Abstract
The intrinsically disordered scaffold proteins AFF1/4 and the transcription elongation factors ELL1/2 are core components of the super elongation complex required for HIV-1 proviral transcription. Here we report the 2.0-Å resolution crystal structure of the human ELL2 C-terminal domain bound to its 50-residue binding site on AFF4, the ELLBow. The ELL2 domain has the same arch-shaped fold as the tight junction protein occludin. The ELLBow consists of an N-terminal helix followed by an extended hairpin that we refer to as the elbow joint, and occupies most of the concave surface of ELL2. This surface is important for the ability of ELL2 to promote HIV-1 Tat-mediated proviral transcription. The AFF4–ELL2 interface is imperfectly packed, leaving a cavity suggestive of a potential binding site for transcription-promoting small molecules.
The host super elongation complex (SEC) is hijacked by HIV-1 for viral transcription. Here the authors present the structure of RNA polymerase elongation factor ELL2 bound to the intrinsically disordered scaffold protein AFF4, identifying an ELL2 surface important for HIV-1 transcription.
Curing AIDS is a major global health goal. AIDS is caused by the human immunodeficiency virus (HIV), which has proved exceptionally difficult to eradicate1,2. The principal obstacle to HIV eradication is the persistence in patients of a reservoir of cells harbouring latent provirus integrated within the genome3. Clinical interest in the reactivation of latent HIV1,2 has brought renewed attention to the mechanism of transcriptional regulation of the HIV provirus. Latency is regulated at the levels of epigenetic silencing, and transcription initiation and elongation4. Transcription elongation, which is promoted by the HIV Tat protein and TAR RNA sequence, is the best understood of these mechanisms. The functions of HIV Tat and TAR in promoting elongation are completely dependent on their ability to hijack the host super elongation complex (SEC)5,6,7.
The SEC consists of the Cyclin-dependent kinase CDK9 and Cyclin T (CycT1 or T2), together known as P-TEFb8; one of either of the intrinsically disordered (ID) scaffold proteins AFF1 or AFF4 (refs 5, 6); one of either ENL or AF9; and one of either of the RNA polymerase elongation factors ELL1 or ELL2 (refs 5, 9, 10). The reason that Tat is such a powerful activator of HIV-1 transcription lies in its ability to pack two distinct transcriptional elongation factors P-TEFb and ELL1/2 into a single SEC complex, where the two factors can synergistically stimulate a single RNA Pol II elongation complex5,7. AFF1/4 is >1,100 residues long and is the principal scaffold that holds the SEC together11. AFF1/4 consists almost entirely of predicted ID regions (IDRs). AFF1 and AFF4 function in transcription elongation by virtue of various peptide motifs interspersed throughout their sequences, much like many other ID signalling and regulatory proteins that have come under intensive study12,13. The AFF1- and ELL2-containing version of the SEC is the most important in the promotion of proviral elongation, despite its low abundance14.
The structure of P-TEFb lacking the C-terminal IDR of CycT1 has been determined in complex with HIV-1 Tat15 and the N-terminal 60 residues of AFF4 (refs 16, 17). This structure shows that AFF4 residues 32–67 bind as an extended strand followed by two α-helices to the CycT1 surface. Nuclear magnetic resonance studies showed that AFF4 residues 761–774 fold into a β-strand that combines with two strands of the AF9 AFF4-binding domain to generate a three-stranded β-sheet18. The structures of the P-TEFb and AF9 complexes with AFF4 revealed two of the three known interfaces used by AFF4 in assembly of the SEC. In this study, we set out to visualize the last of the three known interfaces critical for AFF4 function, its binding site for ELL1/2.
Progress in characterizing the AFF4 interface with ELL2 has been slower than for the P-TEFb and ENL/AF9 interfaces, in part because the AFF4-binding domain of ELL2 is poorly soluble and prone to aggregation. Here we work with a fusion construct such that a stable obligate complex between ELL2 and AFF4 is formed. This fusion-based tethered complex is stable and soluble enough to be crystallized. The crystal structure confirms that the AFF4-binding domain of ELL2 has an occludin fold, as predicted from the sequence homology19. It shows that the IDR consisting of AFF4 residues 301–351 (hereafter referred to as AFF4ELLBow for ELL1/2 binding) folds up into a helix and elbow joint arrangement that makes extensive contacts with the occludin domain of ELL2 (hereafter ELL2Occ). These results complete the structural picture of how AFF1/4 engages its three known partners in the SEC.
Results
Mapping the AFF4ELLBow and ELL2Occ interaction
Following the initial mapping of the AFF4 and ELL2 interaction sites to approximately residues 318–337 of the former and 519–640 of the latter20 (Fig. 1a), we sought to isolate a stable form of this monomeric complex for crystallization (Supplementary Fig. 1a). It was difficult to obtain diffraction-quality crystals of ELL2Occ constructs with AFF4ELLBow fragments because of the propensity of the ELL2 fragment to aggregate over time. We reasoned that fusion of AFF4ELLBow and ELL2Occ fragments might protect the AFF4-binding epitope on ELL2Occ from aggregation. Constructs were generated for both AFF4ELLBow–(Gly-Ser)4–ELL2Occ and ELL2Occ–(Gly-Ser)4– AFF4ELLBow. The ELL2Occ–(Gly-Ser)4–AFF4ELLBow dimerized in solution, while AFF4ELLBow–(Gly-Ser)4–ELL2Occ was monomeric (Supplementary Fig. 1b). Given that the unfused fragments were monomeric, we concluded that the dimerization of ELL2Occ–(Gly-Ser)4–AFF4ELLBow represented a domain-swapping artifact (Supplementary Fig. 1c) and focused efforts on AFF4ELLBow–(Gly-Ser)4–ELL2Occ.
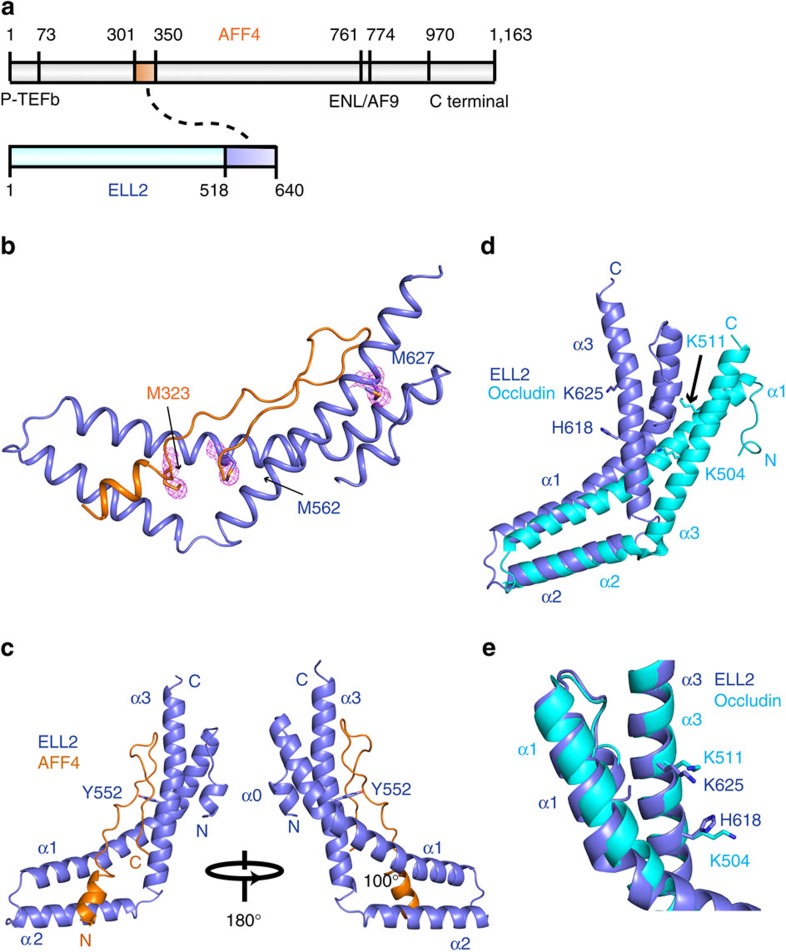
Figure 1. Crystal structure of the AFF4 ELLBow in complex with the occludin homology domain of ELL2.
