Abstract
The Vsa proteins are associated with the virulence of the murine respiratory pathogen Mycoplasma pulmonis. The antigens consist of a conserved N-terminal region that is combined with one of several different variable C-terminal regions comprised of tandem repeats. M. pulmonis strains that produce VsaA with about 40 tandem repeats do not adhere to polystyrene or erythrocytes and are highly resistant to complement killing. Strains that produce VsaA with three tandem repeats adhere strongly to polystyrene and erythrocytes and are highly susceptible to complement killing. We report here that the resistance to complement lysis was not due to a lack of activation of the complement cascade. Isolation and analysis of M. pulmonis strains that produced Vsa proteins other than VsaA (VsaG and VsaI) with either long or short repeat regions indicated that adherence to polystyrene and resistance to complement were dependent on the length of the repeat region but not on the Vsa type. Furthermore, M. pulmonis Vsa variants were susceptible to the polypeptide pore-forming molecule gramicidin D, independent of the Vsa type and length. Collectively, the data indicate the Vsa proteins nonspecifically mediate M. pulmonis surface interactions and function to sterically hinder access of complement to the mycoplasma cell membrane while permitting access of smaller molecules.
Mycoplasmas cause acute and chronic diseases of the joints, the respiratory tract, the reproductive tract, and other tissues in animals and humans. Although not formally proven, their persistence in the presence of an intense inflammatory response is attributed in part to their ability to evade the immune system by antigenic variation (5, 11, 32). The variable surface antigens (Vsa proteins) of Mycoplasma pulmonis, the causative agent of murine respiratory mycoplasmosis, exhibit a high rate of size and phase variation (10−2 to 10−3 per CFU per generation) that is postulated to contribute to immune evasion (32). The Vsa proteins modulate the ability of the mycoplasma to adhere to polystyrene, to adsorb red blood cells (33), to adsorb mycoplasma virus P1 (8), to resist complement-mediated killing (25), and to grow in culture (10). The vsa locus of M. pulmonis strain UAB CT codes for a repertoire of seven different phase-variable Vsa proteins (VsaA, VsaC, VsaE, VsaF, VsaG, VsaH, and VsaI). Phase variation of vsa gene expression results when site-specific DNA inversions combine one of the seven vsa genes with the vsa expression site (2, 24, 26). Vsa size variation occurs from loss or gain in the number of tandem repeat units in the 3′ repetitive region via slipped-strand DNA replication.
As mycoplasmas lack a cell wall, they should be susceptible to the direct effects of lytic agents that act on cell membranes. Investigations have shown that the susceptibility of M. pulmonis to complement lysis is inversely correlated to the number of C-terminal tandem repeat units in its VsaA protein (25). Strains that produce VsaA containing about 40 tandem repeat units (VsaA-R40; a long form of VsaA) are resistant to complement, while strains that produce a short form of VsaA that contains three tandem repeat units (VsaA-R3) or VsaH (containing no repeat units) are highly susceptible to complement. In addition to complement susceptibility, hemadsorption and polystyrene adherence are inversely associated with the length of the VsaA protein. Microbial resistance to the killing and opsonizing effects of complement or a deficiency in complement in the host provide microorganisms with mechanisms and opportunities that enhance survival and virulence (1, 12, 18). As mycoplasma-induced diseases have contributing immune factors (3, 4), the interactions between mycoplasmas and complement are an important aspect of immune evasion and the mycoplasma-induced inflammatory process. To better understand how the Vsa proteins contribute to surface interactions, the complement susceptibility, polystyrene adherence phenotypes, and complement-activating abilities of strains that produced short and long forms of several Vsa proteins were examined. The results indicated that the surface properties of the mycoplasma are modulated nonspecifically by changes in the length of the Vsa proteins.
MATERIALS AND METHODS
Mycoplasma strains and characterization.
