Abstract
Osteoporosis is a metabolic skeletal disease characterized by an imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. We examined the beneficial effect of shock waves (SW) alone or in combination with raloxifene (RAL) on bone loss in ovariectomized rats (OVX). Sixteen weeks after surgery, OVX were treated for five weeks with SW at the antero-lateral side of the right hind leg, one session weekly, at 3 Hz (EFD of 0.33 mJ/mm2), or with RAL (5 mg/kg/die, per os) or with SW+RAL. Sera, femurs, tibiae and vertebrae were sampled for following biochemical and histological analysis. SW, alone or combined with RAL, prevented femur weight reduction and the deterioration of trabecular microarchitecture both in femur and vertebrae. All treatments increased Speed of Sound (SoS) values, improving bone mineral density, altered by OVX. Serum parameters involved in bone remodeling (alkaline phosphatase, receptor activator of nuclear factor kappa-B ligand, osteoprotegerin) and osteoblast proliferation (PTH), altered by ovariectomy, were restored by SW and RAL alone or in combination. In tibiae, SW+RAL significantly reduced cathepsin k and TNF-α levels, indicating the inhibition of osteoclast activity, while all treatments significantly increased runt-related transcription factor 2 and bone morphogenetic-2 expression, suggesting an increase in osteoblastogenic activity. Finally, in bone marrow from tibiae, SW or RAL reduced PPARγ and adiponectin transcription, indicating a shift of mesenchymal cells toward osteoblastogenesis, without showing a synergistic effect. Our data indicate SW therapy, alone and in combination with raloxifene, as an innovative strategy to limit the hypoestrogenic bone loss, restoring the balance between bone formation and resorption.
Introduction
Osteoporosis is a metabolic skeletal disease characterized by low bone mass, deterioration of bone micro-architecture and increased fracture risk [1]. The prominent prevalence of osteoporosis in Europe (approximately 21% of women aged 50–84 years) and increased mortality rate in patients with osteoporotic fractures represent a clinical emergency [2, 3]. Postmenopausal women exhibit the major risk to develop osteoporosis, suggesting that the role of estrogen is critical in its pathogenesis [4]. Indeed, raloxifene, a selective estrogen receptor modulator (SERM), has been approved for the prevention and treatment of postmenopausal osteoporosis, especially because of its capability to prevent or reduce vertebral fractures [5]. However, the efficacy of this drug in reducing or preventing non-vertebral fractures is strongly limited [6].
The osteoporosis progression is due to an imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. The recovery of this balance represents the rationale underlying the two more recent two anti-osteoporotic strategies: the inhibition of bone resorption and turnover, and the stimulation of bone formation [7, 8]. Cathepsin k, a protease abundantly expressed in osteoclasts and in actively resorbing osteoclasts, has been identified as a novel pharmacological target to counteract osteoporosis by reducing the organic bone matrix degradation. In particular, two cathepsin k inhibitors, odanacatib and ONO-5334, have been recently used in clinical trials [9, 10].
To date, the only available agents that stimulate bone formation are the whole molecule parathormone (PTH, 1–84) or its fragment, the teriparatide (1–34). Although PTH increases bone formation through an increase in bone remodeling, its effect is transient and decreases with time [11, 12]. Moreover, the use of PTH analogues in the clinical practice is limited by their cost and potential side effects [13].
In the early 80s, shock waves (SW) were used for kidney and urinary stone lithotripsy [14]. Afterwards, SW therapy has been used for the treatment of other orthopedic diseases, accelerating bone healing [15], callus formation [16] and delayed or non-union of long bone fractures [17]. In addition, SW have been shown to promote the regeneration of alveolar bone in a rodent model of periodontitis [18]. This therapy is considered a safe and highly versatile tool to enhance the time of tissue regeneration, in particular on tendon and muscle tissues, also showing immediate antalgic and anti-inflammatory effects [19].
The biological effects of the SW therapy in bone have been recently examined [20]. Indeed, Van der Jagt et al. [21] have demonstratedt hat single application of SW has a light beneficial effect in a rat model of ovariectomy-induced osteoporosis, increasing trabecular bone volume and reducing bone loss. Additionally, this research group showed that a single application on tibia induces anabolic effects in cortical bone in normal [22] and osteoporotic rats, especially when SW treatment was combined with anti-resorptive alendronate therapy [23]. However, the bone biochemical mechanisms underlying the anti-osteoporotic effects of SW are still overlooked.
The purpose of this study was to evaluate the modulation of serum parameters and tissue markers of bone resorption and bone formation in ovariectomized rats after repeated SW therapy, alone or in combination with raloxifene.
Materials and methods
Animals
Female Sprague Dawley rats (Harlan Italy, San Pietro al Natisone, Udine, Italy) were housed in stainless steel cages in a room kept at 22±1°C with a 12:12 h light-dark cycle. All procedures involving the animals were carried out in accordance with the Institutional Guidelines and complied with the Italian D.L. no.116 of January 27, 1992 of Ministero della Salute and associated guidelines in the European Communities Council Directive of November 24, 1986 (86/609/ECC). All animal procedures reported herein were approved by the Institutional Animal Care and Use Committee (CSV) of University of Naples Federico II under protocol no. 2013/0040366. Prior to sample and tissue collection, animals were euthanized by an intraperitoneal injection of a cocktail of ketamine/xylazine, followed by cervical dislocation to minimize pain. All efforts were made to minimize animal suffering.