(a) Schematic of the interactions of the AFF4 intrinsically disordered protein (IDP) scaffold with its partners in the SEC. The highlighted boxes within AFF4 and ELL2 represent the co-crystallized elements described below. Other regions of AFF4 are annotated for binding to P-TEFb, AF9/ENL and the novel C-terminal ELL1/2-binding site described below. (b) Se anomalous difference peaks and overall structure of the complex. The Se substructure map is displayed at a contour level of 2σ(magenta). (c) Two views of the overall structure of the complex, with AFF4 in orange and ELL2 in light blue. (d,e) Comparison of ELL2 and occludin C-terminal domain showing that the folds are similar, but ELL2 is more sharply bent. Helix α3 from both ELL2 and occludin are aligned, the structurally and functionally conserved residues are shown in stick. ELL2 is shown in light blue while Occludin is shown in cyan. All structural figures in this paper are made with Pymol.
Structure of the AFF4ELLBow complex with ELL2Occ
The structure of the AFF4ELLBow–ELL2Occ complex was determined by Selenomethionyl (SeMet) multiwavelength anomalous dispersion (MAD) phasing (Fig. 1b; Supplementary Fig. 2; Table 1). Helix α1 (residues 538–578) of ELL2 bends inward at Tyr552 by 30° such that the C-terminal part of α1 (553–578) packs against α2 (Fig. 1c). Helices α2 (584–602) and α3 (607–638) of ELL2 are oriented at an angle of ∼100° with respect to each other such that both pack along the length of the long, bent helix α1 (Fig. 1c). The structure confirms that ELL2Occ has a similar arch-shaped three-helix fold as the C-terminal domain of occludin19. The ELL2Occ and occludin C-terminal domain (pdb entry 1XAW) structures can be superimposed with an root mean squared deviation of 4.0 Å for 104 residue pairs (Fig. 1d). The main differences are in the α2–α3 connector and in the mutual orientation of these two helices. The α2–α3 angle is steeper in ELL2Occ than in occludin. A minor difference is that ELL2Occ has an extra single-turn helix, denoted α0, at its N terminus.
Table 1. Statistics of crystallographic data reduction and refinement.
| Native | SeMet (Se peak) | SeMet (Se high remote) | |
|---|---|---|---|
| Data collection | |||
| Space group | P212121 | P212121 | P212121 |
| Unit cell parameters | |||
| a, b, c (Å) | 52.866, 57.324, 61.805 | 52.641, 57.422, 61.338 | 52.641, 57.422, 61.338 |
| α, β, γ (°) | 90.000, 90.000, 90.000 | 90.000, 90.000, 90.000 | 90.000, 90.000, 90.000 |
| Wavelength (Å) | 1.12709 | 0.9797 | 0.9569 |
| Resolution (Å) | 50.00–2.51 (2.60–2.51) | 50.000–2.10 (2.14–2.10) | 50.00–2.10 (2.18–2.10) |
| No. of reflections | 36,437 | 159,481 | 159,642 |
| Completeness (%) | 99.1 (92.3) | 100.0 (100.0) | 100 (100) |
| Redundancy | 5.4 (4.0) | 14.1 (13.9) | 14.1 (14.2) |
| Rsym | 0.139 (0.917) | 0.748(0.141) | 0.132 (0.72) |
| <I>/<σ(I)> | 10.83 (1.05) | 33.7 (3.71) | 20.59 (4.06) |
| CC1/2 | 0.701 | 0.919 | 0.925 |
| Refinement | |||
| Resolution (Å) | 41.92–2.003(2.075–2.003) | ||
| Rwork/Rfree (%) | 19.71/24.83(28.04/40.22) | ||
| Average B-factor (Å) | 40.79 | ||
| R.m.s.d from ideality | |||
| Bond length (Å) | 0.003 | ||
| Bond angle (°) | 0.57 | ||
| Ramachandran plot (%) | |||
| Favoured | 98 | ||
| Allowed | 2 | ||
| Outliers | 0 | ||
R.m.s.d, root mean squared deviation.
Values in parentheses are for the highest-resolution shell. Rfree was calculated with 5% of the reflections selected in the thin shell.
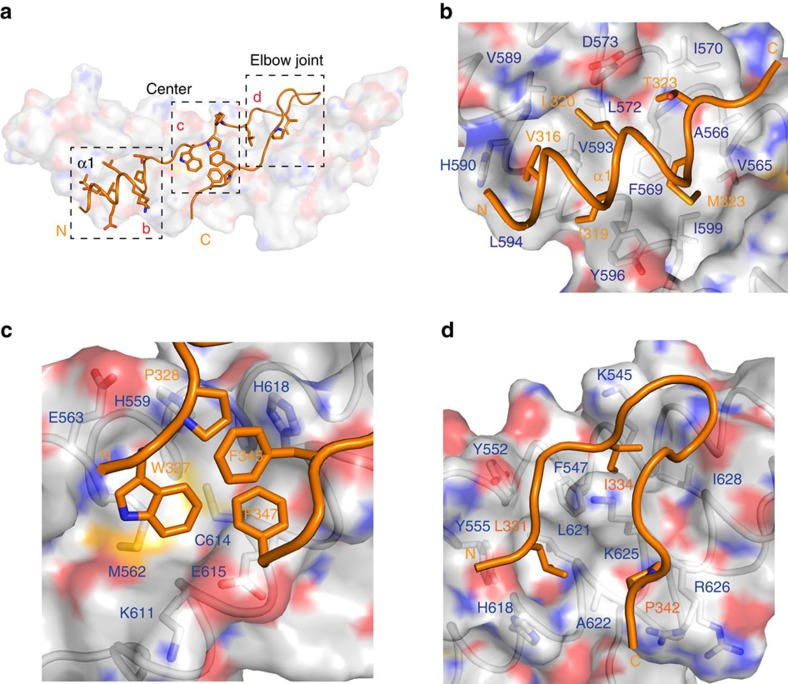
AFF4ELLBow is ordered over residues 314–349 and buries 1,535 Å2 of solvent-accessible surface area. Fully 37% of the entire solvent-accessible surface area of AFF4ELLBow is buried in the contact. The AFF4ELLBow sequence folds into several distinct regions. It begins with helix α1 (315–324), is followed by an extended hydrophobic sequence (325–327), a polyproline segment (328–330), an extended region that doubles back on itself in what we refer to as the ELLBow joint (331–343), and a second extended hydrophobic sequence (344–349) (Fig. 2a). The fusion construct contains 17 residues that are not visualized in electron density. These include AFF4 350–351, followed by 8 Gly-Ser linker residues and ELL2 residues 519–524. These 17 residues are more than adequate to span the 15 Å gap between the carbonyl carbon of AFF4 residue 349 and the amide nitrogen of ELL2 residue 525 in the structure. Hydrophobic side chains of AFF4ELLBow α1, including Val316, Ile319, Leu320 and Met323, are buried in a hydrophobic groove formed by the C-terminal half of ELL2Occ α1 and α2 (Fig. 2b). These helices of ELL2Occ contribute residues Val565, Phe569, Ile570, Leu572, Asp573, Val589, His590, Tyr596, Leu594 and Ile599 to the AFF4 α1-binding site (Fig. 2b). ELL2Occ buries 1,315 Å2 of solvent-accessible surface area, corresponding to 15% of its total surface area.
