Abstract
The study of the immune response to Toxoplasma gondii has provided numerous insights into the role of T cells in resistance to intracellular infections. However, the complexity of this eukaryote pathogen has made it difficult to characterize immunodominant epitopes that would allow the identification of T cells with a known specificity for parasite antigens. As a consequence, analysis of T-cell responses to T. gondii has been based on characterization of the percentage of T cells that express an activated phenotype during infection and on the ability of these cells to produce cytokines in response to complex mixtures of parasite antigens. In order to study specific CD4+ T cells responses to T. gondii, recombinant parasites that express a truncated ovalbumin (OVA) protein, in either a cytosolic or a secreted form, were engineered. In vitro and in vivo studies reveal that transgenic parasites expressing secreted OVA are able to stimulate T-cell receptor-transgenic OVA-specific CD4+ T cells to proliferate, express an activated phenotype, and produce gamma interferon (IFN-γ). Furthermore, the adoptive transfer of OVA-specific T cells into IFN-γ−/− mice provided enhanced protection against infection with the OVA-transgenic (but not parental) parasites. Together, these studies establish the utility of this transgenic system to study CD4+-T-cell responses during toxoplasmosis.
Toxoplasma gondii is an obligate intracellular eukaryote parasite that is capable of infecting all nucleated cells in a diverse array of species. Infection with this parasite leads to the innate production of interleukin-12 by accessory cells and to the subsequent initiation of protective immunity characterized by a strongly polarized Th1-type response and the production of gamma interferon IFN-γ that controls parasite replication (4). The importance of T cells in the control of infection is perhaps best illustrated by patients with primary or acquired deficiencies in T-cell function who develop clinical toxoplasmosis (9). In addition, murine models that lack functional T cells are highly susceptible to T. gondii, while the adoptive transfer of T cells from an immunized animal can confer protection against challenge with a virulent strain in a naive host (11, 20, 25, 33). Although the study of immune responses to T. gondii has provided numerous insights into the role of T cells in resistance to intracellular infections, the complexity of this eukaryotic pathogen has made it difficult to characterize immunodominant epitopes that might allow identification of T cells that are specific for parasite antigens. As a consequence, analysis of T-cell responses to T. gondii has been based on characterization of a fraction of T cells that express an activated phenotype during infection and on the ability of these cells to produce cytokines in response to complex mixtures of parasite antigens.
For many bacterial and viral pathogens, the presence of well-characterized, endogenous immunodominant epitopes has allowed for thorough studies of the in vivo generation of T-cell responses during infection. For example, De Boer et al. recently described the initiation of CD4+- and CD8+-T-cell responses in response to infection with lymphocytic choriomeningitis virus, using six defined CD8+-T-cell epitopes and two CD4+-T-cell epitopes (3). The immunome, or sum of known antigenic determinants responsible for T-cell responses to Listeria monocytogenes, is also well characterized, and major histocompatibility complex (MHC) class I- and MHC class II-restricted epitopes have been used to study several aspects of the T-cell response during infection, including the generation of memory T cells (18). However, for complex eukaryotic pathogens, such as Trypanosoma cruzi, studies of clonotypic response have proved to be more challenging (12, 19, 29). The immune response to T. gondii is likely to be similarly complex, and although several major antigens recognized by the immune system have been identified, little work has been done on epitope specificity (5, 13, 14). As a consequence, it has been difficult to study antigen-specific responses in these systems or to define the full repertoire of potential T-cell receptor (TCR) specificities induced by these complex organisms.
One strategy to study defined antigen-specific responses during infection has been to introduce exogenous antigens of known T-cell receptor specificity into the pathogen genome, enabling “tracking” of the antigen-specific immune response by using TCR-transgenic T cells. OVA-specific T-cell responses to exogenously introduced ovalbumin antigen have been useful in several pathogen systems, including T. cruzi, Salmonella enterica serovar Typhimurium, and Listeria (16, 26, 35). As many genetic tools and strategies have been developed to study T. gondii (1, 28), similar approaches are also feasible for this pathogen. Indeed, recent studies have demonstrated that recombinant T. gondii secreting exogenous antigen can induce a CD8+-T-cell response (2, 17), that such responses can be tracked by using class I tetramers, and that antigen-specific CD8+ T cells persist in the brains of mice with toxoplasmic encephalitis (17).
Considering the critical role of CD4+ T cells in the immune response to T. gondii, we sought to determine whether a comparable transgenic system could be developed to track CD4+-T-cell responses. Recombinant parasites have been engineered to express a truncated form of the protein ovalbumin (OVA) as either a cytosolic or a secreted protein. OVA is expressed under the control of a strong, constitutive promoter derived from the T. gondii α-tubulin gene (23). This protein contains a defined CD4+-T-cell epitope recognized by DO11.10 TCR-transgenic T cells (DO11). Both in vitro and in vivo studies show that parasites expressing a cytoplasmic form of OVA fail to stimulate a strong OVA-specific response but that those expressing the secreted form of OVA stimulate DO11 CD4+ T cells to proliferate, express an activated phenotype, and produce IFN-γ. Furthermore, the adoptive transfer of OVA-specific T cells into IFN-γ−/− mice provides enhanced protection against infection with the OVA-transgenic (but not parental) parasites. Together, these studies demonstrate that OVA-transgenic T. gondii provides a useful tool to address specific questions about the immune response to Toxoplasma, as well as more global questions about the CD4+-T-cell response to intracellular pathogens.
