Abstract
Previous literature indicates that both hypoactivity and hyperactivity of the HPA axis may be related to conduct disorder and externalizing behaviors in young children. Using a longitudinal sample of 283 typically-developing children, the current study examined both the concurrent and the longitudinal association between HPA functioning and externalizing behavior problems, such as conduct problems. Diurnal cortisol rhythms and externalizing problems were assessed at ages 6 and 9. Results suggest that concurrent HPA functioning is not significantly related to externalizing behavior at ages 6 or 9. However, more blunted cortisol rhythms at age 6 (less change across the day from morning to evening) predicted a greater increase in externalizing behavior between age 6 and age 9 than did steeper cortisol rhythms. Further analyses revealed that this association was driven by conduct problems and aggressive behavior, rather than attention problems. The relationship between HPA functioning and subsequent externalizing behavior in children adds to the limited longitudinal work on this topic, suggesting that the association changes over time. These results may serve to clarify the inconsistencies in the cross-sectional literature, particularly with respect to young school-age children.
Keywords: conduct disorder, aggression, HPA axis, cortisol
1.1. Introduction
Externalizing behaviors, such as attention problems, rule breaking, and aggressive behavior, during early childhood are associated with a host of negative outcomes. Conduct disorder, specifically, is one of the more severe externalizing disorders, associated with such problems as substance abuse, theft, lower educational attainment, and poor physical health that significantly impair the functioning of affected individuals and their families, and have high costs for society at large (McMahon, 1994; Moffitt, 1993; Kim-Cohen et al., 2014; Rolon-Arroyo et al., 2014). The majority of studies have reported that hypoactivity of the hypothalamus-pituitary-adrenal (HPA) axis, characterized by blunted diurnal rhythms of cortisol, is associated with conduct problems and externalizing behaviors in school-aged children and adolescents (e.g., Alink et al., 2008; Bernard et al., 2015; Martin et al., 2014). However, some studies have reported contradictory results, suggesting that externalizing behavior may have no relationship to HPA axis activity, or that problem behavior may be positively associated with levels of cortisol in young children (Alink et al., 2008; Fairchild et al., 2008; Klimes-Dougan, Hastings, Granger, Usher & Zahn-Waxler et al., 2001; Van Bokhoven et al., 2005). Using a longitudinal design, the current study seeks to examine both the concurrent and longitudinal association between childhood externalizing behaviors, particularly those related to conduct problems, and HPA axis functioning.
1.1.1 Diurnal Rhythms of Cortisol
The HPA axis regulates a host of biological processes including the body’s response to stress and maintenance of the circadian rhythm. As an end product of the HPA axis, cortisol provides an informative measure of HPA axis functioning. Not only is cortisol released in direct response to acute stressors, but secretion follows a regular diurnal rhythm (i.e., highest soon after awakening, and lowest at the end of the day; Van Cauter, 1990). The diurnal rhythm is evident by three months of life (Price et al.,1983), but continues to become more pronounced and more similar to an adult-like rhythm between the ages of 5 and 8 months (de Weerth & van Geert, 1999). Healthy HPA axis functioning promotes protective and adaptive outcomes such as the replenishment and management of energy or metabolism, retention of memories, and maintenance of the immune system (McEwen & Seeman, 1999). In contrast with the typical steep decline in cortisol from morning to evening, some individuals show a flattened or blunted pattern of cortisol across the day, characterized by morning levels that are atypically low and evening levels that are atypically high (Gunnar & Vazquez, 2001).
1.1.2 HPA Axis Functioning and Concurrent Externalizing Behavior
Much of the research on school-aged children and adolescents suggests that low levels of cortisol at various times during the day are significantly associated with conduct disorder (Kariyawasam, Zaw & Handley, 2002; Oosterlaan et al., 2005; Pajer et al., 2001; Pajer et al., 2006; Vanyukov et al., 1993). Further, both blunted (or smaller) cortisol awakening responses (CAR, the difference between cortisol at waking to 30 minutes post-waking) and blunted change across the day are associated with delinquency and/or a diagnosis of conduct disorder (Popma et al., 2007). Hawes et al. (2009) found that children with higher levels of callous-unemotional traits, an important feature and potential subtype of conduct disorder (Frick et al., 2014), were more likely to have blunted cortisol and severe antisocial behavior than children with lower levels of callous-unemotional traits.
Although there is a wealth of evidence suggesting that hypocortisolism is related to problem behavior in school-aged children, a number of studies have found no association, or that this relationship may be reversed in younger children (Alink et al., 2008; Fairchild et al., 2008; Klimes-Dougan, Hastings, Granger, Usher & Zahn-Waxler, 2001; Van Bokhoven et al., 2005). This literature suggests that HPA axis activity and its relation to externalizing behavior may change over time in young children age. In fact, a meta-analysis by Alink et al. (2008) found that the relationship between basal cortisol and general externalizing behavior in children was moderated by age; externalizing behavior was associated with higher cortisol levels in preschool-aged children, and with lower cortisol levels in elementary school-aged children.
1.1.3 Longitudinal Studies
Previous work on the assessment of cortisol and externalizing behavior in children has typically used cross-sectional rather than longitudinal data. Given the mixed findings in younger children, it is unclear whether externalizing problems are associated with lower levels of cortisol across development, or whether externalizing behavior is associated with hypercortisolism in early childhood, but hypocortisolism in older childhood and adolescence. Further, it is unknown whether cortisol levels predict changes in behavior problems, behavior problems predict changes in cortisol levels, or both influence each other over time. As discussed in Saridjan (2014), the longitudinal examination of diurnal cortisol rhythm and problem behavior is necessary in order to disentangle the direction of these effects.