Figure 2. AFF4ELLBow–ELL2Occ interaction surfaces.
(a) Overview of the main binding determinants of the AFF4 ELLBow. (b) The first helix of the ELLBow (orange) binds in a hydrophobic groove on ELL2 (grey). The key residues are shown in a stick model. (c) The central cluster, in which hydrophobic residues of the ELLBow pack against ELL2 and one another, and are supplemented by polar interactions. Water molecules are shown as red spheres. (d) The ELLBow joint.
AFF4ELLBow is centred on Trp327, which forms extensive hydrophobic interactions with the side chains of ELL2 residues His559, Met562, Cys614 and Glu615. The Trp327 indole nitrogen also forms a water-mediated hydrogen bond with the Tyr607 hydroxyl. This cluster of residues is completed by the side chains of AFF4 Pro328, Phe345 and Phe347 (Fig. 2c). Collectively, this cluster forms an extensive interaction network, in which AFF4ELLBow folds up not only against ELL2 but also against itself.
In the AFF4ELLBow joint, the side chain of Leu331 sticks into a pocket comprising Tyr552, Tyr555, His618, Leu621 and Ala622 of the N-terminal half of ELL2Occ α1 and α3. The side chain of Ile334 packs against the side chains of Lys545, Phe547, Lys625 and Leu628. At the distal end of ELLBow joint, Pro342 falls into a shallow cavity composed of Ala622, Lys625 and Arg626 (Fig. 2d).
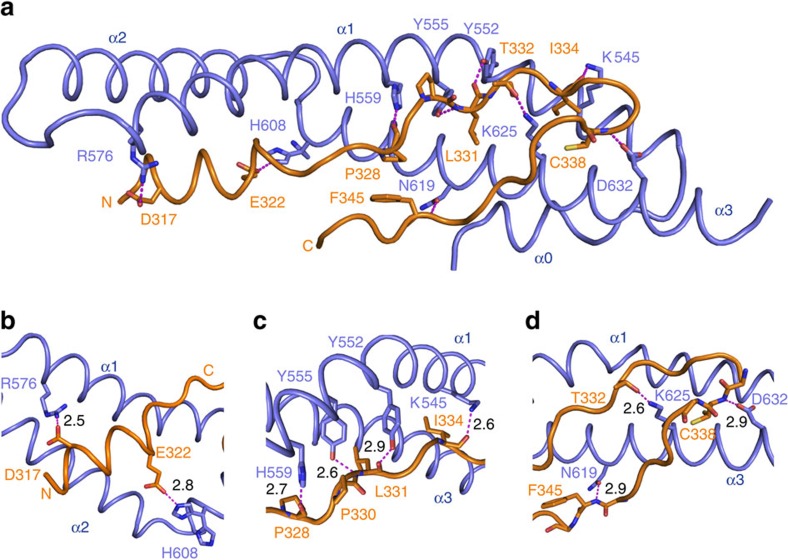
A number of hydrogen bonds are observed in the complex (Fig. 3a). In AFF4ELLBow α1, the side chains of Asp317 and Arg576 of ELL2 form a bidentate salt bridge with one another (Fig. 3b). Glu322 of AFF4 forms a 2.8 Å salt bridge with one of the two observed rotamers of His608 of ELL2 (Fig. 3b). In the central cluster, the carbonyl group of Pro328 forms a 2.7 Å hydrogen bond with the side chain of His559 of ELL2 (Fig. 3c). Moving into the ELLBow joint, the main-chain amide and carbonyl of AFF4 Leu331 form hydrogen bonds with the hydroxyl oxygens of Tyr552 and Tyr555, respectively, of ELL2. A 2.6 Å hydrogen bond is formed between Thr332 of AFF4 and Lys625 of ELL2 (Fig. 3d). The Ile334 carbonyl accepts a hydrogen bond from the side chain of Lys545. The Cys338 main-chain amide donates a hydrogen bond to the side chain of Asp632. The main-chain amide of Phe345 forms a 2.9 Å hydrogen bond with the side chain of Gln619 (Fig. 3d).
Figure 3. Hydrogen bonding in the AFF4ELLBow–ELL2Occ complex.
(a) Overview of the network of hydrogen bonds. The residues involved in the hydrogen bonding are shown in stick. Hydrogen bonds are shown as magenta-coloured dashed lines. (b–d) Details of the hydrogen bonds. The length of the hydrogen bonds is indicated next to the dashed lines.
