Abstract
The purpose of this systematic review and meta-analysis was to determine the effects of supervised resistance and/or aerobic training physical activity interventions on performance-based measures of physical functioning among community-dwelling older adults, and to identify factors impacting intervention effectiveness. Diverse search strategies were used to identify eligible studies. Standardized mean difference effect sizes (d, ES) were synthesized using a random effects model. Moderator analyses were conducted using subgroup analyses and meta-regression. Twenty-eight studies were included. Moderator analyses were limited by inconsistent reporting of sample and intervention characteristics. The overall mean ES was 0.45 (k=38, p=<.01), representing a clinically meaningful reduction of 0.92 seconds in the Timed Up and Go for treatment versus control. More minutes per week (p<.01) and longer intervention session duration (p<0.01) were associated with larger effects. Interventions were especially effective among frail participants (d=1.09). Future research should clearly describe sample and intervention characteristics and incorporate frail populations.
Introduction
Older adults experience high rates of functional decline, hospitalization, and subsequent disability (Anderson, 2010; Centers for Disease Control and Prevention, 2013; U.S. Census Bureau, 2014). The Disablement Model initially proposed by Nagi (Nagi, 1991) and refined by Verbrugge and Jette (Verbrugge & Jette, 1994), suggests pathology related to chronic illness leads to physical impairments that contribute to functional limitations, resulting in disability. The model posits interventions targeting intra-individual and extra-individual factors may moderate progression to disability (Nagi, 1991; Verbrugge & Jette, 1994).
One such intra-individual factor is physical activity (PA) behavior. PA is defined as any bodily movement that increases energy expenditure above a basal level and encompasses various activities including walking and other endurance training, strength training, flexibility training, and exercises to improve balance (Centers for Disease Control and Prevention, 2011). PA contributes to the prevention of functional limitation and early disability among older adults (Chodzko-Zajko et al., 2009; V. S. Conn, 2010; V. S. Conn, Hafdahl, Minor, & Nielsen, 2008; Cotter & Lachman, 2010). For example, high-intensity resistance training can prevent aging-related muscle weakness and frailty (Fiatarone et al., 1994; Morganti et al., 1995). Additionally, aerobic exercise such as walking, can improve aerobic capacity and physical function (PF) (Moore-Harrison, Speer, Johnson, & Cress, 2008), which could help older adults stay independent in their daily activities and self-care (Arnett, Laity, Agrawal, & Cress, 2008).
Engaging in regular PA, in the form of resistance and aerobic training, can help older adults maintain and improve aspects of PF (Chodzko-Zajko et al., 2009; He & Baker, 2004; Hillsdon, Brunner, Guralnik, & Marmot, 2005); however, the magnitude of effect of diverse resistance and aerobic PA interventions on performance-based physical function (PF) outcomes among community-dwelling older adults is yet unknown. Most importantly, the characteristics of resistance and aerobic PA interventions most effective in improving composite PF measures among older adults are not yet clear because no prior systematic reviews and meta-analyses have quantitatively explored moderators of PA intervention effectiveness on these types of measures. Addressing these gaps is critical to efficiently advancing this area of science from PA intervention development and testing to translation into practice of the most effective resistance and aerobic PA interventions on older adult PF.
Prior meta-analyses examining the effect of these PA interventions on PF outcomes have been limited in scope and methodology and inadequately address the research questions for this study. For example, search strategies for seven meta-analyses were critically limited to five online databases or less (Chou, Hwang, & Wu, 2012; de Vries et al., 2012; Gine-Garriga et al., 2010; Gu & Conn, 2008; Howe, Rochester, Neil, Skelton, & Ballinger, 2011; Nicola & Catherine, 2011; Yamamoto, Hotta, Ota, Mori, & Matsunaga, 2015). The most common databases searched were Medline, PubMed, and CINAHL, all of which house similarly indexed citations. Limited database searching diminishes the diverse and comprehensive nature of literature searching critical to rigorous meta-analysis work. Moreover, few researchers searched for unpublished literature (Liu & Latham, 2011; Lopopolo, Greco, Sullivan, Craik, & Mangione, 2006), creating potential publication bias (Dickersin, 2006). Some meta-analyses focused only on frail populations (Chou et al., 2012; de Vries et al., 2012; Gine-Garriga et al., 2010) or only on cognitively impaired populations (Gates, Fiatarone Singh, Sachdev, & Valenzuela, 2013; Potter, Ellard, Rees, & Thorogood, 2011), while others excluded studies with participants with chronic illnesses common among older adults (Tschopp, Sattelmayer, & Hilfiker, 2011), limiting generalizability of study findings. Several prior meta-analyses restricted analyses to specific PF outcomes, such as balance (Howe et al., 2011; Orr, 2010), gait speed (Lopopolo et al., 2006), limb strength (Borde, Hortobágyi, & Granacher, 2015; Raymond, Bramley-Tzerefos, Jeffs, Winter, & Holland, 2013), or cardiorespiratory fitness (Lemura, von Duvillard, & Mookerjee, 2000). Additionally, two meta-analyses combined measures of PF with measures of disability (Gu & Conn, 2008; Liu & Latham, 2011). In the Disablement Model, PF and disability are conceptually distinct; therefore, measures of PF, which assess the individual’s functional limitation in a controlled environment, should be analyzed separately from measures of disability, which assess the individual’s ability to do activities in a socioecological context (Guralnik & Ferrucci, 2003; Jette, 2003; Verbrugge & Jette, 1994).
The purpose of this study was to determine the effects of PA interventions on performance-based, composite measures of PF among community-dwelling older adults. The following research questions guided this study:
What is the overall effect size of supervised resistance and/or aerobic PA interventions on performance-based, composite measures of PF community-dwelling among adults age 65 and older?
Do supervised resistance and/or aerobic PA intervention effects on PF outcomes vary based on intervention characteristics (e.g., dose, intervention components)?
Do supervised resistance and/or aerobic PA intervention effects on PF outcomes vary based on sample characteristics (e.g., gender, baseline body mass index, health status)?
Do supervised resistance and/or aerobic PA intervention effects on PF outcomes vary based on study characteristics (e.g., risk of bias)?
Methods
Standard and accepted meta-analysis methods were used to conduct this study (Borenstein, Hedges, Higgins, & Rothstein, 2009; Cooper, Hedges, & Valentine, 2009). In addition, this meta-analysis was guided by PRISMA reporting standards (Moher, Liberati, Tetzlaff, & Altman, 2009).
Eligibility criteria
Published or unpublished reports of intervention studies were eligible if they 1) written in English; 2) conducted from 1960–2015; 3) tested a supervised PA intervention; 4) involving resistance and/or aerobic training; 5) used two-group, treatment versus control pre-posttest design; 6) included community-dwelling or independent-living participants 7) aged 65 or older, or with a mean age of the entire sample of 70; 8) used performance-based, composite measures of PF to report PF outcomes; and 9) provided adequate data to calculate an effect size including sample size and outcome statistic. Supervised PA interventions were selected for this meta-analysis because PA dose is observed and verified by the supervising interventionist. An accurate dose of PA can help clarify the link between amounts of PA needed to optimize PF among older adults.
For this study, and in accordance with the Disablement Model (Nagi, 1991; Verbrugge & Jette, 1994), PF was conceptually defined as an individual’s ability to perform discrete physical actions through the integration of multiple body systems (Jette, 2003). PF is operationally defined via aggregated assessments of functional limitation, or restrictions of physical performance at the individual level using performance-based, composite measures of PF (Nagi, 1991). These instruments are multidimensional, objective measures involving a battery of tests evaluating multiple components of PF, as in the Short Physical Performance Battery, Functional Fitness Test, Physical Performance Test, Continuous Scale Physical Functional Performance (Freiberger et al., 2012; Guralnik & Ferrucci, 2003). Studies using measures of single components of PF (e.g., chair rise test) and measures of disability (e.g., basic and instrumental activities of daily living) were excluded.