All of the strains used in this study were derived from M. pulmonis strain CT (Table 1). The mycoplasmas were grown in mycoplasma broth supplemented with 10% heat-inactivated whole horse serum (HyClone) as described previously (6). Cultures containing 107 CFU/ml (or 109 CFU/ml for complement deposition and activation assays) in broth supplemented with 10% glycerol were stored at −80°C in 1.5-ml aliquots. Strains CT182-R3 and CT228 are previously described (25, 27, 28) mutants that contain transposon Tn4001T (9) inserted in their genomes. In strain CT182-R3, which produces a VsaA protein with three tandem repeats, Tn4001T truncates the last codon of the vsaH gene. Tn4001T disrupts the gene encoding the HvsR recombinase that catalyzes vsa gene rearrangements in strains CT228 (27) and CTG535, resulting in phase-locked strains that produce VsaA and VsaG, respectively. Strains CTG-R5 and CTG-R40 are VsaG-producing mutants that were isogenically derived from strain CTG535. M. pulmonis strain CT-H.8 and CTI-R4 were isolated from the trachea of a BALB/cAnNTac mouse (Taconic, Germantown, N.Y.) 14 days postinoculation with M. pulmonis strain CT, and CTI-R40 was isolated from the lungs of a C57BL/6J (Jackson Laboratory, Bar Harbor, Maine) mouse infected with strain CT (A. M. Denison and K. Dybvig, unpublished data).
TABLE 1.
Summary of characteristics of M. pulmonis strains used in this study
| Strain | Vsa type | Vsa repeat length | PAa | Complement susceptibility | Gramicidin susceptibility | Comment or reference |
|---|---|---|---|---|---|---|
| CT182-R3 | A | R3 | + | + | + | 25 |
| CT228 | A | R40 | − | − | + | 25 |
| CTG535 | G | R40 | NDb | ND | ND | Phase-locked parent of CTG-R5 and R40 (this study) |
| CTG-R5 | G | R5 | + | + | + | Isogenic sibling of CTG-R40 (this study) |
| CTG-R40 | G | R40 | − | − | + | Isogenic sibling of CTG-R5 (this study) |
| CTI-R4 | I | R4 | + | + | + | This study |
| CTI-R40 | I | R40 | − | − | + | This study |
| CT-H.8 | H | No tandem repeats | + | + | + | This study |
PA, polystyrene adherence.
ND, not done.
The particular vsa gene that occupied the vsa expression site of each M. pulmonis strain under study was determined by semiquantitative PCR using methods and primers previously described (14). To determine the length of the vsa gene, a forward primer that binds within the vsa expression site (primer o.6666 [14]) and a vsa-specific reverse primer (5′-ATAAGCGATTACCTTAACAGCC-3′ for vsaI; 5′-ATTTATCACATCAAAGAAGCGG-3′ for vsaG) that anneals 3′ of the vsa gene were used to amplify the tandem repeat region of the expressed vsaG or vsaI gene in its entirety. The nucleotide sequences of the resulting PCR products were analyzed with the ABI PRISM model 377 software package (DNA Synthesis and Sequencing Facility, Iowa State University, Ames).
Polystyrene adherence.
Twenty-five-microliter aliquots of frozen starter stocks of the M. pulmonis strains were used to inoculate 3 ml of mycoplasma broth in a 25-cm2 tissue culture flask (Falcon 353082). After overnight growth at 37°C, the broth was pipetted from the flask (nonadherent phase), the flask was gently washed three times with 5 ml of phosphate-buffered saline (PBS) at room temperature, and the attached mycoplasmas were dislodged from the bottom of the flask (adherent phase) with a cell scraper (Falcon 353085) into 1 ml of mycoplasma broth. The nonadherent and adherent phases were assayed for CFU to determine the percentage of CFU that adhered to the flask.
Complement killing assays.
Complement killing of M. pulmonis strains was previously described (25). Briefly, approximately 104 CFU of a mycoplasma strain in 50 μl of broth was incubated with 50 μl of a 20% concentration of guinea pig complement (Colorado Serum Company, Denver, Colo.), heat-inactivated (HIA) complement, or normal saline (N/S; 0.9% NaCl). Mg2+ and Ca2+ were supplemented to the reaction mixtures at a final concentration of 5 and 1 mM, respectively. After incubation in a water bath at 37°C for 30 min, the tubes were placed on ice, serially diluted in mycoplasma broth, and assayed for CFU. Several replicate tubes were assayed in all cases. The data for each replicate tube were represented as the fraction of CFU recovered after complement treatment relative to the CFU surviving treatment with N/S. Data were analyzed by one-way analysis of variance, with pairwise multiple comparisons performed with the Student-Newman-Keuls method.
Immunoblot analysis.
Mycoplasma proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose (Bio-Rad). All immunological reactions were performed at room temperature as previously described (32). The Vsa-specific monoclonal antibody (MAb) 7.1-2 (immunoglobulin G1 [κ]) (31, 32) was diluted 1:500, and the alkaline phosphatase-conjugated secondary sheep anti-murine immunoglobulin G (Serotec) was diluted 1:2,000. The Vsa protein bands were visualized by development of the immunoblots with 5-bromo-4-chloro-3-indolyl phosphate (Sigma) as the substrate.