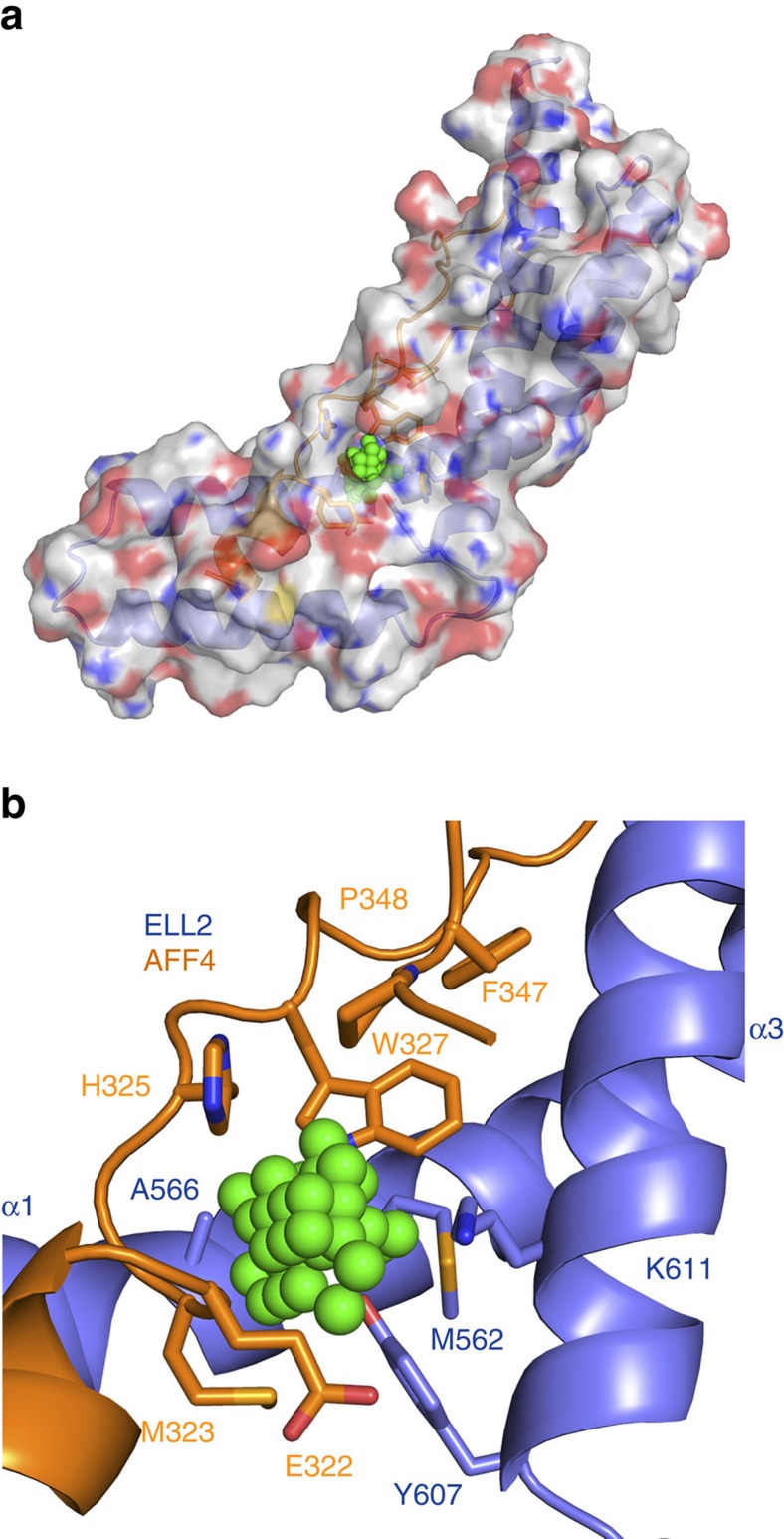
The AFF4ELLBow–ELL2Occ complex was screened for cavities using POCASA 1.1 (POcket-CAvity Search Application)21 with a probe radius of 3 Å. Of the five largest cavities located, one is an internal cavity at the AFF4ELLBow–ELL2Occ interface (Fig. 4a). The cavity is 36 Å3 in volume and is connected to the exterior by a narrow mouth (Fig. 4a). It is lined by the aliphatic part of Glu322, Met323, His325, Trp327, Phe347 and Pro348 of AFF4, and by Met562, Ala566, Tyr607 and the aliphatic part of Lys611 of ELL2 (Fig. 4b). These residues are in or adjoin the central cluster part of the interface.
Figure 4. Cavity at the AFF4ELLBow–ELL2Occ interface.
(a) Overall view of the cavity that might work as a potential drug-binding site. Molecules of AFF4ELLBow:ELL2Occ complex are shown in translucent cartoon and surface model colours are light blue, ELL2; orange, AFF4; and green, dummy atoms (b) Close-up of the cavity region as represented and coloured in a.
Function of the AFF4ELLBow interface with ELL2Occ in binding
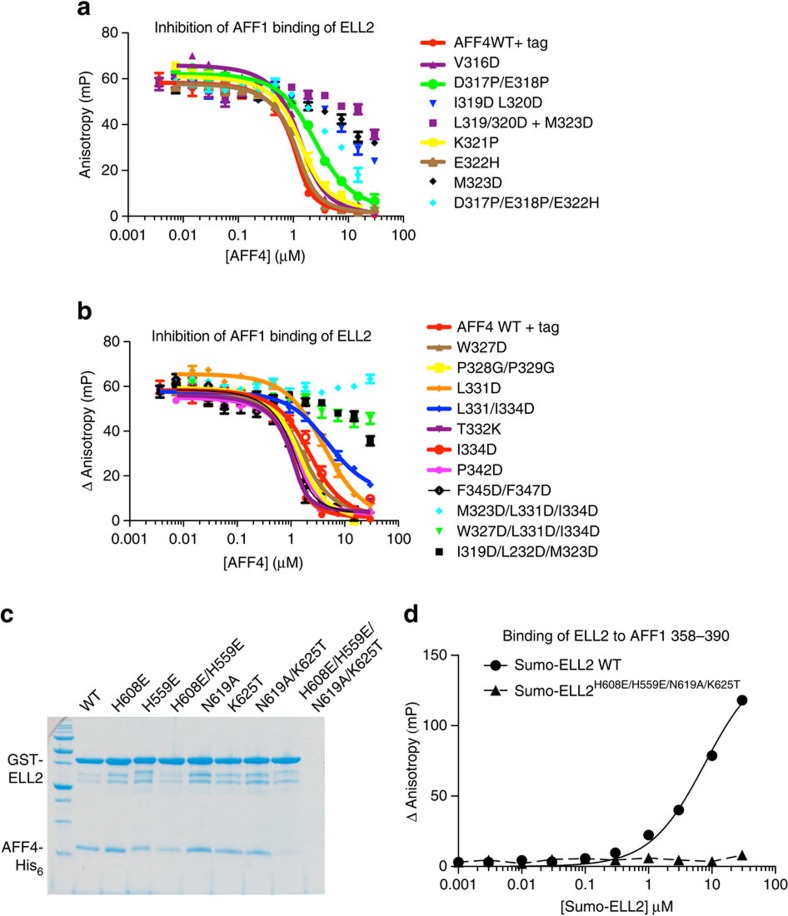
To validate whether the observed structural interface corresponded to the mode of binding of AFF4ELLBow and ELL2Occ in solution, we carried out a series of mutant peptide binding assays using fluorescence polarization. We considered this particularly critical given the use of the fusion construct to obtain crystals. The assay monitored the displacement of fluorescently labelled wild-type AFF1ELLBow peptide by unlabelled mutant peptides 301–351. The unlabelled wild-type peptide in this system has Kd=86 nM (Supplementary Table 1; Fig. 5a). The AFF4 hydrophobic residues Val316, Ile319, Leu320, Met323, Trp327, Leu331, Ile334 and Pro342, were mutated to Asp to maximally destabilize hydrophobic interactions. Consistent with expectation, mutation of multiple hydrophobic residues to Asp resulted in large decreases in affinity. The double mutant I319D/L320D reduced affinity by >25-fold (Supplementary Table 1; Fig. 5a). The Kd for the triple mutant I319D/L320D/M323D was immeasurable due to weak binding, but >3 μM, representing a ∼50-fold loss of affinity (Supplementary Table 1; Fig. 5a). The same was true of two other triple hydrophobic mutants tested, M323D/L331D/I334D and W327D/L331D/I334D (Supplementary Table 1; Fig. 5b). The single mutant M323D has the largest effect of any single-amino-acid change, with a reduction in affinity of >25-fold (Supplementary Table 1; Fig. 5b). Moving closer to the centre of the AFF4ELLBow, L331D and I334D reduce affinity by ∼20- and 8-fold, respectively (Supplementary Table 1; Fig. 5b). This highlights the role of hydrophobic residues in AFF4ELLBow helix α1 and immediately C terminal to it in the central cluster, as the critical anchor points and affinity determinants.