Ovariectomy, Shock Waves (SW) and pharmacological treatment
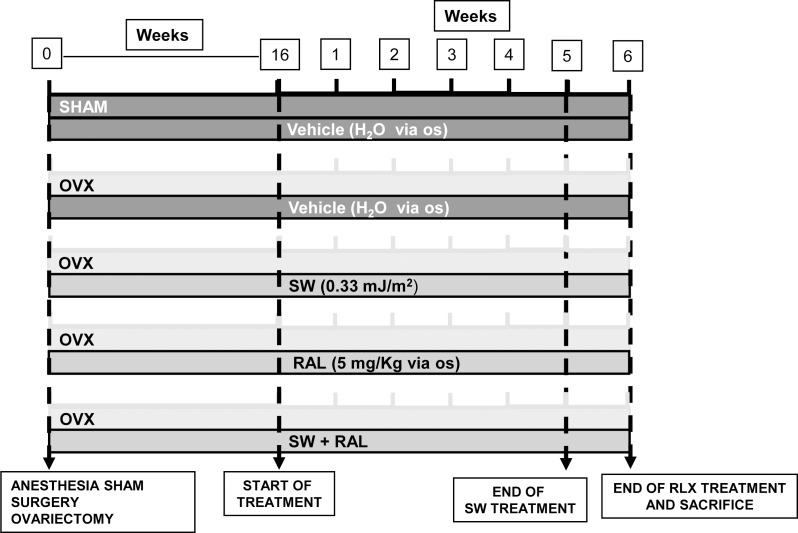
The experimental design is illustrated in Fig 1. At the onset of the study, female rats (mean body weight of the cohort: 216.4 ± 1.6 g) were bilaterally ovariectomized (OVX) under anesthesia (100 mg kg-1 ketamine plus 5 mg kg-1xylazine ip). The sham-operated (SHAM) animals were subjected to the same general surgical procedure as OVX groups except for ovarian excision. After surgery, SHAM and OVX animals received topical antibiotic treatment (with a preparation containing 0,05 g of neomycin sulfate and 9,95 g of sulfathiazole, for five days).
Fig 1. Experimental scheme.
At the onset of the study, female rats were bilaterally ovariectomized (OVX) while the sham-operated (SHAM) animals were used as controls. After sixteen weeks, rats were divided into five groups: 1) SHAM, receiving drug vehicle; 2) OVX, receiving drug vehicle; 3) OVX treated with SW once a week for five weeks; 4) OVX, receiving RAL (5 mg/kg/die per os for five days a week for six weeks); and 5) OVX rats treated with SW in combination with RAL.
Sixteen weeks after surgery, necessary time for the development of osteoporotic features [24], rats were divided into the following five groups (n = 6 animals each group): 1) SHAM, control rats receiving per os drug vehicle; 2) OVX, rats receiving vehicle; 3) OVX+SW, rats treated with SW one weekly session for five times; 4) OVX+RAL, rats receiving raloxifene (5 mg/kg/die, Lilly Research Laboratories, Indianapolis, USA) per os five days a week for six weeks; and 5) OVX+SW+RAL, rats treated with SW in combination with the SERM.
Briefly, every week all rats receiving SW were anesthetized with a mix of oxygen and 2% isoflurane, and the right hind leg was shaved and an ultrasonic gel was applied, as coupling media. The SW applicator was placed at the antero-lateral side of the right thigh and each session of SW treatment consisted in the application of 1000 electro-magnetically generated SW at 3 Hz with an energy flux densities (EFD) of 0.33 mJ/mm2 (Duolith® SD1-Storz Medical AG, Tagerwilen, Switzerland). The focus diameter of the Duolith was 4 mm Full Width at Half Maximum (FWHM) at the energy used. Non-SW treated animals were also undergone an anesthesia and shaving without SW application. All animals were euthanized one week after 5th SW application, while last administration of raloxifene was performed 2 h before killing. During necropsy, we confirmed the effectiveness of OVX surgery, through the evaluation of uterus weight. Subsequently, bone tissues (treated and contralateral femurs, tibiae, vertabrae) were sampled and surrounding tissues (skin, muscle and tendons) were removed. Tibiae were immediately frozen in liquid nitrogen for following molecular analyses of bone tissue and bone marrow, whereas femurs and lumbar vertebrae were fixed in 10% Neutral Buffered Formalin (NBF) and subjected to histomorphological examination. Before fixing, femurs were dried to check their weight, distinguishing SW treated bone from SW-untreated ones.
Body weight gain and fat mass
Throughout the experimental period, body weight was assessed once per week and body weight gain was calculated as difference between the last measure and the body weight recorded at the beginning of the experiment. Lastly, values of body weight gain during time were cumulated and expressed as area under curve (AUC). At the start of necropsy procedure, bioelectrical impedance analysis was used to assess the rat body composition with the BIA 101analyzer, modified for the rat (Akern, Florence, Italy). Fat-free mass was calculated using the bioelectrical impedance analysis (50 kHz) prediction equation of Ilagan et al. [25], and fat mass content was determined as the difference between body weight and fat-free mass.
Analysis of serum parameters
At the start of necropsy procedure, rats were anesthetized by enflurane and blood was collected by cardiac puncture. Sera were obtained by centrifugation at 1500 x g at 4°C for 15 min, and stored at -70°C for later biochemical and hormonal determinations. Osteoprotegerin (OPG), Receptor activator of nuclear factor kappa-B ligand (RANKL), and alkaline phosphatase (ALP) were measured by Enzyme-Linked ImmunoSorbent Assay (ELISA) kits for rats purchased from Cloud Clone Corp. (Houston, TX, USA), while rat intact PTH (iPHT) levels were determined by an ELISA kit obtained from MyBioSource Inc. (San Diego, CA, USA). All procedures were performed following the manufacturer’s protocol.
Histological analysis and ultrasonometry
Extracted bones (femurs and vertebrae) were fixed for 3 days in 10% neutral buffered formalin. Then, the fixed tissues were decalcified with a 4% trichloroacetic acid solution and embedded in paraffin. Each femur was cut at a 4 micrometer sections through the longitudinal axis, while each vertebra was cut through the transversal axis. Finally, hematoxylin and eosin (H&E) staining was performed for their morphological evaluation. Slides were analyzed using an optic microscope Nikon Eclipse E600. A blind examination of the sections based on primary histological analysis of bone (modified from Dempster et al. [26]) was made independently by two veterinary pathologists (OP and TBP) at high (20X) and low (10X) magnification.