MATERIALS AND METHODS
Parasite and cell cultures.
Tachyzoites derived from the avirulent Prugniaud strain of T. gondii (ΔHXGPRT knockout parasites) were kindly provided by D. Soldati (Imperial College, London, United Kingdom). All parasites were maintained by serial passage in human foreskin fibroblast (HFF) cell monolayers in Dulbecco's modified Eagle's medium (Gibco, Carlsbad, Calif.) containing 10% fetal bovine serum as previously described (28). Before in vivo or in vitro infections, tachyzoites were purified from HFF cells by filtration through a 3.0-μm-pore-size filter (Nuclepore, Clifton, N.J.) and washed in phosphate-buffered saline (PBS).
Molecular methods.
Clonal transgenic lines were engineered to constitutively express a truncated form of OVA (amino acids [aa] 140 to 386) in either the parasite cytosol or the parasitophorous vacuole (PV). Vectors were based on Bluescript pKS(+) (Stratagene, La Jolla, Calif.) and contain the following elements: (i) the T. gondii TUB1 promoter (23), terminating at a BglII site upstream of the initiation codon; (ii) coding sequences (as described below) terminating in an AvrII site, cloned in frame with OVA aa 140 to 386 terminating in a PstI site; (iii) a 3′ untranslated region from the T. gondii dihydrofolate reductase-thymidylate synthase gene, terminating in a NotI site (27); and (iv) a chloramphenicol acetyltransferase (CAT)-selectable marker expressed under the control of 5′ and 3′ untranslated regions derived from the T. gondii SAG1 gene, which encodes the parasite's major surface antigen, P30 (15).
The P30 signal peptide was used to target OVA into the PV through the dense granule secretory pathway (7, 32). Plasmid ptubP30-OVA/sagCAT was constructed by replacing the green fluorescent protein (GFP) domain of ptubP30-GFP/sagCAT (32) with OVA via AvrII/PstI restriction digests. Cytosolic expression of OVA was engineered by fusing the first 25 aa from CAT to OVA (21). Construct ptubCAT-GFP/sagCAT was engineered by first replacing GFP in pdhfrCAT-GFP/sagCAT (32) with OVA via AvrII/PstI restriction, followed by transfer of the CAT-OVA-3′dhfr fragment in place of P30-OVA-3′dhfr in ptubP30-OVA/sagCAT via BglII/NotI restriction digests. Transfections were performed by electroporation as previously described (28), using 2 × 107 tachyzoites and 70 μg of NotI-linearized plasmid DNA in a 2-mm-gap cuvette (BTX; 1.5-kEV pulse, 24 Ω). Stable transgenic cells were selected in the presence of 20 μM chloramphenicol, and parasite clones were isolated by limiting dilution after drug selection.
Immunofluorescence assays and microscopy.
For immunolabeling of OVA, parasite-infected HFF cell monolayers grown on 22-mm-diameter glass coverslips were fixed in 4% (wt/vol) paraformaldehyde in PBS and permeabilized in 0.2% (vol/vol) Triton X-100. Immunofluorescence assays were performed with rabbit anti-chicken ovalbumin antibody (Bethyl Laboratories, Inc., Montgomery, Tex.) diluted 1:1,000 in 10% (vol/vol) fetal bovine serum-0.1% Triton X-100 (in PBS) and AlexaFluor488-conjugated goat anti-rabbit immunoglobulin G antibody (Molecular Probes, Eugene, Oreg.). Fluorescence was detected with a Zeiss Axiovert 35 microscope equipped with a 100-W Hg vapor lamp, appropriate barrier-emission filters, and an interline transfer chip charge-coupled device camera (Hamamatsu, Bridgewater, N.J.). Images were captured, colored, and contrast adjusted by using Openlab software (Improvision, Lexington, Mass.).
Mice.
Female BALB/c IFN-γ-deficient and BALB/c mice (age 6 to 8 weeks) were obtained from The Jackson Laboratory (Bar Harbor, Maine). DO11.10 TCR-transgenic BALB/c mice (22) were originally obtained from Philip Scott and bred within the University Laboratory Animal Resources facility of the University of Pennsylvania. CD4+ T cells from these mice express an α-TCR that recognizes a chicken ovalbumin peptide (aa 332 to 339) in the context of I-Ad. All mice were maintained under specific-pathogen-free conditions in accordance with institutional guidelines.
Adoptive cell transfer and T. gondii infection.