Figure 5. Contributions of AFF4 ELLBow interactions to binding in solution.
(a) Binding of AFF4ELLBow wild type (WT) and mutants in the N-terminal α-helix to Sumo-ELL2Occ. Sumo-ELL2Occ binding to fluorescently labelled AFF1ELLBow peptide 358–390 is competitively inhibited by increasing amounts of AFF4ELLBow, as described in experimental procedures. Error bars reflect the s.e. from three experimental replicates in a,b. (b) Binding of AFF4ELLBow WT and mutants in the central cluster and elbow joint, assayed as in a. (c) GST fusions of the indicated ELL2Occ mutants were immobilized and their ability to pull-down His6-tagged WT AFF4ELLBow assessed. An uncropped version of this pull-down gel is shown in Supplementary Fig. 4. (d) Direct binding of WT and mutant Sumo-ELL2 to fluorescently labelled AFF1ELLBow peptide. The assay was performed in triplicate. The s.e. from the three replicates in d is smaller than the symbols used to plot the data points.
Hydrophobic residues of the central cluster make smaller contributions than those highlighted above. W327D reduces affinity fourfold, while F345D/F347D reduces it by less than twofold. P342D led to a similar threefold drop (Supplementary Table 1; Fig. 5b). These more modest contributions may reflect that these side chains are partially solvent accessible in the AFF4ELLBow–ELL2Occ complex. Moreover, their interactions are made in part with other residues within the AFF4ELLBow such that they could potentially make residual hydrophobic interactions even in unbound AFF4. The polyproline helix does not seem to have a major role in affinity, with the double 328–329 Pro–Gly mutant reducing affinity only by a factor of three (Supplementary Table 1; Fig. 5b).
The interface has a significant polar component, with some hydrophilic residues contributing substantially to binding, and others less so. The AFF4ELLBow α1 mutant D317P/E317P was designed to disrupt hydrogen bonding, involving Asp317 and to introduce helix breaker mutants in α1. This mutation lowered affinity by 10-fold (Supplementary Table 1; Fig. 5a). The charge reversal mutation E322H reduced affinity by less than twofold (Supplementary Table 1; Fig. 5a).
It proved impossible to purify hydrophobic to Asp mutants in the AFF4-binding site of ELL2Occ because these proteins were insoluble when expressed in Escherichia coli. Presumably, this is because these hydrophobic residues also contribute to the hydrophobic core of the ELL2Occ fold. It was, however, possible to purify ELL2Occ polar mutants in the binding site. We examined the roles of ELL2 His559, His608, Asn619 and Lys625 by pull-down assay (Fig. 5c). Single mutants H559E, H608E, N619A and K625T had no apparent effect on binding by pull-down. However, the quadruple mutant H559E/H608E/N619A/K625T completely abrogated binding both in the pull-down assay (Fig. 5c) and in a fluorescence polarization binding assay (Fig. 5d). This validates the role of these residues in the interface in solution.
The AFF4ELLBow and ELL2Occ interface in vivo
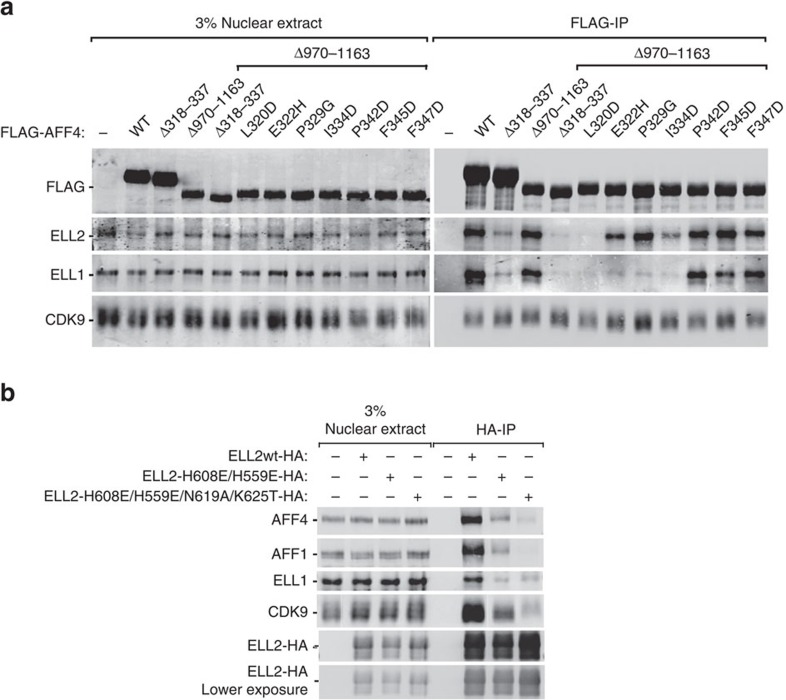
It had previously been shown that the AFF4 sequence 318–337 was sufficient for ELL2 binding20 and that AFF4 can heterooligomerize AFF1 via its C-terminal domain22. To prevent the endogenous AFF1 from rescuing the mutant construct, function was tested in the context of a deletion of the C-terminal sequence 970–1,163. Double deletion of AFF4 residues 318–337 and 970–1,163 abrogated the interaction between AFF4 and ELL1/2 completely (Fig. 6a). To determine if single residues within AFF4ELLBow contributed to binding and function in cells, point mutants were constructed in the context of AFF4 Δ970–1,163. ELL1 contains a C-terminal domain homologous to that of ELL2, hence binding to ELL1 was also tested. L320D was most effective, blocking both ELL1 and ELL2, consistent with its very strong effect on binding in vitro (Fig. 6a; Supplementary Table 1). E322H, P329G and I334D partially blocked ELL2 binding, but completely knocked out ELL1 binding, consistent with their intermediate effects on in vitro peptide binding. Both ELL1 and ELL2 bound robustly to the mutants P324D, F345D and F347D, consistent with their two- to threefold effects on binding in vitro (Fig. 6a; Supplementary Table 1).
Figure 6. Role of the ELLBow in AFF4 interactions with ELL1/2 in nuclear extracts.
(a,b) Nuclear extracts (NE) were prepared from HEK 293T cells transfected with the indicated plasmids and subjected to immunoprecipitation (IP) with anti-FLAG (a) or anti-HA (b) agarose beads. The NE inputs and IP eluates were examined by immunoblotting for the presence of the various proteins indicated on the left. A shorter exposure of the ELL2-HA panel is also shown at the bottom of b. Uncropped versions of the blots are shown in Supplementary Fig. 5.
To determine if the AFF4-binding site on ELL2 was functional in cells, polar mutants were inserted into ELL2 alleles and these were transfected into HeLa cells.
We avoided testing hydrophobic mutants of ELL2, since we had previously found that these destabilized the ELL2 structure. HA-tagged ELL2H559E/H608E and ELL2H559E/H608E/N619A/K625T were expressed at essentially wild-type levels in HeLa cells (Fig. 6b). Wild-type HA-ELL2 pulled down AFF1, AFF4 and ELL1 from extracts. ELL2H559E/H608E has sharply reduced binding to AFF1, AFF4 and ELL1. ELL2H559E/H608E/N619A/K625T has only trace binding to AFF1 and AFF4 in extracts. These findings support that the structural interface is responsible for the interaction of ELL2 with both AFF1 and AFF4 in cells.