Complement component C3 deposition assays.
M. pulmonis cells were incubated with 20% DBA/2NCr serum supplemented with 5 mM Mg2+ and 1 mM Ca2+ at 37°C in a final reaction volume of 100 μl. The reactions were then diluted by the addition of 900 μl of PBS-EDTA (PBS containing 2.5 mM EDTA; 4°C). Cells were harvested by centrifugation (12,000 × g) and washed two additional times in PBS-EDTA. The proteins were subjected to Western analysis with goat anti-murine C3 and alkaline phosphatase-conjugated, rabbit anti-goat (Immunology Consultant Laboratory, Newbury, Oreg.) as primary and secondary antibodies, respectively. In control experiments, HIA DBA/2NCr serum was used as the complement source. The relative amounts of the 65- and 46-kDa C3 degradation fragments on the blots were determined using the ImageJ gel analysis function (version 1.29x; National Institutes of Health).
Bystander hemolytic assays.
When a complement activation surface (the mycoplasmas) results in the formation of the complement terminal lytic complex (membrane attack complex [MAC]) in close proximity to erythrocytes, soluble MAC will diffuse to and lyse the erythrocytes (18). The soluble hemoglobin released to the reaction supernatants (read as the optical density at 415 nm) is used to determine the percentage of erythrocytes lysed. A total of 108 CFU of mycoplasmas in 180-μl reaction mixtures containing 5% guinea pig serum and 1% sheep erythrocytes in N/S were incubated for 30 min at 37°C. The unlysed erythrocytes were removed from the reactions by centrifugation (3,000 × g), and 75-μl aliquots of the supernatants were transferred to the wells of 96-well microtiter plates containing 100 μl of water. The absorbance at 415 nm (A415), as determined with an enzyme-linked immunosorbent assay plate reader, was the indicator of hemoglobin release. The percentage of erythrocytes lysed (EL) was calculated as follows: EL = (AN − AS)/(A100 − A0) × 100, where AN was defined as the A415 of reactions performed in normal guinea pig serum and AS was the A415 of control reactions performed in N/S. One hundred percent erythrocyte lysis (A100) was determined by incubating the erythrocytes in water rather than N/S. Zero percent lysis (A0) was determined by incubating the erythrocytes in N/S only. Control reactions were done in the presence of 5% HIA guinea pig serum as the complement source or N/S as a negative control for lysis.
MICs of gramicidin D.
The MICs of gramicidin D for the growth of mycoplasmas were determined as previously described (19). For the determination of the MIC, the mycoplasmas (104 CFU) were incubated in a final volume of 100 μl of mycoplasma broth in 1.5-ml microcentrifuge tubes in the presence of twofold dilutions of gramicidin D from 160 to 0 ng/ml and at 200 and 2,000 ng/ml. A change in the color of the phenol red indicator in the growth medium indicated bacterial growth. The MIC was defined as the lowest gramicidin concentration that completely inhibited mycoplasma growth after 7 days.
RESULTS
Characterization of Vsa variants derived from M. pulmonis strain CT.
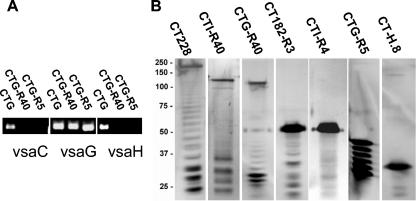
Alternative vsa genes are expressed in M. pulmonis when site-specific DNA inversions switch the particular vsa gene that occupies the vsa expression site. As our group has previously shown, subpopulations of cells expressing alternative vsa genes are detectable after 40 cycles of PCR (14, 27). As determined by vsa-specific PCR analysis (Fig. 1A), strain CTG produced primarily VsaG and contained subpopulations of cells producing alternate Vsa proteins. Interruption of the hvsR gene has been shown to result in mutants that can no longer undergo DNA inversions at the vsa locus and are subsequently unable to phase vary their Vsa proteins (27). Strain CTG535 was derived from strain CTG (9) and contained Tn4001T inserted within the hvsR gene at nucleotide position 656,564 of the complete M. pulmonis genome sequence (http://genolist.pasteur.fr/MypuList/). The HvsR protein has 248 amino acids, extending from nucleotide position 656,832 to 656,089. In strain CTG535, HsvR would be truncated to only 89 amino acids. Strains CTG-R40 and CTG-R5 are filter clones of CTG535. PCR analysis of CTG-R40 and CTG-R5 with vsa-specific primer pairs (14, 24) confirmed that these strains are phase locked, as predicted from the hvsR disruption, and have the vsaG gene occupying the expression site (Fig. 1A). These data indicate that strains CTG535, CTG-R40, and CTG-R5 produce VsaG exclusively.