Information Sources and Search Strategies
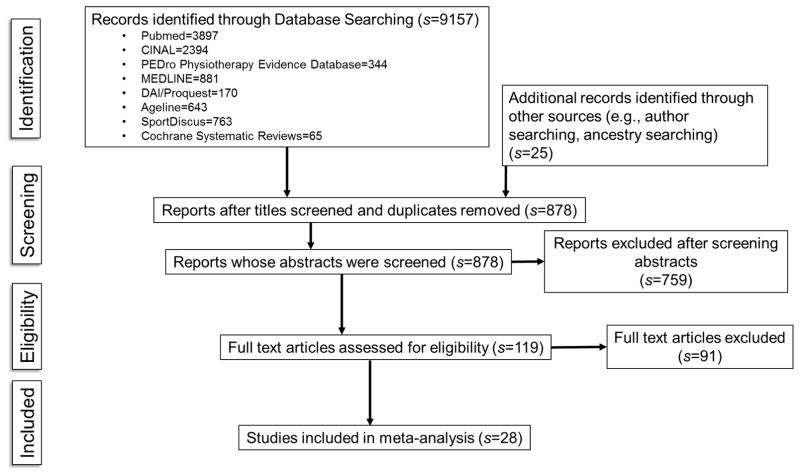
Sampling for this meta-analysis involved an extensive search of the relevant PA intervention literature to avoid the bias of narrow or limited searches. An expert health sciences reference librarian was consulted to develop and refine search strategies for the following online databases: MEDLINE, PubMed, CINAHL, SportDiscus, PEDro Physiotherapy Evidence Database, The Cochrane Library, Dissertation Abstracts International/Proquest, Ageline. Example of search terms developed in conjunction with the health sciences reference librarian and used for MEDLINE were: “physical activity or exercise,” “resistance or strength*,” aerobic or endurance,” “function,” “older adult*or elder*.” Citations retrieved from database searching were exported to a reference manager to review for duplicates. Titles and abstracts of citations remaining after elimination of duplicates were reviewed for eligibility criteria, then full-text reports were retrieved for eligible citations (Figure 1).
Figure 1.
Flow Diagram of Study Selection
Data Extraction
Data were extracted using a codebook developed from prior relevant research (Brown, Upchurch, & Acton, 2003; Chase, 2014; Conn, Valentine, & Cooper, 2002) and expert consultation. The codebook was pilot tested on 10 randomly selected studies to identify any possible missed themes or problem coding items prior to use on the entire sample (Wilson, 2009). Two, doctorally-prepared, trained coders independently performed data extraction. Code sheets were compared, and discrepancies were discussed until consensus was achieved.
Study level information, including year, authors, geographic location, funding, and publication status, were collected. Data related to study design (e.g., type of control group, randomization) and sample characteristics (e.g., mean age, percent female, race/ethnicity, health status, frailty status) were also collected when available. We captured intervention characteristics related to training load (e.g., number of repetitions and sets, intensity), intervention dose (e.g., number of sessions, session duration, frequency of sessions per week, minutes per week), whether the intervention was conducted in a community (e.g., senior center, public gym) or clinical (e.g., rehabilitation facility, hospital gym) setting, interventionist type (e.g., physical therapist, nurse), and specific types of supervised exercise (e.g., walking, body weight exercises). Additional intervention characteristics collected included: targeted muscle groups (e.g., upper extremity, lower extremity), equipment used, and distance walked. Data necessary to calculate effect sizes were extracted (e.g., sample sizes, mean PF outcome values, measures of variability). Corresponding authors were contacted for any missing data related to outcomes statistics.
Risk of Bias
Several strategies were used to manage risk of bias for this meta-analysis (Valentine, 2009). To manage primary study quality, we 1) only included studies with a two-group treatment versus control pre-posttest design; 2) only included studies using an objective measure of PF; and 3) incorporated elements of the PEDro Scale (e.g., allocation concealment, blinding of subjects, interventionists, and data collectors, intent-to-treat analysis) into the codebook to allow for empirical examination of primary study quality using moderator analysis (Maher, Sherrington, Herbert, Moseley, & Elkins, 2003). An analysis of publication bias was also conducted to determine unintended exclusion of unpublished research.
Analyses
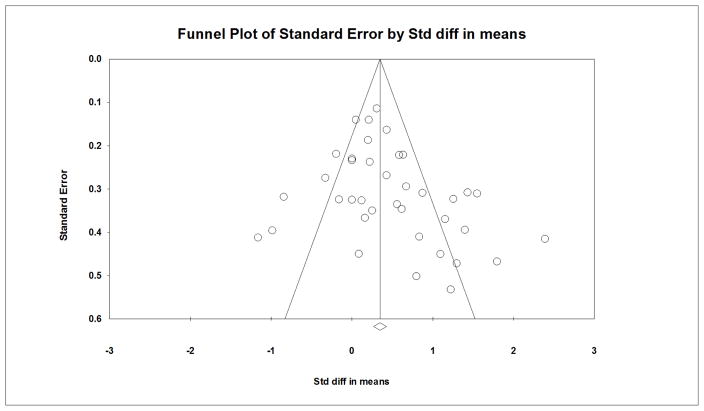
Comprehensive Meta-Analysis Software, Version 3 was used to conduct all analyses (Borenstein, Hedges, Higgins, & Rothstein, 2015). For studies in which multiple treatment groups, but only one control group was employed, the control group was evenly divided into smaller groups for comparison to each treatment group (Higgins & Green, 2011). This method allows for inclusion of all data for studies with multiple treatment arms. Although control group estimates remain unchanged using this method, associated variances would be larger in our meta-analyses (Cuijpers, van Straten, & Smit, 2006; Higgins & Green, 2011). Standardized mean difference effect sizes (Cohen’s d, ESs), defined as the treatment group mean minus the control group mean divided by the pooled standard deviation, were calculated for each included study (Borenstein et al., 2009; Cooper et al., 2009). Values were then weighted by the inverse of the variance to account for sample size and adjust for bias. ESs were synthesized using a random effects model to account for between- and within-study variation. No reports included correlation data to link pre-posttest scores; however, a moderate correlation (r=0.80) was assumed as all pre-posttest data were collected from the same individuals in each study. The overall mean ES was converted to an original metric (e.g., performance-based function score) to facilitate clinical interpretation of effect size findings (Lipsey & Wilson, 2000). The metric selected for conversion was the most utilized measure across all studies. Publication bias was assessed subjectively by constructing a funnel plot of standard errors by standardized difference in means for each comparison.
Homogeneity of variance was tested among effect sizes using Q and I2 statistics. The Q statistic quantifies overall observed heterogeneity of effects, and I2 represents the proportion of heterogeneity due to true differences in effects across studies (Borenstein et al., 2009). Heterogeneity across studies was expected given the variety of study and sample characteristics among PA intervention studies. This inherent heterogeneity allows for exploratory moderator analyses to determine sample, study, and intervention characteristics which may be linked with better PF outcomes (Berlin, 1995; Colditz, Burdick, & Mosteller, 1995; Thompson, 1994). Moderator analyses were conducted on variables for which five or more comparisons were present. This decision was based on meta-analyses with similar research foci (Cuijpers et al., 2006; Lee, Soeken, & Picot, 2007; Shiri, Falah-Hassani, Viikari-Juntura, & Coggon, 2016; Stretton, Mudge, Kayes, & McPherson, 2016). Meta-analytic analogs of ANOVA and regression were used for dichotomous variables (e.g., presence or absence of exercise types) and continuous variables (e.g., intervention dose), respectively.