Naive CD3+ T cells were purified from DO11 BALB/c mice. Cell suspensions from spleens and lymph nodes were depleted of erythrocytes by using 0.86% (wt/vol) ammonium chloride (Sigma, St. Louis, Mo.), and T cells were isolated by using CD3+-specific columns (R&D Systems, Minneapolis, Minn.), as described by the manufacturer. The CD4+-T-cell population was ∼93% DO11 CD4+ and exhibited a naive phenotype characterized by small size, high levels of CD62L, and low levels of CD44 and CD25.
To assess the ability of transgenic parasites to induce CD4+-T-cell proliferation, naive DO11 T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) as previously described (24). CFSE is distributed equally between daughter cells during mitotic division, thereby diminishing fluorescence intensity by 50% with each round of proliferation and enabling analysis of a population's proliferative history. Each BALB/c or IFN-γ-deficient recipient received 2 × 106 CFSE-labeled cells in 0.2 ml of PBS by retro-orbital intravenous injection. Animals with adoptively transferred cells were inoculated intraperitoneally at 24 h posttransfer with 200 μl of PBS containing 104 control Prugniaud parasites (Pru) or parasites expressing OVA in either the parasitophorous vacuole (P30-OVA) or the cytosol (CAT-OVA). IFN-γ-deficient animals were immunized at 24 h posttransfer with OVA peptide (20 μg/50 μl in each flank) in incomplete Freund's adjuvant, boosted with OVA in incomplete Freund's adjuvant at day 7, and challenged at day 14 posttransfer with 104 tachyzoites of either Pru or P30-OVA strain parasites.
Preparation of dendritic cells and in vitro infection.
Bone marrow-derived dendritic cells (BMDCs) were prepared as previously described (31), and dendritic cell purity was determined to be 83 to 89% CD11c+ by fluorescence-activated cell sorter (FACS) analysis. For stimulation and/or infection, 5 × 105 BMDCs were incubated at 37°C in 5% CO2 for 24 h with OVA protein (2 μg/ml), Pru, CAT-OVA, or P30-OVA-transgenic parasites (1:1 ratio), resulting in an infection rate of ∼51 to 67%. Cells were washed once with PBS to remove free parasites, and CFSE-labeled DO11 CD4+ T cells were isolated and labeled as described above and added to dendritic cells in a ratio of 10:1. After 96 h, proliferation was assessed by flow cytometry.
Flow cytometry.
Single-cell suspensions were made from the spleens and from draining (mesenteric, periaortal, and mediastinal) and nondraining (inguinal, brachial, and cervical) lymph nodes harvested 7 days postinfection from BALB/c mice with adoptively transferred DO11 CD3+ cells, transgenic BALB/c mice, and control T. gondii-infected BALB/c mice. Cells were stained and analyzed directly ex vivo for proliferation, using CFSE as well as surface expression of activation markers CD62L (allophycocyanin conjugated) and CD4+ (peridin chlorophyll a protein conjugated) and the DO11 antiidiotypic antibody KJ126 (phycoerythrin conjugated).
Isolation and culture of splenocytes for ex vivo recall assays.
Spleens from mice with adoptively transferred cells inoculated with Pru or transgenic CAT-OVA and P30-OVA T. gondii were isolated at day 7 postinfection and dissociated into single-cell suspensions. After erythrocyte depletion, 105 cells per well were plated in 96 well, round-bottom plates (Costar, Acton, Mass.) and incubated at 37° for 72 h with OVA protein (2 μg/ml) or soluble Toxoplasma antigen (STAg) (25 μg/ml). Supernatants from recall cultures and from serum were assayed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) analysis.
RESULTS
Engineering of OVA-transgenic Prugniaud T. gondii.
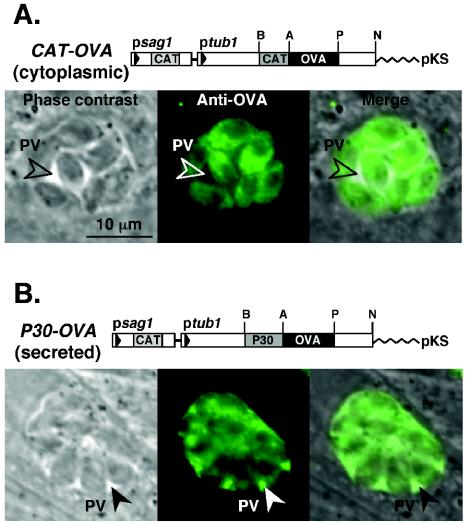
In order to produce transgenic parasites expressing OVA (in either the secreted or cytoplasmic form), constructs were engineered in which the first 25 aa of CAT (ptub1CAT-OVA) or the P30 signal peptide (ptub1P30-OVA) was fused upstream of the OVA-coding sequence (Fig. 1, top panels). Expression of these chimeric proteins was controlled by the T. gondii α-tubulin gene promoter, which resulted in strong constitutive expression (21, 23). Prugniaud strain T. gondii tachyzoites were transfected with these expression plasmids, selected for chloramphenicol resistance, and cloned by limiting dilution (see Materials and Methods). Multiple independent clonal lines were screened by immunofluorescence to assess OVA expression levels and subcellular compartmentalization. As expected, CAT-OVA-transgenic parasites expressed OVA in the parasite cytosol (Fig. 1A, bottom panels), while P30-OVA (Fig. 1B, bottom panels) secreted OVA via the dense granule secretory pathway into the parasitophorous vacuole (7). It is important to note that these proteins were not detected in the cytoplasm of infected cells, consistent with previous studies that indicate discrete compartmentalization of the parasitophorous vacuole and host cell cytoplasm. One clone of each strain was selected for further study.
