Abstract
G protein-coupled receptor kinases (GRKs) mediate desensitization of agonist-occupied G protein-coupled receptors (GPCRs). Here we report that GRK5 contains a DNA-binding nuclear localization sequence (NLS) and that its nuclear localization is regulated by GPCR activation, results that suggest potential nuclear functions for GRK5. As assessed by fluorescence confocal microscopy, transfected and endogenous GRK5 is present in the nuclei of HEp2 cells. Mutation of basic residues in the catalytic domain of GRK5 (between amino acids 388 and 395) results in the nuclear exclusion of the mutant enzyme (GRK5ΔNLS), demonstrating that GRK5 contains a functional NLS. The nuclear localization of GRK5 is subject to dynamic regulation. Calcium ionophore treatment or activation of Gq-coupled muscarinic-M3 receptors promotes the nuclear export of the kinase in a Ca2+/calmodulin (Ca2+/CaM)-dependent fashion. Ca2+/CaM binding to the N-terminal CaM binding site of GRK5 mediates this effect. Furthermore, GRK5, but not GRK5ΔNLS or GRK2, binds specifically and directly to DNA in vitro. Consistent with their presence in the nuclei of transfected cells, all the GRK4, but not GRK2, subfamily members contain putative NLSs. These results suggest that the GRK4 subfamily of GRKs may play a signaling role in the nucleus and that GRK4 and GRK2 subfamily members perform divergent cellular functions.
G protein-coupled receptor kinases (GRKs) comprise a family of seven serine/threonine protein kinases that phosphorylate agonist-bound, activated, G protein-coupled receptors (GPCRs). GRK-mediated GPCR phosphorylation initiates β-arrestin binding, receptor uncoupling from the G protein, and targeting of the β-arrestin-receptor complex to a clathrin-coated pit for internalization. The receptor may then be degraded or returned to the cell surface for a further round of signaling (reviewed in reference 4).
Seven mammalian GRKs have been identified, which are divided into three subfamilies on the basis of sequence homology and the regulatory mechanisms controlling their activity (reviewed in reference 24): the GRK1-like subfamily, GRK1 (or rhodopsin kinase) and GRK7; the GRK2-like subfamily, GRK2 (β-adrenergic kinase) and GRK3 (β-adrenergic kinase 2); and the GRK4-like subfamily, GRK4, GRK5, and GRK6. Four splice variants of GRK4 (α, β, γ, and δ) and three splice variants of GRK6 (A, B, and C) have been identified (24).
GRKs are regulated by several mechanisms, including modulation of their subcellular localization, kinase activity, and expression (reviewed in reference 21). In many instances, regulation of GRK activity is subfamily specific. PKC phosphorylation results in activation of GRK2 but inhibition of GRK5 activity (21). Additionally, the three GRK subfamilies display differential affinities for calcium binding proteins. GRK1 binds Ca2+/recoverin, and GRK4α, GRK5, and GRK6A, -B and -C bind Ca2+/calmodulin (Ca2+/CaM) with high affinity (reviewed in reference 32). In contrast, GRK2 exhibits an approximately 40-fold lower affinity for Ca2+/CaM (32). The binding of Ca2+/CaM prevents the interaction of GRKs with the plasma membrane, thereby inhibiting GRK-mediated GPCR phosphorylation, although GRK-mediated phosphorylation of soluble substrates is unaffected (3, 9).
The emergence of GRK gene deletion and transgenic mouse models has helped reveal the specificity of GPCR regulation by GRKs in vivo. Heterozygous GRK2 knockout mice exhibit supersensitivity to cardiac β-adrenergic stimulation (15), while GRK3 knockout mice exhibit supersensitivity to olfactory stimuli (22) and muscarinic cholinergic airway responsiveness (36). GRK5 knockout mice exhibit impaired desensitization of responses mediated by the muscarinic M2 receptor (12, 35), and GRK6 knockout mice show impaired desensitization of postsynaptic D2-like dopamine receptors (11).
In addition to their established role in mediating GPCR desensitization, recent lines of evidence suggest additional cellular functions of these enzymes. Nonreceptor substrates identified for the GRKs include tubulin (1), synucleins (30), the ribosomal protein P2 (10), the inhibitory gamma subunit of the type 6 retinal cGMP phosphodiesterase (37), and epithelial Na+ channels (7). GRK2 has also been shown to interact with the GRK interactor family of proteins, GTPase-activating proteins for ADP ribosylation factor 6, a small GTP-binding protein (26). The functional relevance of many of these GRK-mediated non-GPCR phosphorylation events remains somewhat unclear. However, GRK2-mediated phosphorylation of the inhibitory gamma subunit of cGMP phosphodiesterase has been demonstrated to play a role in mediating epidermal growth factor receptor-mediated activation of ERK in HEK293 cells (37), and GRK2-mediated epithelial Na+ channel phosphorylation inhibits Nedd4-2-mediated Na+ channel ubiquitination and inhibition (7). These observations suggest that in addition to their characterized signal-attenuating functions, the GRKs also function as signal propagators in a cellular context.
In this study we report the identification of a functional nuclear localization sequence (NLS) in GRK5, a member of the GRK4 subfamily of GRKs. The nuclear localization of GRK5 is regulated in a GPCR and Ca2+/CaM-dependent fashion, and its NLS mediates DNA binding in vitro. These findings hint at previously unsuspected nuclear functions for this enzyme. Furthermore, potential NLSs are found in other GRK4, but not GRK2, subfamily members, suggesting divergent cellular roles for these GRK subfamilies.
MATERIALS AND METHODS
Cell culture.
CHO cells stably expressing the muscarinic M3 receptor (a generous gift of Andrew Tobin, University of Leicester) were maintained in modified Eagle medium alpha without nucleosides (GIBCO) containing 10% fetal calf serum (Sigma) and penicillin and streptomycin (100 IU of penicillin and 100 μg of streptomycin/ml; Sigma) at 37°C, 5% CO2. All other cells were maintained in Dulbecco's modified Eagle medium (GIBCO) containing 10% fetal calf serum (Sigma) and penicillin and streptomycin (100 IU of penicillin and 100 μg of streptomycin/ml; Sigma) at 37°C, 5% CO2.
cDNA constructs.
GRK5ΔNLS; pRK5-GRK5 (23) was used as a template to create GRK5ΔNLS using the Quikchange site-directed mutagenesis kit (Stratagene). Basic residues located between amino acids 388 to 395 were mutated to alanine residues using the following primers: sense primer, 5′-C CAG TCG CCC TTC CGC GGC GCT GCT GAG GCT GTG GCT GCT GAG GAG GTG GAC CGC CGG-3′; antisense primer, 5′-CCG GCG GTC CAC CTC CTC AGC AGC CAC AGC CTC AGC AGC GCC GCG GAA GGG CGA CTG G-3′. Nucleotides in bold encode the mutated amino acids. Integrity of the GRK5ΔNLS construct was confirmed by direct DNA sequencing. The plasmids for pRK5-GRK5NTPB (23), pRK5-GRK5CTPB (23), pRK5-GRK2 (25), pcDNA1-GRK3 (18), pRK5-GRK4α (28), pBK(Δ)-GRK6A (13), pEYFP-CaMBP/m, and pEYFP-CaMBP/n (17, 38) have been described previously. pEYFP-CaMBP constructs were generous gifts of Marcia A. Kaetzel and John Dedman (University of Cincinnati College of Medicine) and David Sacks (Harvard Medical School). pRK5-GRK5K215R was a generous gift from Robert J. Lefkowitz (HHMI/Duke University Medical Center).