Results
Study Characteristics
Figure 1 depicts the flow of study selection. Twenty-eight studies were described by 34 reports. Eleven studies had multiple treatment groups resulting in a total of 41 comparisons for analysis. Descriptions of study characteristics are available in the Supplementary Table. Studies were published from 1999–2015 and varied in country of origin with 13 studies from Europe (Boshuizen, Stemmerik, Westhoff, & Hopman-Rock, 2005; Capodaglio, Capodaglio Edda, Facioli, & Saibene, 2007; de Vreede, Samson, van Meeteren, Duursma, & Verhaar, 2005; E Freiberger, Haberle, Spirduso, & Zijlstra, 2012; Freiberger et al., 2013; Gine-Garriga et al., 2010; Granacher, Lacroix, Muehlbauer, Roettger, & Gollhofer, 2013; Jorgensen, Laessoe, Hendriksen, Nielsen, & Aagaard, 2013; Puggaard, 2003; Sousa, Mendes, Abrantes, Sampaio, & Oliveira, 2014; Stiggelbout, Popkema, Hopman-Rock, de Greef, & van Mechelen, 2004; Uusi-Rasi et al., 2015; Zech et al., 2012); eight from the United States (Brandon, Boyette, Gaasch, & Lloyd, 2000; Brandon, Boyette, Lloyd, & Gaasch, 2004; M. Brown et al., 2000; Cress et al., 1999; Mangione, Craik, Palombaro, Tomlinson, & Hofmann, 2010; Miszko et al., 2003; Villareal et al., 2011; Villareal, Banks, Sinacore, Siener, & Klein, 2006); three from South America (Bunout et al., 2006; de Andrade et al., 2013; Lustosa et al., 2011); two from Asia (So et al., 2013; Yamada et al., 2011); and two from Australia (Baker et al., 2007; Foley, Hillier, & Barnard, 2011). All but four studies (Brandon et al., 2000; Granacher et al., 2013; Sousa et al., 2014; Yamada et al., 2011) had funding.
Sample Characteristics
Sample characteristics are presented in Table 1. Samples were generally small with a median control group size of 19 participants and median treatment group size of 22 participants. Median mean age of study samples was 75.92. Median percent female of study samples was 70.66%. Median percent participants that were racially or ethnically diverse could not be calculated due because only one study reported race or ethnicity of participants (Villareal et al., 2011). Participants were overweight, with a median mean body mass index of 27.55 kg/m2. Only ten studies provided health status of participants. Five studies included only healthy participants (Brandon et al., 2000, 2004; Bunout et al., 2006; Capodaglio et al., 2007; Puggaard, 2003), and five studies included frail older adults (Boshuizen et al., 2005; M. Brown et al., 2000; Gine-Garriga et al., 2010; Villareal et al., 2011, 2006). Additional sample characteristics, such as baseline activity status, cognitive status, and use of assistive devices were inconsistently reported.
Table 1.
Study Characteristics
| Sample Characteristics | ||||
|---|---|---|---|---|
|
| ||||
| k | Min | Median | Max | |
| Mean age (years) | 38 | 65 | 75.92 | 85 |
| Total sample size | 41 | 18 | 46 | 318 |
| Percentage women | 40 | 0 | 70.66 | 100 |
| Mean BMI (kg/m2) | 20 | 22.3 | 27.55 | 39 |
| Mean weight (kg) | 22 | 49.9 | 72.88 | 49.90 |
|
| ||||
| Intervention Characteristics | ||||
|
| ||||
| Session duration (minutes) | 38 | 20 | 60 | 90 |
| Sessions per week | 41 | 1 | 2 | 3 |
| Minutes per week | 41 | 40 | 120 | 270 |
| Days over which intervention was delivered | 39 | 63 | 112 | 732 |
| Total number of intervention sessions | 39 | 10 | 36 | 313.2 |
Note. k = number of comparisons, or groups within a report, for which data were available. Some reports may have multiple treatment groups, resulting in multiple comparisons within one report.
Intervention Characteristics
Intervention characteristics are presented in Table 1. Of the studies reporting the type of interventionist, almost all interventions were conducted by an exercise specialist (k=16) or a physical therapist (k=6). Interventions were conducted over a median of 112 days. The median number of intervention sessions was 36, and sessions lasted a median of 60 minutes per session. The median frequency per week of sessions was two, with median minutes per week of 120. Only 12 of the 28 studies met the current World Health Organization global recommendation of 150 minutes of PA per week (Chodzko-Zajko et al., 2009; World Health Organization, 2016).
Eighteen studies used resistance-training only; whereas the remainder employed combination resistance and aerobic training. For studies utilizing resistance training, a majority of studies reported high intensity and progressive training. The types of resistance exercises were poorly described across primary studies. The median number of repetitions per resistance exercise was 10, and the median number of sets was 2.75. Minutes of rest between sets was inconsistently reported. Six studies reported using walking for aerobic exercise. Intensity of aerobic exercise was generally described as low to moderate.
Intervention Effects
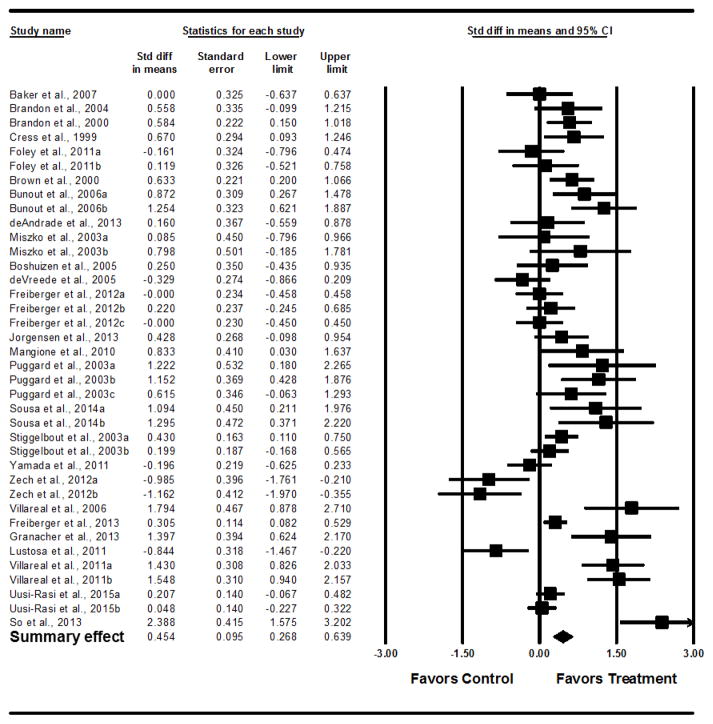
ESs were calculated from 2,608 participants. Supervised PA interventions were significantly effective in improving performance-based, composite measures of PF (d=0.62; 95% CI= 0.40–0.84). The analysis with two outlier studies with the largest ESs removed reduced the ES to 0.45, (95% CI= 0.27–.064) (Figure 2). An ES of 0.45 equates to a reduction in Timed Up and Go time by 0.92 seconds for participants in the treatment group compared to those in the control group. An examination of the funnel plot for publication bias demonstrated some smaller, negative studies may have been excluded from the analysis (Figure 3)
Figure 2.