FIG. 1.
PCR and Western analysis of M. pulmonis strain CT Vsa variants. (A) vsa-specific PCR analysis of M. pulmonis strains CTG, CTG-R40, and CTG-R5. Shown are the vsa-specific PCR products, observed by agarose gel electrophoresis, from reactions with the vsaC-, vsaG-, and vsaH-specific primer sets. (B) Western blot analysis of M. pulmonis proteins (10 μg per lane) detected with Vsa-specific MAb 7.1-2.
The Vsa protein produced by each strain was examined by a combination of Western analysis to assess the protein size and PCR analysis to determine which gene occupied the vsa expression site. Western analysis (Fig. 1B) using the Vsa-specific MAb showed the typical Vsa ladder pattern (32, 33) with the apparent molecular mass of the uppermost (and major) protein band of about 200 kDa (strain CT228), 120 kDa (strain CTG-R40), 110 kDa (strain CTI-R40), 55 kDa (strains CT182-R3 and CTI-R4), 42 kDa (strain CTG-R5), and 32 kDa (strain CT-H.8) (Fig. 1). For large Vsa proteins with an apparent molecular mass greater than 100 kDa, the precise number of repeats in the tandem repeat region was not determined because the repeat region of the gene was large and problematic to sequence. Such proteins are labeled with the suffix R40 to denote many tandem repeats, possibly as many as 40. For smaller Vsa proteins, the tandem repeat region of the gene was PCR amplified and sequenced. The Vsa types of the strains under study (Table 1) were such that long (R40) and short (R < 10) forms of the VsaA, -G, and -I proteins were identified. The number of repeats is denoted with the suffix RX, where X refers to the number of repeats.
M. pulmonis strains CT182-R3 and CT228 activate complement component C3.
The differences in complement resistance between strains producing the R40 and R3 forms of VsaA (strains CT228 and CT182-R3, respectively) could result from different levels of activation of the complement pathway. The immunological detection of complement component C3 deposition onto a bacterial surface is an indicator of complement activation (18, 22). Initial experiments to detect guinea pig C3 on the surface of CT182-R3 by either Western blot analysis or immunofluorescence microscopy failed, presumably as the complement lysis of this strain prevented the centrifugal recovery of sufficient cell membrane needed for these analyses. Therefore, DBA/2 mouse serum was used for subsequent analyses, as it is deficient in complement component 5 (C5) activity and lacks the ability to activate and form MAC. It does have functional C3 (34).
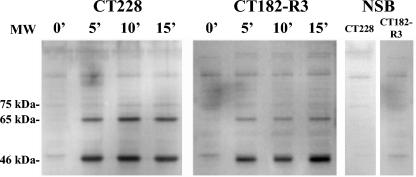
Western analysis (Fig. 2) of strains CT228 (VsaA-R40) and CT182-R3 (VsaA-R3) that were reacted with 10% DBA/2 serum revealed C3 activation fragments that migrated at 65 kDa (consistent with the size of the C3 beta-fragment) and 46 kDa (consistent with the size of factor I-mediated cleavage fragments of activated C3) (23). As determined by image analysis of the C3 protein bands on the blots, the ratio of the relative amounts of the 65-kDa band to the 46-kDa band for CT228 (0.495; n = 3; standard mean error = 0.03) was about twice that for CT182-R3 (0.266; n = 3; standard mean error = 0.004). C3 deposition was not detected when HIA serum was used. That these fragments were present in similar quantities in both CT228 and CT182-R3 indicated that the surfaces of both strains activate complement.
FIG. 2.
Western analysis of complement component C3 deposition on M. pulmonis detected with goat anti-murine C3, as shown in this time course analysis of M. pulmonis strains CT228 and CT182-R3 incubated with DBA/2NCr serum for 0, 5, 10, and 15 min. The panel labeled NSB represents the strains incubated in N/S and is a control for nonspecific binding of the detection antibodies.
Bystander hemolysis is induced by CT182-R3 and CT228.