Immunofluorescence.
HEp2 cells were grown to 60 to 70% confluency prior to transfection by electroporation in HEBS buffer (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM d-glucose), using two 450-V, 125-μF pulses (Gene Electropulser II; Bio-Rad) and 0.5 μg of the relevant cDNA. CHO and Cos-7 cells at approximately 50% confluency were transfected using Gene Juice (Novagen) according to the manufacturer's instructions. Twenty-four hours posttransfection, cells were treated as described in the figure legends. Cells treated with calcium ionophore were incubated with 25 μM A23187 (Calbiochem) for 15 min, unless otherwise indicated, in medium supplemented with 0.6 mM CaCl2 at 37°C, 5% CO2. Acetylcholine (ACh) (Sigma) was used at a concentration of 100 μM for 5 min, and the phospholipase C inhibitor U73122 (Sigma) was used at 1 μM for 1 h unless otherwise specified. Following treatment, cells were fixed in 4% paraformaldehyde (TAAB)-phosphate-buffered saline (PBS) for 20 min and quenched for 10 min in 0.27% NH4Cl-0.37% glycine in PBS. Two percent bovine serum albumin (First Link UK Ltd.)-0.2% saponin (Sigma)-PBS was subsequently used to block and permeabilize fixed cells. Primary antibody incubations with mouse anti-GRK4-6 antibody (Upstate), mouse anti-GRK2/3 antibody (Upstate) at a 1:300 dilution, or a rabbit anti-muscarinic-M3 antibody at a 1:50 dilution (Andrew Tobin, University of Leicester) were performed for 1 h at room temperature. Cells were subsequently washed with 2% bovine serum albumin-0.2% saponin-PBS and incubated with alexa-fluor 594/488 donkey anti-mouse or anti-rabbit immunoglobulin G (Molecular Probes) at a 1:800 dilution for 45 min at room temperature. Yellow fluorescent protein (YFP) constructs were not subject to immunostaining. After washing, coverslips were mounted on slides in 90% glycerol (Sigma)-PBS-3% N-propyl-galate (Sigma). Essentially the same protocol was used for the detection of endogenous GRK5 in HEp2 cells with the following exceptions; a rabbit anti-GRK5 (C20) antibody (Santa Cruz) was used at a 1:200 dilution, and the primary antibody incubation was performed overnight at 4°C. Additionally, the fluorescently labeled secondary antibodies described above were replaced with a tyramide signal amplification kit (Molecular Probes), which was used according to the manufacturer's instructions.
Confocal images were taken at room temperature, using a Bio-Rad MRC 1024 laser scanning confocal system with Nikon Plan Apo ×60 oil immersion lens and Optiphot 2 microscope using Bio-Rad Lasersharp 2000 software to acquire the images. Images were optimized for contrast in Adobe Photoshop, but no further manipulations were made.
DNA binding assay.
Cos-7 cells expressing GRK5, GRK5ΔNLS, or GRK2 were lysed in a solution containing 10 mM HEPES (pH 7.4), 0.1% Triton X-100, 0.1 M NaCl, 10% glycerol, and 0.05 mM EDTA supplemented with protease inhibitors and lysates and clarified by centrifugation at 13,000 × g for 20 min. Cell lysates (10 μg of mock-transfected, GRK5, and GRK5ΔNLS cells and 5 μg of GRK2 cells) or purified GRK5 (150 ng) were incubated with 25 μl of native DNA-cellulose (Amersham), single-stranded DNA-cellulose (Sigma), poly(A)-Sepharose 4B (Amersham), or cellulose (Sigmacell Cellulose type 50; Sigma) in 100 μl of cold binding buffer (10 mM HEPES [pH 7.4], 1 mM MgCl2, 0.1% Triton X-100, 3 mM dithiothreitol, 0.1 M NaCl, 0.05 mM EDTA) for 1 h at 4°C. For competition assays, GRK5 was preincubated with the stated amounts of DNA (sonicated, calf thymus; Amersham) or RNA (calf liver type IV; Sigma) for 30 min on ice and then added to 25 μl of native DNA-cellulose. Following incubation, the resin was washed four times with 1 ml of binding buffer, and the amount of GRK retained on the resin was determined by Western blot analysis. Blots were developed with ECL (Amersham) and films quantified using a densitometer (Bio-Rad). Significance was determined by performing a t test.
GRK5 kinase assays.
Kinase assays were performed with a buffer containing 20 mM Tris-HCl (pH 7.5), 2.0 mM EDTA, 10 mM MgCl2, and 1 mM dithiothreitol containing 50 μM ATP (∼6,000 cpm/pmol) in a total volume of 25 μl. Purified rod outer segments (ROS), prepared as described in reference 20 (1 μg), or purified tubulin (cytoskeleton; 10 μg) was included as a GRK substrate where appropriate.
When measuring DNA- or RNA-mediated inhibition of GRK5 or GRK5ΔNLS activity, transfected Cos-7 cell lysates were used as the source of GRK5. Cos-7 cells were transfected with 2 μg of the appropriate cDNA, using Gene Juice (Novagen) according to the manufacturer's instructions. Twenty-four hours posttransfection, cells were lysed in 1 ml of buffer (20 mM HEPES [pH 7.2], 250 mM NaCl, 10 mM EDTA, 0.02% Triton X-100 supplemented with protease inhibitors) by polytron homogenization (two 15-s pulses), and lysates were clarified by centrifugation at 13,000 × g for 10 min. Equivalent rhodopsin kinase activities of GRK5 and GRK5ΔNLS were used in the assays, corresponding to ∼0.6 and ∼1.0 μg of total protein, respectively. For each assay condition, the kinase activity present in an equivalent amount of protein from a mock-transfected lysate was determined and subtracted from that obtained with the GRK5-expressing lysate.
The activity comparison of GRK5, GRK5ΔNLS, GRK5NTPB, GRK5CTPB, and GRK5K215R (see Fig. 2B) was performed with partially purified transfected Cos-7 cell extracts. GRK5NTPB is expressed at approximately 20% of the level of the other GRK5 constructs, and detecting GRK5NTPB-mediated tubulin phosphorylation above a background of endogenous kinases proved difficult without this enrichment step. Cells transfected with the appropriate cDNA were lysed as described above. Lysates (∼2 mg of protein) were diluted twofold in buffer A (20 mM HEPES [pH 7.2], 2 mM EDTA, supplemented with protease inhibitors) and loaded onto 0.5-ml S-Sepharose columns by gravity flow. The columns were washed sequentially with 2.5 ml of buffer A, buffer A plus 400 mM NaCl, and buffer A plus 1 M NaCl. Wild-type GRK5 and mutant kinases eluted in the 1 M NaCl wash were subsequently desalted and concentrated in Nanosep 30K centrifugal devices (Pall Life Sciences). A total of three separate transfections and three S-Sepharose columns were run for each enzyme. Before activity was assayed, the individual purifications were pooled and the amount of GRK5/unit volume was quantified by Western blot analysis. The amount of total protein assayed was adjusted such that equivalent amounts of all kinases were used in the reactions. This ranged between 200 and 300 ng of protein with the exception of GRK5NTPB-expressing lysates, where approximately five times more protein was used. Assays using equivalent amounts of protein derived from a partially purified mock-transfected lysate were used to determine endogenous kinase activity, which was subsequently subtracted from the relevant kinase-expressing lysate activity value.