FIG. 1.
Transgenic T. gondii parasites expressing OVA. Transfection vectors were engineered to express OVA in the parasite cytoplasm (Panel A.; ptubCAT-OVA/sagCAT), or secreted into the parasitophorous vacuole (Panel B.; ptubP30-OVA/sagCAT). For antigen expression in the cytoplasm, OVA-coding sequences were fused downstream of the first 19 amino acids of CAT (to enhance expression levels). For secreted antigen expression, OVA was fused downstream of the signal sequence derived from the parasite's major glycosyl-phosphatidylinositol-anchored surface antigen, P30. Phase-contrast and immunofluorescence images show HFFs infected with either CAT-OVA- or P30-OVA-transgenic parasites. CAT-OVA-transgenic cells show expression in the parasite cytoplasm (nucleus outlined in shadow) but not secreted into the PV. P30-OVA-transgenic cells secrete OVA into the PV (via dense granules, visible as small vesicles with the parasites). Bar, 10 μm.
In vitro activation of CD4+ OVA-specific T cells by transgenic parasites expressing OVA.
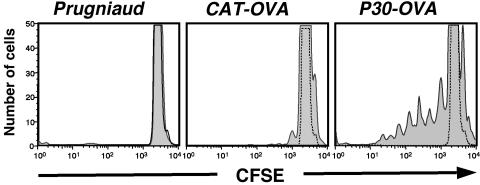
To determine whether the OVA protein expressed by these transgenic parasites is capable of activating CD4+ T cells, bone marrow-derived dendritic cells were infected with different clones of T. gondii at a 1:1 ratio and incubated with CFSE-labeled CD4+ DO11+ T cells in vitro. Proliferation was assayed after 4 days by examining CFSE levels in DO11+ T cells identified by T-cell receptor staining with the anticlonotypic antibody KJ126. Dendritic cells infected with the parental Pru or CAT-OVA-transgenic (expressing antigen in the cytosol) parasites failed to stimulate DO11+-T-cell proliferation (Fig. 2, left and center panels). In contrast, dendritic cells infected with transgenic P30-OVA parasites (expressing antigen in the PV) exhibited significant proliferation (Fig. 2, right panel), comparable to the proliferative response observed when BMDCs were pulsed with 2 μg of OVA protein per ml (data not shown). These studies demonstrate that neither Pru- nor CAT-OVA-transgenic parasites induce DO11+ T cells to proliferate in vitro, whereas parasites that express OVA in the PV are able to stimulate proliferation of DO11+ CD4+ T cells.
FIG. 2.
Proliferation of DO11+ CD4+ T cells induced by in vitro culture with bone marrow-derived dendritic cells infected with parental Prugniaud-strain parasites (left), CAT-OVA-transgenic cells (center), or P30-OVA-transgenic cells (right). Only cultures infected with parasites expressing secreted OVA stimulated DO11 cell proliferation, as assessed by the dilution of CFSE staining.
Activation of CD4+ T cells by OVA-expressing T. gondii in vivo.