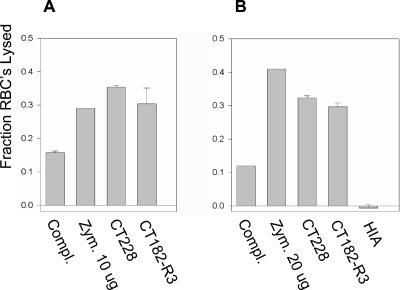
A bystander hemolytic assay was used to determine whether strains CT182-R3 and CT228 activated the complement pathway to the extent that MAC was formed. Zymosan A, a strong activator of the complement pathway, and complement alone were used as positive and negative controls, respectively. The results of two representative experiments, shown as the fraction of erythrocytes lysed in a reaction (Fig. 3), indicated that strains CT228 and CT182-R3 induce the complement lytic system to produce functional MACs. All pairwise comparisons between the mycoplasmas and the controls showed significant differences. The strain that produced VsaA-R40 induced about the same MAC activity (36% [Fig. 3A] and 32% [Fig. 3B] RBC lysis; n = 3 in each experiment) as did the strain that produced VsaA-R3 (30% [Fig. 3A] and 29% [Fig. 3B] RBC lysis; n = 3 in each experiment). Hemolysis ranged from 12 to 16% in reactions where mycoplasmas were excluded, indicating that complement alone produced a background level of bystander activity. Bystander hemolysis was not detected when HIA guinea pig serum was used as the complement source, indicating that the mycoplasmas did not exhibit any intrinsic hemolytic activity under the reaction conditions. These data indicate that the resistance to complement of strain CT228 is not due to a reduced level of complement activation.
FIG. 3.
Bystander hemolysis assays of M. pulmonis strains CT228 and CT182-R3: results of two representative assays. The results are represented as the mean (+ standard error of the mean; n = 3) of the fraction of erythrocytes (RBC) lysed. Guinea pig serum and RBC were incubated in the absence of mycoplasmas (complement only [Compl.]), in the presence of zymosan A (Zym.), and in the presence of M. pulmonis strain CT228 or CT182-R3. The combined results of separate reactions of CT228 or CT182-R3 incubated with RBC and HIA guinea pig serum (n = 6) are shown. (A) Mycoplasmas at 5 × 107 CFU per reaction mixture and zymosan A used at 10 μg per reaction mixture. (B) Mycoplasmas at 108 CFU per reaction mixture and zymosan A at 20 μg per reaction mixture.
Resistance to complement killing and adherence to polystyrene are independent of the Vsa type (A, G or I) and dependent on the length of the Vsa repeat region.
Resistance to complement, in the presence of complement activation, suggested that production of a long form of the Vsa protein may protect the mycoplasma surface from MAC. Complement killing assays were done to determine whether strains that produced long forms of VsaI and VsaG proteins were similarly resistant to complement lysis. Table 2 shows the percent CFU recovered from reaction mixtures where M. pulmonis strains that produced different forms of Vsa were incubated with normal guinea pig serum (without the addition of M. pulmonis-specific antibody) as the complement source. Less than 6% of the CFU of strains CT182-R3, CTG-R5, and CTI-R4 and 41% of the CFU of CT-H.8 were recovered from the reaction mixtures. In contrast, all of strain CT228, 87% of strain CTG-R40, and 72% of strain CTI-R40 were recovered from the reaction mixtures. This indicated that strains that produced a short form of the Vsa protein were killed more efficiently than strains that produced a long (R40) form of the Vsa protein (P < 0.001). Killing was abolished by heat inactivation of the serum (P < 0.001). The differences in killing between strains CT182-R3, CTG-R5, and CTI-R4 were not significant. However, the killing of strain CT-H.8 differed significantly from that of all of the other strains.
TABLE 2.
Complement killing and polystyrene adherence of M. pulmonis strain UAB CT-derived Vsa variants
| CT strain | % Recovered (SE)a after treatment with:
|
Polystyrene adherence
|
|||
|---|---|---|---|---|---|
| Complement | HIA serum | nb | % Attached (SE) | n | |
| CT182-R3 | 6 (3.4) | 88 (7.0) | 9 | 87 (1.7) | 3 |
| CT228 | 110 (7.5) | 111 (12.2) | 6 | 2 (1.0) | 3 |
| CT-H.8 | 41 (4.0) | 112 (6.8) | 6 | 73 (4.5) | 3 |
| CTI-R4 | 3 (0.7) | 103 (10.2) | 9 | 77 (2.2) | 3 |
| CTI-R40 | 72 (3.9) | 99 (7.0) | 6 | 10 (1.8) | 3 |
| CTG-R5 | 3 (1.7) | 114 (8.4) | 6 | 93 (2.4) | 3 |
| CTG-R40 | 87 (5.5) | 98 (9.8) | 6 | 9 (0.8) | 3 |
SE, mean standard error.
n, number of replicates.