Forest Plot of Pre-posttest Physical Function Outcomes, Outliers Removed
Figure 3.
Funnel Plot for Pre-posttest Analysis, Outliers Removed
Moderator Analyses
Study and Sample characteristics
Moderator analyses findings for study and sample characteristics are presented in Tables 2 and 3. Interventions appeared to be more effective in improving PF among frail participants (d=1.09) than participants who were not frail (d=0.35). The percent sample that was female and baseline mean BMI did not impact intervention effectiveness.
Table 2.
Subgroup Analyses
| Variable | k0a | k1b | d0a (95% CI) | d1b (95%CI) | QB |
|---|---|---|---|---|---|
| Risk of Bias Characteristics | |||||
|
| |||||
| Allocation concealment | 32 | 6 | 0.52 (0.31–0.73) | 0.11 (−0.11–0.33) | 7.01* |
| Blinded assessor | 14 | 24 | 0.66 (0.45–0.86) | 0.32 (0.07–0.58) | 4.03* |
| Participants individually randomized | 24 | 14 | 0.35 (0.14–0.55) | 0.69 (0.28–1.10) | 2.18 |
| Intention to treat analysis | 24 | 14 | 0.46 (0.20–0.71) | 0.45 (0.18–0.72) | 0.00 |
| True control group | 17 | 21 | 0.65 (0.40–0.91) | 0.28 (0.02–0.54) | 4.17* |
|
| |||||
| Study and Sample Characteristics | |||||
|
| |||||
| Frail participants | 33 | 5 | 0.35 (0.17–0.54) | 1.09 (0.55–1.64) | 6.38* |
|
| |||||
| Intervention Characteristics | |||||
|
| |||||
| Study conducted in a community location | 30 | 8 | 0.57 (0.32–81) | 0.20 (0.04–0.36) | 6.04* |
| Study conducted in a clinical location | 31 | 7 | 0.58 (0.40–0.77) | −0.18 (−0.71–0.35) | 7.19* |
| High exercise intensity | 8 | 13 | 0.48 (−0.14–1.10) | 0.46 (0.11–0.81) | 0.00 |
| Exercise progressed in intensity | 8 | 20 | 0.86 (0.35–1.37) | 0.27 (−0.01–0.55) | 3.97* |
| Resistance-only exercise | 19 | 19 | 0.61 (0.37–0.85) | 0.29 (−0.00–0.58) | 2.78 |
|
| |||||
| Resistance Exercise Characteristics | |||||
|
| |||||
| Upper extremity exercises | 10 | 20 | 0.04 (−0.41–0.49) | 0.56 (0.32–0.81) | 4.00* |
| Upper leg exercises | 5 | 21 | 0.33 (−0.06–0.72) | 0.28 (0.01–0.56) | 0.04 |
| Lower leg exercises | 12 | 15 | 0.63 (0.25–1.01) | 0.03 (−0.22–0.29) | 6.46* |
| Used exercise bands | 21 | 14 | 0.32 (0.01–0.57) | 0.62 (0.28–0.96) | 1.89 |
| Used free weights | 25 | 9 | 0.61 (0.35–0.88) | 0.08 (−0.16–0.33) | 7.91* |
| Used exercise machines | 20 | 15 | 0.50 (0.23–0.77) | 0.35 (0.04–0.67) | 0.50 |
| Used body weight | 17 | 18 | 0.53 (0.23–0.84) | 0.35 (0.08–0.62) | 0.75 |
Note. k=number of studies; d=standardized mean difference calculated under random-effects model; QB = Q between.
0 refers to studies missing the variable of interest.
1 refers to studies containing the variable of interest.
p<.05.
Table 3.
Moderator Analyses of Continuous Variables
| Variable | Number of studies | B | SE | p |
|---|---|---|---|---|
| Percent sample women | 35 | −0.004 | 0.006 | 0.47 |
| Mean BMI (kg/m2) | 17 | 0.069 | 0.051 | 0.18 |
| Percent sessions actually attended by participants | 13 | 0.019 | 0.020 | 0.34 |
| Frequency of interventions sessions (days/week) | 38 | 0.222 | 0.134 | 0.10 |
| Session duration (minutes) | 35 | 0.021 | 0.006 | <0.01 |
| Number of intervention minutes per week | 35 | 0.005 | 0.002 | <0.01 |
| Number of repetitions for resistance exercises | 21 | 0.130 | 0.047 | <0.01 |
| Number of sets for resistance exercises | 23 | 0.195 | 0.113 | 0.09 |
| Days over which intervention delivered | 36 | 0.000 | 0.000 | 0.37 |
| Total number of sessions | 36 | 0.003 | 0.002 | 0.07 |
Note. B= unstandardized meta-regression coefficient for slope; SE=standard error of B; p= statistical significance for B; BMI=body mass index
Risk of bias
Moderator analyses results of study design and risk of bias are presented in Table 2. Studies that did not employ allocation concealment had larger effects (d=0.52) compared to studies that did use allocation concealment (d=0.11). Studies with blinded assessors had significantly smaller ESs (d=0.32) than studies that did not blind assessors (d=0.66). Similarly, studies utilizing a true control group had significantly smaller effects (d=0.28) than studies not using a true control group (d=0.65). Individually randomizing participants and using intention to treat analysis did not significantly impact intervention effects.
Intervention Characteristics
Tables 2 and 3 show findings from the moderator analyses of intervention characteristics. Interventions that were conducted in the community setting were significantly less effective (d=0.20) than studies not conducted in a community setting (d=0.57). Similarly, interventions conducted in a clinical setting (d=−0.18) were less effective than interventions not conducted in this type of setting (d=0.58). There was no statistically significant difference in effects between interventions conducted in a clinical versus community-based setting. Although intervention intensity did not appear to impact intervention effects, maintaining a consistent intensity throughout the duration of the intervention demonstrated larger effects (d=0.86) than progressively increasing intensity (d=0.27). Number of repetitions of resistance exercises was associated with intervention effectiveness (p<0.01); whereas number of sets was not (p=0.09). There was no significant difference in effects between interventions that only focused on resistance exercises and interventions that combined resistance and aerobic exercises. Interventions using upper extremity exercises demonstrated larger effects on PF outcomes (d=0.56) compared to interventions that did not use these types of exercise (d=0.04). Interventions without lower leg exercises were more effective (d=0.62) than interventions with these types of exercises (d=0.03). Most types of exercise equipment did not impact intervention effectiveness; however, interventions in which participants used free weights were less effective (d=0.08) than interventions that did not use this type of equipment (d=0.61).
Intervention Dose
More intervention minutes per week (p<0.01) was associated with larger intervention effects. In contrast, the number of intervention sessions per week was not associated with larger intervention effects (p=0.10). Longer duration of intervention sessions was also associated with larger intervention effects (p<0.01). The total duration of the intervention (p=0.37) and the total number of sessions (p=.07) were not significantly associated with effects on PF outcomes.
Discussion
Supervised resistance and/or aerobic PA interventions significantly improved performance-based, composite PF outcomes among community-dwelling older adults. These positive findings are similar to past meta-analyses looking at PA intervention effects on single components of PF among older adults, including mobility (Chou et al., 2012; de Vries et al., 2012), strength (Ada, Dorsch, & Canning, 2006; Nicola & Catherine, 2011), and balance (Chou et al., 2012; Howe et al., 2011). The overall mean ES of supervised PA interventions on performance-based, composite PF represented a reduction in TUG time of 0.92 seconds. Recent research suggests a reduction in TUG time by 0.8–1.4 seconds demonstrates a clinically significant improvement in PF (Wright, Cook, Baxter, Dockerty, & Abbott, 2011). Additionally, small differences in TUG time may also improve fall risk (Arnold & Faulkner, 2007; Hess & Woollacott, 2005; Shumway-Cook, Brauer, & Woollacott, 2000). Shumway and colleagues (2000) found that an older adult who completed the TUG in 13 seconds time had a 69% probability of being a faller; whereas completing the TUG in 14 seconds increased that probability to 83%.