FIG. 2.
The nuclear export of GRK5 requires the interaction of Ca2+/CaM with the N-terminal CaM-binding site of the kinase. (A) Sequestration of cytosolic CaM does not affect the Ca2+-dependent nuclear export of GRK5. HEp2 cells were cotransfected with GRK5 and YFP-tagged CaMBP/m. Twenty-four hours posttransfection, cells were either left untreated (a and c) or treated with A23187 (b and d), and expressed proteins were visualized as described in Methods. (B) Sequestration of nuclear CaM inhibits Ca2+-dependent nuclear export of GRK5. HEp2 cells cotransfected with GRK5 and YFP-tagged CaMBP/n were treated as described for panel (A) Panels a and c show the distribution of, respectively, GRK5 and CaMBP/n in untreated cells, and panels b and d show the distribution of GRK5 and CaMBP/n in ionophore-treated cells. (C) The cellular distribution of GRK5 (a), GRK5NTPB (c), GRK5CTPB (e), and GRK5K215R (g) expressed in HEp2 cells was visualized by fluorescence confocal microscopy immunofluorescence as described in Materials and Methods. Where indicated, cells were treated with calciumionophore (A23187) prior to fixation (b, d, f, and h). Scale bars, 10 μM. (D and E) Rhodopsin and tubulin kinase activities of wild-type and mutant GRK5s. Partially purified Cos-7 cell lysates expressing GRK5, GRK5ΔNLS, GRK5NTPB, GRK5CTPB, and GRK5K215R were assayed for their ability to phosphorylate the GPCR substrate rhodopsin (D) and the soluble substrate tubulin (E) as described in Materials and Methods. The results shown for each enzyme represent the average activities ± standard errors of the means for three separate transfections and partial purifications that were subsequently pooled and assayed in triplicate. Assays were performed using equivalent amounts of GRK5 protein as determined by quantitation of Western blots, using a densitometer.
GRK5 was overexpressed and purified from baculovirus-infected SF9 cells according to previously published procedures (27). Seventy-five nanograms of purified GRK5 was used in each assay.
All reactions were incubated at 30°C for 10 min; when rhodopsin was used as a substrate, samples were illuminated during this incubation period. Reactions were stopped by addition of an equal volume of sample loading buffer (8% sodium dodecyl sulfate, 25 mM Tris-HCl [pH 6.5], 10% glycerol, 5% mercaptoethanol, 0.003% bromophenol blue) and electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels. The dried gels were analyzed and quantified, using a phosphorimager (Bio-Rad), and significance was determined by performing a t test.
RESULTS
GRK5 contains a functional nuclear localization sequence.
Expression of GRK2 and GRK5 in a variety of mammalian cell lines (human epithelial 2 [HEp2], Chinese hamster ovary [CHO], HeLa, Cos-7, and human embryonic kidney 293 cells) reveals distinct cellular distributions for these two related kinases. GRK2 is cytoplasmic and is excluded from the nucleus, whereas GRK5 is ubiquitously distributed throughout the cell (Fig. 1A, panels a and b). In an attempt to account for this differential distribution, we searched for a potential NLS in GRK5 (5). A putative NLS was identified in the catalytic domain of GRK5 between amino acids 388 and 395 (388R K E K V K R E395). Mutation of basic amino acids in this sequence to alanine residues (388A A E A V A A E395) resulted in the nuclear exclusion of the mutant kinase (GRK5ΔNLS) (compare Fig. 1A, panels b and c). The cytoplasmic distribution of the GRK5ΔNLS mutant suggests that GRK5 contains a functional NLS, a somewhat surprising finding since no nuclear substrates or functions for the GRKs have been identified to date.
FIG. 1.
GRK5 contains a functional NLS and exhibits calcium-dependent nuclear export. (A) GRK5, but not GRK2 or GRK5ΔNLS, can be detected in the nuclei of transfected HEp2 cells. The cellular distribution of transiently transfected GRK2 (a), GRK5 (b), and GRK5ΔNLS (c) expressed in HEp2 cells was visualized by immunofluorescence as described in Materials and Methods. (B) The effect of calcium ionophore treatment on the subcellular distribution of GRK5 and GRK5ΔNLS. HEp2 cells overexpressing GRK5 were untreated (a) or treated with the calcium ionophore A23187 (b). In an analogous fashion, panels c and d show the distribution of GRK5ΔNLS in untreated and ionophore-treated cells, respectively. (C) Subcellular distribution of endogenous GRK5 in HEp2 cells, visualized by immunofluorescence as previously described. Where indicated, cells were treated with calcium ionophore, A23187, prior to fixation. Scale bars, 10 μM.
Calcium ionophore treatment promotes the nuclear export of GRK5.
To determine if the nuclear localization of GRK5 is modulated by extrinsic signals, HEp2 cells transfected with GRK5 or GRK5ΔNLS were subjected to various stimuli. Calcium ionophore (A23187) treatment stimulated nuclear export of GRK5 (compare Fig. 1B, panels a and b) without affecting GRK5ΔNLS distribution (Fig. 1B, panels c and d). The transfected GRK5 (Fig. 1B) and endogenous GRK5 (Fig. 1C) display similar subcellular distributions and calcium ionophore-dependent nuclear export. As assessed by Western blot analysis, the polyclonal anti-GRK5 antibody (Santa Cruz) used here and in a previous study (40) to detect endogenous GRK5 recognizes expressed GRK5, but not GRK6, in lysates of transfected cells (data not shown). Taken together, these results indicate that both transfected and endogenous GRK5 is exported from the nucleus in a Ca2+-dependent fashion.
The nuclear export of GRK5 is calmodulin dependent.
The cellular actions of calcium are largely mediated through a family of calcium binding proteins, of which CaM is the major calcium sensor. Since GRK5 binds Ca2+/CaM with high affinity (equilibrium dissociation constant of ∼8 nm) (16), we investigated a potential role for CaM in mediating the calcium-dependent nuclear export of GRK5. A palmitoylated or unmodified YFP-tagged peptide encoding the CaM-binding sequence of rabbit skeletal muscle myosin light chain kinase was used to sequester, respectively, cytosolic membrane-bound (CaMBP/m) or nuclear (CaMBP/n) CaM (17, 38). Calcium-dependent nuclear export of transfected GRK5, is unaffected by coexpression of CaMBP/m. Figure 2A shows the subcellular distribution of coexpressed GRK5 and CaMBP/m in untreated (a and c) or ionophore-treated (b and d) cells. In contrast to cytosolic CaM sequestration, expression of CaMBP/n, and thereby sequestration of nuclear CaM, inhibits ionophore-dependent nuclear export of GRK5 (Fig. 2B, panels a and b show, respectively, the distribution of GRK5 in untreated and ionophore-treated cells, and panels c and d show CaMBP/n expression in the same cells). These results indicate that Ca2+/CaM mediates calcium ionophore-dependent nuclear export of GRK5 and suggest that the interaction of nuclear Ca2+/CaM with nuclear GRK5 is required for this process.