The ultrasound measurements (QUS) were performed using a DBM Sonic 1200 (IGEA, Carpi, Italy) with an electronic high-precision caliper (± 0,02 mm) where two ultrasound probes (diameter: 12 mm) are mounted: one probe generates the ultrasound (1,25MHz) and the other one receives the ultrasound beam after it has crossed the bone specimen. To obtain the values of speed of sound (SoS, m/s), we used an ultrasonic contact gel along the longitudinal axis of animal femurs. After four measurements, the speed of sound (SoS, m/s) was calculated after four measurements in order to reduce repositioning errors, as previously described [27].
RNA extraction and Real-time semi-quantitative PCR
Tibiae were dissected from the rats and cleaned with 0.1 M ice-cold PBS, pH 7.2. For OVX+SW and OVX+RAL+SW groups, both contralateral and SW-treated tibiae were used. The epiphysis of each bone was excised and the bone marrow was collected [28]. Bone tissues were snap-frozen in liquid nitrogen to make them breakable. Total RNA was extracted using TRIzol Reagent (Bio-Rad Laboratories), according to the manufacturer’s instructions. cDNA was synthesized using a reverse transcription kit (NucleoSpin®, MACHEREY-NAGEL GmbH & Co, Düren, Germany) from 2 μg total RNA. PCRs were performed with a Bio-Rad CFX96 Connect Real-time PCR System instrument and software (Bio-Rad Laboratories). The PCR conditions were 15 min at 95°C followed by 40 cycles of two-step PCR denaturation at 94°C for 15 s, annealing at 55°C for 30 s and extension at 72°C for 30 s. Each sample contained 1–100 ng cDNA in 2X QuantiTect SYBRGreen PCR Master Mix and primers, TNF-α, RANKL, OPG, Bone morphogenetic 2 (Bmp2), runt-related transcription factor 2 (RUNX2), cathepsin k, Peroxisome proliferator-activated receptor gamma (PPARγ) and adiponectin (both by Qiagen, Hilden, Germany) in a final volume of 50 μl. The relative amount of each studied mRNA was normalized to GAPDH as housekeeping gene, and data were analyzed according to the 2-ΔΔCT method.
Statistical analysis
All data were presented as mean ± SEM. Statistical analysis was performed by ANOVA test for multiple comparisons, followed by Bonferroni’s test. Statistical significance was set at P <0.05.
Results
Body parameters
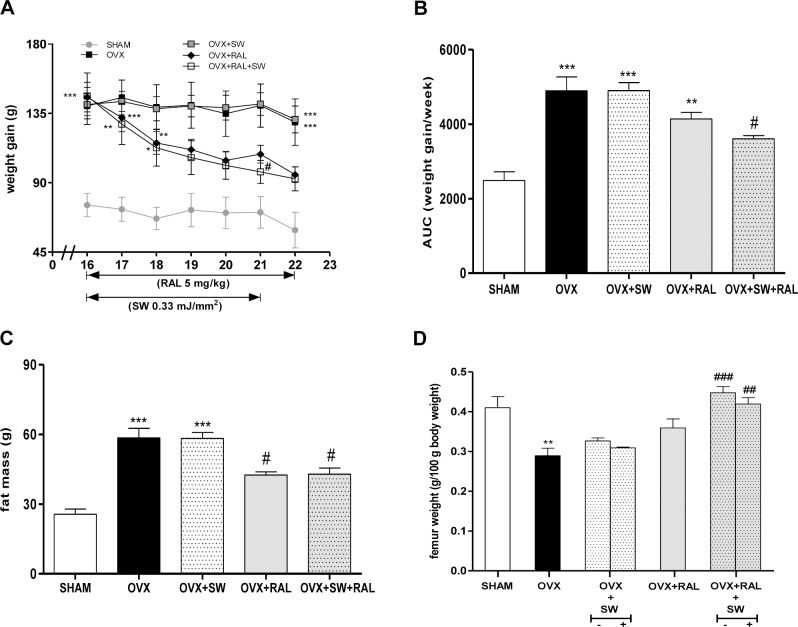
As depicted in Fig 2A, weight gain of OVX+RAL+SW group decreased during time and was significantly lower than OVX rats after 5 weeks of the combined treatment (21th week). RAL treatment of OVX rats showed a decreasing trend in body weight gain compared to OVX group. Weight gain of OVX+SW did not differ significantly from OVX group. All these data were confirmed by AUC of weight gain during the treatment time (Fig 2B). As shown in Fig 2C, fat mass, measured at 22th week, was significantly increased in the OVX rats, compared with the SHAM animals, while the treatment with RAL or its association with SW reduced it.
Fig 2.
Effects of SW and RAL treatment on body weight gain (A), also expressed as AUC of cumulative weight gain during time (B), fat mass (C) and femur weight (D) are shown. Body weight gain was monitored every week, during all experimental time. Femur weight was determined in contralateral (-) and SW-treated (+) of OVX+SW and OVX+RAL+SW animals. Values are expressed as means ± SEM (n = 6); *P <0.05, **P <0.01 and ***P <0.001 compared with SHAM; #P <0.05, ##P <0.01 and ###P <0.001 compared with OVX.
The femurs of rats were weighed after excision, discerning the SW treated from untreated bone (Fig 2D). The SW+RAL associated treatment significantly improved the remarkable loss of bone weight of OVX animals.