Role of the interface in proviral transactivation
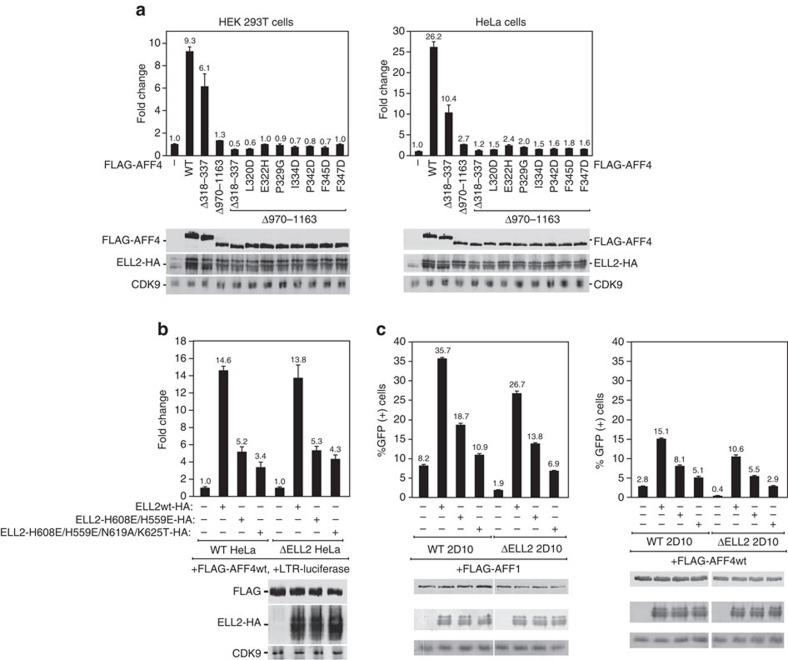
Overexpression of AFF4 stimulates proviral transcription by ∼5–9-fold and ∼26-fold in HEK 293 T and HeLa cells, respectively (Fig. 7a). Deletion of the C-terminal ELL1/2-binding domain almost completely blocked transactivation (Fig. 7a). The residual activity of AFF4 Δ970–1,163 was so low that meaningful results could not be obtained for transactivation phenotypes of these mutants (Fig. 7a). The abundance of the SEC complex appears to be limiting for transactivation such that overexpression of ELL2 in the presence of extra AFF4 promotes transcription by a factor of 14 (Fig. 7b). Polar mutants in the AFF4-binding site of ELL2Occ were tested for their effects on transcription. ELL2H559E/H608E and ELL2H559E/H608E/N619A/K625T had threefold and fivefold less transactivation activity, respectively, than wild type. Very similar three- to fourfold effects are seen in Jurkat 2D10 cells (Fig. 7c). These observations strongly support a functional role for the AFF4ELLBow-binding site on ELL2Occ in transactivation.
Figure 7. The ELLBow-binding site of ELL2 is important for HIV-1 long terminal repeat (LTR) transcription.
(a,b) Luciferase activities were measured and analysed in extracts of cells transfected in triplicate with the HIV-1 LTR-luciferase construct together with the combinations of plasmids expressing wild-type and mutant ELL2-HA and FLAG-AFF4 as indicated. Each of the ELL2 and AFF4 plasmids was transfected at 1 μg per well. The activities in the control groups were set to 1. The error bars represent mean ±s.d. from triplicate wells. An aliquot of each cell extract was examined by immunoblotting for presence of the various proteins labelled on the left. (c) Jurkat 2D10 cells were nucleofected in triplicates with plasmids expressing AFF1 (top) or AFF4 (bottom) alone or in combination with ELL2wt or its mutants as indicated. 48 h post nucleofection, the percentages of GFP+ cells were measured by flow cytometry and plotted. The error bars represent mean ±s.d.'s. The levels of the indicated proteins in nucleofected cells were determined by immunoblotting. Uncropped versions of the blots are shown in Supplementary Fig. 6.
Discussion
The crystallization of the AFF4ELLBow–ELL2Occ complex rounds out our structural-level understanding of how the AFF4 scaffold recruits its three known partners in the SEC, P-TEFb, ENL/AF9 and ELL1/2. The limited solubility of ELL2Occ made this a more challenging target for crystallization, hence the necessity for the fusion approach. When using protein chimeras as a basis for structure solution, it is particularly critical to validate the findings in solution and in functional assays. One area that remains to be further explored is the relationship between ELL1/2 binding to AFF1/4 and the putative heterodimerization mediated by the C-terminal domains of AFF1/4. Binding assays in vitro, pull-downs from nuclear extracts, and proviral transactivation assays present a unified, consistent picture that validates the structural results.
The structure confirms the decade-old prediction that the C-terminal domains of ELL1/2 would have the same fold as the occludin ZO-1-binding domain. Occludin is a transmembrane tight junction protein that has no known involvement in transcription. It is not clear why this protein and ELL1/2 should share a domain uniquely present in this small set of otherwise unrelated proteins. In the initial analysis of the occludin structure, it was proposed that another tight junction protein, ZO-1, bound to a basic patch at the concave center of the arch19. This patch of occludin includes Lys504 and Lys511, which correspond structurally to the functionally important His618 and Lys625 in the AFF1/4-binding site of ELL2 (Fig. 1e). Subsequently, another report proposed that ZO-1 bound elsewhere, at one tip of the occludin domain arch. Despite these uncertainties, the structural similarities are extensive enough to suggest a common evolutionary origin and related protein-binding functions for the three-helical domains of occludin and ELL1/2.
The bromodomain and extraterminal protein inhibitor JQ1 (ref. 23) and related latency-reversing agents promote reactivation of HIV-1 from latency via P-TEFb24,25,26,27,28. New classes of HIV-1 latency-reversing agents are being sought in the context of HIV eradication strategies2. We observed a cavity at the AFF4–ELL2 interface that appears likely to be present also in the AFF1–ELL2 complex relevant to proviral activation14, on the basis of the complete identity of the AFF1 and AFF4 residues involved. If so, this could provide an avenue for the design of new SEC activators with JQ1-like effects on latency, but acting by an orthogonal molecular mechanism.