Binding of Ca2+/CaM to the amino-terminal CaM-binding site of GRK5 is required for nuclear export.
Two CaM binding sites have been identified in GRK5, located between residues 20 to 39 in the N terminus and residues 540 to 578 in the C terminus of the enzyme (16). These regions of the kinase contain clusters of basic and hydrophobic residues characteristic of CaM-binding sites identified in other CaM target proteins (31). To determine if Ca2+/CaM-mediated nuclear export of GRK5 is dependent on the direct interaction of activated CaM with GRK5, the calcium-dependent nuclear export of mutant GRK5 constructs lacking the N-terminal polybasic (GRK5NTPB) or C-terminal polybasic (GRK5CTPB) CaM binding sites was examined. Mutation of basic residues in the N-terminal (GRK5NTPB; Fig. 2C, panels c and d) but not C-terminal (GRK5CTPB; Fig. 2C, panels e and f) CaM binding site of GRK5 results in a mutant form of the kinase which is almost exclusively nuclear and whose distribution is unaffected by ionophore treatment. These findings suggest that the direct interaction of Ca2+/CaM with the N-terminal CaM-binding site of GRK5 is responsible for mediating nuclear export. Furthermore, the more-nuclear localization of GRK5NTPB than of GRK5 in untreated cells suggests that under basal conditions, endogenous Ca2+/CaM binding to this site may contribute to the ubiquitous distribution of the wild-type kinase (compare panels a and c of Fig. 2C).
The abilities of GRK5, GRK5ΔNLS, GRK5NTPB, GRK5CTPB, and GRK5K215R (a catalytically inactive form of the kinase) to phosphorylate light-activated rhodopsin (Fig. 2D) and tubulin (Fig. 2E) were assessed using partially purified Cos-7 cell lysates expressing these kinases. GRK5NTPB, although approximately 50% less active than wild-type GRK5, has activity against both substrates similar to that of GRK5CTPB, a mutant form of the kinase which exhibits ionophore-dependent nuclear export (Fig. 2D and 2E). These results suggest that impaired CaM binding, rather than impaired kinase activity, most likely explains the inability of GRK5NTPB to exit the nucleus in a calcium-dependent fashion (Fig. 2C, panels c and d). Indeed, the catalytically inactive mutant of GRK5 (GRK5K215R) (Fig. 2D and E) is exported from the nucleus following ionophore treatment (Fig. 2C, panels g and h), demonstrating that Ca2+/CaM-dependent nuclear export of GRK5 does not require kinase activity. Furthermore, the observation that GRK5ΔNLS has kinase activity comparable to that of wild-type GRK5 (Fig. 2D and E) suggests that the nuclear exclusion of this mutant enzyme (Fig. 1B, panel c) is not simply a consequence of mutation-induced gross structural abnormalities.
Activation of a Gq-coupled receptor promotes the nuclear export of GRK5.
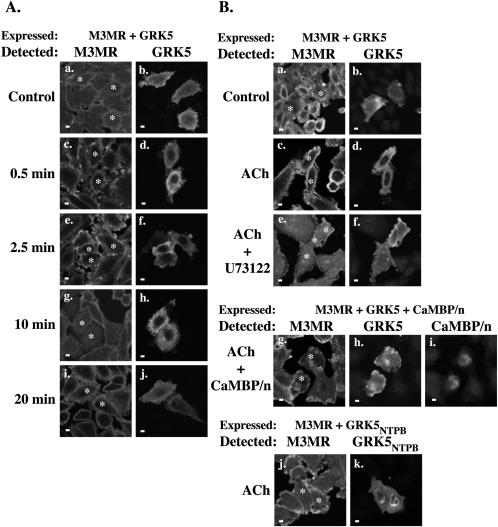
Given that Ca2+/CaM regulates GRK5 subcellular localization, we hypothesized that GPCRs that increase intracellular levels of calcium may promote the nuclear export of GRK5. To this end, the distribution of GRK5 was examined in CHO cells stably expressing the Gq-coupled muscarinic-M3 receptor (CHO-M3MR) and transiently expressing GRK5. Agonist stimulation of M3MR with acetylcholine (ACh) promotes the rapid and reversible nuclear export of GRK5 (Fig. 3A; left panels show M3MR, and right panels show GRK5 staining). Three lines of evidence indicate that M3MR-mediated nuclear export of GRK5 is Ca2+/CaM dependent. Firstly, U73122-mediated inhibition of phospholipase C, and thus inositol trisphosphate production, inhibits M3MR-dependent nuclear export of GRK5 (Fig. 3B, compare panels c and d [plus ACh] with e and f [plus ACh, plus U73122]). These results suggest that Gq-mediated increases in cellular calcium levels are important for this process. Second, coexpression of GRK5 and the nuclear CaM sequestrant (CaMBP/n) in this M3MR-expressing cell line prevents agonist-dependent GRK5 redistribution (Fig. 3B, panels g, h, and i). Third, GRK5NTPB, a mutant form of the kinase lacking the N-terminal CaM-binding site, fails to exit the nucleus following M3MR activation (Fig. 3B, panels j and k). Together these results indicate that activation of a Gq-coupled GPCR can induce the cytoplasmic redistribution of GRK5 via a mechanism that requires the interaction of Ca2+/CaM with the N-terminal CaM-binding site of GRK5.
FIG. 3.
Muscarinic-M3 receptor activation promotes the nuclear export of GRK5. (A) CHO-M3MR cells were transfected with GRK5 and treated with ACh for the indicated times (min). M3MR and GRK5 in coexpressing cells were visualized by immunofluorescence. Asterisks indicate cotransfected cells. Scale bars, 10 μM. (B) CHO-M3MR cells were transfected with GRK5 (a to f), GRK5, and CaMBP/n (g, h, and i) or GRK5ΔNTPB (j and k). Transfected cells were subsequently left untreated (Control) or treated with ACh for 5 min, and GRK5 and M3MR were detected as previously described. Where indicated (U73122), cells were pretreated with 1 μM U73122 for 1 h prior to agonist treatment. Asterisks indicate cotransfected cells. Scale bars, 10 μm.
GRK5 binds DNA in vitro.
In an attempt to elucidate a potential role for nuclear GRK5, the functions of proteins containing NLSs of similar sequence were examined. Somewhat surprisingly, the NLS of GRK5 shares sequence homology with the DNA binding NLSs of homeobox-containing transcription factors (5). One hundred thirty-two proteins were identified as containing an NLS conforming to the same consensus sequence as that found in GRK5 (R[R/K]X[K/R]X[R/K]2[D/E]) (5). All of these proteins contained a homeodomain, and of the 119 demonstrated to bind DNA, 116 contain the NLS as part of the DNA binding site (5). To assess whether GRK5 binds DNA in vitro, Cos-7 cell lysates expressing GRK5, GRK5ΔNLS, and GRK2 were incubated with native DNA-cellulose, single-stranded DNA (ssDNA)-cellulose, or poly(A)-4B-Sepharose. GRK5, but not GRK5ΔNLS or GRK2, binds native and ssDNA-cellulose in vitro (Fig. 4A). Approximately 25% of the applied GRK5 was retained on both native and single stranded DNA cellulose (Fig. 4A). In marked contrast, very little GRK5ΔNLS and GRK2 bound these resins (∼4% of the load) (Fig. 4A). No significant binding to poly(A)-4B-Sepharose was detected for any of the kinases (Fig. 4A), suggesting that a nonspecific ionic interaction is unlikely to account for the binding of GRK5 to DNA. Consistent with these observations, DNA but not RNA inhibits the binding of GRK5 to native DNA-cellulose (Fig. 4B). The addition of 20 μg of DNA or RNA to the binding assay results in, respectively, an 80% ± 8% or a 22% ± 15% reduction in binding of GRK5 to immobilized DNA (P < 0.01). No detectable binding of GRK5 to the cellulose support was observed under these conditions (Fig. 4B), reinforcing the suggestion that the interaction of GRK5 with DNA is specific.