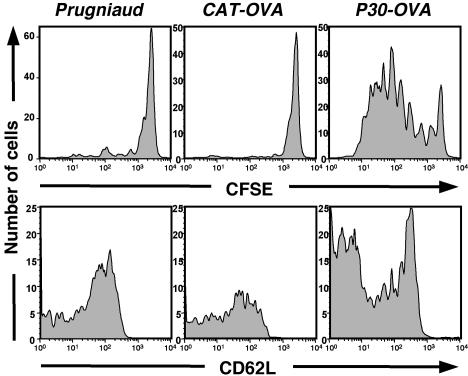
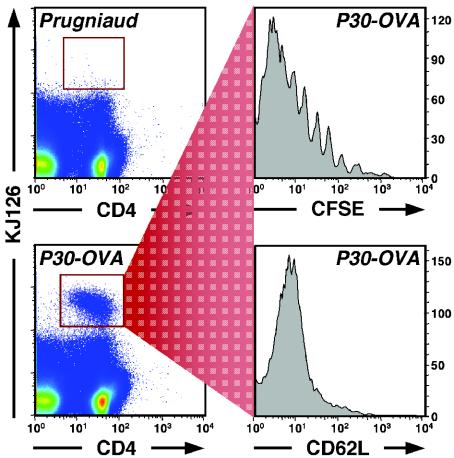
The ability of OVA-transgenic parasites to activate a naive DO11 CD4+-T-cell population in vivo was assayed by examining proliferation and expansion of effector cells, changes in surface phenotype, and cytokine production. BALB/c mice were adoptively transferred intravenously with 2 × 106 CFSE-labeled DO11 CD4+ T cells and infected 1 to 2 days later with Pru, CAT-OVA, or P30-OVA parasites, and the spleens and lymph nodes were collected on day 7 postinfection. The KJ126 antibody, which recognizes DO11 T cells, was used in combination with anti-CD4 to identify OVA-specific T cells by FACS. CD4+ KJ126+ cells were examined for CFSE levels to determine proliferation, as well as changes in surface expression of the activation markers CD62L, CD44, and CD25. Intracellular staining and ELISAs were performed to examine the production of IFN-γ. As shown in Fig. 3, at 7 days postinfection, animals with adoptively transferred cells and infected with Pru (left panels) or CAT-OVA parasites (center panels) showed only a small population of KJ126+ DO11 T cells (∼1,200 and 800 cells in the whole population, respectively) in the draining lymph nodes. High levels of CFSE (top row) and high levels of CD62L (bottom row) indicate that these cells were not activated (similar results were obtained for the activation markers CD44 and CD25 [data not shown]). In contrast, mice challenged with Prugniaud-transgenic parasites secreting P30-OVA (right panels) showed a marked increase in the number of DO11+ T cells in the draining lymph node (∼3,500 cells). In preliminary studies, similar results were also seen with transgenic RH strain parasites expressing the same P30-OVA construct (data not shown). Analysis of the cells from mice infected with P30-OVA-transgenic cells revealed multiple rounds of proliferation as assessed by CFSE staining (top right panel), and a large percentage of cells expressed an activated phenotype, as assessed by decreased expression of CD62L (bottom right panel) and increased levels of CD44 and CD25 (not shown).
FIG. 3.
Proliferation and activation of DO11+ T cells induced in vivo in the draining mesenteric lymph nodes of animals infected with parental Prugniaud parasites (left), CAT-OVA-transgenic cells (center), or P30-OVA-transgenic cells (right). Upper panels show CFSE fluorescence; lower panels show expression of CD62L.
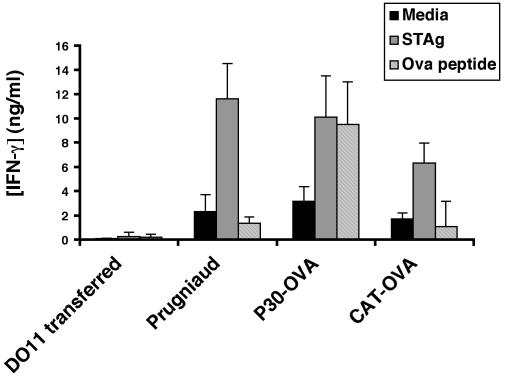
To assess the ability of these cells to make IFN-γ, splenocytes from uninfected mice or mice infected with Pru, CAT-OVA, or P30-OVA parasites, were restimulated in vitro with OVA peptide or STAg, and levels of IFN-γ were measured by ELISA, as shown in Fig. 4. Splenocytes from uninfected mice that had been adoptively transferred with DO11 cells failed to produce IFN-γ in response to either T. gondii or OVA antigen, consistent with the naive status of these T cells. Splenocytes from animals infected with the parental Pru parasites or CAT-OVA parasites did not produce significant levels of IFN-γ when stimulated with OVA peptide but did respond to STAg. In contrast, splenocytes from animals infected with OVA-secreting parasites produced high levels of IFN-γ in response to either STAg or OVA peptide. In summary, these results indicate that infection with transgenic parasites secreting OVA protein can lead to the specific activation of DO11 T cells in vivo, as demonstrated by their expansion, expression of an activated phenotype, and ability to produce IFN-γ.
FIG. 4.
Production of IFN-γ by restimulated splenocytes recovered from DO11-transferred, IFN-γ−/− controls and from animals infected with parental Prugniaud or P30-OVA- or CAT-OVA-transgenic cells in response to medium, STAg, or OVA peptide. Only splenocytes from animals infected with transgenic parasites expressing secreted OVA were able to respond to OVA peptide. Error bars indicate standard deviations.
Functional activity of OVA-specific CD4+ T cells in IFN-γ−/− mice.