Bone histology and serum remodeling markers
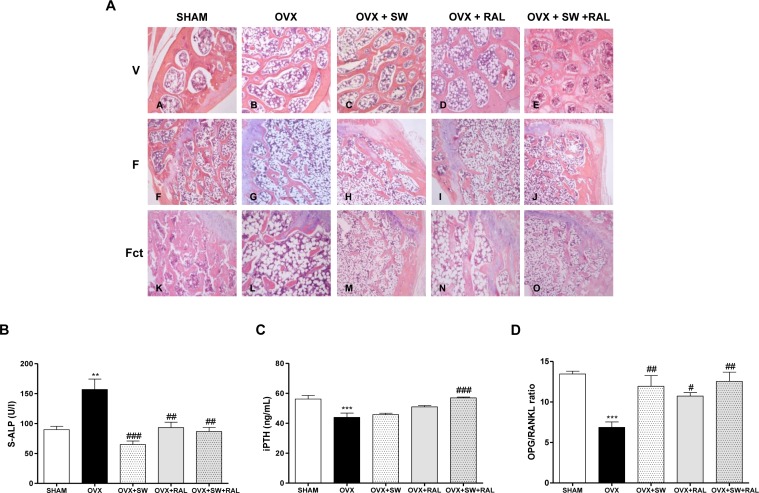
We evaluated histomorphology of vertebrae (V) and femurs (F) excised from all rats. Trabecular thickness and organization, and adipose tissue infiltration in bone marrow were examined (Fig 3A). OVX rats showed, as expected, a reduced thickness and organization of vertebra and femur trabeculae, and an increased amount of adipose tissue infiltration compared with SHAM group. In particular in vertebrae, SW and its combination with RAL induced a reduction of adipose tissue infiltration and an improvement of trabecular thickness and organization compared with OVX. The same parameters were also analyzed on femur metaphyses, distinguishing SW treated and contralateral limb (Fct). Thickness and organization of metaphyseal trabeculae were remarkably ameliorated by SW or RAL alone and notably their combination restored trabecular structure as that of SHAM, without difference between SW treated and contralateral limb.
Fig 3.
Effects of SW and RAL treatment on histological features of vertebrae (V), femural metaphyseal bone (F) and contralateral femurs (Fct) of SHAM (A,F,K), OVX (B,G,L), OVX+SW (C,H,M), OVX+RAL (D,I,N) and OVX+SW+RAL (E,J,O) groups (panel A). Bone sections were stained with H&E to visualize trabecular thickness and organization, and adipose tissue infiltration in bone marrow. Effects of SW and RAL treatments on bone markers in serum: ALP (panel B), iPTH (panel C) and OPG/RANKL ratio (panel D). Values are expressed as means ± SEM (n = 6); **P <0.01 and ***P <0.001 compared with SHAM; #P <0.05, ##P <0.01 and ###P <0.001 compared with OVX.
By the quantitative ultrasound measurement, we calculated the SoS of the femur of all animals. OVX rats showed a significant decrease of SoS than SHAM group, improved by all treatments (SHAM, 1931± 6,85; OVX, 1826 ± 4,251 ***; OVX+SW, 1867 ± 9,291 #; OVX+RAL, 1887 ± 13,58 ###; OVX+SW+RAL, 1864 ± 5,37 #; *** p<0,001 vs SHAM; # p<0,05 and ### p<0,001 vs OVX).
Serum parameters involved in bone formation or osteoblast proliferation, S-ALP and iPTH respectively, were examined (Fig 3B and 3C). Both markers were modulated by all treatments and in particular SW+RAL was able to significantly restore their levels. Moreover, we showed that treatments with SW, RAL and their combination increased OPG/RANKL ratio showing a reduction of the osteoclastogenic process (Fig 3D).
SWs alone or combined with RAL modulate genes involved in bone formation and resorption
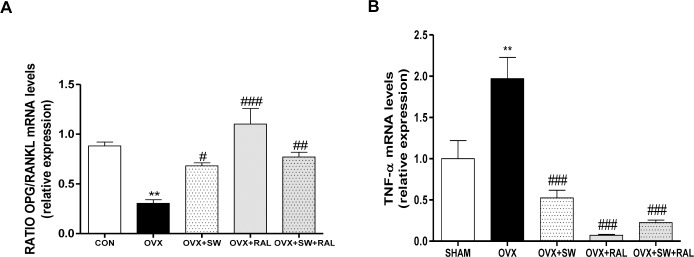
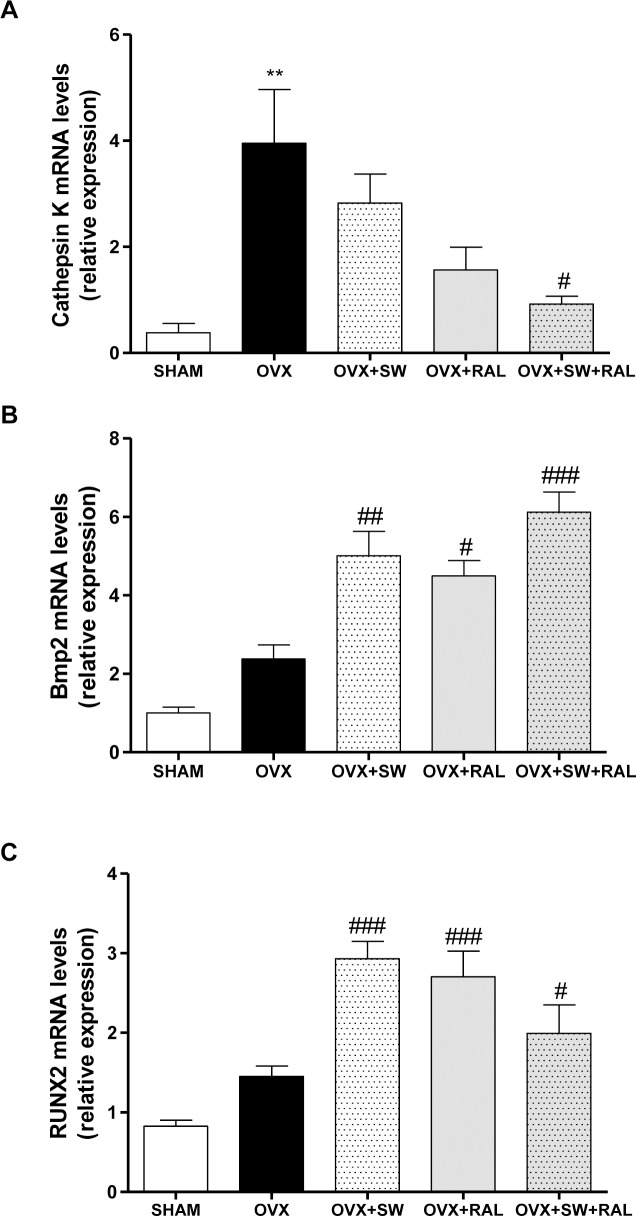
To confirm the involvement of OPG/RANKL in the effect of SW and RAL, we also evaluated their mRNA expression by Real Time PCR in tibiae. OVX group showed a significant reduction of OPG/RANKL ratio compared with SHAM, that was significantly increased by SW and RAL, alone or in combination (Fig 4A), indicating a reduction in bone resorption. Consistently, OVX animals showed a significant increase of TNF-α which stimulates osteoclast proliferation, while all treatments remarkably reduced its transcription (Fig 4B). Fig 5 showed the mRNA expression of cathepsin K, Bmp2, andRUNX2. OVX caused a marked increase of cathepsin K compared with SHAM; SW and RAL alone showed a trend of enzyme reduction that reached significance when combined (Fig 5A). Moreover, SW and RAL alone or combined increased Bmp2 mRNA expression(Fig 5B), while SW or RAL alone increased RUNX2 transcription, that was less affected by their combination(Fig 5C).