The strains that were efficiently killed by complement also adhered strongly to the polystyrene surface of tissue culture flasks. More than 70% of the cell populations of M. pulmonis strains CT-H.8, CT182-R3, CTG-R5, and CTI-R4 remained adhered to the polystyrene surface of the tissue culture flasks (Table 1). Less than 10% of the cells in cultures of strains CT182-R40, CTG-R40, and CTI-R40 remained adhered to the polystyrene.
M. pulmonis is susceptible to lysis by gramicidin D.
We sought to determine whether different Vsa-producing strains would differ in susceptibility to the pore-forming polypeptide gramicidin D. MIC assays indicated that all of the M. pulmonis strains were inhibited at a concentration range of 5.0 to 40 ng/ml without an apparent dependency on the Vsa type or length of the Vsa repeat region. The MIC of gramicidin D for Mycoplasma mycoides strain GM9 was greater than 2,000 ng/ml, thus validating that our assays were within a working range where resistance could be detected.
DISCUSSION
Resistance to the cytolytic effects of complement contributes to the virulence of many microorganisms (12). Animal models indicate that colonization, dissemination (22), and immunopathology (16, 29) are some of the pathological features that are dependent to some extent on the complement system. Our group previously showed that the length of the VsaA repeat region was inversely associated with the susceptibility of M. pulmonis to complement killing (25). This previous study also showed that an M. pulmonis strain that produced VsaH, which contains no tandem repeats, was susceptible to complement killing, but the complement susceptibilities of strains that produced other Vsa types were not examined. In the present study, we determined that the conversion of complement component C3 to activated cleavage products occurred about equally on M. pulmonis cells that produced either the R40 or the R3 forms of VsaA. Also, these two strains both activated complement in a bystander hemolysis assay. Therefore, the complement resistance of cells producing VsaA-R40 was not due to a reduced level of activation of the complement cascade. This suggests that complement resistance may result from an inability of MAC to access the mycoplasma cell membrane. Such inhibition may depend on steric interactions and/or charge effects mediated by the long forms of the Vsa proteins.
M. pulmonis strains that adhered to polystyrene (producing VsaI-R4, VsaA-R3, VsaG-R5, or VsaH) had a relatively short Vsa tandem repeat region and were susceptible to complement killing independent of the particular Vsa protein (A, G, H, or I) that was produced. These data indicate that the length of the C terminus of the Vsa protein, independent of the Vsa type, may nonspecifically shield the mycoplasma cell membrane from MAC. Furthermore, all of the M. pulmonis strains tested were highly susceptible to the effects of the polypeptide pore-forming molecule, gramicidin D, regardless of whether they were producing a long or short form of the Vsa protein. This is consistent with a shielding model in which long forms of the Vsa protein exclude large macromolecules from the mycoplasmal surface and allow smaller molecules (e.g., gramicidin) access to the cell membrane. Dybvig et al. (10) showed that Vsa affects growth of M. pulmonis, and our own observations confirm that strains that produce the longer form of Vsa grow more slowly on agar (unpublished data). Some macromolecules that provide M. pulmonis with nutrients (e.g., amino acids or nucleotides) may have diminished access to degradative enzymes on the mycoplasma surface if Vsa is large. Accordingly, in the absence of host defenses such as complement, mycoplasmas that have a shortened Vsa protein and less shielding may have a selective advantage over cells producing a large Vsa protein. This may account for the loss of virulence that occurs when M. pulmonis is passaged in vitro (7, 13).
Although steric interactions mediate resistance to complement in some bacteria (20, 30), other bacterial strategies to confer resistance to complement include inhibition of early complement activation (1), inhibition of MAC formation, or shedding of bacterial surface structures that have MAC bound to them (15). The detection of the 46-kDa C3 degradation products on cells producing VsaA-R40 or VsaA-R3 is consistent with factor H or I inactivation of the early complement cascade. However, C3 degradation could result from proteases native to the M. pulmonis surface. As strain CT182-R3 appeared to degrade the 65-kDa C3 activation fragment to a greater extent than did strain CT228, it is plausible that C3 components may be degraded on the basis of their access to the mycoplasma surface. Nonetheless, the bystander hemolysis and complement killing assays indicated that complement activation was sufficient to lyse the strains that produced the short form of the Vsa protein.
