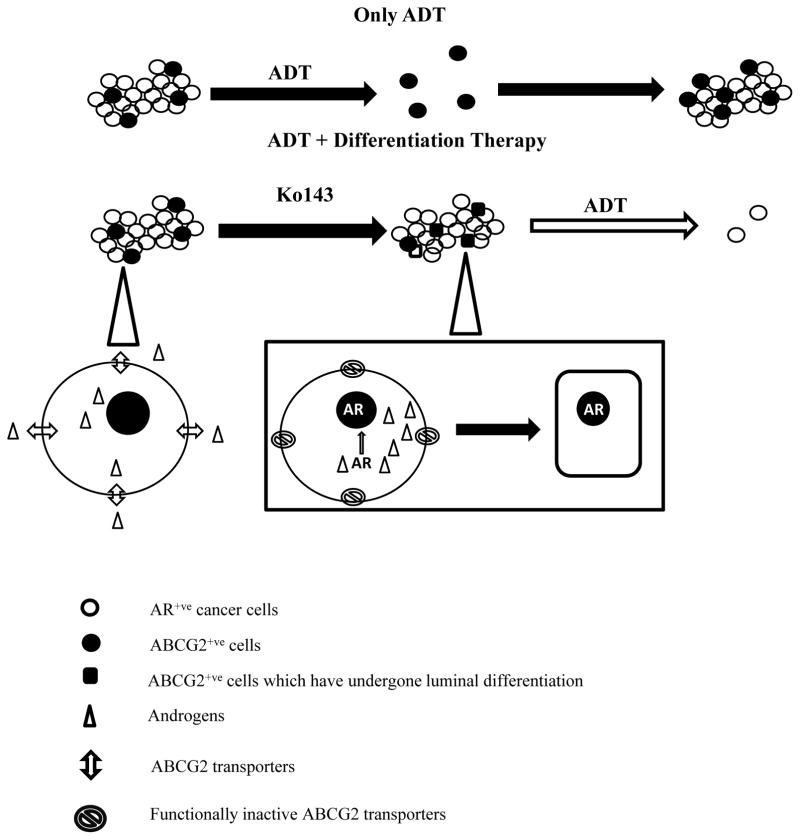
Figure 7. Schematic representation of our proposed model.
ADT targets only the AR+ve cancer cells whereas a small population of AR−ve prostate cancer stem cells survives and over time proliferates to give rise to CRPC. Combining differentiation therapy with ADT would in theory target the AR−ve stem cell as well as the AR+ve cancer cell population. In our model, we show that the ABCG2 transporters efflux androgens. Inhibiting this ABCG2-mediated androgen efflux would induce the nuclear translocation of AR and consequently force the prostate cells to differentiate to an ADT-sensitive luminal phenotype. Subjecting the tumor to ADT after the introduction of Ko143 would then target both, the pre-existing cancer cells as well as the newly differentiated prostate cancer stem cells.
The important role played by prostate cancer stem cells in the etiology of CRPC have sparked a lot of interest in differentiation therapy as an adjuvant to ADT as reviewed in [28]. However, initial attempts at applying differentiation therapy in prostate cancer management failed to yield significant results [29, 30]. The failure of differentiation therapy could be attributed to the focus on bulk tumor response not the prostate cancer stem cell population. Additionally, most of the studies evaluated differentiation therapy in the LnCaP cell line, which is primarily comprised of luminal-differentiated cells [31, 32]. In contrast, our study was conducted in the ABCG2-enriched undifferentiated subpopulation derived from both normal and tumorigenic prostate cell lines. Future studies will test the direct role of ABCG2 in delaying/minimizing the progression to CRPC will be assessed in mouse models that closely mimic the disease progression in humans, such as the CWR22 and LuCaP 35 xenografts which progress from an androgen-dependent to an androgen-independent phenotype.
Since we could successfully induce luminal differentiation in the HPr-1-AR and CWR-R1 cell lines and CWR-R1 xenografts, our approach could be applied in the clinical setting, in both hormone naïve as well as castration recurrent scenario. Our proposed model relies on restored AR activity to induce differentiation, while this seems counterintuitive to the current standard of care, which focuses on inhibiting AR activity, we intend to highlight the dual role of AR, in inducing differentiation and growth retardation in PSCs and inducing proliferation in AR expressing cancer cells. Ko143 is proposed to restore AR activity and induce luminal differentiation only in the small population of ABCG2-expressing PSCs, while the bulk of the ABCG2 negative cancer cells will not be affected. Additionally, we demonstrated that inhibiting the ABCG2-mediated androgen efflux in the CWR-R1 cells increases only the full length AR expression (which subsequently causes luminal differentiation) despite the presence of AR variants in the ABCG2 negative cell population (Supplemental Figure 3). Blocking AR activity after Ko143 administration is proposed to target the newly differentiated stem cells as well as the pre-existing AR expressing cancer cells (Figure 7). The presence of androgens is important for this approach. Hence, in the hormone naïve setting, Ko143 could be administered during intermittent ADT, when the androgen sensitivity is restored, to delay the progression to CRPC or prior to Enzalutamide in the CRPC setting, when androgens are synthesized by intratumoral steroidogenesis to improve survival. However, further investigation is needed to fully understand the significance of targeting ABCG2 for differentiation therapy. Although ABCG2 expression enriches for PSCs, it is likely that a sub-population of stem cells lack ABCG2 expression, thus it is important to evaluate the percentage of ABCG2 negative stem cells in the prostate and assess their contribution to the etiology of CRPC. Additionally, it would be interesting to study the expression profile of ABCG2 as prostate cancer progresses from hormone naïve to CRPC and the effects of ADT and AR antagonists on ABCG2 expression.