Although the number of studies is limited, extant longitudinal work on this topic has been informative. In a population-based sample, Saridjan (2014) found that high diurnal HPA axis activity in infancy was predictive of later internalizing problems in preschool. However, these researchers did not find any relationship between HPA axis activity in infancy and externalizing behavior in preschool-aged children. In another longitudinal study, Shoal et al. (2003) found that low resting cortisol in 10- to 12-year-olds predicted elevated physical and verbal aggression at 15 to 17 years of age, with this pathway mediated by low self-control. Similarly, McBurnett et al. (2000) found that lower cortisol at age 10 and age 12 were associated with aggressive behavior at age 12. However, neither McBurnett et al. (2000) nor Shoal et al. (2003) measured the diurnal rhythm of cortisol; rather, each used only one or two samples in all analyses. More importantly, neither study controlled for pre-existing aggressive behavior at the first assessment period. Without measuring pre-existing aggression, it is impossible to determine whether cortisol predicts change in behavior problems, and only correlational inferences are warranted.
Finally, Ruttle et al. (2011) examined whether there was a change in the relationship between externalizing behavior and HPA axis functioning over time in a sample of 96 children. Children were assessed first at 6.3–10.8 (M=7.7) years of age, and then again at 9.3–13.5 (M=10.87) years. Both concurrent and longitudinal analyses suggested that externalizing behavior was related to a blunted diurnal cortisol. However, their results suggested that the longitudinal relationship between early externalizing disorders and later blunted cortisol was slightly stronger than the concurrent relationship during childhood. This work, combined with the inconsistent literature on problem behavior and HPA axis functioning in younger children, suggests that the HPA axis may develop and change over time. Using a longitudinal design, the current study seeks to examine both the concurrent and longitudinal association between HPA axis functioning and childhood externalizing behaviors, particularly those related to conduct problems.
1.1.4 The Current Study
The current study aimed to investigate both the concurrent and longitudinal association between childhood externalizing behaviors, particularly those related to conduct problems and aggression, and HPA axis functioning. In order to understand this relationship in normally developing children, the current sample is community-based and consists of both boys and girls at two time points (age 6 and age 9). Externalizing behaviors were measured at both ages using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), and saliva samples were collected in the morning and evening at both time points to assess the diurnal rhythm of cortisol. Based on previous literature, we predicted that we would find a different relationship when externalizing disorders and HPA axis functioning are analyzed concurrently, than when analyzed longitudinally; concurrent analyses at age 6 may reveal a weaker, or even positive, relationship between externalizing behaviors and cortisol slope. However, when analyzed longitudinally, we hypothesized that a more blunted rhythm of cortisol from the morning to the evening, or a less pronounced decline, would predict increases in externalizing behavior over time. Further, given the longitudinal nature of our data, we were also able to investigate directionality of this relationship (i.e., does HPA axis functioning predict externalizing behavior, or does externalizing behavior predict HPA axis functioning?) Given that most of the previous evidence on externalizing disorders and HPA axis functioning has focused on conduct problems and aggression, we predicted that the CBCL scales related to conduct problems and aggressive behaviors, rather than those related to attention problems, would drive the association between cortisol and externalizing behavior.
2.1 Methods
2.1.1 Study Population
The original sample consisted of 815 families with a child between the ages of 3 and 4 and an English-speaking biological parent (see Olino, Klein, Dyson, Rose, & Durbin, 2010 for details). Participants were identified through a commercial mailing list and screened by telephone. Exclusion criteria included the existence of a significant medical condition or a developmental disability in the child. Of the families deemed eligible, 66.4% (N=541) entered into the study. Analyses indicated that there were no differences in child sex, race/ethnicity, parental marital status, education or employment status between families who agreed to participate and those who declined.
Around 3 years later, 470 children (86.9% of the original sample) participated in a follow-up assessment (Time 1 for the current study). The mean age of the children at this follow-up was 6.08 years old, ranging from 5.8–7.5 (SD = 0.41). During this assessment wave, parents were asked to assist their children in collecting morning and bedtime saliva samples across 2 days to assess cortisol levels. 288 children participated in this aspect of the study.
Children were assessed again approximately three years later (Time 2 for the current study), when they were 9 years old, ranging from 8.4–11 (M = 9.3, SD = 0.4). Over 82% (446) of the original sample participated in this third assessment, with 420 of these participants providing saliva samples. Given that the current study aimed to examine longitudinal associations in diurnal cortisol, only children who participated in the collection of salivary cortisol at both time-points were included in the present analyses, for a total sample size of 283 (comprising 98.3% of those with saliva samples at Time 1).
The majority of children in our sample were identified as White (84.5%), followed by 11.3% Black, 3.9% Asian and 0.4% Native American. Further, 13% of our sample were identified as Hispanic. In all, 89% of the parents of the children were married at age 6.
2.1.2 Child Psychopathology Measure
Mothers completed the CBCL (Achenbach & Rescorla, 2001) at both assessments (age 6 and age 9). The CBCL is a widely used parent questionnaire for assessing both internalizing and externalizing problems in children between the ages of 6 and 18 years. The scale consists of 113 items and parents choose from responses “0 = not true,” “1 = somewhat or sometimes true,” and “2 = very true or often true.” For the current study, we were primarily interested in the higher-order factor for Externalizing behavior, as well as its associated subscales (i.e., Syndrome scales: Attention Problems, Rule-Breaking Behavior, Aggressive Behavior; DSM-oriented scales: Attention Deficit Hyperactivity Disorder Problems, Oppositional Defiant Problems, Conduct Problems). The CBCL has high levels of reliability and validity; for example, test-retest reliability for Externalizing Problems is .92 (Achenbach & Rescorla, 2001). For our sample, Cronbach’s alpha for Externalizing Problems is also high at .88. Given high comorbidity of externalizing and internalizing behaviors in childhood (Angold et al., 1999), we also included the higher-order factor for Internalizing problems to examine the specificity of associations.