Our moderator analyses revealed several interesting findings related to intervention delivery and dose. The type of setting in which supervised PA interventions were conducted did not significantly impact gains in PF. Thus, clinicians prescribing resistance and/or aerobic exercise may be generally confident that supervised exercise in either clinical or community settings will improve their older clients’ PF. Regarding intervention dose, most supervised PA intervention studies included in this meta-analysis incorporated PA doses less than the World Health Organization’s global recommendations for PA dose (World Health Organization, 2016). Some countries’ specific PA recommendations are not similar to the global recommendations, which may account for the variation in intervention dose across included studies. Nevertheless, more intervention minutes per week and longer duration of intervention sessions were positively associated with greater intervention effects. Both characteristics contribute to greater exposure to the supervised PA intervention, and may be important considerations when developing future interventions. Thus, researchers should consider testing the effects of supervised PA interventions that at least meet the World Health Organization’s global recommendations of weekly PA dose.
Various aspects of training load were also examined in this meta-analysis. Studies generally reported training load variables for resistance exercise, but not for aerobic exercise. We found that more repetitions of resistance exercise, but not number of sets, was associated with larger intervention effects. Other recent meta-analyses examining muscle strength outcomes with resistance training suggest that number of sets does not significantly impact strength gains among older adults (Borde, Hortobágyi, & Granacher, 2015; Silva, Oliveira, Fleck, Leon, & Farinatti, 2014). However, both meta-analyses examined studies with healthier samples. Future research might compare different training loads among older adults with chronic conditions to determine the most effective combinations in this population.
Regarding intensity, we found that interventions that progressed in intensity were not associated with larger effects. Four of the eight comparisons that did not include progressive intensity did not report intensity level. Additionally, some information regarding repetitions, sets, and types of exercises for the eight comparisons were missing; therefore, it is difficult to determine what potential factors from these studies may have influenced this finding. Clearer descriptions of PA intervention characteristics are needed in future primary work. Future research might compare different levels of intensity and stable or progressive intensity to better understand this finding.
Interventions that included upper extremity resistance exercises were associated with greater intervention effects. In contrast, interventions including lower leg resistance exercises were associated with smaller effects. There is substantial evidence supporting lower extremity exercise in improving PF and preventing disability (Bean et al., 2010; Portegijs et al., 2008; Puthoff, Janz, & Nielson, 2008; Puthoff & Nielsen, 2007); therefore, these exercises should continue to be studied and also recommended in the clinical setting, in addition to upper extremity exercises. Findings from this meta-analysis may be due to the types of performance-based, composite measures employed by eligible studies. Except for the Short Physical Performance Battery, several of the studies’ performance-based, composite measures of PF primarily involved tests of upper extremity function (e.g., Continuous Scale Physical Performance, Physical Performance Test). Thus, interventions using upper extremity exercises may have resulted in better outcomes for these measures. Researchers conducting PA interventions should consider the focus of each type of PA intervention and match performance-based, composite PF measures with appropriate measure that reflect target outcomes. Moreover, additional study into the impact of improving these measures on additional health outcomes, such as quality of life, symptoms, and disability progression needs further attention.
Regarding sample characteristics, supervised PA interventions conducted among frail older adults were especially effective in improving PF. The number of comparisons for this analysis was small; however, these findings are similar to a previous meta-analysis of physical exercise therapy effects among older adults with physical disability and multi-morbidity (de Vries et al., 2012). Clinicians and researchers may be hesitant to recommend and conduct PA interventions among frail older adults; however, this group may greatly benefit from exercise (Chodzko-Zajko et al., 2009; Fiatarone et al., 1994; Moore-Harrison et al., 2008; Morganti et al., 1995). Future research should seek to determine which exercises and exercise doses may be safest and most beneficial for this population.
Some risk of bias may have been present across primary studies. Most included studies did not randomize participants nor conceal allocation. Studies without allocation concealment had larger effects, a finding that may reflect experimenter bias (Gliner, Morgan, & Leech, 2009). Bias related to the lack of employing a true control group or blinding assessors was not present, as most studies had these attributes. In the moderator findings, presence of a true control group or blinded assessor, both of which safeguard internal validity in experimental studies (Liu, LaValley, & Latham, 2011), were associated with smaller effects,. Researchers should consider multiple strategies to reduce risk of bias in future PA interventions.
Limitations
This study has some important limitations. Primary studies inconsistently and incompletely described sample and study characteristics, limiting moderator analyses. Some variables of interest could not be included due to small numbers of comparisons. Additionally, key sample characteristics, such as race and ethnicity and health of study participants could not accurately be summarized across studies. Meta-analyses are observational studies, and this study’s findings are intended to guide future research and inform clinical practice. The moderator analyses findings should be considered exploratory and interpreted in light of the small number of comparisons for some analyses. The intent of the moderator analyses was to uncover potentially important characteristics of supervised resistance and/or aerobic PA interventions that may contribute to greater improvements in PF among older adults. The findings from the moderator analyses could be incorporated into future primary research testing supervise PA interventions effects on PF among older adults.
We sought to include data representative of the wide breadth and depth of eligible studies. To include all data from studies with multiple treatment groups, we chose to evenly divide the control group of these studies into smaller groups to compare to each treatment group. Although this methodology has been described for handling multiple treatment groups, the resulting comparisons remain correlated (Higgins & Green, 2011). Also, given the larger variances produced by splitting the control group, this method could introduce greater error in the meta-analysis. Despite these limitations, this methodology permits examination of heterogeneity across multiple treatment arms, contributing important data to identifying effective intervention components.
Evaluation of publication bias demonstrated some smaller studies with negative findings may have been missing from this meta-analysis despite employing diverse searches strategies. By limiting the type of outcome PF measure in the inclusion criteria, we may have excluded some studies that could contribute to study findings. However, given the vast and diverse measures for PF available in the research literature, precisely defining the PF outcome was essential to the scope of and resources available for the study.
Conclusion
Despite these limitations, this systematic review and meta-analysis contributes to the growing body of research literature focusing on PA interventions to improve PF outcomes among community-dwelling older adults. Supervised resistance and/or aerobic PA interventions significantly improve PF outcomes, which can have clinically important effects. Future PA intervention research should include clear descriptions of sample and intervention characteristics, attend to recommended guidelines for PA behavior, and include frail populations.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by Mizzou Alumni Association Richard Wallace Faculty Incentive Grant and University of Missouri Research Council Grant from the University of Missouri; and the National Institute Of Nursing Research of the National Institutes of Health under Award Number T32NR009356 (Chase, postdoctoral fellow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Jo-Ana D. Chase, Assistant Professor, S343 School of Nursing, University of Missouri, Columbia, MO 65211.
Lorraine J. Phillips, Associate Professor, S414 Sinclair School of Nursing, University of Missouri, Columbia, MO 65211.
Marybeth Brown, Professor Emeritus, 891 Clark Hall, University of Missouri, Columbia, MO 65211.