FIG. 4.
The NLS of GRK5 binds DNA in vitro. (A) GRK5, but not GRK5ΔNLS or GRK2, binds DNA in vitro. Lysates of cells expressing GRK5, GRK5ΔNLS, or GRK2 were incubated with native DNA-cellulose, ssDNA-cellulose, or poly(A)-4B-Sepharose, and after extensive washing the amount of bound GRK was determined by Western blot analysis. A representative Western blot of one such experiment is shown. L, 25% of total lysate loaded; B1, native DNA-cellulose-bound GRK; B2, ssDNA cellulose-bound GRK; B3, poly(A)-4B-Sepharose-bound GRK. Quantification of Western blot analyses from multiple experiments is represented graphically. The y axis represents the percentage of loaded GRK5 that remained bound to the resin. The data shown represent the mean values ± standard errors of the mean for at least three experiments. **, P < 0.01. (B) DNA, but not RNA, inhibits the interaction of GRK5 with native DNA-cellulose. Lysates (10 μg) of Cos-7 cells overexpressing GRK5 were incubated with native DNA-cellulose, following a 30-min preincubation with the indicated amounts of DNA or RNA. Additionally, the interaction of GRK5 with cellulose was examined. After extensive washing, the amount of resin-associated GRK5 was determined by Western blot analysis. The amount of GRK5 bound to native DNA-cellulose in the absence of DNA or RNA addition, 24% of the total GRK5 loaded, was normalized to 100%. The data shown represent the means ± standard errors of the means for at least four separate determinations. **, P < 0.01. (C) DNA inhibits GRK5, but not GRK5ΔNLS, activity. Cos-7 cell lysates expressing GRK5 or GRK5ΔNLS were assayed for their ability to phosphorylate light-activated rhodopsin in the presence of the indicated amounts of DNA (black columns) or RNA (shaded columns). Kinase assays were performed as described in Materials and Methods. The activities of the wild-type and mutant kinases in the absence of DNA or RNA were equivalent and were normalized to 100% activity. The results shown represent the means ± standard errors of the means for three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) DNA inhibits the kinase activity of purified GRK5. Kinase assays were performed as described for panel C and inMaterials and Methods, with the exception that purified GRK5, rather than a GRK5-expressing cell lysate, was used as the source of the kinase activity. The amount of purified GRK5 added to the assay was adjusted such that its rhodopsin kinase activity in the absence of DNA addition, 100% activity, was similar to that observed with GRK5-expressing lysates under similar conditions. The results shown represent the means ± standard errors of the means for three experiments. *, P < 0.05; ***, P < 0.001. (E) Purified GRK5 binds directly to DNA. Assays were performed as described for panel B, except that 150 ng of purified GRK5 rather than 10 μg of GRK5-expressing cell lysate was used in the binding assay. The amount of GRK5 bound to native DNA-cellulose in the absence of DNA was normalized to 100% and was 40% of the total GRK5 loaded. The results shown represent the means ± standard errors of the means for at least five experiments. *, P < 0.05; ***, P < 0.001.
Additional evidence supporting the hypothesis that GRK5 binds specifically to DNA via its NLS is provided in Fig. 4C. Cos-7 cell lysates expressing GRK5 or GRK5ΔNLS were assessed for their ability to phosphorylate light-activated rhodopsin in the presence of increasing concentrations of DNA or RNA. As shown in Fig. 4C, when assayed at equivalent rhodopsin kinase activities, GRK5 is more potently inhibited by DNA than its mutant counterpart (Fig. 4C, black columns). The addition of 150 ng of DNA inhibits GRK5 activity by 93% ± 0.2% and GRK5ΔNLS activity by 28% ± 5% (P < 0.001), results which suggest that DNA binding is mediated by the NLS of GRK5. RNA is a much less effective inhibitor of GRK5 activity than DNA; 150 ng of RNA inhibits GRK5 activity by 58% ± 4% (Fig. 4C, left side, shaded columns). GRK5ΔNLS activity is unaffected by RNA addition over the range of concentrations used (Fig. 4C, right side, shaded columns).
Together, the results shown in Fig. 4A, B, and C indicate that GRK5 binds specifically to DNA in vitro and that the NLS of GRK5 is required for this interaction.
Since cell lysates expressing GRK5 were used for these experiments, however, we could not exclude the possibility that the binding of GRK5 to DNA is indirect. To determine if GRK5 binds directly to DNA, we assayed the rhodopsin kinase activity of purified GRK5 (∼93% pure) in the presence of increasing concentrations of DNA and RNA (Fig. 4D) and assessed the ability of the purified kinase to bind native DNA-cellulose (Fig. 4E). Consistent with the results obtained with lysates expressing GRK5, DNA potently inhibits the kinase activity of purified GRK5 (Fig. 4D, black columns). RNA proved a much less effective inhibitor of the purified enzyme, the addition of 200 ng of DNA or RNA resulting in, respectively, a 93% ± 1% and 55% ± 3% inhibition of kinase activity (P < 0.001). These results, which indicate that GRK5 binds directly and specifically to DNA, are supported by experiments in which the interaction of purified GRK5 with native DNA-cellulose was examined. As shown in Fig. 4E, purified GRK5 binds specifically to native DNA-cellulose, and its binding is inhibited more potently by DNA than by RNA (Fig. 4E).
Overall, these results demonstrate that GRK5 binds directly to DNA in vitro via its NLS and provocatively suggest hitherto-unsuspected functions for this enzyme.
GRKs of the GRK1 and GRK4 subfamilies contain putative NLSs.
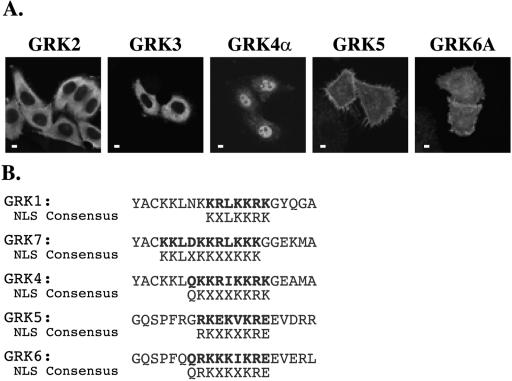
The members of the GRK1 and GRK4, but not GRK2, subfamilies are predicted to contain putative NLSs, shown in Fig. 5B (5). Consistent with this observation, GRK5, GRK4α and GRK6A but not GRK2 or GRK3 can be detected in the nuclei of HEp2 cells expressing these kinases (Fig. 5A). The NLS of GRK5, located towards the C terminus of the catalytic domain of the enzyme, is essentially identical in position and sequence to the putative NLS of GRK6, suggesting that GRK6 may also contain a functional DNA-binding NLS. In contrast, the predicted NLS of GRK4 is distinct from that of GRK5 and GRK6, is located within the N terminus of the catalytic domain, and is homologous to the predicted NLS of the GRK1 subfamily (Fig. 5B). The NLSs of GRK1, -7, and -4 are not predicted to bind DNA (5). The fact that GRK2 and -3 are excluded from the nucleus and do not contain an NLS suggests divergent functions for the GRK2 and GRK1/4 subfamilies. Furthermore, the distinct locations and predicted DNA binding properties of the putative NLSs within the GRK1 and -4 subfamilies may reflect different nuclear functions of these enzymes.