2.1.3 Salivary Cortisol measurements
Saliva samples were collected twice per day over 2 consecutive days at age 6 and 3 consecutive days at age 9, resulting in a total of 4 samples per child at age 6 and 6 samples at age 9. Parents were asked to assist their children in taking one sample 30 minutes after awakening in the morning, and a second sample 30 minutes before going to bed at night. These two time-points were chosen to in order to measure the morning peak in cortisol, which occurs about 30 minutes post-waking, and the lowest point, which occurs before bedtime. Mean sampling time for morning samples at the Age 6 assessment was 8:11a.m., EST (SD = 48 minutes) and mean sampling time for evening samples at the Age 6 assessment was 8:10 p.m., EST (SD = 96 minutes). For participants at the Age 9 assessment, mean sampling times were 7:53 a.m., EST (SD = 51 minutes), and 8:51 pm, EST (SD = 85 minutes). Participants were asked to passively drool into a small straw which fed into a polypropylene tube, which was labeled with the date and time. Parents instructed their children not to eat, drink, or brush their teeth for 30 minutes prior to sample collection. Saliva samples were sent back to our laboratory via mail or picked up by research assistants and then frozen at −20° C. Samples were then packaged on dry-ice and shipped to Trier, Germany for cortisol assays, which were conducted in duplicate using a time-resolved immunoassay with fluorometric detection (DELFIA). Intra-assay coefficients of variation (CVs) were between 4.0% and 6.7%, and inter-assay CVs ranged from 7.1% to 9.0%.
2.1.4. Statistical analyses
Consistent with previous research, morning and evening cortisol values showed a positively skewed distribution. We applied a log10 transformation to the data to yield unskewed values. Cortisol values that exceeded values of 3 standard deviations above the mean were considered outliers; we excluded 12 individual cortisol samples. These deleted scores were treated as missing values, but children were included in analyses if they had least one valid cortisol sample. Therefore, no participants were excluded at this step (only individual samples). Next, compliance with study procedure was determined. Participants were considered non-compliant if they took the morning saliva sample more than 15 minutes before or after the designated time of collection. Applying these criteria, 10 cortisol samples at age 6 were excluded, and 9 cortisol samples at age 9 were excluded (all from different participants). Again, no participants were excluded at this step (only individual samples). Thus, despite excluding some samples as outliers or due to noncompliance, we were able to retain the full sample of 283 participants for analyses.
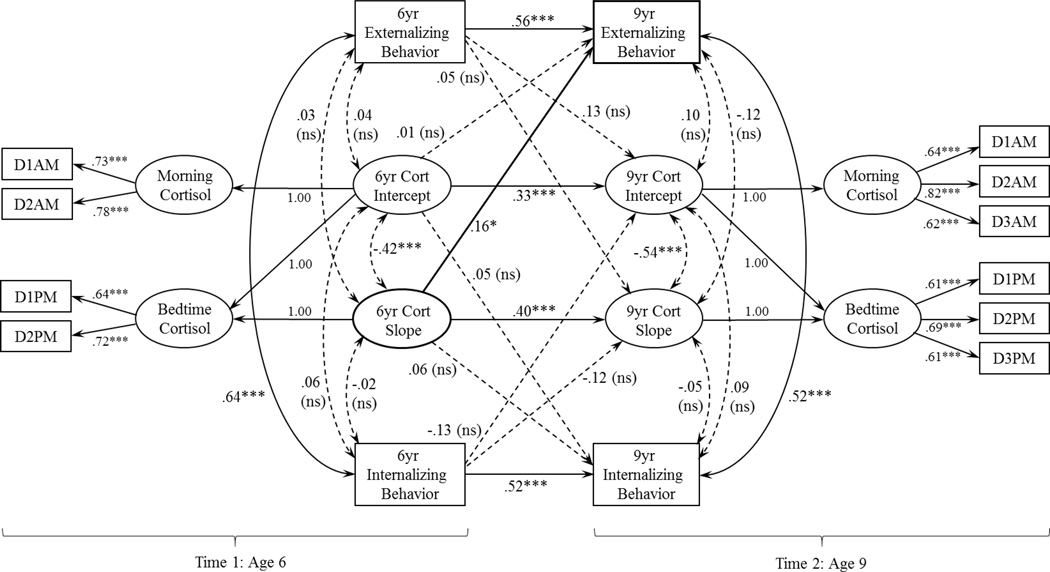
We used MPlus 7.11 (Muthén and Muthén 1998–2013) for the primary analyses. Morning (AM) and evening (PM) cortisol were modeled as latent variables with log-transformed cortisol values as indicators; for age 6, the latent variables for AM and PM cortisol each had two indicators (Day 1 and Day 2), and for age 9, the latent variables for AM and PM cortisol each had three indicators (Days 1, 2, and 3). The diurnal rhythm (i.e., change across the day from AM to PM) was modeled as a latent change score, following procedures used previously (Bernard et al., 2015; Kertes et al., 2008; McArdle & Hamagami, 2001). At each time point, the cortisol intercept reflects the average AM cortisol level, and the cortisol slope (i.e., the latent change score) reflects the change in cortisol across the day (i.e., Bedtime – Wake-up), with more negative values reflecting a steeper decline. Our primary aim was to examine the direction of association between diurnal cortisol rhythms and externalizing behavior from age 6 to age 9. Thus, we simultaneously modeled regression pathways between cortisol (i.e., slope and intercept) and externalizing behavior at age 6 and cortisol and externalizing behavior at age 9. We also estimated concurrent correlations between cortisol and externalizing behavior at both ages. Concurrent and longitudinal pathways between cortisol and internalizing behavior were also estimated. The model was estimated using maximum likelihood estimation and absolute fit was assessed with χ2, χ2 to df ratio (χ2/df), the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and the Tucker-Lewis index (TLI). Model fit was determined based on commonly used criteria, including a non-significant χ2 test of model fit, relative χ2 (i.e., ratio of χ2 to degrees of freedom) in the 2-1 range (Byrne, 1989; Carmines & McIver, 1981), RMSEA < .08, CFI >.90, and TLI > .90 (Browne & Cudeck, 1993; Chen et al., 2005). The full model is shown in Figure 1.