The implications of mycoplasmas activating the complement system while remaining resistant to complement are significant. Complement activation products have immunomodulatory effects, such as inducing cytokine release (17) and mast cell degranulation (and subsequent histamine release) and, therefore, they have the potential to alter the immune response to an infection or exacerbate existing lung disease (16, 21). As complement activation results in opsonization, interactions with phagocytes provide the host with a redundant mechanism by which it could clear mycoplasmas. The findings presented in this study provide substantial support of the concept that Vsa variation contributes to mycoplasma pathogenicity. Thus, understanding the mechanisms by which mycoplasmas can evade the complement cascade will lead to a better understanding of their pathological mechanisms.
TABLE 3.
MICs of gramicidin D for M. pulmonis strains with variant Vsa types
| CT strain | n | MIC (ng/ml) |
|---|---|---|
| CT182-R3 | 12 | 10-40 |
| CT228 | 7 | 20-40 |
| CT-H.8 | 6 | 10 |
| CTI-R4 | 3 | 5 |
| CTI-R40 | 3 | 10-20 |
| CTG-R5 | 3 | 10 |
| CTG-R40 | 3 | 10 |
| M. mycoides GM9 | 4 | >2,000 |
Acknowledgments
We thank A. J. Szalai (Department of Medicine, University of Alabama at Birmingham) for his critique of this work and P. Caldwell for her technical assistance.
This work was supported by Public Health Service grant GM51126 to K.D. and training grant award T32 HL 07553 to A.M.D. from the National Institutes of Health.
Editor: J. T. Barbieri
REFERENCES
- 1.Alitalo, A., T. Meri, L. Ramo, T. S. Jokiranta, T. Heikkila, I. J. T. Seppala, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhugra, B., L. L. Voelker, N. Zou, H. Yu, and K. Dybvig. 1995. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol. Microbiol. 18:703-714. [DOI] [PubMed] [Google Scholar]
- 3.Cartner, S. C., J. R. Lindsey, J. Gibbs-Erwin, G. H. Cassell, and J. W. Simecka. 1998. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect. Immun. 66:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartner, S. C., J. W. Simecka, J. R. Lindsey, G. H. Cassell, and J. K. Davis. 1995. Chronic respiratory mycoplasmosis in C3H/HeN and C57BL/6N mice: lesion severity and antibody response. Infect. Immun. 63:4138-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citti, C., and R. Rosengarten. 1997. Mycoplasma genetic variation and its implication for pathogenesis. Wien. Klin. Wochenschr. 109:562-568. [PubMed] [Google Scholar]
- 6.Davidson, M. K., J. R. Lindsey, R. F. Parker, J. G. Tully, and G. H. Cassell. 1988. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect. Immun. 56:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, J. K., R. B. Thorp, R. F. Parker, H. White, D. Dziedzic, J. D'Arcy, and G. H. Cassell. 1986. Development of an aerosol model of murine respiratory mycoplasmosis in mice. Infect. Immun. 54:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dybvig, K., J. Alderete, H. L. Watson, and G. H. Cassell. 1988. Adsorption of mycoplasma virus P1 to host cells. J. Bacteriol. 170:4373-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dybvig, K., C. T. French, and L. L. Voelker. 2000. Construction and use of derivatives of transposon Tn4001 that function in Mycoplasma pulmonis and Mycoplasma arthritidis. J. Bacteriol. 182:4343-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dybvig, K., J. W. Simecka, H. L. Watson, and G. H. Cassell. 1989. High-frequency variation in Mycoplasma pulmonis colony size. J. Bacteriol. 171:5165-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybvig, K., and L. L. Voelker. 1996. Molecular biology of mycoplasmas. Annu. Rev. Microbiol. 50:25-57. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furr, P. M., and D. Taylor-Robinson. 1993. Factors influencing the ability of different mycoplasmas to colonize the genital tract of hormone-treated female mice. Int. J. Exp. Pathol. 74:97-101. [PMC free article] [PubMed] [Google Scholar]