FIG. 5.
Members of the GRK1 and -4, but not GRK2, subfamilies contain putative NLSs. (A) The subcellular localization of GRK family members when overexpressed in HEp2 cells as determined by fluorescence confocal microscopy. Scale bars, 10 μm. (B) Regions of the catalytic domains of GRK subfamily members containing putative NLSs, indicated in bold. The consensus NLS sequences are shown below each GRK sequence. Amino acid sequences shown are as follows: GRK1, Y216 to A235; GRK7, Y217 to A236; GRK4α/γ, Y213 to A232; GRK4β/δ, Y181 to A200; GRK5, G381 to R400; GRK6, G381 to L400.
DISCUSSION
GRKs phosphorylate and desensitize activated GPCRs and thereby play an important role in regulating intracellular signal transduction cascades. The identification of nonreceptor substrates for these kinases suggests that they may play a more diverse, and direct, role in regulating signaling than previously appreciated. In this study we demonstrate that GRK5, a member of the GRK4 subfamily of GRKs, contains a DNA-binding NLS. Furthermore, the nuclear localization of GRK5 is regulated in a GPCR-dependent fashion. Agonist occupancy of the M3MR, which elevates intracellular calcium levels and activates the calcium sensor protein, CaM, promotes nuclear export of GRK5. The binding of activated CaM to the N terminus of GRK5 is required for this export event. By regulating the nuclear localization of GRK5 and thus, possibly, the ability of the kinase to bind DNA in cells, Gq-coupled GPCRs could regulate potential nuclear functions of this kinase. That other GRKs contain putative NLSs and can be detected in the nuclei of transfected cells suggests that a signaling role in the nucleus may be a common feature of some members of this kinase family.
In this study, transfected and endogenous GRK5 was detected in the nuclei of HEp2 cells using fluorescence confocal microscopy (Fig. 1). Similarly, Yi and colleagues have reported endogenous GRK5 in the nuclei of rat cardiac myocytes (40). Treatment of these cells with 12-o-tetradecanoylphorbol-13-acetate (TPA), a strong PKC activator, results in a more pronounced nuclear localization of the endogenous kinase (40). GRK5 is itself a PKC substrate (40), and the authors propose that PKC-mediated phosphorylation of GRK5 may lead to its nuclear accumulation. Conversely, in this study the binding of Ca2+/CaM to the N-terminal CaM-binding site of GRK5 is demonstrated to result in nuclear export of the kinase (Fig. 2B). Combined, these observations suggest that multiple regulatory mechanisms control the intracellular, and specifically nuclear, localization of GRK5.
The binding of Ca2+/CaM to GRK5 potently inhibits GRK5-mediated GPCR phosphorylation (50% inhibitory concentration, ∼50 nM) (32). Ca2+/CaM binding prevents the interaction of GRK5 with both phospholipids and activated receptors without directly affecting catalytic activity (32). Of the two identified CaM-binding sites in GRK5, the C-terminal site appears to be principally responsible for Ca2+/CaM-mediated inhibition of membrane-located substrate phosphorylation (16). Our study suggests a distinct role for the N-terminal CaM-binding site of GRK5, since this site is responsible for CaM-dependent nuclear export of the kinase (Fig. 2B). The N-terminal CaM-binding site of GRK5 is coincident with a phosphatidylinositol-4,5-bisphosphate (PIP2)-binding site, and the interaction of these two ligands with GRK5 is likely to be competitive (9). Calcium ionophore treatment would not be anticipated to modulate intracellular PIP2 levels yet promotes the nuclear export of GRK5. This, coupled with the observation that a CaM sequestrant inhibits nuclear export, leads us to propose that CaM rather than PIP2 binding to the N terminus of GRK5 is responsible for mediating these effects. A role for PIP2, potentially antagonistic to that of CaM, in regulating the nuclear localization of this kinase cannot be ruled out, however.
As described previously, Ca2+/CaM binding inhibits GRK5-mediated GPCR phosphorylation. Additionally, CaM stimulates autophosphorylation of GRK5, which further contributes to impaired receptor phosphorylation by inhibiting membrane localization of the kinase (29). The effects of CaM binding on GRK5-mediated GPCR phosphorylation coupled with the observation that PKC-mediated GRK5 phosphorylation directly inhibits the catalytic activity of the kinase (29) suggest that agonist-occupied Gq-coupled receptors and thus the M3MR represent poor substrates for this kinase. Three lines of evidence are consistent with this hypothesis. First, overexpression of catalytically inactive GRK6 but not GRK5 inhibits M3MR receptor desensitization induced by endogenous GRKs in human neuroblastoma SH-SY5Y cells (39). Second, mice lacking the GRK5 gene exhibit no difference in M3MR-mediated agonist-induced contraction of airway smooth muscle compared to wild-type littermates (35). Third, overexpression of GRK5 in CHO cells stably expressing the M3MR fails to promote agonist-dependent internalization of the receptor (Fig. 3A, left panels). Although apparently not a GRK5 substrate, the M3MR does play a role in regulating the subcellular localization of GRK5. Following activation of the M3MR, GRK5 is translocated to the cytosol (Fig. 3A); potential nuclear functions of this kinase may thus be negatively regulated. Since DNA inhibits GRK5 activity (Fig. 4C and D), putative nuclear functions of GRK5 would be expected to be kinase independent and presumably related solely to the ability of the enzyme to bind DNA.
How might binding of Ca2+/CaM to the N terminus of GRK5 promote nuclear export of the kinase? GRK5 binds DNA via its NLS in vitro, and it is tempting to speculate that DNA binding in a cellular setting may contribute to the nuclear retention of this enzyme. Binding of Ca2+/CaM to the N terminus of GRK5 may dissociate GRK5 from DNA, facilitating its nuclear export. A number of scenarios for how this may be affected can be envisaged. Ca2+/CaM binding may sterically inhibit the interaction of GRK5 and DNA, alter the conformation of the enzyme such that the DNA-binding NLS is masked and thereby inactivated, or, alternatively, it could prevent DNA binding by stimulating autophosphorylation of the kinase. Calreticulin, a calcium binding protein originally identified in the lumen of the endoplasmic reticulum, binds directly to the DNA-binding domain of glucocorticoid receptors (6). This interaction inhibits DNA binding and mediates nuclear export of the nuclear hormone receptor (6). In a similar vein, Ca2+/CaM binds to the DNA binding domain of members of the basic helix-loop-helix family of transcription factors to inhibit their interaction with DNA (14). The possibility exists that the NLS of GRK5 is itself a Ca2+/CaM-binding domain, the accessibility of which is regulated by the interaction of Ca2+/CaM with the N terminus of the enzyme. The regulatory mechanisms modulating GRK5/DNA binding and the proteins required for the nuclear export of this kinase remain to be established.