Figure 1.
Path diagram with standardized coefficients for model with age 6 diurnal cortisol rhythm (6yr Cort Slope) predicting age 9 externalizing behavior (9yr Externalizing Behavior). D1AM-D3AM and D1PM-D3PM represent log-transformed morning cortisol indicators and bedtime cortisol indicators, respectively. Model fit statistics indicated good fit: χ2 (59) = 77.74, p = .052; χ2/df = 1.32, RMSEA = .03, CFI = .97, TLI = .95, *p < .05, ***p < .001, ns = non-significant
In order to examine whether particular types of externalizing behavior were driving effects, follow-up analyses were conducted by re-estimating the full model using the subscales on the CBCL externalizing factor and the DSM externalizing problem scales in place of the higher-order externalizing behavior factor. Finally, we examined whether gender moderated the association between cortisol slope and externalizing behavior.
3.1 Results
Table 1 shows descriptive statistics for cortisol at both time points and Table 2 shows bivariate correlations among key study variables. Bivariate correlations suggest that more blunted cortisol rhythms at age 6 are correlated with increased conduct behavior (r = .17, p = .009) and increased rule-breaking behavior (r = .16, p = .014) from age 6 to 9.
Table 1.
Descriptive Statistics for Cortisol Data
| Time of sample | Cortisol value (in ug/dl) |
Log-transformed cortisol value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | Min | Max | M (SD) | Min | Max | M (SD) | Min | Max | |
| Age 6 (n = ) | ||||||||||
| AM- Day 1 | 237 | 8:11 (0:58) | 5:37 | 11:30 | 7.72 (4.75) | .18 | 27.76 | .79 (.33) | −.74 | 1.44 |
| AM- Day 2 | 235 | 8:12 (0:53) | 4:55 | 10:45 | 8.26 (5.01) | .31 | 27.39 | .83 (.31) | −.51 | 1.44 |
| PM- Day 1 | 270 | 8:14 (1:40) | 8:36 | 11:30 | 1.19 (1.60) | .100 | 14.15 | −.17 (.45) | −1.00 | 1.15 |
| PM- Day 2 | 265 | 8:04 (2:02) | 7:45 | 11:00 | 1.08 (1.36) | .100 | 9.52 | −.19 (.43) | −1.00 | .98 |
| Age 9 (n = ) | ||||||||||
| AM- Day 1 | 206 | 7:52 (0:53) | 5:00 | 11:20 | 10.01(5.2) | .12 | 35.15 | .93 (.28) | −.92 | 1.55 |
| AM- Day 2 | 203 | 7:53 (0:49) | 6:15 | 11:20 | 9.99 (4.66) | 1.81 | 27.81 | .95 (.22) | .26 | 1.44 |
| AM- Day 3 | 199 | 7:53 (0:51) | 6:10 | 11:15 | 10.24 (5.95) | .16 | 45.48 | .94 (.29) | −.80 | 1.66 |
| PM- Day 1 | 216 | 8:49 (1:30) | 6:15 | 1:00 | .65 (.52) | .00 | 2.52 | −.30 (.33) | −1.70 | .40 |
| PM- Day 2 | 225 | 8:52 (1:34) | 6:45 | 12:37 | .64 (.58) | .00 | 3.83 | −.32 (.33) | −1.07 | .58 |
| PM- Day 3 | 226 | 8:57 (1:38) | 6:58 | 12:15 | .69 (.58) | .00 | 6.94 | −.28 (.33) | −1.05 | .60 |
Table 2.
Bivariate Correlations among Cortisol Variables at Age 6 and 9 and Change in CBCL Externalizing Behavior Subscales
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Age 6 Cort AM |
-- | ||||||||||
| 2. | Age 6 Cort PM |
.11 | -- | |||||||||
| 3. | Age 6 Cort PM-AM |
−.95** | .23** | -- | ||||||||
| 4. | Age 9 Cort AM |
.09 | .09 | −.07 | -- | |||||||
| 5. | Age 9 Cort PM |
−.10 | .23** | .16* | .23** | -- | ||||||
| 6. | Age 9 Cort PM-AM |
−.13 | −.07 | .11 | −.99** | −.11 | -- | |||||
| 7. | Δ Attention Problems |
.05 | .01 | −.06 | −.02 | .05 | .03 | -- | ||||
| 8. | Δ Aggressive Behavior |
−.04 | .09 | .06 | −.01 | .02 | .01 | .37** | -- | |||
| 9. | Δ Rule- breaking |
−.14* | .05 | .16* | .03 | .03 | −.03 | .34** | .50** | -- | ||
| 10. | Δ AD/HD | .10 | .02 | −.10 | −.03 | .03 | .03 | .81** | .45** | .33** | -- | |
| 11. | Δ ODD | .01 | .10 | .02 | −.02 | −.01 | .02 | .26** | .83** | .30** | .30** | -- |
| 12. | Δ Conduct | −.15* | .06 | .17** | .02 | .01 | −.01 | .34** | .62** | .90** | .33** | .35** |
p < .05,
p < .01
Delta (Δ) represents a change score for each CBCL subscale (Age 9 – Age 6)
For the full model testing the longitudinal associations between cortisol and the composite measure of externalizing behavior, fit indices demonstrated good fit to the data: the χ2 test of model fit was non-significant, χ2 (59) = 77.74, p = .052, χ2/df = 1.32, RMSEA = .03, CFI = .97, and TLI = .95. Results demonstrated that concurrent associations between diurnal cortisol and externalizing behavior at each time-point were non-significant. Diurnal cortisol at age 6 predicted externalizing behavior age at 9, β = .16, p = .034, accounting for earlier externalizing behavior (See Figure 1). Specifically, more blunted (i.e., flatter) cortisol rhythms predicted higher externalizing behavior, than steeper cortisol rhythms. In contrast, externalizing behavior at age 6 did not predict diurnal cortisol rhythms at age 9, β =.05, p = .69, accounting for earlier diurnal cortisol. These findings provide support that early flattened diurnal cortisol rhythms predict an increase in later behavior problems, but not vice versa. These effects were specific to the diurnal slope rather than morning levels. Both cortisol rhythms and externalizing behavior demonstrated moderate to strong stability across time: cortisol slope, β = .40, p < .001, externalizing behavior, β = .56, p < .001. Internalizing behavior was not associated concurrently or longitudinally with cortisol. Table 3 presents model-estimated parameters for the full model.