To explore whether the ability of OVA-secreting parasites to activate DO11 CD4+ T cells is biologically relevant, a system was developed to determine whether the ability of these cells to make IFN-γ results in protective immunity. CFSE-labeled DO11+ T cells (2 × 106) were transferred into IFN-γ-deficient BALB/c mice, and these animals were then immunized with OVA peptide in incomplete Freund's adjuvant at 24 h posttransfer and boosted at day 7. As expected, this protocol resulted in an expanded population of KJ126+ CD4+ cells in lymphoid tissues (data not shown). One week later, animals were infected with either the parental Pru or P30-OVA parasites, and the response of the DO11 T cells was monitored at day 7 after infection. FACS analysis of spleen and lymph node cells from these mice revealed that very few KJ126+ CD4+ cells were found in the draining lymph nodes after infection with the parental Pru parasites (Fig. 5, top left panel). In contrast, mice infected with the P30-OVA parasites developed a large population of KJ126+ CD4+ T cells (Fig. 5, bottom left panel). In the P30-OVA infected animals, the KJ126+ CD4+ T cells proliferated extensively (Fig. 5, top right panel) and were activated as shown by decreased levels of CD62L (Fig. 5, bottom right panel). No detectable IFN-γ was detected by ELISA in the sera of mice infected with the parental Pru parasites, but P30-OVA-infected animals expressed ∼300 pg of IFN-γ per ml (standard deviation 103 pg/ml; n = 8).
FIG. 5.
Expansion and activation of DO11 T cells in IFN-γ−/− animals. FACS analysis of lymph nodes from DO11-transferred, immunized IFN-γ−/− mice infected with parental Prugniaud parasites (top left) or P30-OVA-transgenic cells (bottom left) is shown. Animals infected with parasites secreting P30-OVA developed a substantial KJ126+ CD4+-T-cell population (red box), which was analyzed for proliferation based on CFSE staining (top right) and for activation based on CD62L expression (bottom right).
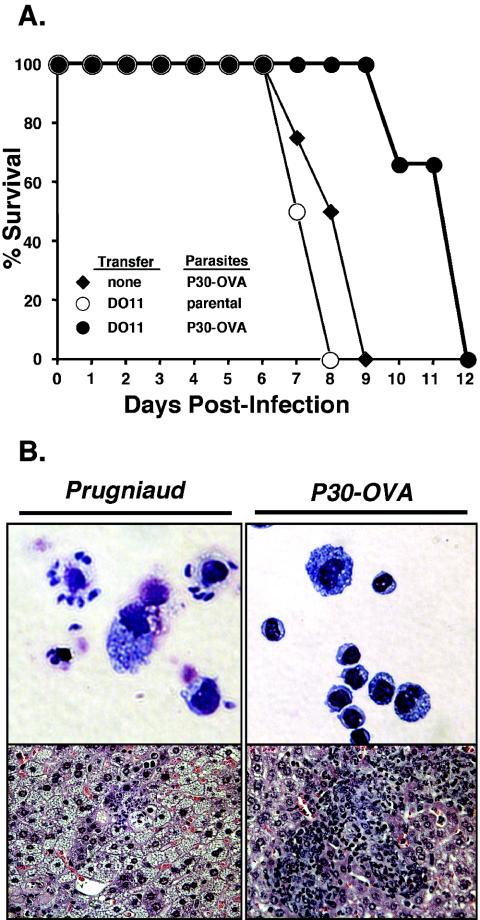
The transfer of DO11+ T cells into IFN-γ-deficient mice and subsequent vaccination with OVA failed to protect animals from the parental strain of T. gondii, and animals succumbed to infection within ∼8 days (Fig. 6A). Morbidity was associated with a high parasite burden and a lack of inflammatory response in the tissues (Fig. 6B, left panels). In contrast, immunized mice infected with P30-OVA parasites survived for several days longer than those infected with the parental Pru strain (Fig. 6A), exhibited a lower parasite burden, and showed a granulomatous inflammatory response in the liver, heart, lung, and spleen (Fig. 6B, right panels, and data not shown). Differences in the parasite burdens of animals infected with Pru parasites versus those infected with P30-OVA-transgenics were not due to reduced infectivity or fitness, as the parasite burden and mortality in nonadoptively transferred IFN-γ-deficient mice were indistinguishable for the two strains. In sum, these studies demonstrate that DO11+ T cells stimulated in response to infection with P30-OVA parasites constitute an activated effector population capable of producing IFN-γ, reducing parasite burden, and providing enhanced protection.
FIG. 6.
Protection of IFNγ−/− mice by DO11 T cells. Panel A: Survival of IFN-γ−/− mice after infection with P30-OVA-transgenic cells (♦) or after adoptive transfer with DO11 cells and infection with parental Prugniaud parasites (○) or P30-OVA-transgenic cells (•). DO11 cells provided significant protection against parasites expressing secreted OVA. Panel B: Peritoneal exudate cells (cytospins) and liver histopathology demonstrate reduced parasite burden in DO11-transferred animals infected with P30-OVA-transgenic cells (right) versus parental Prugniaud strain parasites (left).