References
- Ada L, Dorsch S, Canning C. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Australian Journal of Physiotherapy. 2006;52(4):241–248. doi: 10.1016/s0004-9514(06)70003-4. [DOI] [PubMed] [Google Scholar]
- Anderson G. Chronic care: Making the case for ongoing care. Baltimore, MD: Johns Hopkins University; 2010. Retrieved from https://folio.iupui.edu/bitstream/handle/10244/807/50968chronic.care.chartbook.pdf?sequence=1. [Google Scholar]
- Arnett SW, Laity JH, Agrawal SK, Cress ME. Aerobic reserve and physical functional performance in older adults. Age and Ageing. 2008;37(4):384–389. doi: 10.1093/ageing/afn022. http://doi.org/10.1093/ageing/afn022. [DOI] [PubMed] [Google Scholar]
- Arnold CM, Faulkner RA. The history of falls and the association of the timed up and go test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatrics. 2007;7:17. doi: 10.1186/1471-2318-7-17. http://doi.org/10.1186/1471-2318-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MK, Kennedy DJ, Bohle PL, Campbell DS, Knapman L, Grady J, … Fiatarone Singh MA. Efficacy and feasibility of a novel tri-modal robust exercise prescription in a retirement community: a randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55(1):1–10. doi: 10.1111/j.1532-5415.2006.01031.x. http://doi.org/10.1111/j.1532-5415.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- Bean J, Kiely D, LaRose S, Goldstein R, Frontera W, Leveille S. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? Journal of the American Geriatrics Society. 2010;58(12):2363–8. doi: 10.1111/j.1532-5415.2010.03155.x. http://doi.org/http://dx.doi.org/10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JA. Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. American Journal of Epidemiology. 1995;142(4):383–387. doi: 10.1093/oxfordjournals.aje.a117645. [DOI] [PubMed] [Google Scholar]
- Borde R, Hortobágyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Medicine (Auckland, NZ) 2015;45(12):1693–1720. doi: 10.1007/s40279-015-0385-9. http://doi.org/10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 1. Wiley; 2009. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Comprehensive Meta-Analysis (Version 3) Englewood, NJ: Biostat; 2015. [Google Scholar]
- Boshuizen HC, Stemmerik L, Westhoff MH, Hopman-Rock M. The effects of physical therapists’ guidance on improvement in a strength-training program for the frail elderly. Journal of Aging and Physical Activity. 2005;13(1):5–22. doi: 10.1123/japa.13.1.5. [DOI] [PubMed] [Google Scholar]
- Brandon L, Boyette L, Gaasch D, Lloyd A. Effects of lower extremity strength training on functional mobility in older adults. Journal of Aging & Physical Activity. 2000;8(3):214–227. [Google Scholar]
- Brandon L, Boyette LW, Lloyd A, Gaasch DA. Resistive Training and Long-Term Function in Older Adults. Journal of Aging and Physical Activity. 2004;12(1):10–28. doi: 10.1123/japa.12.1.10. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Archives of Physical Medicine and Rehabilitation. 2000;81(7):960–965. doi: 10.1053/apmr.2000.4425. http://doi.org/10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- Brown SA, Upchurch SL, Acton GJ. A framework for developing a coding scheme for meta-analysis. Western Journal of Nursing Research. 2003;25(2):205–222. doi: 10.1177/0193945902250038. [DOI] [PubMed] [Google Scholar]
- Borde R, Hortobágyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Medicine (Auckland, NZ) 2015;45(12):1693–1720. doi: 10.1007/s40279-015-0385-9. http://doi.org/10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunout D, Barrera G, Leiva L, Gattas V, de la Maza M, Avendano M, Hirsch S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Experimental Gerontology. 2006;41(8):746–52. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Capodaglio P, Capodaglio Edda M, Facioli M, Saibene F. Long-term strength training for community-dwelling people over 75: impact on muscle function, functional ability and life style. Journal of Applied Physiology. 2007;100(5):535–42. doi: 10.1007/s00421-006-0195-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Physical activity for everyone: Glossary of terms. 2011 Retrieved September 29, 2012, from http://www.cdc.gov/physicalactivity/everyone/glossary/
- Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention, United States Department of Health and Human Services; 2013. Retrieved from http://www.cdc.gov/features/agingandhealth/state_of_aging_and_health_in_america_2013.pdf. [Google Scholar]
- Chase JD. Interventions to increase physical activity among older adults: A meta-analysis. The Gerontologist. 2014 doi: 10.1093/geront/gnu090. http://doi.org/10.1093/geront/gnu090. [DOI] [PMC free article] [PubMed]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. http://doi.org/10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Archives of Physical Medicine and Rehabilitation. 2012;93(2):237–244. doi: 10.1016/j.apmr.2011.08.042. http://doi.org/10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. American Journal of Epidemiology. 1995;142(4):371–382. doi: 10.1093/oxfordjournals.aje.a117644. [DOI] [PubMed] [Google Scholar]
- Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Annals of Behavioral Medicine. 2010;39(2):128–38. doi: 10.1007/s12160-010-9172-x. http://doi.org/http://dx.doi.org/10.1007/s12160-010-9172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Minor MA, Nielsen PJ. Physical activity interventions among adults with arthritis: meta-analysis of outcomes. Seminars in Arthritis and Rheumatism. 2008;37(5):307–316. doi: 10.1016/j.semarthrit.2007.07.006. http://doi.org/10.1016/j.semarthrit.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2002;24(3):190–200. doi: 10.1207/S15324796ABM2403_04. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. Russell Sage Foundation; 2009. [Google Scholar]
- Cotter KA, Lachman ME. Psychosocial and behavioural contributors to health: age-related increases in physical disability are reduced by physical fitness. Psychology & Health. 2010;25(7):805–820. doi: 10.1080/08870440902883212. http://doi.org/10.1080/08870440902883212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress M, Buchner D, Questad K, Esselman P, deLateur B, Schwartz R. Exercise: effects on physical functional performance in independent older adults. Journals of Gerontology Series A-Biological Sciences. 1999;54(5):242–8. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: a meta-analysis of randomized controlled trials. International Journal of Geriatric Psychiatry. 2006;21(12):1139–1149. doi: 10.1002/gps.1620. http://doi.org/10.1002/gps.1620. [DOI] [PubMed] [Google Scholar]
- De Andrade LP, Gobbi LTB, Coelho FGM, Christofoletti G, Costa JLR, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: a controlled trial. Journal of the American Geriatrics Society. 2013;61(11):1919–1926. doi: 10.1111/jgs.12531. http://doi.org/10.1111/jgs.12531. [DOI] [PubMed] [Google Scholar]
- De Vreede PL, Samson MM, van Meeteren NLU, Duursma SA, Verhaar HJJ. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. Journal of the American Geriatrics Society. 2005;53(1):2–10. doi: 10.1111/j.1532-5415.2005.53003.x. http://doi.org/10.1111/j.1532-5415.2005.53003.x. [DOI] [PubMed] [Google Scholar]
- De Vries NM, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Staal JB, Nijhuis-van der Sanden MWG. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Research Reviews. 2012;11(1):136–149. doi: 10.1016/j.arr.2011.11.002. http://doi.org/10.1016/j.arr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Dickersin K. Publication Bias: Recognizing the Problem, Understanding Its Origins and Scope, and Preventing Harm. In: HRR Co-Chair, AJS Co-Author, MB DALPI, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. John Wiley & Sons, Ltd; 2006. pp. 9–33. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/0470870168.ch2/summary. [Google Scholar]