Table 3.
Model-estimated Parameters for Full Model of Longitudinal Associations between Diurnal Cortisol Rhythms and Externalizing Behavior
| Effect | Unstandardized Estimate |
SE | Est/SE | p | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Measurement model factor loadings | ||||||
| Age 6 Log-transformed AM Cort | ||||||
| Day 1 | 0.97 | 0.02 | 39.91 | 0.000 | 0.93 | 1.02 |
| Day 2 | 1.00 | -- | -- | -- | -- | -- |
| Age 6 Log-transformed PM Cort | ||||||
| Day 1 | 0.93 | 0.12 | 7.85 | 0.000 | 0.70 | 1.16 |
| Day 2 | 1.00 | -- | -- | -- | -- | -- |
| Age 9 Log-transformed AM Cort | ||||||
| Day 1 | 0.99 | 0.02 | 44.51 | 0.000 | 0.94 | 1.03 |
| Day 2 | 1.00 | -- | -- | -- | -- | -- |
| Day 3 | 0.98 | 0.02 | 51.08 | 0.000 | 0.94 | 1.02 |
| Age 9 Log-transformed PM Cort | ||||||
| Day 1 | 0.89 | 0.07 | 13.75 | 0.000 | 0.76 | 1.02 |
| Day 2 | 1.00 | -- | -- | -- | -- | -- |
| Day 3 | 0.87 | 0.07 | 13.25 | 0.000 | 0.75 | 1.01 |
| Longitudinal direct effects | ||||||
| Age 9 Externalizing behavior, Regressed ON | ||||||
| Age 6 Cort slope | 2.48 | 1.22 | 2.04 | 0.042 | 0.09 | 4.88 |
| Age 6 Cort intercept | 0.14 | 1.34 | 0.10 | 0.918 | −2.49 | 2.77 |
| Age 6 Ext behavior | 0.48 | 0.04 | 11.95 | 0.000 | 0.40 | 0.56 |
| Age 9 Internalizing behavior, Regressed ON | ||||||
| Age 6 Cort slope | 0.95 | 1.23 | 0.77 | 0.441 | −1.46 | 3.35 |
| Age 6 Cort intercept | 0.86 | 1.37 | 0.63 | 0.527 | −1.81 | 3.54 |
| Age 6 Int behavior | 0.49 | 0.04 | 11.03 | 0.000 | 0.40 | 0.58 |
| Age 9 Cortisol slope, Regressed ON | ||||||
| Age 6 Cort slope | 0.33 | 0.09 | 3.61 | 0.000 | 0.15 | 0.51 |
| Age 6 Ext behavior | −0.002 | 0.006 | 0.40 | 0.690 | −0.01 | 0.01 |
| Age 6 Int behavior | −0.006 | 0.006 | −1.082 | 0.279 | −0.02 | 0.01 |
| Age 9 Cortisol intercept, Regressed ON | ||||||
| Age 6 Cort intercept | 0.25 | 0.06 | 4.08 | 0.000 | 0.13 | 0.37 |
| Age 6 Ext behavior | 0.004 | 0.003 | 1.24 | 0.214 | −0.002 | 0.01 |
| Age 6 Int behavior | −0.005 | 0.004 | −1.30 | 0.194 | −0.01 | 0.002 |
| Path model: Concurrent associations | ||||||
| Age 6 Externalizing behavior, correlated WITH | ||||||
| Age 6 Cort slope | 0.06 | 0.15 | 0.37 | 0.710 | −0.24 | 0.35 |
| Age 6 Cort intercept | 0.05 | 0.11 | 0.49 | 0.621 | −0.16 | 0.26 |
| Age 9 Externalizing behavior, correlated WITH | ||||||
| Age 9 Cort slope | −0.11 | 0.09 | −1.19 | 0.234 | −0.29 | 0.07 |
| Age 9 Cort intercept | 0.07 | 0.06 | 1.14 | 0.255 | −0.05 | 0.18 |
| Age 6 Internalizing behavior, correlated WITH | ||||||
| Age 6 Cort slope | −0.04 | 0.13 | −0.28 | 0.779 | −0.29 | 0.21 |
| Age 6 Cort intercept | 0.07 | 0.09 | 0.80 | 0.424 | −0.11 | 0.25 |
| Age 9 Internalizing behavior, correlated WITH | ||||||
| Age 9 Cort slope | −0.05 | 0.09 | −0.57 | 0.572 | −0.22 | 0.12 |
| Age 9 Cort intercept | 0.06 | 0.06 | 1.10 | 0.271 | −0.05 | 0.17 |
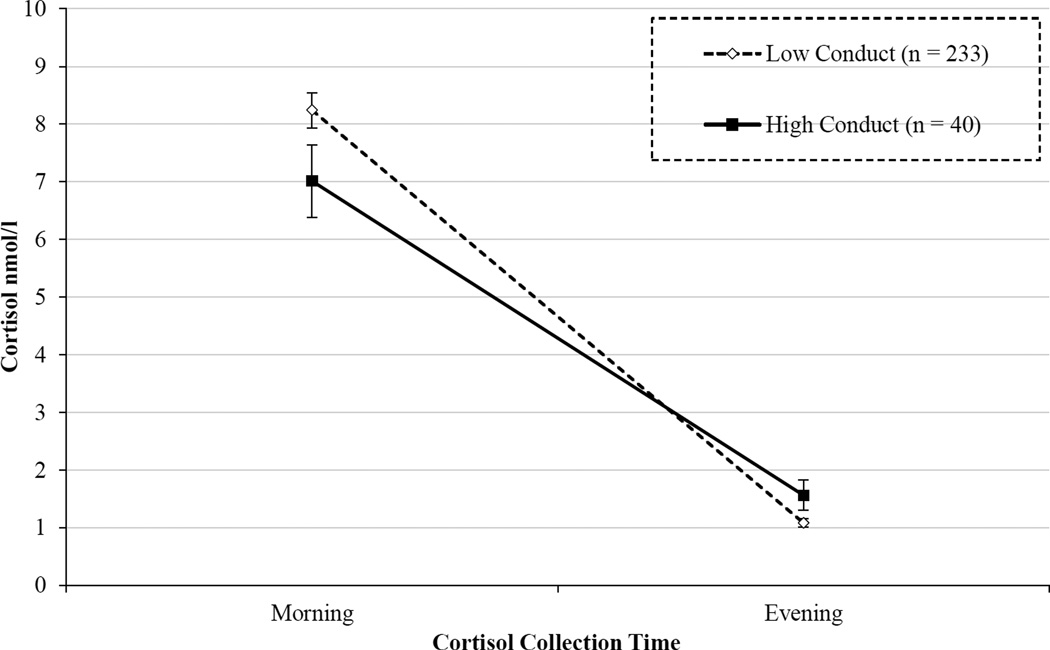