Fig 4. Effect of SW and RAL treatments on bone resorption parameters in tibiae.
mRNA of OPG/RANKL ratio (A) and TNF-αare shown (B). For OVX+SW and OVX+RAL+SW groups, contralateral and SW-treated tibiae were used. Values are expressed as means ± SEM (n = 6); **P <0.01 compared with SHAM; #P <0.05, ##P <0.01 and ###P <0.001 compared with OVX.
Fig 5.
Effects of SW and RAL treatments on bone remodeling balance: cathepsin K (A), Bmp2 (B) and RUNX2 (C) mRNA expression are shown. For OVX+SW and OVX+RAL+SW groups, contralateral and SW-treated tibiae were used Values are expressed as means ± SEM (n = 6); **P <0.01 compared with SHAM; #P <0.05, ##P <0.01 and ###P <0.001 compared with OVX.
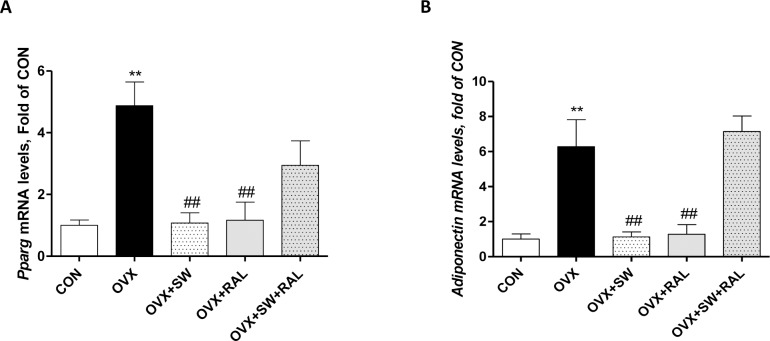
Modulation of PPARγ and adiponectin transcription in tibial bone marrow by SW alone or combined with RAL
Hypoestrogenism in rodent, as well as in human, was found to be associated with increased bone marrow adiposity [29, 30]. Consistently, we showed an increase in PPARγ and adiponectin mRNAs in bone marrow of tibiae from OVX (Fig 6A and 6B). Both treatments either SW or RAL significantly reduced the transcription of both genes. Conversely, SW and RAL combination did not significantly modify OVX-induced increase in PPARγ and adiponectin mRNAs, although a trend of decrease of PPARγ expression was found.
Fig 6. Modulation of PPARγ and adiponectin transcription in tibial bone marrow by SW alone or combined with RAL.
PPARγ (A) and adiponectin (B) mRNA expression are shown. For OVX+SW and OVX+RAL+SW groups, contralateral and SW-treated tibiae were used. Values are expressed as means ± SEM (n = 6); **P <0.01 compared with SHAM; ##P <0.01 compared with OVX.
Discussion
In the present study we show the beneficial effect of SW therapy, alone or in combination with raloxifene, inducing bone formation and reducing bone resorption impaired by OVX.
Postmenopausal hypoestrogenism triggers the events involved in the osteoporotic process: massive bone resorption entailing loss of bone mass and architecture and failure to replace lost bone due to remarkable reduction of bone formation [4]. The ovariectomized rat is a standard preclinical model in the development of anti-osteoporosis therapies, reproducing molecular and biochemical alterations of the pathology [24, 31].
Our hypothesis regarding the benefits of SW treatment in osteoporosis was suggested by previous data demonstrating that a single SW application could be effective increasing trabecular bone volume and reducing bone loss [21], especially when it was combined with anti-resorptive alendronate therapy [23]. Here, we demonstrate the efficacy of repeated SW applications alone or combined with raloxifene (for 6 weeks) on osteoporosis induced by long term ovariectomy. As previously described [31], and here confirmed, 22 weeks ovariectomy clearly led to a significant increase in body weight and fat mass, and raloxifene reduced fat mass by its estrogenic activity. In our experimental conditions RAL, administered when ovariectomy-induced hypoestrogenism was defined, reduced also significantly weight gain only in association with SW treatment.
Interestingly, when RAL is associated with SW, a synergistic effect is also shown on femur weight since this parameter raised significance only in SW+RAL-treated rats both in SW treated and contralateral femur, indicating a lower bone loss than in OVX animals. In addition, all treatments were able to increase the values of SoS reduced by OVX, indicating an improvement of bone mineral density (BMD).As previously demonstrated, QUS technique is significantly comparable to the dual X-ray absorptiometry (DXA), the main used technology for BMD evaluation [32].