Notably, there is some evidence linking GRK5 expression to changes in gene expression (8). Cardiac hypertrophy, the physiological response of the heart to an increased work load, is associated with transcriptional activation of genes encoding embryonic markers, including atrial natriuretic factor (ANF), α-skeletal actin, and β-myosin heavy chain (reviewed in reference 2). Transgenic mice expressing a constitutively activated mutant of the α1B-adrenergic receptor in the heart exhibit myocardial hypertrophy and have elevated diacylglycerol content and ventricular ANF expression (8, 19). Interestingly, concomitant expression of GRK5 did not affect myocardial diacylglycerol content but did significantly attenuate constitutively active mutant α1B-adrenergic receptor-induced hypertrophy and ANF expression (8). Thus, the cardiac overexpression of GRK5, while not affecting α1B-adrenergic receptor/Gq coupling, does apparently inhibit cardiac gene transcription. It is tempting to speculate that the ability of GRK5 to bind DNA may explain its differential effects on receptor function and gene transcription in this model system.
Endogenous GRK5 displays a more nuclear localization in cardiac myocytes derived from spontaneously hypertensive heart failure (SHHF) rats than in age-matched control animals (40). The SHHF rat represents a genetic model for cardiac hypertrophy, and the more nuclear localization of GRK5 in these cells may reflect either, a role for GRK5 in hypertrophic gene expression or, alternatively, an adaptive response to hypertrophy. The second explanation would serve to reconcile the results obtained using SHHF rats with those obtained using transgenic mice expressing constitutively active α1B-adrenergic receptor in the heart, where coexpression of GRK5 inhibits gene expression.
In addition to GRK5, all other members of the GRK1 and -4 subfamilies contain NLS consensus sequences. The putative NLS identified in the three splice variants of GRK6 is almost identical to that of GRK5 and is located in an analogous position within the catalytic domain of the kinase (Fig. 5B). Homology between the NLSs of these two kinases suggests that GRK6 binds DNA in vitro and may indicate that GRK5 and -6 have similar functions in the nucleus. However, GRK6 has a lower affinity for Ca2+/CaM than GRK5, and in contrast to the case with GRK5, endogenous levels of GRK6 have been shown to mediate Gq-coupled receptor desensitization (34, 39). These observations suggest that potentially similar nuclear functions of these kinases may be subject to differential modes of regulation.
The predicted NLS of GRK4 is distinct from that of GRK5 and -6 and is homologous to the predicted NLS of the GRK1 subfamily (Fig. 5B). In contrast to the NLS of GRK5 and -6, those of GRK1, -7, and -4 are not predicted to bind DNA (5). Four splice variants of GRK4 have been identified (GRK4α, -β, -γ, and -δ). However, only the longest (GRK4α), has been shown to phosphorylate light-activated rhodopsin in vitro (28, 33). It is tempting to speculate that GRK4β, -γ, and -δ may function as nuclear kinases and not serve as GPCR kinases at all. Of the GRK4 splice variants, only GRK4α has been shown to bind to Ca2+/CaM in vitro, even though by homology with GRK5 it might be predicted that GRK4β possesses a C-terminal, and GRK4γ possesses an N-terminal, CaM binding site (28, 33). Ca2+/CaM-mediated regulation of the nuclear localization of GRK4, if any, would thus be anticipated to be splice variant specific. It may be predicted that since the NLS of GRK4 is not predicted to bind DNA, the nuclear functions of this kinase may differ from those of GRK5 and -6.
The identification of a DNA binding NLS in GRK5 and putative NLSs in the other GRK1 and -4 subfamily members suggests that in addition to their characterized role in mediating GPCR desensitization, the GRK1 and -4 subfamilies may have additional and differential cellular functions.
Acknowledgments
We thank Marcia A. Kaetzel, John Dedman, and David Sacks for the CaM binding protein and cDNAs, Andrew Tobin for the anti-muscarinic M3 antibody and CHO-M3 stable cell line, and Robert J. Lefkowitz for the GRK5K215R construct.
This work was funded by grants from the Wellcome Trust (J.A.P. and M.G.H.S.) and Medical Research Council (L.R.J.).
REFERENCES
- 1.Carman, C. V., T. Som, C. M. Kim, and J. L. Benovic. 1998. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J. Biol. Chem. 273:20308-20316. [DOI] [PubMed] [Google Scholar]
- 2.Chien, K. R. 1999. Stress pathways and heart failure. Cell 98:555-558. [DOI] [PubMed] [Google Scholar]
- 3.Chuang, T. T., L. Paolucci, and A. De Blasi. 1996. Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J. Biol. Chem. 271:28691-28696. [DOI] [PubMed] [Google Scholar]
- 4.Claing, A., S. A. Laporte, M. G. Caron, and R. J. Lefkowitz. 2002. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Prog. Neurobiol. 66:61-79. [DOI] [PubMed] [Google Scholar]
- 5.Cokol, M., R. Nair, and B. Rost. 2000. Finding nuclear localization signals. EMBO Rep. 1:411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFranco, D. B. 2001. Nuclear export: DNA-binding domains find a surprising partner. Curr. Biol. 11:R1036-R1037. [DOI] [PubMed] [Google Scholar]
- 7.Dinudom, A., A. B. Fotia, R. J. Lefkowitz, J. A. Young, S. Kumar, and D. I. Cook. 2004. The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc. Natl. Acad. Sci. USA 101:11886-11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckhart, A. D., S. J. Duncan, R. B. Penn, J. L. Benovic, R. J. Lefkowitz, and W. J. Koch. 2000. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ. Res. 86:43-50. [DOI] [PubMed] [Google Scholar]
- 9.Freeman, J. L., E. M. Cruz, T. D. Pollard, R. J. Lefkowitz, and J. A. Pitcher. 1998. Regulation of G protein-coupled receptor kinase 5 (GRK5) by actin. J. Biol. Chem. 273:20653-20657. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, J. L., P. Gonzalo, J. A. Pitcher, A. Claing, J. P. Lavergne, J. P. Reboud, and R. J. Lefkowitz. 2002. Beta 2-adrenergic receptor stimulated, G protein-coupled receptor kinase 2 mediated, phosphorylation of ribosomal protein P2. Biochemistry 41:12850-12857. [DOI] [PubMed] [Google Scholar]
- 11.Gainetdinov, R. R., L. M. Bohn, T. D. Sotnikova, M. Cyr, A. Laakso, A. D. Macrae, G. E. Torres, K. M. Kim, R. J. Lefkowitz, M. G. Caron, and R. T. Premont. 2003. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38:291-303. [DOI] [PubMed] [Google Scholar]