Next, we conducted separate follow-up analyses for the CBCL subscales that assess more specific domains of externalizing behavior. We ran one model including the empirically-based syndrome subscales (i.e., Attention Problems, Rule-breaking Behavior, Aggressive Behavior), and a second model including the DSM-oriented subscales (i.e., AD/HD Problems, Oppositional Defiant Problems, Conduct Problems). By simultaneously including scales assessing different aspects of externalizing behavior (i.e., attention problems and aggressive behavior), we could determine which dimensions of externalizing behavior were driving effects. As displayed in Table 4, flatter cortisol rhythms predicted higher Aggressive behavior and Conduct Problems than steeper cortisol rhythms. Figure 2 provides a graphic illustration of the results for Conduct Problems. In contrast, cortisol rhythms at age 6 did not significantly predict scores on subscales for Attention Problems, Rule-Breaking Behavior, AD/HD Problems or Oppositional Defiant Problems. These results suggest that the association found between early blunted cortisol rhythms and externalizing behavior was driven primarily by behaviors characterized by aggression and conduct problems, rather than attention problems or less severe forms of non-compliance.
Table 4.
Model-estimated Parameters for Longitudinal Associations between Age 6 Diurnal Cortisol Rhythms and Age 9 CBCL Subscales
| Effect | Unstandardized Estimate |
SE | Est/SE | p | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age 9 CBCL Empirically Based Syndrome Scales, Regressed ON Age 6 Cortisol Slope | ||||||
| 1. Attention Problems | −0.11 | 0.68 | −0.15 | 0.88 | −1.45 | 1.24 |
| 2. Rule-Breaking Behavior | 1.64 | 0.87 | 1.88 | 0.060 | −0.07 | 3.35 |
| 3. Aggressive Behavior | 0.90 | 0.40 | 2.24 | 0.025 | 0.11 | 1.68 |
| Age 9 CBCL DSM-Oriented Scales, Regressed ON Age 6 Cortisol Slope | ||||||
| 4. AD/HD Problems | −0.17 | 0.60 | −0.29 | 0.775 | −1.34 | 1.00 |
| 5. Oppositional Defiant Problems | 0.82 | 0.44 | 1.86 | 0.063 | −0.05 | 1.68 |
| 6. Conduct Problems | 0.93 | 0.44 | 2.12 | 0.034 | 0.07 | 1.80 |
Note. Unstandardized estimates represent the regression coefficients between age 6 cortisol slope and age 9 CBCL externalizing subscales. To obtain these estimates, two separate models were estimated, one for the empirically based syndrome scales, and one for DSM-oriented scales. All other pathways from the longitudinal model (Figure 1) were retained in these analyses, except those to internalizing behavior variables, which were non-significant in the full model. For simplicity, only the estimates between age 6 cortisol slope and age 9 externalizing subscales are presented here.
Figure 2.
Age 6 diurnal cortisol rhythms of 9-year-old children with low versus high conduct problems. Children in the Low Conduct group scored < 3 and children in the High Conduct group score ≥ 3 on the CBCL Conduct Problems subscale. Children with more severe conduct problems at age 9 showed a more blunted diurnal cortisol rhythm across the day than children who have no/low conduct problems at age 9. Statistical analyses used a continuous measure of conduct problems, but groups were created for purposes of graphing.
Finally, we examined whether the effect of cortisol slope on externalizing behavior was moderated by gender by adding Gender (male = 0, female = 1) and a Gender × Cortisol slope (at age 6) interaction term to the model. The interaction term was non-significant, p = .13, indicating that gender did not moderate the effect of age 6 cortisol on age 9 externalizing behavior.