The capability of both treatments in restoring bone architecture was also evidenced by histological analysis. All SW-based treatments were more effective than RAL in reversing histological features of osteoporosis showed in OVX rats, not only in vertebrae but also in treated and contralateral femurs. The evaluation of trabecular architecture in osteoporotic metaphyseal femur shows its considerable improvement after repeated weekly applications of SW. This effect was more evident after combined therapy. We also observed an increase of adipose infiltration in OVX group compared to control and treated groups, not only in femur but also in vertebrae. No differences between treated and contralateral limbs were noticed in SW alone or combined with RAL, supporting the hypothesis of a systemic effect of SW therapy.
Many key factors regulate the balance between bone resorption and formation. Osteoclastogenesis is stimulated by the binding between RANK and RANKL, expressed in osteoclast progenitor cells and osteoblasts, respectively. RANKL-RANK interaction is prevented by the natural RANKL inhibitor, OPG. Clinical and experimental studies demonstrated that estrogens increase mRNA and protein expression of OPG and decrease RANKL and macrophage colony-stimulating factor (M-CSF) expression [33, 34]. In our experimental model, we demonstrate that not only RAL, but also SW and their association inhibit osteoclastogenesis, modulating OPG/RANKL ratio both in serum and bone tissue, remarkably altered by 22 week ovariectomy. Moreover, all treatments significantly reduce the TNF-α mRNA expression in tibiae, and this cytokine, as well as IL-1 and PGE2, has been identified as regulator for osteoclast activity [35]. TNF-α presents anti-apoptotic activity on osteoclasts, increasing their lifespan [36] and synergistically with RANKL stimulates osteoclastogenic activity [37], inducing osteoclast formation and bone resorption both directly and increasing the sensitivity of maturing osteoclasts to RANKL. TNF-activated pathways and mechanisms involved in bone remodeling remain partially unclear; in fact, a recent review summarized the paradoxical role of TNF-α on bone homeostasis and bone remodeling imbalance involved in systemic/vertebral osteoporosis [38]. Other studies will be necessary to explain the lack of synergistic effect between SW and RAL on TNF-α in bone. A possible competitive effect between the two treatments, by unidentified endogenous endocrine or paracrine factors on bone, can not be excluded and should be clarified.
On the other hand, the association SW+RAL significantly reduces cathepsin k levels, a prominent lysosomal cysteine protease that is involved in bone degradation of extracellular matrix proteins, such as elastin and collagen [39]. Few years ago, it was demonstrated that two cathepsin K inhibitors prevented bone loss with similar efficacy to that of alendronate in estrogen-deficient rabbits, but, unlike bisphosphonates, had no suppressive effect on bone-formation rate at trabecular and cortical bone sites suggesting better long term benefits than the current standard of cure [40]. To date, odanacatib and ONO-5334 are currently in clinical development, as new reliable strategy for osteoporosis treatment [41].
Approximately thirty Bmps members of transforming growth factor-β superfamily have been identified and characterized, among which Bmp2 is recognized as a pivotal signal in regulating osteoblastogenesis [42]. Several studies using transgenic mice have shown that Bmp2 functions as a fundamental component of the inherent regenerative capacity of bone [43]. Bmps are used locally for the treatment of non-union fractures and proteosome inhibitors enhancing Bmp2 expression may have an effect on bone tissue and have been proposed as potential anabolic therapies [8]. The involvement of NO pathway in SW osteogenic effect was previously reported in vitro [44], evidencing an increase in Bmp2 and RUNX2 transcription in marrow stromal cells of hips with osteonecrosis. Here, we evidenced that SW and their association with RAL increase mRNA Bmp2, indicating their effects on bone regeneration, compromised in OVX rats. This data was strengthened by the significant increase in serum iPTH level induced by SW plus RAL, supporting the stimulatory effect on bone formation of the combined therapy.
During bone remodeling, beyond the resorptive role of osteoclasts, new bone is formed by osteoblasts derived from mesenchymal stem cells [45]. These latter ones could also differentiate into adipose cells, as it can occur during bone loss through an expansion of adipose tissue in the bone marrow [46]. Among factors having contrary effects on both differentiation pathways,RUNX2 is master transcription factor for osteogenesis [47], while PPARγ is designated to induce adipogenesis [48]. Interestingly, SW and RAL not only markedly increased the transcription of RUNX2 in bone, but also reduced PPARγ levels in bone marrow, indicating the shift of MSCs from adipogenesis to osteogenesis. This hypothesis was confirmed by the reduction of adiponectin in bone marrow from SW and RAL treated animals. In fact, clinical studies showed that high levels of adiponectin are associated with a reduction of bone mineral content and body mineral density [49, 50], that also confirmed in mouse models [51]. Clinical postmenopausal estrogen insufficiency was found to be associated with increased bone marrow adiposity [30], while the observed increase in marrow adipogenesis could be prevented or reversed by estrogen replacement both in humans and rats [29]. Our data confirm previous results showing that adipogenesis induced by OVX is a reversible process which can be corrected by estrogen, phytoestrogens, or raloxifene treatments [52], and for the first time we demonstrate the reduction of adipogenesis in rat bone marrow after repeated SW application. Interestingly, the lack of the synergic or additive effects by SW and RAL on adipogenesis/osteogenesis balance could represent a limit of their interplay; indeed, a possible competitive effect by unidentified endogenous endocrine or paracrine factors involved in their respective mechanism of action at bone marrow level can be considered.