The initial discovery of ABCG2 and its role in multidrug resistance sparked a lot of interest in the ABC transporters. However, the subsequent lack of clinical benefit and neurotoxicity associated with ABCG2 inhibitors led to a lot of disappointment [33]. Despite the lack of clinical benefit associated with first and second generation ABCG2 inhibitors, Ko143 has shown immense potential in preclinical studies; In addition to being the most specific ABCG2 inhibitor to date, it is also well tolerated in mice at extremely high dosages [34] and for a long period of time, as confirmed by our study. Most of the studies conducted on clinically targeting ABCG2 have focused on targeting drug resistance by administering ABCG2 inhibitors in combination with chemotherapeutic drugs to increase the bioavailability of drugs [35–37]. However, combination therapy is associated with increased toxicity since ABCG2 is expressed in organs that influence drug excretion. We propose a novel use of Ko143 in differentiation therapy, which entails administering Ko143 as a single agent in the presence of endogenous androgens, thus potentially minimizing the toxicity associated with combination therapy. In conclusion, we provide a strategy to induce luminal differentiation in the PSCs by targeting the ABCG2 transporters and sensitize them to ADT.
Supplementary Material
Acknowledgments
Funding
This study was supported by the NIH RO1CA095367 (W.J. Huss), Pharmacology & Therapeutics Departmental Funding (W.J. Huss), Mark Diamond Research Fund (N.G. Sabnis) and the NCI Cancer Center Support Grant (P30CA016056) to RPCI supported Mouse Tumor Model Resource and Laboratory Animal Resources.
We thank Drs. Barbara Foster, Michael Moser, James Mohler, Kalyan Gangavarapu, Mugdha Samant, and Vineet Dhiman, for insightful discussions about the in vivo experiments, sphere formation assays, and cell line. We thank Ellen Karasik and Bryan Gillard for their technical assistance with the in vivo experiments. We thank the following cores at RPCI supported by NCI Cancer Center Support Grant (P30CA016056): Laboratory Animal Resources (LAR) and the Mouse Tumor Model Resource (MTMR) Core.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- 1.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya S, Das A, Mallya K, Ahmad I. Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling. J Cell Sci. 2007;120(Pt 15):2652–2662. doi: 10.1242/jcs.008417. [DOI] [PubMed] [Google Scholar]
- 3.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565(1–3):6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 5.Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP, et al. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol. 2011;195(1):147–163. doi: 10.1083/jcb.201103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apati A, Orban TI, Varga N, Nemeth A, Schamberger A, Krizsik V, et al. High level functional expression of the ABCG2 multidrug transporter in undifferentiated human embryonic stem cells. Biochim Biophys Acta. 2008;1778(12):2700–2709. doi: 10.1016/j.bbamem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, et al. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res. 2008;103(8):825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew G, Timm EA, Jr, Sotomayor P, Godoy A, Montecinos VP, Smith GJ, et al. ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate. Cell cycle (Georgetown, Tex. 2009;8(7):1053–1061. doi: 10.4161/cc.8.7.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer research. 2005;65(15):6640–6650. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- 10.Pascal LE, Oudes AJ, Petersen TW, Goo YA, Walashek LS, True LD, et al. Molecular and cellular characterization of ABCG2 in the prostate. BMC urology. 2007;7:6. doi: 10.1186/1471-2490-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer MJ, Smit FP, Sedelaar JP, Schalken JA. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol Med. 2011;17(7–8):657–664. doi: 10.2119/molmed.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colabufo NA, Pagliarulo V, Berardi F, Contino M, Inglese C, Niso M, et al. Bicalutamide failure in prostate cancer treatment: involvement of Multi Drug Resistance proteins. Eur J Pharmacol. 2008;601(1–3):38–42. doi: 10.1016/j.ejphar.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Foster BA, Gangavarapu KJ, Mathew G, Azabdaftari G, Morrison CD, Miller A, et al. Human prostate side population cells demonstrate stem cell properties in recombination with urogenital sinus mesenchyme. PLoS One. 2013;8(1):e55062. doi: 10.1371/journal.pone.0055062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J, Lee SO, Liang L, Luo J, Huang CK, Li L, et al. Targeting the unique methylation pattern of androgen receptor (AR) promoter in prostate stem/progenitor cells with 5-aza-2′-deoxycytidine (5-AZA) leads to suppressed prostate tumorigenesis. J Biol Chem. 2012;287(47):39954–39966. doi: 10.1074/jbc.M112.395574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Li Q, Liu X, Liu C, Liu R, Rycaj K, et al. Defining a Population of Stem-like Human Prostate Cancer Cells That Can Generate and Propagate Castration-Resistant Prostate Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling MT, Chan KW, Choo CK. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J Endocrinol. 2001;170(1):287–296. doi: 10.1677/joe.0.1700287. [DOI] [PubMed] [Google Scholar]