4.1 Discussion
Our concurrent findings demonstrated no significant associations between HPA axis functioning and externalizing behavior at age 6 or at age 9. However, our longitudinal findings demonstrated that, after controlling for age 6 externalizing behavior, blunted cortisol rhythms at age 6 (i.e., less change across the day from morning to evening, measured as a latent change score) predicted externalizing behavior problems at age 9. These results suggest that blunted cortisol predicts an increase in externalizing behavior from age 6 to age 9. Importantly, this relationship was unidirectional; the path from age 6 externalizing behavior to age 9 cortisol was not significant. In order to test the specificity of these findings to externalizing problems, we included internalizing behavior in the model. None of the concurrent or longitudinal associations between internalizing behaviors and HPA axis functioning were significant. In addition, follow-up analyses indicated that the association between cortisol levels and externalizing behavior was driven specifically by conduct disorder problems and aggressive behavior, rather than AD/HD and oppositional defiant problems, and attention problems and rule-breaking behavior.
Taken together, these findings advance previous research, particularly in young school-age children, by (1) clarifying the directionality of the longitudinal association between blunted cortisol and increased externalizing behavior (i.e., early blunted cortisol predicts later increases in externalizing behavior) and (2) indicating that HPA axis functioning does not have a consistent association with externalizing behavior, as we did not observe concurrent association between HPA axis functioning and externalizing behavior at either age 6 or age 9.
Similar to the results reported by Ruttle et al. (2011), we found that longitudinal associations emerged more consistently than concurrent links. Although Ruttle et al. (2011) found that externalizing behavior was related to blunted cortisol both concurrently and longitudinally, the longitudinal findings appeared to be stronger. We found that concurrent associations were nonsignificant (both at ages 6 and 9), but a blunted cortisol slope at age 6 predicted an increase in externalizing problems at age 9. These results are consistent with a meta-analysis by Miller, Chen & Zhou (2007), which suggests that time may be an important factor in the relationship between chronic stressors and HPA functioning, furthering the argument that concurrent associations may differ from longitudinal associations.
Based on our findings, we cannot draw conclusions about the initial onset of externalizing behaviors; however, we can conclude that HPA axis blunting is a predictor of increases in such behavior. Children who show increases in aggressive behavior and conduct problems at a young age may be more severe than those who first exhibit these behaviors in adolescence. Lahey et al. (1998) found that boys who met criteria for CD before age 10 (early onset) were 8.7 times more likely to exhibit conduct disorder characterized by aggressive behaviors than those who had a later onset. Much of the work on early antisocial behavior has been guided by Moffitt’s (1993) theory, proposing that there are two distinct types of antisocial behavior: (1) Life Course Persistent, present soon after birth and persistent throughout the life-span, accompanied by deficits in neuropsychological abilities and exacerbated adverse environments, and (2) Adolescent-Limited, with antisocial behaviors that are only present during adolescence, are not accompanied by neuropsychological deficits, and do not persist throughout adulthood. In the current study, a blunted diurnal cortisol rhythm was evident in children from the general population whose conduct problems and aggression increased over time; this may represent a different or more severe group than children whose externalizing behavior emerges or increases at a later age.
Although no studies to our knowledge have identified the mechanism by which blunted HPA axis activity leads to increases in externalizing behavior, there are a number of possible explanations for the direction of this relationship. One hypothesis is that children with blunted diurnal cortisol rhythms are chronically under-stimulated and under-aroused, leading them to seek out more stimulation, thereby engaging in aggressive or antisocial behaviors (Kruesi et al., 1989; Zuckerman, 1979). This theory suggests that children seek to raise their arousal levels by engaging in externalizing or rule-breaking behavior. A second explanation is that this under-arousal may lead children to experience less anxiety in the face of dangerous situations, and therefore feel less afraid of the consequences of acting out (Raine, 2002). Combined, these theories suggest that decreased HPA axis activity may create psychological states of under-arousal and fearlessness, both of which may lead a child to engage in more rule-breaking behaviors.
Our findings, demonstrating that blunted diurnal cortisol predicts increases in externalizing psychopathology, but not concurrent behavior, adds to a body of literature with potential clinical implications. If findings are replicated among children showing clinically elevated externalizing behavior, the present findings may provide clues regarding mechanisms that could lead to developing more sensitive and specific biomarkers that may provide a basis for targeted intervention. Early preventive interventions, then, could be targeted towards these children at risk for more severe externalizing problems. Notably, preventive interventions that support positive parenting have been shown to normalize diurnal cortisol rhythms. For example, the Attachment and Biobehavioral Catch-up (ABC) intervention, which aimed to increase sensitive parenting during infancy and toddlerhood for children who experience early adversity, led to steeper morning-to-evening cortisol rhythms than a control intervention (Bernard et al., 2015a; Bernard et al., 2015b). The results of the current study suggest that, by normalizing cortisol rhythms, such interventions may further prevent or reduce severe externalizing behaviors in later childhood, a hypothesis that warrants further study.
Our study benefited from a number of strengths, including the large sample size, longitudinal design, and collection of multiple saliva samples to assess diurnal rhythm of cortisol. Further, the sophisticated statistical approach was well-suited to the research question in both modeling diurnal rhythms via latent change scores and using a cross-lagged design to examine directionality. However, there were also a number of limitations that should be considered. First, as only a limited number of children displayed significant conduct problems and aggressive behavior (only 6% of the sample fell in the borderline and clinical range) we cannot generalize our findings to a clinical population. Second, although the CBCL has shown good concurrent validity with other instruments like the DSM-IV checklist (Hudziak, 1998), DSM clinical diagnoses in children’s case records (Achenbach & Rescorla, 2001), and semi-structured diagnostic interviews (Kaufman, et al., 1997), we relied only on a parent-report questionnaire to assess children’s behavior problems.