- Fiatarone M, O’Neill E, Ryan N, Clements K, Solares G, Nelson M, … Evans W. Exercise training and nutritional supplementation for physical frailty in very elderly people. Journal of Medicine. 1994;330(25):1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Foley A, Hillier S, Barnard R. Effectiveness of once-weekly gym-based exercise programmes for older adults post discharge from day rehabilitation: a randomised controlled trial. Journal of Sports Medicine. 2011;45(12):978–86. doi: 10.1136/bjsm.2009.063966. http://doi.org/http://dx.doi.org/10.1136/bjsm.2009.063966. [DOI] [PubMed] [Google Scholar]
- Freiberger E, Blank WA, Salb J, Geilhof B, Hentschke C, Landendoerfer P, … Siegrist M. Effects of a complex intervention on fall risk in the general practitioner setting: a cluster randomized controlled trial. Clinical Interventions in Aging. 2013;8:1079–1088. doi: 10.2147/CIA.S46218. http://doi.org/10.2147/CIA.S46218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberger E, de Vreede P, Schoene D, Rydwik E, Mueller V, Frändin K, Hopman-Rock M. Performance-based physical function in older community-dwelling persons: a systematic review of instruments. Age and Ageing. 2012;41(6):712–721. doi: 10.1093/ageing/afs099. http://doi.org/10.1093/ageing/afs099. [DOI] [PubMed] [Google Scholar]
- Freiberger E, Haberle L, Spirduso W, Zijlstra G. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: a randomized controlled trial. Journal of the American Geriatrics Society. 2012;60(3):437–46. doi: 10.1111/j.1532-5415.2011.03859.x. http://doi.org/http://dx.doi.org/10.1111/j.1532-5415.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The Effect of Exercise Training on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Meta-analysis of Randomized Controlled Trials. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. http://doi.org/10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Gine-Garriga M, Guerra M, Pages E, Manini T, Jimenez R, Unnithan V. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. Journal of Aging. 2010;18(4):401–24. doi: 10.1123/japa.18.4.401. [DOI] [PubMed] [Google Scholar]
- Gliner JA, Morgan GA, Leech NL. Research methods in applied settings: an integrated approach to design and analysis. 2. New York: Routledge; 2009. [Google Scholar]
- Granacher U, Lacroix A, Muehlbauer T, Roettger K, Gollhofer A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology. 2013;59(2):105–113. doi: 10.1159/000343152. http://doi.org/10.1159/000343152. [DOI] [PubMed] [Google Scholar]
- Gu MO, Conn VS. Meta-analysis of the effects of exercise interventions on functional status in older adults. Research in Nursing & Health. 2008;31(6):594–603. doi: 10.1002/nur.20290. http://doi.org/10.1002/nur.20290. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L. Assessing the building blocks of function: utilizing measures of functional limitation. American Journal of Preventive Medicine. 2003;25(3 Suppl 2):112–121. doi: 10.1016/s0749-3797(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Hess J, Woollacott M. Effect of high-intensity strength-training on functional measures of balance ability in balance-impaired older adults. Journal of Manipulative. 2005;28(8):582–90. doi: 10.1016/j.jmpt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- He XZ, Baker DW. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. American Journal of Public Health. 2004;94(9):1567–1573. doi: 10.2105/ajph.94.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. 16.5.4 How to include multiple groups from one study. Retrieved from http://handbook.cochrane.org/chapter_16/16_5_4_how_to_include_multiple_groups_from_one_study.htm. [Google Scholar]
- Hillsdon MM, Brunner EJ, Guralnik JM, Marmot MG. Prospective study of physical activity and physical function in early old age. American Journal of Preventive Medicine. 2005;28(3):245–250. doi: 10.1016/j.amepre.2004.12.008. http://doi.org/10.1016/j.amepre.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. The Cochrane Database of Systematic Reviews. 2011;(11):CD004963. doi: 10.1002/14651858.CD004963.pub3. http://doi.org/10.1002/14651858.CD004963.pub3. [DOI] [PMC free article] [PubMed]
- Jette AM. Assessing disability in studies on physical activity. American Journal of Preventive Medicine. 2003;25(3 Suppl 2):122–128. doi: 10.1016/s0749-3797(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen M, Laessoe G, Hendriksen U, Nielsen C, OBF, Aagaard P. Efficacy of nintendo wii training on mechanical leg muscle function and postural balance in community-dwelling older adults: a randomized controlled trial. Journals of Gerontology Series A: Biological Sciences & Medical Sciences. 2013;68(7):845–852. doi: 10.1093/gerona/gls222. http://doi.org/10.1093/gerona/gls222. [DOI] [PubMed] [Google Scholar]
- Lee J, Soeken K, Picot SJ. A meta-analysis of interventions for informal stroke caregivers. Western Journal of Nursing Research. 2007;29(3):344–356. doi: 10.1177/0193945906296564. http://doi.org/10.1177/0193945906296564. [DOI] [PubMed] [Google Scholar]
- Lemura L, von Duvillard S, Mookerjee S. The effects of physical training of functional capacity in adults. Ages 46 to 90: a meta-analysis. Journal of Sports Medicine. 2000;40(1):1–10. [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- Liu C, Latham N. Can progressive resistance strength training reduce physical disability in older adults? A meta-analysis study. Disability & Rehabilitation. 2011;33(2):87–97. doi: 10.3109/09638288.2010.487145. http://doi.org/http://dx.doi.org/10.3109/09638288.2010.487145. [DOI] [PubMed] [Google Scholar]
- Liu C, LaValley M, Latham N. Do unblinded assessors bias muscle strength outcomes in randomized controlled trials of progressive resistance strength training in older adults? Journal of Physical Medicine. 2011;90(3):190–6. doi: 10.1097/PHM.0b013e31820174b3. http://doi.org/http://dx.doi.org/10.1097/PHM.0b013e31820174b3. [DOI] [PubMed] [Google Scholar]
- Lopopolo RB, Greco M, Sullivan D, Craik RL, Mangione K. Effect of Therapeutic Exercise on Gait Speed in Community-Dwelling Elderly People: A Meta-analysis. Physical Therapy. 2006;86(4):520–540. [PubMed] [Google Scholar]
- Lustosa L, Silva J, Coelho F, Pereira D, Parentoni A, Pereira L. Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: a randomized crossover trial. Revista Brasileira de Fisioterapia. 2011;15(4):318–24. http://doi.org/S1413-35552011000400010. [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Physical Therapy. 2003;83(8):713–721. [PubMed] [Google Scholar]
- Mangione K, Craik R, Palombaro K, Tomlinson S, Hofmann M. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. Journal of the American Geriatrics Society. 2010;58(10):1911–7. doi: 10.1111/j.1532-5415.2010.03076.x. http://doi.org/http://dx.doi.org/10.1111/j.1532-5415.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of Strength and Power Training on Physical Function in Community-Dwelling Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(2):M171–M175. doi: 10.1093/gerona/58.2.m171. http://doi.org/10.1093/gerona/58.2.M171. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed) 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Harrison T, Speer E, Johnson F, Cress M. The effects of aerobic training and nutrition education on functional performance in low socioeconomic older adults. Journal of Geriatric Physical Therapy. 2008;31(1):18–23. doi: 10.1519/00139143-200831010-00004. [DOI] [PubMed] [Google Scholar]
- Morganti CM, Nelson ME, Fiatarone MA, Dallal GE, Economos CD, Crawford BM, Evans WJ. Strength improvements with 1 yr of progressive resistance training in older women. Medicine and Science in Sports and Exercise. 1995;27(6):906–912. [PubMed] [Google Scholar]