In conclusion, our study demonstrates the beneficial anti-osteoporotic effects of SW therapy, alone or in combination with raloxifene, evidencing their strengths and limits. The mechanisms of these effects can be a result of the increase of bone formation and the reduction of bone resorption. Combined or multi-target therapies are a common approach in the treatment of chronic and multifactorial diseases, such as osteoporosis. SW therapy is considered a non-invasive therapeutic modality with effectiveness, convenience, and safety; also replacing surgery with no surgical risks in many orthopedic disorders [20, 53] and it may represent an innovative strategy to limit the progression of osteoporosis.
Acknowledgments
We thank Mr. Giovanni Esposito and Mr. Angelo Russo for animal care and assistance.
We also thank GUACCI s.p.a. and MAGGIORE Pharmacy for contribution in reagents/materials.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by Storz Medical AG, Tagerwilen, Switzerland. This external company provided an economical support for fulfillment of the study, contributing to purchase of laboratory equipment. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript which did not contribute to experimental model design, experiment realization, data analysis, results interpretation and manuscript writing. There was no additional external funding received for this study.
References
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–8. Epub 2006/06/20. 10.1016/S0140-6736(06)68891-0 [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. Epub 2012/10/20. PubMed Central PMCID: PMC3587294. 10.1007/s00198-012-2074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin KH, Friis-Holmberg T, Hermann AP, Abrahamsen B, Brixen K. Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res. 2013;28(8):1701–17. Epub 2013/04/18. 10.1002/jbmr.1956 [DOI] [PubMed] [Google Scholar]
- 4.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–25. Epub 2005/12/03. PubMed Central PMCID: PMC1297264. 10.1172/JCI27071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reginster JY. Antifracture efficacy of currently available therapies for postmenopausal osteoporosis. Drugs. 2011;71(1):65–78. Epub 2010/12/24. 10.2165/11587570-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148(3):197–213. Epub 2007/12/19. [DOI] [PubMed] [Google Scholar]
- 7.Ng KW, Martin TJ. New therapeutics for osteoporosis. Curr Opin Pharmacol. 2014;16:58–63. Epub 2014/04/05. 10.1016/j.coph.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Appelman-Dijkstra NM, Papapoulos SE. Novel approaches to the treatment of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2014;28(6):843–57. Epub 2014/11/30. 10.1016/j.beem.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Engelke K, Fuerst T, Dardzinski B, Kornak J, Ather S, Genant HK, et al. Odanacatib treatment affects trabecular and cortical bone in the femur of postmenopausal women: results of a two-year placebo-controlled trial. J Bone Miner Res. 2015;30(1):30–8. Epub 2014/06/06. 10.1002/jbmr.2292 [DOI] [PubMed] [Google Scholar]
- 10.Eastell R, Nagase S, Small M, Boonen S, Spector T, Ohyama M, et al. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J Bone Miner Res. 2014;29(2):458–66. Epub 2013/07/23. 10.1002/jbmr.2047 [DOI] [PubMed] [Google Scholar]
- 11.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. Epub 2001/05/11. 10.1056/NEJM200105103441904 [DOI] [PubMed] [Google Scholar]
- 12.Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–39. Epub 2007/03/07. [DOI] [PubMed] [Google Scholar]
- 13.Dobnig H. A review of teriparatide and its clinical efficacy in the treatment of osteoporosis. Expert Opin Pharmacother. 2004;5(5):1153–62. Epub 2004/05/25. 10.1517/14656566.5.5.1153 [DOI] [PubMed] [Google Scholar]
- 14.Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982;127(3):417–20. Epub 1982/03/01. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Yang KD, Ko JY, Huang CC, Huang HY, Wang FS. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide. 2009;20(4):298–303. Epub 2009/03/14. 10.1016/j.niox.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Narasaki K, Shimizu H, Beppu M, Aoki H, Takagi M, Takashi M. Effect of extracorporeal shock waves on callus formation during bone lengthening. J Orthop Sci. 2003;8(4):474–81. Epub 2003/08/05. 10.1007/s00776-003-0664-4 [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Chen HS, Chen CE, Yang KD. Treatment of nonunions of long bone fractures with shock waves. Clin Orthop Relat Res. 2001;(387):95–101. Epub 2001/06/13. [DOI] [PubMed] [Google Scholar]
- 18.Sathishkumar S, Meka A, Dawson D, House N, Schaden W, Novak MJ, et al. Extracorporeal shock wave therapy induces alveolar bone regeneration. J Dent Res. 2008;87(7):687–91. Epub 2008/06/25. [DOI] [PubMed] [Google Scholar]
- 19.Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16(19):2366–72. Epub 2009/07/16. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JH, Wang CJ. Biological mechanism of shockwave in bone. Int J Surg. 2015. Epub 2015/06/30. [DOI] [PubMed] [Google Scholar]
- 21.van der Jagt OP, van der Linden JC, Schaden W, van Schie HT, Piscaer TM, Verhaar JA, et al. Unfocused extracorporeal shock wave therapy as potential treatment for osteoporosis. J Orthop Res. 2009;27(11):1528–33. Epub 2009/05/15. 10.1002/jor.20910 [DOI] [PubMed] [Google Scholar]
- 22.van der Jagt OP, Piscaer TM, Schaden W, Li J, Kops N, Jahr H, et al. Unfocused extracorporeal shock waves induce anabolic effects in rat bone. J Bone Joint Surg Am. 2011;93(1):38–48. Epub 2011/01/07. 10.2106/JBJS.I.01535 [DOI] [PubMed] [Google Scholar]
- 23.van der Jagt OP, Waarsing JH, Kops N, Schaden W, Jahr H, Verhaar JA, et al. Unfocused extracorporeal shock waves induce anabolic effects in osteoporotic rats. J Orthop Res. 2013;31(5):768–75. Epub 2012/12/15. 10.1002/jor.22258 [DOI] [PubMed] [Google Scholar]