Children with early increases in conduct problems are at higher risk of exhibiting aggressive and antisocial behaviors as adults, and represent a significant cost to society. Our findings that a blunted diurnal cortisol rhythm at age 6 predicted an increase in externalizing behavior at age 9 suggest that there may be utility in using our findings to provide clues for additional research into noninvasive biological markers for early detection of risk, thereby providing an opportunity for targeted prevention efforts.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health R01 MH069942
References
- Achenbach TM, Rescorla L. ASEBA school-age forms & profiles. Burlington: Aseba; 2001. [Google Scholar]
- Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of child psychology and psychiatry. 1999;40(01):57–87. [PubMed] [Google Scholar]
- Bernard K, Dozier M, Bick J, Gordon MK. Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Development and Psychopathology. 2015a;27:829–841. doi: 10.1017/S095457941400073X. doi.org/10.1017/S095457941400073X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Hostinar CE, Dozier M. Intervention Effects on Diurnal Cortisol Rhythms of Child Protective Services–Referred Infants in Early Childhood Preschool Follow-up Results of a Randomized Clinical Trial. JAMA pediatrics. 2015b;169(2):112–119. doi: 10.1001/jamapediatrics.2014.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Zwerling J, Dozier M. Effects of early adversity on young children's diurnal cortisol rhythms and externalizing behavior. Developmental psychobiology. 2015 doi: 10.1002/dev.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136–136. [Google Scholar]
- Byrne BM. Multigroup comparisons and the assumption of equivalent construct validity across groups: Methodological and substantive issues. Multivariate Behavioral Research. 1989;24(4):503–523. doi: 10.1207/s15327906mbr2404_7. [DOI] [PubMed] [Google Scholar]
- Carmines EG, McIver JP. Analyzing models with unobserved variables: Analysis of covariance structures. Social measurement: Current issues. 1981:65–115. [Google Scholar]
- Chen FF, Sousa KH, West SG. Teacher's corner: Testing measurement invariance of second-order factor models. Structural equation modeling. 2005;12(3):471–492. [Google Scholar]
- de Weerth C, van Geert P, Hoijtink H. Intraindividual variability in infant behavior. Developmental Psychology. 1999;35(4):1102. doi: 10.1037//0012-1649.35.4.1102. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological psychiatry. 2008;64(7):599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Annual Research Review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of child Psychology and Psychiatry. 2014;55(6):532–548. doi: 10.1111/jcpp.12152. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and psychopathology. 2001;13(03):515–538. doi: 10.1017/s0954579401003066. Retrieved from http://journals.cambridge.org/action/displayJournal?jid=DPP. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Current opinion in psychiatry. 2009;22(4):357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wade S. Endocrine correlates of stress vulnerability. Psychotherapy and psychosomatics. 1993;60(1):8–17. doi: 10.1159/000288675. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ. DSM-IV Checklist for childhood disorders. Burlington, VT: Research Center for Children, Youth, and Families, University of Vermont. 1998 [Google Scholar]
- Kariyawasam SH, Zaw F, Handley SL. Reduced salivary cortisol in children with comorbid attention deficit hyperactivity disorder and oppositional defiant disorder. Neuroendocrinology Letters. 2002;23(1):45–48. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Development and psychopathology. 2008;20(02):473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Arseneault L, Caspi A, Tomás MP, Taylor A, Moffitt TE. Validity of DSM- IV conduct disorder in 4½–5-year-old children: A longitudinal epidemiological study. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.162.6.1108. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and psychopathology. 2001;13(03):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Schmidt ME, Donnelly M, Hibbs ED, Hamburger SD. Urinary free cortisol output and disruptive behavior in children. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(3):441–443. doi: 10.1097/00004583-198905000-00024. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Quay HC, Applegate B, Shaffer D, Waldman I, Bird HR. Validity of DSM-IV Subtypes of Conduct Disorder Based on Age of Onset. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(4):435–442. doi: 10.1097/00004583-199804000-00022. [DOI] [PubMed] [Google Scholar]
- Martin CG, Kim HK, Bruce J, Fisher PA. Child diurnal cortisol rhythms, parenting quality, and externalizing behaviors in preadolescence. Psychoneuroendocrinology. 2014;40:170–180. doi: 10.1016/j.psyneuen.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. 2001 [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57(1):38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McMahon RJ. Diagnosis, assessment, and treatment of externalizing problems in children: The role of longitudinal data. Journal of Consulting and Clinical Psychology. 1994;62(5):901. doi: 10.1037//0022-006x.62.5.901. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133(1):25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological review. 1993;100(4):674. [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide, seventh ed.(Los Angeles, CA) 1998. 2013 [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry research. 2005;134(1):1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. doi.org/10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31(10):1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk CA, Rinne T, van den Brink W, Knol DL, Doreleijers TA. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological psychiatry. 2007;61(3):405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood. 1983;58(6):454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of abnormal child psychology. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rolon-Arroyo B, Arnold DH, Harvey EA. The predictive utility of conduct disorder symptoms in preschool children: A 3-year follow-up study. Child Psychiatry & Human Development. 2014;45(3):329–337. doi: 10.1007/s10578-013-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DBD, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridjan NS, Velders FP, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. The longitudinal association of the diurnal cortisol rhythm with internalizing and externalizing problems in pre-schoolers. The Generation R Study. Psychoneuroendocrinology. 2014;50:118–129. doi: 10.1016/j.psyneuen.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(9):1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven I, Van Goozen SHM, Van Engeland H, Schaal B, Arseneault L, Seguin JR, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. Journal of neural transmission. 2005;112(8):1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Hormone Research in Paediatrics. 1990;34(2):45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: associations with cortisol. Psychiatry research. 1993;46(1):9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking. Corsini Encyclopedia of Psychology. 1979 [Google Scholar]