DISCUSSION
The studies presented here demonstrate that Prugniaud strain transgenic T. gondii (type II, low virulence) can be engineered to express recombinant ovalbumin in the parasite cytosol or secreted into the parasitophorous vacuole (Fig. 1). Only secreted OVA (produced by the P30-OVA-transgenic parasites) stimulated OVA-specific CD4+-T-cell expansion, activation, and production of IFN-γ (Fig. 2 to 4). The effector population generated in IFN-γ-deficient animals with adoptively transferred naive DO11+ T cells and infected with P30-OVA parasites (Fig. 5) provides functional evidence that this system can be used to study Toxoplasma-induced CD4+ primary immune responses. The enhanced protection seen in these animals (Fig. 6) is likely mediated by a reduced parasite burden due to increased IFN-γ production, although this small population of antigen-specific CD4+ T cells was insufficient for long-term resistance.
The finding that secreted, but not cytoplasmic, OVA was the most efficient stimulus for a CD4+-T-cell response is in agreement with a previous report that β-galactosidase was capable of inducing a β-galactosidase-specific CD8+-T-cell response only when directed into the parasitophorous vacuole in transgenic T. gondii (17). The impact of antigen localization on the priming of a CD4+ immune response has also been demonstrated in Salmonella, where secreted antigen induces protection while cytosolic antigen does not (8). In contrast, studies with the intracellular pathogen L. monocytogenes found that both cytosolic and secreted recombinant antigens can induce CD4+ responses; however, it remains to be seen whether this activation can lead to protection (6). These disparities may be ascribed to differences in the interactions of pathogens with the antigen presentation pathway based on where the pathogen resides, i.e., the host cytosol (Listeria), phagosomes (Salmonella), or the parasitophorous vacuole (Toxoplasma). With T. gondii, most of the secreted antigen is retained within the parasitophorous vacuole (30) (Fig. 1), and it is likely that the bulk of foreign antigen is released only upon host cell lysis, whereupon it becomes available for cross-presentation. Therefore, during toxoplasmosis, the lysis of host cells may generate the first pool of foreign antigens available for presentation and thereby influence the hierarchy of which parasite antigens are recognized. Recent studies have also indicated that parasite-derived antigen is capable of escaping from the parasitophorous vacuole and entering the host cell cytoplasm, suggesting that infected cells may also be capable of presenting antigen (Boris Striepen, personal communication).
The ability to track in vivo immune responses to pathogen-derived antigens has advanced our understanding of the relationship between naïve and memory/effector T cells, while providing new insights into the mechanisms by which different pathogens evoke distinct immune responses. Recombinant pathogens have had an important role in these studies. For example, transgenic Listeria expressing OVA or the lymphocytic choriomeningitis virus-derived antigens have been valuable for studies ranging from the examination of memory cell development during infection to how this bacterium evades the immune response (10, 34). To date, our understanding of how T. gondii directs an in vivo immune response, and the generation of different populations of memory/effector cells, has been limited by the inability to track T-cell responses to parasite antigens. The studies presented here demonstrate that an important tool has been developed to examine clonotypic immune responses to MHC-restricted, T. gondii-expressed antigens. Although continuous in vitro passage has diminished the ability of these parasites to form bradyzoite tissue cysts in vivo, new parasite lines capable of efficient differentiation, and exhibiting stage-specific OVA expression, are currently under development. These reagents should provide new insights into the development and regulation of the CD4+-T-cell response during toxoplasmosis, information that will be essential for vaccine development.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI42334, AI28724, and Parasitology Training Grant AI07532) and the State of Pennsylvania. D.R. is an Ellison Medical Foundation Senior Scholar in Global Infectious Diseases.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Boothroyd, J. C., M. Black, S. Bonnefoy, A. Hehl, L. J. Knoll, I. D. Manger, E. Ortega-Barria, and S. Tomavo. 1997. Genetic and biochemical analysis of development in Toxoplasma gondii. Philos. Trans. R. Soc. London B 352:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charest, H., M. Sedegah, G. S. Yap, R. T. Gazzinelli, P. Caspar, S. L. Hoffman, and A. Sher. 2000. Recombinant attenuated Toxoplasma gondii expressing the Plasmodium yoelii circumsporozoite protein provides highly effective priming for CD8+ T cell-dependent protective immunity against malaria. J. Immunol. 165:2084-2092. [DOI] [PubMed] [Google Scholar]
- 3.De Boer, R. J., D. Homann, and A. S. Perelson. 2003. Different dynamics of CD4(+) and CD8(+) T cell responses during and after acute lymphocytic choriomeningitis virus infection. J. Immunol. 171:3928-3935. [DOI] [PubMed] [Google Scholar]