Methods
Cloning and protein purification
DNAs for ELL2 fragments and AFF4–ELL2 fusions were subcloned into pGST-parallel2, and DNAs for AFF4 peptide fragments were subcloned into pRSFduet-1 and pHis-parallel2. Plasmids expressing FLAG-tagged wild-type AFF4 and HA-tagged wild-type ELL2 were generated using the primers described in Supplementary Table 2 (ref. 5). The plasmids expressing mutant versions of AFF4 and ELL2 were generated by PCR mutagenesis. The mutant constructs were verified by DNA sequencing. All proteins were expressed in E. coli BL21-gold (DE3) cells (Agilent Technologies). After induction with 0.2 mM isopropyl-β-D-thiogalactoside overnight at 16 °C, the cells were pelleted by centrifugation at 4,000g for 10 min. Cell pellets were lysed in 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5 mM TCEP-HCl and 1 mM phenylmethylsulphonyl fluoride by ultrasonication. The lysate were centrifuged at 25,000g for 1 h at 4 °C. The supernatants for ELL2 and its fusions were loaded onto GS4B resin at 4 °C, target proteins were eluted and the eluate applied to a Hi Trap Q HP column. Peak fractions were collected and digested with tobacco etch virus protease at 4 °C overnight. Tobacco etch virus and GST were removed by loading the solution onto Ni-NTA and GS4B columns, respectively. Target proteins were further purified on a Superdex 200 16/60 column equilibrated with 25 mM Tris-HCl pH 8.0, 150 mM NaCl and 0.5 mM TCEP-HCl. The peak fractions were collected and flash-frozen in liquid N2 for storage. The supernatant of AFF4 was loaded onto Ni-NTA resin at 4 °C, eluted with an imidazole gradient, and applied to a Superdex 75 16/60 column equilibrated with 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5 mM TCEP-HCl. SeMet protein was expressed in E. coli BL21-gold (DE3) cells grown in M9 minimal medium supplemented with 5% LB medium. An amount of 0.2 mM isopropyl-β-D-thiogalactoside and 100 mg selenomethionine were added when the OD600 reached 1.0. Cells were pelleted by centrifugation at 4,000g for 10 min after overnight induction at 16 °C. SeMet AFF4301–351–(Gly-Ser)4–ELL2519–640 was prepared as above and SeMet incorporation verified by mass spectrometry.
Crystallization of the AFF4ELLBow–ELL2Occ fusion
The purified fusion construct AFF4 (301–351)–(Gly-Ser)4–ELL2 (519–640) was concentrated to 10 mg ml−1 with a 10 kD centrifugal filter (Millipore). Crystals were grown by hanging-drop vapour diffusion at 19 °C. The protein solution was mixed with well buffer composed of 0.2 M NaCl, 10 mM MgCl2, 0.3 M Na3 citrate, 0.2 M Na thiocyanate and 0.1 M Hepes pH 7.4. Crystals appeared in 24 h and grew to full size in 5 days. Crystals were flash-frozen with liquid N2 in well buffer. SeMet crystals were grown in the same condition as native crystals. Native data were collected on BL7-1 at Stanford Synchrotron Radiation Lightsource. Native crystals diffracted to 2.5 Å and data were collected at a wavelength of 1.1271 Å. The structure was solved using data from SeMet crystals as described in the main text.
Pull-down assays
Mutants of ELL2 (519–640) and AFF4 (300–351) were purified as described above. The concentration of proteins and peptides was determined by ultraviolet absorption at 260–280 nm. A measure of 9 μM GST-ELL2 and 20 μM His6-AFF4 were incubated with GS4B resin at 4 °C for 2 h in 80 μl of 25 mM Tris-HCl pH 8.0, 150 mM NaCl and 0.5 mM TCEP-HCl. The resin was washed three times with the incubation buffer. Then, the resin was boiled in 30 μl 1 × SDS loading buffer at 95 °C for 5 min before being applied to SDS–polyacrylamide gel electrophoresis for analysis.
Fluorescence polarization
Protein binding was measured using the fluorescence anisotropy of a 33-residue segment of AFF1 (residues 358–390), following procedures similar to those used previously to characterize AFF4 binding to P-TEFb17. AFF1 358–390 are almost identical to AFF4 318–350 with only three amino-acid changes between the two homologues. The AFF1 peptide C-FAM-GABA-EILKEMTHSWPPPLTAIHTPSTAEPSKFPFPTK-amide was synthesized (University of Utah DNA/Peptide Facility), where FAM indicates 5-carboxyfluoroscein and GABA indicates a γ-amino-butyric acid spacer. Competition titration experiments with unlabelled His-tagged AFF4 protein 301–351 were performed using 2 μM Sumo-ELL2 in 25 mM HEPES pH 7.5, 100 mM NaCl, 10% glycerol, 0.05% NP-40, 0.5 mM TCEP and 5 nM fluorescent peptide. A Victor 3V (Perkin Elmer) multi-label plate reader was used to measure fluorescence anisotropy. Three experimental replicates were carried out for each curve. Binding curves were fit to a formula describing competitive binding of two different ligands to a protein29 using Prism version 5.0c (Graphpad Software).
Co-immunoprecipitation
Approximately 2 × 107 HEK 293T cells (UC Berkeley Cell Culture facility) in two 145-mm dishes were transfected by plasmids expressing the wild-type or mutant FLAG-AFF4 or ELL2-HA (20 μg each). Forty-eight hour after transfection, the cells were harvested and swollen in 4 ml hypotonic buffer A (10 mM HEPES-KOH (pH 7.9), 1.5 mM MgCl2 and 10 mM KCl) for 5 min and then centrifuged at 362g for 5 min. The cells were then disrupted by grinding 20 times with a Dounce tissue homogenizer in 2 ml buffer A, followed by centrifugation at 3,220g for 10 min to collect the nuclei. The nuclei were then extracted in 400 μl buffer C (20 mM HEPES-KOH (pH 7.9), 0.42 M NaCl, 25% glycerol, 0.2 mM EDTA, 1.5 mM MgCl2, 0.4% NP-40, 1 mM dithiothreitol and 1 × protease inhibitor cocktail) on ice for 30 min, followed by centrifugation at 20,800g for 30 min. The supernatant (nuclear extracts (NE)) was then mixed with 10 μl of anti-FLAG agarose (A2220 Sigma) or anti-HA agarose (A2095 Sigma) and rotated at 4 °C overnight. The beads were then washed three times with buffer D (20 mM HEPES-KOH (pH 7.9), 0.3 M KCl, 15% glycerol, 0.2 mM EDTA and 0.4% NP-40), and eluted with 30 μl 0.1 M glycine-HCl (pH 2.0). For western blot, 3% of the NE input and 50% of the immunoprecipitation eluate were loaded into each NE and immunoprecipitation lane, respectively. Primary antibodies used for western blots are: mouse anti-FLAG (F1804, Sigma), rat anti-HA (11867423001, Roche), rabbit anti-human ELL2 (A302–505A, Bethyl), rabbit anti-human ELL1 (A301–645A, Bethyl), rabbit anti-human AFF1 (A302–344A, Bethyl), mouse anti-human AFF4 (ab57077, Abcam) and mouse anti-human α-tubulin (CP06, EMD CHEMICALS). Secondary antibodies used for western blots are: goat anti-mouse-680 nm (A-21057, Invitrogen), goat anti-rabbit-680 nm (A-21076, Invitrogen) and goat anti-rat-800 nm (612-132-120, Rockland). For endogenous proteins except α-tubulin, the primary antibodies were diluted to 1 μg ml−1, for FLAG/HA tags and α-tubulin, the primary antibodies were diluted 5,000-fold. Secondary antibodies were diluted 10,000-fold.
Luciferase reporter assay
Approximately 6 × 105 HEK 293T cells or 4 × 105 HeLa cells (UC Berkeley Cell Culture facility) in six-well plates were transfected in triplicate by plasmids expressing FLAG-AFF4 and/or ELL2-HA (1 μg each) with the HIV-1 LTR-luciferase construct (0.1 μg). Forty-eight hours after transfection, the cells were collected and lysed in 1 × reporter lysis buffer (E3971 Promega), followed by centrifugation at 20,800g for 1 min. Luciferase activities in the supernatant were measured using the Luciferase Assay System (E1501 Promega) on a Lumat LB 9501 luminometer.