- 12.Gainetdinov, R. R., L. M. Bohn, J. K. Walker, S. A. Laporte, A. D. Macrae, M. G. Caron, R. J. Lefkowitz, and R. T. Premont. 1999. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron 24:1029-1036. [DOI] [PubMed] [Google Scholar]
- 13.Hall, R. A., R. F. Spurney, R. T. Premont, N. Rahman, J. T. Blitzer, J. A. Pitcher, and R. J. Lefkowitz. 1999. G protein-coupled receptor kinase 6A phosphorylates the Na+/H+ exchanger regulatory factor via a PDZ domain-mediated interaction. J. Biol. Chem. 274:24328-24334. [DOI] [PubMed] [Google Scholar]
- 14.Hermann, S., J. Saarikettu, J. Onions, K. Hughes, and T. Grundstrom. 1998. Calcium regulation of basic helix-loop-helix transcription factors. Cell Calcium 23:135-142. [DOI] [PubMed] [Google Scholar]
- 15.Jaber, M., W. J. Koch, H. Rockman, B. Smith, R. A. Bond, K. K. Sulik, J. Ross, Jr., R. J. Lefkowitz, M. G. Caron, and B. Giros. 1996. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. USA 93:12974-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levay, K., D. K. Satpaev, A. N. Pronin, J. L. Benovic, and V. Z. Slepak. 1998. Localization of the sites for Ca2+-binding proteins on G protein-coupled receptor kinases. Biochemistry 37:13650-13659. [DOI] [PubMed] [Google Scholar]
- 17.Li, L., Z. Li, and D. B. Sacks. 2003. Calmodulin regulates the transcriptional activity of estrogen receptors. Selective inhibition of calmodulin function in subcellular compartments. J. Biol. Chem. 278:1195-1200. [DOI] [PubMed] [Google Scholar]
- 18.Menard, L., S. S. Ferguson, L. S. Barak, L. Bertrand, R. T. Premont, A. M. Colapietro, R. J. Lefkowitz, and M. G. Caron. 1996. Members of the G protein-coupled receptor kinase family that phosphorylate the beta2-adrenergic receptor facilitate sequestration. Biochemistry 35:4155-4160. [DOI] [PubMed] [Google Scholar]
- 19.Milano, C. A., P. C. Dolber, H. A. Rockman, R. A. Bond, M. E. Venable, L. F. Allen, and R. J. Lefkowitz. 1994. Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 91:10109-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papermaster, D. S., and W. J. Dreyer. 1974. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry 13:2438-2444. [DOI] [PubMed] [Google Scholar]
- 21.Penn, R. B., A. N. Pronin, and J. L. Benovic. 2000. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc. Med. 10:81-89. [DOI] [PubMed] [Google Scholar]
- 22.Peppel, K., I. Boekhoff, P. McDonald, H. Breer, M. G. Caron, and R. J. Lefkowitz. 1997. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J. Biol. Chem. 272:25425-25428. [DOI] [PubMed] [Google Scholar]
- 23.Pitcher, J. A., Z. L. Fredericks, W. C. Stone, R. T. Premont, R. H. Stoffel, W. J. Koch, and R. J. Lefkowitz. 1996. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J. Biol. Chem. 271:24907-24913. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher, J. A., N. J. Freedman, and R. J. Lefkowitz. 1998. G protein-coupled receptor kinases. Annu. Rev. Biochem. 67:653-692. [DOI] [PubMed] [Google Scholar]
- 25.Pitcher, J. A., J. J. G. Tesmer, J. L. R. Freeman, W. D. Capel, W. C. Stone, and R. J. Lefkowitz. 1999. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J. Biol. Chem. 274:34531-34534. [DOI] [PubMed] [Google Scholar]
- 26.Premont, R. T., A. Claing, N. Vitale, J. L. Freeman, J. A. Pitcher, W. A. Patton, J. Moss, M. Vaughan, and R. J. Lefkowitz. 1998. Beta 2-adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA 95:14082-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Premont, R. T., W. J. Koch, J. Inglese, and R. J. Lefkowitz. 1994. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J. Biol. Chem. 269:6832-6841. [PubMed] [Google Scholar]
- 28.Premont, R. T., A. D. Macrae, R. H. Stoffel, N. Chung, J. A. Pitcher, C. Ambrose, J. Inglese, M. E. MacDonald, and R. J. Lefkowitz. 1996. Characterization of the G protein-coupled receptor kinase GRK4. J. Biol. Chem. 271:6403-6410. [DOI] [PubMed] [Google Scholar]
- 29.Pronin, A. N., and J. L. Benovic. 1997. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J. Biol. Chem. 272:3806-3812. [DOI] [PubMed] [Google Scholar]
- 30.Pronin, A. N., A. J. Morris, A. Surguchov, and J. L. Benovic. 2000. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem. 275:26515-26522. [DOI] [PubMed] [Google Scholar]
- 31.Rhoads, A. R., and F. Friedberg. 1997. Sequence motifs for calmodulin recognition. FASEB J. 11:331-340. [DOI] [PubMed] [Google Scholar]
- 32.Sallese, M., L. Iacovelli, A. Cumashi, L. Capobianco, L. Cuomo, and A. De Blasi. 2000. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim. Biophys. Acta 1498:112-121. [DOI] [PubMed] [Google Scholar]
- 33.Sallese, M., S. Mariggio, G. Collodel, E. Moretti, P. Piomboni, B. Baccetti, and A. De Blasi. 1997. G protein-coupled receptor kinase GRK4. Molecular analysis of the four isoforms and ultrastructural localization in spermatozoa and germinal cells. J. Biol. Chem. 272:10188-10195. [DOI] [PubMed] [Google Scholar]
- 34.Vroon, A., C. J. Heijnen, R. Raatgever, I. P. Touw, R. E. Ploemacher, R. T. Premont, and A. Kavelaars. 2004. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J. Leukoc. Biol. 75:698-704. [DOI] [PubMed] [Google Scholar]
- 35.Walker, J. K., R. R. Gainetdinov, D. S. Feldman, P. K. McFawn, M. G. Caron, R. J. Lefkowitz, R. T. Premont, and J. T. Fisher. 2004. G protein-coupled receptor kinase 5 regulates airway responses induced by muscarinic receptor activation. Am. J. Physiol. Lung Cell Mol. Physiol. 286:L312-L319. [DOI] [PubMed] [Google Scholar]
- 36.Walker, J. K., K. Peppel, R. J. Lefkowitz, M. G. Caron, and J. T. Fisher. 1999. Altered airway and cardiac responses in mice lacking G protein-coupled receptor kinase 3. Am. J. Physiol. 276:R1214-R1221. [DOI] [PubMed] [Google Scholar]
- 37.Wan, K. F., B. S. Sambi, R. Tate, C. Waters, and N. J. Pyne. 2003. The inhibitory gamma subunit of the type 6 retinal cGMP phosphodiesterase functions to link c-Src and G-protein-coupled receptor kinase 2 in a signaling unit that regulates p42/p44 mitogen-activated protein kinase by epidermal growth factor. J. Biol. Chem. 278:18658-18663. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., B. Campos, G. A. Jamieson, Jr., M. A. Kaetzel, and J. R. Dedman. 1995. Functional elimination of calmodulin within the nucleus by targeted expression of an inhibitor peptide. J. Biol. Chem. 270:30245-30248. [DOI] [PubMed] [Google Scholar]
- 39.Willets, J. M., R. Mistry, S. R. Nahorski, and R. A. Challiss. 2003. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol. Pharmacol. 64:1059-1068. [DOI] [PubMed] [Google Scholar]
- 40.Yi, X. P., A. M. Gerdes, and F. Li. 2002. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension 39:1058-1063. [DOI] [PubMed] [Google Scholar]