- 4.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dlugonska, H., K. Dytnerska, G. Reichmann, S. Stachelhaus, and H. G. Fischer. 2001. Towards the Toxoplasma gondii proteome: position of 13 parasite excretory antigens on a standardized map of two-dimensionally separated tachyzoite proteins. Parasitol. Res. 87:634-637. [DOI] [PubMed] [Google Scholar]
- 6.Guzman, C. A., D. Saverino, E. Medina, D. Fenoglio, B. Gerstel, A. Merlo, G. Li Pira, F. Buffa, T. Chakraborty, and F. Manca. 1998. Attenuated Listeria monocytogenes carrier strains can deliver an HIV-1 gp120 T helper epitope to MHC class II-restricted human CD4+ T cells. Eur. J. Immunol. 28:1807-1814. [DOI] [PubMed] [Google Scholar]
- 7.Hager, K. M., B. Striepen, L. G. Tilney, and D. S. Roos. 1999. The nuclear envelope serves as an intermediary between the ER and Golgi complex in the intracellular parasite Toxoplasma gondii. J. Cell Sci. 112:2631-2638. [DOI] [PubMed] [Google Scholar]
- 8.Hess, J., I. Gentschev, D. Miko, M. Welzel, C. Ladel, W. Goebel, and S. H. Kaufmann. 1996. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc. Natl. Acad. Sci. USA 93:1458-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israelski, D. M., and J. S. Remington. 1993. Toxoplasmosis in patients with cancer. Clin. Infect. Dis. 17(Suppl. 2):S423-S435. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, J., L. A. Zenewicz, L. R. San Mateo, L. L. Lau, and H. Shen. 2003. Activation of antigen-specific CD8 T cells results in minimal killing of bystander bacteria. J. Immunol. 171:6032-6038. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, L. L. 1992. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect. Immun. 60:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamm, C., M. Skoberne, and G. Geginat. 2003. CD8 T cell immunome analysis of Listeria monocytogenes. FEMS Immunol. Med. Microbiol. 35:235-242. [DOI] [PubMed] [Google Scholar]
- 13.Khan, I. A., K. H. Ely, and L. H. Kasper. 1994. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 152:1856-1860. [PubMed] [Google Scholar]
- 14.Khan, I. A., K. A. Smith, and L. H. Kasper. 1988. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J. Immunol. 141:3600-3605. [PubMed] [Google Scholar]
- 15.Kim, K., D. Soldati, and J. C. Boothroyd. 1993. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science 262:911-914. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, S., and R. L. Tarleton. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J. Immunol. 166:4596-4603. [DOI] [PubMed] [Google Scholar]
- 17.Kwok, L. Y., S. Lutjen, S. Soltek, D. Soldati, D. Busch, M. Deckert, and D. Schluter. 2003. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J. Immunol. 170:1949-1957. [DOI] [PubMed] [Google Scholar]
- 18.Lara-Tejero, M., and E. G. Pamer. 2004. T cell responses to Listeria monocytogenes. Curr. Opin. Microbiol. 7:45-50. [DOI] [PubMed] [Google Scholar]
- 19.Laucella, S. A., M. Postan, D. Martin, B. Hubby Fralish, M. C. Albareda, M. G. Alvarez, B. Lococo, G. Barbieri, R. J. Viotti, and R. L. Tarleton. 2004. Frequency of interferon-gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect. Dis. 189:909-918. [DOI] [PubMed] [Google Scholar]
- 20.Lindberg, R. E., and J. K. Frenkel. 1977. Toxoplasmosis in nude mice. J. Parasitol. 63:219-221. [PubMed] [Google Scholar]
- 21.Matrajt, M., M. Nishi, M. J. Fraunholz, O. Peter, and D. S. Roos. 2002. Amino-terminal control of transgenic protein expression levels in Toxoplasma gondii. Mol. Biochem. Parasitol. 120:285-289. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 23.Nagel, S. D., and J. C. Boothroyd. 1988. The alpha- and beta-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol. Biochem. Parasitol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 24.Parish, C. R. 1999. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol. 77:499-508. [DOI] [PubMed] [Google Scholar]
- 25.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. [DOI] [PubMed] [Google Scholar]
- 27.Roos, D. S. 1993. Primary structure of the dihydrofolate reductase-thymidylate synthase gene from Toxoplasma gondii. J. Biol. Chem. 268:6269-6280. [PubMed] [Google Scholar]
- 28.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27-63. [DOI] [PubMed] [Google Scholar]
- 29.Sartori, A. M., J. E. Neto, E. V. Nunes, L. M. Braz, H. H. Caiaffa-Filho, C. Oliveira Oda, Jr., V. A. Neto, and M. A. Shikanai-Yasuda. 2002. Trypanosoma cruzi parasitemia in chronic Chagas disease: comparison between human immunodeficiency virus (HIV)-positive and HIV-negative patients. J Infect. Dis. 186:872-875. [DOI] [PubMed] [Google Scholar]
- 30.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speirs, K., L. Lieberman, J. Caamano, C. A. Hunter, and P. Scott. 2004. Cutting Edge: NF-kappaB2 is a negative regulator of dendritic cell function. J. Immunol. 172:752-756. [DOI] [PubMed] [Google Scholar]
- 32.Striepen, B., C. Y. He, M. Matrajt, D. Soldati, and D. S. Roos. 1998. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol. Biochem. Parasitol. 92:325-338. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946. [PubMed] [Google Scholar]
- 34.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 35.Yrlid, U., M. Svensson, A. Hakansson, B. J. Chambers, H. G. Ljunggren, and M. J. Wick. 2001. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 69:5726-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]