- Nagi SZ. Disability concepts revisited: Implications for prevention. In: Pope AM, Tarlov AR, editors. Disability in America: Toward a National Agenda for Prevention. Washington, DC: Division of Health Promotion and Disease Prevention, Institute of Medicine. National Academy Press; 1991. pp. 309–327. [Google Scholar]
- Nicola F, Catherine S. Dose-response relationship of resistance training in older adults: a meta-analysis. Journal of Sports Medicine. 2011;45(3):233–4. doi: 10.1136/bjsm.2010.083246. http://doi.org/http://dx.doi.org/10.1136/bjsm.2010.083246. [DOI] [PubMed] [Google Scholar]
- Nicola F, Catherine S. Dose–response relationship of resistance training in older adults: a meta-analysis. British Journal of Sports Medicine. 2011;45(3):233–234. doi: 10.1136/bjsm.2010.083246. http://doi.org/10.1136/bjsm.2010.083246. [DOI] [PubMed] [Google Scholar]
- Orr R. Contribution of muscle weakness to postural instability in the elderly. A systematic review. [Review] [111 refs] Journal of Physical. 2010;46(2):183–220. [PubMed] [Google Scholar]
- Portegijs E, Kallinen M, Rantanen T, Heinonen A, Sihvonen S, Alen M, … Sipila S. Effects of resistance training on lower-extremity impairments in older people with hip fracture. Archives of Physical Medicine & Rehabilitation. 2008;89(9):1667–74. doi: 10.1016/j.apmr.2008.01.026. http://doi.org/http://dx.doi.org/10.1016/j.apmr.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Potter R, Ellard D, Rees K, Thorogood M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. International Journal of Geriatric Psychiatry. 2011;26(10):1000–1011. doi: 10.1002/gps.2641. http://doi.org/10.1002/gps.2641. [DOI] [PubMed] [Google Scholar]
- Puggaard L. Effects of training on functional performance in 65, 75 and 85 year-old women: experiences deriving from community based studies in Odense, Denmark. Journal of Medicine. 2003;13(1):70–6. doi: 10.1034/j.1600-0838.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- Puthoff ML, Janz KF, Nielson D. The relationship between lower extremity strength and power to everday walking behaviors in older adults with functional limitations. Journal of Geriatric Physical Therapy (2001) 2008;31(1):24–31. doi: 10.1519/00139143-200831010-00005. [DOI] [PubMed] [Google Scholar]
- Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Physical Therapy. 2007;87(10):1334–1347. doi: 10.2522/ptj.20060176. http://doi.org/10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- Raymond MJ, Bramley-Tzerefos RE, Jeffs KJ, Winter A, Holland AE. Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Archives of Physical Medicine and Rehabilitation. 2013;94(8):1458–1472. doi: 10.1016/j.apmr.2013.02.022. http://doi.org/10.1016/j.apmr.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Shiri R, Falah-Hassani K, Viikari-Juntura E, Coggon D. Leisure-time physical activity and sciatica: A systematic review and meta-analysis. European Journal of Pain. 2016 doi: 10.1002/ejp.885. n/a–n/a. http://doi.org/10.1002/ejp.885. [DOI] [PMC free article] [PubMed]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Physical Therapy. 2000;80(9):896–903. [PubMed] [Google Scholar]
- Silva NL, Oliveira RB, Fleck SJ, Leon ACMP, Farinatti P. Influence of strength training variables on strength gains in adults over 55 years-old: a meta-analysis of dose-response relationships. Journal of Science and Medicine in Sport/Sports Medicine Australia. 2014;17(3):337–344. doi: 10.1016/j.jsams.2013.05.009. http://doi.org/10.1016/j.jsams.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Sousa N, Mendes R, Abrantes C, Sampaio J, Oliveira J. Effectiveness of combined exercise training to improve functional fitness in older adults: A randomized controlled trial. Geriatrics & Gerontology International. 2014;14(4):892–898. doi: 10.1111/ggi.12188. http://doi.org/10.1111/ggi.12188. [DOI] [PubMed] [Google Scholar]
- So W, Song M, Park Y, Cho B, Lim J, Kim S, Song W. Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: a randomized controlled trial. Aging Clinical and Experimental Research. 2013;25(2):167–174. doi: 10.1007/s40520-013-0032-y. http://doi.org/10.1007/s40520-013-0032-y. [DOI] [PubMed] [Google Scholar]
- Stiggelbout M, Popkema D, Hopman-Rock M, de Greef M, van Mechelen W. Once a week is not enough: effects of a widely implemented group based exercise programme for older adults; a randomised controlled trial. Journal of Epidemiology. 2004;58(2):83–8. doi: 10.1136/jech.58.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton CM, Mudge S, Kayes NM, McPherson KM. Interventions to improve real-world walking after stroke: A systematic review and meta-analysis. Clinical Rehabilitation. 2016 doi: 10.1177/0269215516640863. 0269215516640863. http://doi.org/10.1177/0269215516640863. [DOI] [PubMed]
- Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ (Clinical Research Ed) 1994;309(6965):1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp M, Sattelmayer M, Hilfiker R. Is power training or conventional resistance training better for function in elderly persons? A meta-analysis. Age & Ageing. 2011;40(5):549–56. doi: 10.1093/ageing/afr005. http://doi.org/http://dx.doi.org/10.1093/ageing/afr005. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 65+ in the United States: 2010. 2014 Retrieved August 5, 2014, from http://www.census.gov/content/dam/Census/library/publications/2014/demo/p23-212.pdf.
- Uusi-Rasi K, Patil R, Karinkanta S, Kannus P, Tokola K, Lamberg-Allardt C, Sievänen H. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Internal Medicine. 2015;175(5):703–711. doi: 10.1001/jamainternmed.2015.0225. http://doi.org/10.1001/jamainternmed.2015.0225. [DOI] [PubMed] [Google Scholar]
- Valentine JC. Judging the quality of primary research. In: Cooper HM, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. Russell Sage Foundation; 2009. pp. 122–146. [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine (1982) 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Villareal D, Banks M, Sinacore D, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Archives of Internal Medicine. 2006;166(8):860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- Villareal D, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, … Shah K. Weight loss, exercise, or both and physical function in obese older adults. The New England Journal of Medicine. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. http://doi.org/10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. Systematic Coding; pp. 159–176. [Google Scholar]
- World Health Organization. Physical Activity and Older Adults. 2016 Retrieved May 4, 2016, from http://www.who.int/dietphysicalactivity/factsheet_olderadults/en/
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. The Journal of Orthopaedic and Sports Physical Therapy. 2011;41(5):319–327. doi: 10.2519/jospt.2011.3515. http://doi.org/10.2519/jospt.2011.3515. [DOI] [PubMed] [Google Scholar]
- Yamada M, Aoyama T, Hikita Y, Takamura M, Tanaka Y, Kajiwara Y, … Tanaka B. Effects of a DVD-based seated dual-task stepping exercise on the fall risk factors among community-dwelling elderly adults. Journal. 2011;17(10):768–72. doi: 10.1089/tmj.2011.0054. http://doi.org/http://dx.doi.org/10.1089/tmj.2011.0054. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Hotta K, Ota E, Mori R, Matsunaga A. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: A meta-analysis. Journal of Cardiology. 2015 doi: 10.1016/j.jjcc.2015.09.005. http://doi.org/10.1016/j.jjcc.2015.09.005. [DOI] [PubMed]
- Zech A, Drey M, Freiberger E, Hentschke C, Bauer JM, Sieber CC, Pfeifer K. Residual effects of muscle strength and muscle power training and detraining on physical function in community-dwelling prefrail older adults: a randomized controlled trial. BMC Geriatrics. 2012;12:68. doi: 10.1186/1471-2318-12-68. http://doi.org/10.1186/1471-2318-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.