- 17.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126(1):59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 18.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Libertini SJ, George M, Dandekar S, Tepper CG, Al-Bataina B, et al. Genome-wide analysis of androgen receptor binding and gene regulation in two CWR22-derived prostate cancer cell lines. Endocr Relat Cancer. 2010;17(4):857–873. doi: 10.1677/ERC-10-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31(45):4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33(24):3140–3150. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojo D, Lin X, Wong N, Gu Y, Tang D. Prostate Cancer Stem-like Cells Contribute to the Development of Castration-Resistant Prostate Cancer. Cancers (Basel) 2015;7(4):2290–2308. doi: 10.3390/cancers7040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostaghel EA. Steroid hormone synthetic pathways in prostate cancer. Transl Androl Urol. 2013;2(3):212–227. doi: 10.3978/j.issn.2223-4683.2013.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Bieber MM, Teng NN. Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog. 2014;53(8):625–634. doi: 10.1002/mc.22015. [DOI] [PubMed] [Google Scholar]
- 25.Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/beta-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32(22):2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- 26.Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. The Journal of biological chemistry. 1992;267(2):968–974. [PubMed] [Google Scholar]
- 27.Heer R. Hunterian Lecture. Characterisation of human prostate epithelial progenitor differentiation in response to androgens. Ann R Coll Surg Engl. 2011;93(6):424–428. doi: 10.1308/10.1308/147870811X589245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rane JK, Pellacani D, Maitland NJ. Advanced prostate cancer--a case for adjuvant differentiation therapy. Nat Rev Urol. 2012;9(10):595–602. doi: 10.1038/nrurol.2012.157. [DOI] [PubMed] [Google Scholar]
- 29.Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58(15):3344–3352. [PubMed] [Google Scholar]
- 30.Debruyne FJ, Murray R, Fradet Y, Johansson JE, Tyrrell C, Boccardo F, et al. Liarozole--a novel treatment approach for advanced prostate cancer: results of a large randomized trial versus cyproterone acetate. Liarozole Study Group. Urology. 1998;52(1):72–81. doi: 10.1016/s0090-4295(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 31.Esquenet M, Swinnen JV, Heyns W, Verhoeven G. Control of LNCaP proliferation and differentiation: actions and interactions of androgens, 1alpha,25-dihydroxycholecalciferol, all-trans retinoic acid, 9-cis retinoic acid, and phenylacetate. Prostate. 1996;28(3):182–194. doi: 10.1002/(SICI)1097-0045(199603)28:3<182::AID-PROS5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Gleave ME, Sato N, Sadar M, Yago V, Bruchovsky N, Sullivan L. Butyrate analogue, isobutyramide, inhibits tumor growth and time to androgen-independent progression in the human prostate LNCaP tumor model. J Cell Biochem. 1998;69(3):271–281. [PubMed] [Google Scholar]
- 33.Robey RW, Ierano C, Zhan Z, Bates SE. The challenge of exploiting ABCG2 in the clinic. Curr Pharm Biotechnol. 2011;12(4):595–608. doi: 10.2174/138920111795163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Molecular cancer therapeutics. 2002;1(6):417–425. [PubMed] [Google Scholar]
- 35.Lopez JP, Wang-Rodriguez J, Chang C, Chen JS, Pardo FS, Aguilera J, et al. Gefitinib inhibition of drug resistance to doxorubicin by inactivating ABCG2 in thyroid cancer cell lines. Arch Otolaryngol Head Neck Surg. 2007;133(10):1022–1027. doi: 10.1001/archotol.133.10.1022. [DOI] [PubMed] [Google Scholar]
- 36.Shishido Y, Ueno S, Yamazaki R, Nagaoka M, Matsuzaki T. ABCG2 inhibitor YHO-13351 sensitizes cancer stem/initiating-like side population cells to irinotecan. Anticancer Res. 2013;33(4):1379–1386. [PubMed] [Google Scholar]
- 37.Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets. 2011;12(5):621–630. doi: 10.2174/138945011795378540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.