CRISPR/Cas9-mediated knockout of ELL2 gene in HeLa cells
The procedures and single-guide RNA sequence for generating the HeLa-based ELL2-KO knockout (KO) cell line dELL2 (ref. 14) were as follows. Briefly, forward (5′-CACCGAGCGCCCGGATCGCCGTCT-3′) and reverse(5′-AAACAGACGGCGATCCGGGCGCTC-3′) DNA oligos containing the single-guide RNA sequence targeting the first exon of ELL2 were synthesized, annealed and cloned into the pSpCas9(BB)-2A-Puro vector (Addgene plasmid ID: 48,139), and transfected into HeLa cells, which were then selected by puromycin for 2 days, and diluted to single clones. The KO clone was initially identified by anti-ELL2 immunoblotting (Supplementary Fig. 3), and then verified by Sanger sequencing of the targeted genomic site.
Test of the effects of ELL2 mutants on HIV latency reversal
A total of 2 μg plasmids expressing AFF1/4 alone or in combination with ELL2wt or its mutants were nucleofected into 1 × 106 Jurkat 2D10 cells (gift of J. Karn, Case Western Reserve University)30 using Amaxa kit V and the manufacture's protocols (X-005). GFP+ cells indicating the reversal of HIV latency were measured by flow cytometry 48 h post nucleofection. Three biological repeats were done for each group, with their percentages of GFP+ averaged and s.d.'s calculated to generate the error bars. An aliquot of cells from each group were lysed for immunoblotting with the indicated antibodies.
Data availability
Coordinates and structure factor of the structure reported here have been deposited into the Protein Data Bank with PDB Code: 5JW9. All additional experimental data are available from the corresponding author on reasonable request. The PDB Code 1XAW, UniProt accession codes Q9UHB7 and O00472 were used in this study.
Additional information
How to cite this article: Qi, S. et al. Structural basis for ELL2 and AFF4 activation of HIV-1 proviral transcription. Nat. Commun. 8, 14076 doi: 10.1038/ncomms14076 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures and Supplementary Tables.
Acknowledgments
We thank Xuefeng Ren, James Holton and George Meigs for assistance with data collection and Bo Wan for the construction of the HeLa-based ELL2-KO cell line. This work was supported by NIH grants P50GM082250 (J.H.H.), and NIAID R01AI041757 and R01AI095057 (Q.Z.), and NSFC grant 81671388 (Q.S.). The Minstrel crystal farm was purchased with support from the NIH, S10 OD016268. Beamline 8.3.1 at the Advanced Light Source, LBNL, is supported by the U. C. Office of the President, Multicampus Research Programs and Initiatives grant MR-15-328599 and the Program for Breakthrough Biomedical Research, which is partially funded by the Sandler Foundation. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The Stanford Synchrotron Radiation Lightsource is supported by the U.S.D.O.E. under contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by NIH grant P41GM103393.
Footnotes
The authors declare no competing financial interests.
Author contributions S.Q. performed the structural biological study. Z.L. performed the cell biological experiments. U.S.-G. performed the fluorescence polarization assay. G.S. performed hydrogen-deuterium exchange coupled to mass spectrometry (HDX MS) analysis used to optimize crystallization constructs. J.H.H. and S.Q. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
References
- Archin N. M., Sung J. M., Garrido C., Soriano-Sarabia N. & Margolis D. M. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat. Rev. Microbiol. 12, 750–764 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D. M., Garcia J. V., Hazuda D. J. & Haynes B. F. Latency reversal and viral clearance to cure HIV-1. Science 353, aaf6517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelas D. S. & Greene W. C. An integrated overview of HIV-1 latency. Cell 155, 519–529 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonye U. & Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 454, 328–339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. et al. HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol. Cell 38, 428–438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhian B. et al. HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol. Cell 38, 439–451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. et al. AFF1 is a ubiquitous P-TEFb partner to enable Tat extraction of P-TEFb from 7SK snRNP and formation of SECs for HIV transactivation. Proc. Natl Acad. Sci. USA 111, E15–E24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. H. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20, 2629–2634 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D. et al. Function of leukemogenic mixed lineage leukemia 1 (MLL) fusion proteins through distinct partner protein complexes. Proc. Natl Acad. Sci. USA 108, 15751–15756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. J. et al. The super elongation complex family of rna polymerase II elongation factors: gene target specificity and transcriptional output. Mol. Cell. Biol. 32, 2608–2617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol. Cell 37, 429–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P., Davey N. E., Gibson T. J. & Babu M. M. A million peptide motifs for the molecular biologist. Mol. Cell 55, 161–169 (2014). [DOI] [PubMed] [Google Scholar]
- Csizmok V., Follis A. V., Kriwacki R. W. & Forman-Kay J. D. Dynamic protein interaction networks and new structural paradigms in signaling. Chem. Rev. 116, 6424–6462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lu H. & Zhou Q. A minor subset of super elongation complexes plays a predominant role in reversing HIV-1 latency. Mol. Cell. Biol. 36, 1194–1205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirov T. H. et al. Crystal structure of HIV-1 Tat complexed with human P-TEFb. Nature 465, 747–751 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J. et al. Crystal structure of HIV-1 Tat complexed with human P-TEFb and AFF4. Cell Cycle 13, 1788–1797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Gahmen U. et al. The AFF4 scaffold binds human P-TEFb adjacent to HIV Tat. Elife 2, e00327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach B. I. et al. Leukemia fusion target AF9 is an intrinsically disordered transcriptional regulator that recruits multiple partners via coupled folding and binding. Structure 21, 176–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. H., Fanning A. S., Anderson J. M. & Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J. Mol. Biol. 352, 151–164 (2005). [DOI] [PubMed] [Google Scholar]
- Chou S. et al. HIV-1 Tat recruits transcription elongation factors dispersed along a flexible AFF4 scaffold. Proc. Natl Acad. Sci. USA 110, E123–E131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., ZHou Y., Tanaka I. & Yao M. Roll: a new algorithm for the detection of protein pockets and cavities with a rolling probe sphere. Bioinformatics 26, 46–52 (2010). [DOI] [PubMed] [Google Scholar]
- Yokoyama A., Lin M., Naresh A., Kitabayashi I. & Cleary M. L. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell 17, 198–212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C. et al. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J. Leukocyte Biol. 92, 1147–1154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeeusen K., Xiang Y. H., Fujinaga K. & Peterlin B. M. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J. Biol. Chem. 287, 36609–36616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. et al. Reactivation of Latent HIV-1 by Inhibition of BRD4. Cell Rep. 2, 807–816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle 12, 452–462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. C., Guo J., Wu Y. T. & Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 41, 277–287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X. An exact mathematical expression for describing competitive-binding of 2 different ligands to a protein molecule. FEBS Lett. 360, 111–114 (1995). [DOI] [PubMed] [Google Scholar]
- Pearson R. et al. Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J. Virol. 82, 12291–12303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and Supplementary Tables.
Data Availability Statement
Coordinates and structure factor of the structure reported here have been deposited into the Protein Data Bank with PDB Code: 5JW9. All additional experimental data are available from the corresponding author on reasonable request. The PDB Code 1XAW, UniProt accession codes Q9UHB7 and O00472 were used in this study.