- 14.Gumulak-Smith, J., A. Teachman, A. H. Tu, J. W. Simecka, J. R. Lindsey, and K. Dybvig. 2001. Variations in the surface proteins and restriction enzyme systems of Mycoplasma pulmonis in the respiratory tract of infected rats. Mol. Microbiol. 40:1037-1044. [DOI] [PubMed] [Google Scholar]
- 15.Joiner, K. A., C. H. Hammer, E. J. Brown, R. J. Cole, and M. M. Frank. 1982. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J. Exp. Med. 155:797-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karp, C. L., A. Grupe, E. Schadt, S. L. Ewart, M. Keane-Moore, P. J. Cuomo, J. Köhl, L. Wahl, D. Kuperman, S.Germer, D. Aud, G. Peltz, and M. Wills-Karp. 2000. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat. Immunol. 1:221-226. [DOI] [PubMed] [Google Scholar]
- 17.Marth, T., and B. L. Kelsall. 1997. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neeleman, C., S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nir-Paz, R., M.-C. Prevost, P. Nicolas, A. Blanchard, and H. Wroblewski. 2002. Susceptibilities of Mycoplasma fermentans and Mycoplasma hyorhinis to membrane-active peptides and enrofloxacin in human tissue cell cultures. Antimicrob. Agents Chemother. 46:1218-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rautemma, R., and S. Meri. 1999. Complement-resistance mechanisms of bacteria. Microbes Infect. 1:785-794. [DOI] [PubMed] [Google Scholar]
- 21.Regal, J. F. 1997. Role of the complement system in pulmonary disorders. Immunopharmacology 38:17-25. [DOI] [PubMed] [Google Scholar]
- 22.Ren, B., A. J. Szala, T. Orlanda, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahu, A., and J. D. Lambris. 2001. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180:35-48. [DOI] [PubMed] [Google Scholar]
- 24.Shen, X., J. Gumulak, H. Yu, C. T. French, N. Zou, and K. Dybvig. 2002. Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J. Bacteriol. 182:2900-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons, W. L., and K. Dybvig. 2003. The Vsa proteins modulate susceptibility of Mycoplasma pulmonis to complement, hemadsorption, and adherence to polystyrene. Infect. Immun. 71:5733-5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons, W. L., C. Zuhua, J. I. Glass, J. W. Simecka, G. H. Cassell, and H. L. Watson. 1996. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect. Immun. 64:472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitaraman, R., A. M. Denison, and K. Dybvig. 2002. A unique, bifunctional site-specific DNA recombinase from Mycoplasma pulmonis. Mol. Microbiol. 46:1033-1040. [DOI] [PubMed] [Google Scholar]
- 28.Teachman, A. M., C. T. French, H. Yu, W. L. Simmons, and K. Dybvig. 2002. Gene transfer in Mycoplasma pulmonis. J. Bacteriol. 184:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters, D. M., P. N. Breysse, B. Schofield, and M. Willis-Karp. 2002. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 27:413-418. [DOI] [PubMed] [Google Scholar]
- 30.Ward, C. K., and T. J. Inzana. 1994. Resistance of Actinobaciilus pleuropneumoniae to bacterial antibody and complement is mediated by capsular polysaccharide and blocking antibody specific for lipopolysaccharide. J. Immunol. 153:2110-2121. [PubMed] [Google Scholar]
- 31.Watson, H. L., D. K. Blalock, and G. H. Cassell. 1988. Characterization of the variable V-1 antigen of Mycoplasma pulmonis, p. 529-534. In G. Stanek, G. H. Cassell, J. G. Tully, and R. F. Whitcomb (ed.), Recent advances in mycoplasmology, vol. 20. Gustav Fischer Verlag, Stuttgart, Germany.
- 32.Watson, H. L., L. S. McDaniel, D. K. Blalock, M. T. Fallon, and G. H. Cassell. 1988. Heterogeneity among strains and a high rate of variation within strains of a major surface antigen of Mycoplasma pulmonis. Infect. Immun. 56:1358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson, H. L., X. Zheng, and G. H. Cassell. 1993. Structural variations and phenotypic switching of mycoplasmal antigens. Clin. Infect. Dis. 17:S183-S186. [DOI] [PubMed] [Google Scholar]
- 34.Wetsel, R. A., D. T. Fleischer, and D. L. Haviland. 1990. Deficiency of the murine fifth complement component (C5): a 2-base pair gene deletion in a 5′-exon. J. Biol. Chem. 265:2435-2440. [PubMed] [Google Scholar]