- 24.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–30. Epub 2008/11/14. PubMed Central PMCID: PMC2707131. [PMC free article] [PubMed] [Google Scholar]
- 25.Ilagan J, Bhutani V, Archer P, Lin PK, Jen KL. Estimation of body composition changes during weight cycling by bioelectrical impedance analysis in rats. J Appl Physiol (1985). 1993;74(5):2092–8. Epub 1993/05/01. [DOI] [PubMed] [Google Scholar]
- 26.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. Epub 2012/12/01. PubMed Central PMCID: PMC3672237. 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luisetto G, Camozzi V, De Terlizzi F, Moschini G, Ballanti P. Use of ultrasonography in the diagnosis of osteomalacia: preliminary results on experimental osteomalacia in the rat. J Ultrasound Med. 1999;18(3):225–9. [DOI] [PubMed] [Google Scholar]
- 28.Amend SR, Valkenburg KC, Pienta KJ. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J Vis Exp. 2016;(110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benayahu D, Shur I, Ben-Eliyahu S. Hormonal changes affect the bone and bone marrow cells in a rat model. J Cell Biochem. 2000;79(3):407–15. [DOI] [PubMed] [Google Scholar]
- 30.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19(9):1323–30. PubMed Central PMCID: PMCPMC2652842. 10.1007/s00198-008-0574-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145(7):3115–21. Epub 2004/04/03. en.2004-0129 [pii]. 10.1210/en.2004-0129 [DOI] [PubMed] [Google Scholar]
- 32.Amo C, Revilla M, Hernandez ER, Gonzalez-Riola J, Villa LF, Seco C, et al. Correlation of ultrasound bone velocity with dual-energy X-ray bone absorptiometry in rat bone specimens. Invest Radiol. 1996;31(2):114–7. [DOI] [PubMed] [Google Scholar]
- 33.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A. 2000;97(14):7829–34. Epub 2000/06/28. 130200197 [pii]. PubMed Central PMCID: PMC16630. 10.1073/pnas.130200197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140(9):4367–70. Epub 1999/08/28. 10.1210/endo.140.9.7131 [DOI] [PubMed] [Google Scholar]
- 35.Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol. 2010;54(2):123–32. Epub 2010/05/21. [DOI] [PubMed] [Google Scholar]
- 36.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170(2):427–35. Epub 2007/01/27. PubMed Central PMCID: PMC1851862. 10.2353/ajpath.2007.060834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–68. Epub 2005/11/30. 10.1111/j.0105-2896.2005.00324.x [DOI] [PubMed] [Google Scholar]
- 38.Osta B, Benedetti G, Miossec P. Classical and Paradoxical Effects of TNF-alpha on Bone Homeostasis. Front Immunol. 2014;5:48 PubMed Central PMCID: PMCPMC3923157. 10.3389/fimmu.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troen BR. The role of cathepsin K in normal bone resorption. Drug News Perspect. 2004;17(1):19–28. Epub 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 40.Pennypacker BL, Duong le T, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY, et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011;26(2):252–62. Epub 2010/08/25. 10.1002/jbmr.223 [DOI] [PubMed] [Google Scholar]
- 41.Pietschmann P. Cathepsin K inhibitors: emerging treatment options for osteoporosis. Wien Med Wochenschr. 2015;165(3–4):47 Epub 2015/02/19. 10.1007/s10354-015-0347-8 [DOI] [PubMed] [Google Scholar]
- 42.Wagner N, Weyhersmuller A, Blauth A, Schuhmann T, Heckmann M, Krohne G, et al. The Drosophila LEM-domain protein MAN1 antagonizes BMP signaling at the neuromuscular junction and the wing crossveins. Dev Biol. 2010;339(1):1–13. Epub 2009/12/29. 10.1016/j.ydbio.2009.11.036 [DOI] [PubMed] [Google Scholar]
- 43.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20(5–6):475–80. Epub 2009/11/07. 10.1016/j.cytogfr.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 44.Yin TC, Wang CJ, Yang KD, Wang FS, Sun YC. Shockwaves enhance the osteogenetic gene expression in marrow stromal cells from hips with osteonecrosis. Chang Gung Med J. 2011;34(4):367–74. [PubMed] [Google Scholar]
- 45.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11(2):76–81. Epub 2005/02/08. 10.1016/j.molmed.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 46.Schilling T, Ebert R, Raaijmakers N, Schutze N, Jakob F. Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J Steroid Biochem Mol Biol. 2014;139:252–61. Epub 2012/12/25. 10.1016/j.jsbmb.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 47.Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015;78:202–8. Epub 2015/04/18. 10.1016/j.ijbiomac.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 48.Sadie-Van Gijsen H, Crowther NJ, Hough FS, Ferris WF. The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cell Mol Life Sci. 2013;70(13):2331–49. 10.1007/s00018-012-1211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurimae J, Jurimae T. Plasma adiponectin concentration in healthy pre- and postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol Endocrinol Metab. 2007;293(1):E42–7. 10.1152/ajpendo.00610.2006 [DOI] [PubMed] [Google Scholar]
- 50.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92(4):1517–23. 10.1210/jc.2006-2097 [DOI] [PubMed] [Google Scholar]
- 51.Fu Y, Li R, Zhong J, Fu N, Wei X, Cun X, et al. Adipogenic differentiation potential of adipose-derived mesenchymal stem cells from ovariectomized mice. Cell Prolif. 2014;47(6):604–14. 10.1111/cpr.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somjen D, Katzburg S, Kohen F, Gayer B, Posner GH, Yoles I, et al. The effects of native and synthetic estrogenic compounds as well as vitamin D less-calcemic analogs on adipocytes content in rat bone marrow. J Endocrinol Invest. 2011;34(2):106–10. 10.3275/7108 [DOI] [PubMed] [Google Scholar]
- 53.MC DA, Frairia R, Romeo P, Amelio E, Berta L, Bosco V, et al. Extracorporeal shockwaves as regenerative therapy in orthopedic traumatology: a narrative review from basic research to clinical practice. J Biol Regul Homeost Agents. 2016;30(2):323–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.