Supplemental Digital Content is available in the text
Abstract
The association of latent tuberculosis infection (LTBI) with subsequent cancer remains unclear. We investigated the risk of future cancer among tuberculosis (TB) contacts with or without subsequent TB activation. Using the Taiwan National Health Insurance Research Database, we conducted a nationwide population-based study. TB contacts during 1997 to 2012 were included as the study cohort. Patients with antecedent cancer and TB were excluded. Data from 11,522 TB contacts and 46,088 age-, sex-, and enrollment date–matched subjects during 1997 to 2012 were analyzed. The 2 cohorts were monitored until December 31, 2012 for incidence of cancer and TB infection. LTBI was defined as a TB contact with subsequent TB activation. The primary endpoint was occurrence of newly diagnosed cancer. There was no difference in cancer development between the TB contact cohort and comparison cohort (log-rank test, P = 0.714). After multivariate adjustment, the hazard ratio (HR) for cancer among the LTBI patients was 2.29 [95% confidence interval (CI), 1.26–4.17; P = 0.007]. There was increase in cancer incidences for several specific cancer types, including multiple myeloma (HR 340.28), lung (HR 2.69), kidney and bladder (HR 6.16), hepatobiliary (HR 2.36), and gastrointestinal (HR 2.99) cancers. None of the 136 TB contacts who received isoniazid prophylaxis developed cancer. LTBI patients had a higher risk of future cancer.
INTRODUCTION
Cancer and pulmonary tuberculosis (TB) are major global health concerns and are associated with substantial morbidity and mortality. Cancer affects populations worldwide, and the global burden of cancer is increasing: 14.1 million new cancer cases and 8.2 million cancer-related deaths were reported in 2012, and 20 million new cases are expected in 2020.1 In many countries cancer is the most common cause of death. Worldwide trends show that the burden of cancers associated with environmental risk factors is increasing in developing countries. In Taiwan, 2014 was the 33rd consecutive year in which cancer was the leading cause of death nationwide. Fortunately, the general public is increasingly aware that some cancers can be prevented by avoiding risk factors.
In 2013, there were 9.0 million new cases of active TB worldwide;2 thus, any association between TB and cancer is of great clinical importance. Most (56%) of the 9 million people who developed TB in 2013 live in Southeast Asia or the Western Pacific region. Among infectious diseases in Taiwan, TB has had the highest incidence and mortality rates for decades. In 2012, there were 53 new cases per 100,000 population and 2.7 tuberculosis-related deaths per 100,000 population in the country. A patient with uncontrolled active TB will infect an average of 10 to 15 people per year.2 Latent tuberculosis infection (LTBI) is a state of persistent immune response to TB without evidence of clinically active TB. Only 5% to 10% of people infected with TB will develop clinical manifestations of active TB within 2 years after exposure. About 90% of TB-infected patients have LTBI, and the lifetime chance that LTBI will progress to overt, active TB is only 10%.
TB is a chronic infectious and inflammatory disease. The association between active TB and subsequent cancer development has been investigated for several decades, and systematic reviews3,4 have shown a relation between TB and cancer development. Recent population-based studies found that TB might increase the risks of lung cancer5–7 and other cancers8 (eg, esophageal, head, and neck cancers). Several studies have reported a strong association between tuberculosis and lung cancer. TB-related chronic inflammation and infection is a possible mechanism of cancer pathogenesis. However, the relation between LTBI and subsequent cancer development remains unclear, and no large-scale study has investigated this association. We hypothesized that LTBI increases the risk of cancer development. To evaluate this hypothesis, we conducted a nationwide population-based study of cancer risk among TB contacts and patients with LTBI.
METHODS
Data Source
In this study, we used the National Health Insurance Research Database (NHIRD), which has been collecting almost all Taiwanese medical care data. The Taiwan National Health Insurance (NHI) program is a mandatory universal health insurance program since 1995 (http://nhird.nhri.org.tw/en/index.htm). The National Health Research Institutes (NHRI) administers the Taiwan NHI program and records the entire insurance claims database, which is referred to as the NHIRD. The NHI program covers inpatient, outpatient, and emergency care, as well as medical procedures, surgical procedures, and prescription drugs. The NHIRD has detailed health care data and covered all inpatient and ambulatory medical claims for more than 99% of the entire Taiwanese population.9 To keep individual information confidential, personal information that could identify individual patients is encrypted. The Longitudinal Health Insurance Database (LHID) is a representative database of 1 million patients. Three subsets of the NHIRD, LHID 2000, LHID 2005, and LHID 2010, which contains claims data of 1-million beneficiaries randomly selected from the NHIRD in 2000, 2005, and 2010, respectively. The present study was conducted by analyzing the LHID 2005 from 1996 to 2012. The NHI Database of Catastrophic Illness is a subset of the NHIRD that records comprehensive health care information on all patients with catastrophic illnesses like malignancies. Patients with catastrophic illnesses are exempted from copayments under the NHI program. Numerous studies have also been published using data from the NHI program.
Study Cohort
Using data from the Taiwan NHIRD, we conducted a retrospective cohort study of the period from January 1, 1996 through December 31, 2012. In this cohort study, we selected subjects aged 20 years or older who were newly diagnosed with TB contact between January 1, 1996 and December 31, 2012. TB contact was defined as an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code of V01.1 with at least 1 chest radiographic examination or an ICD-9-CM code of 795.5. In this study, we enrolled adults newly classified as a TB contact as the study cohort. Patients who had received a diagnosis of cancer (ICD-9-CM codes 140-208) or TB disease (ICD-9-CM codes 011-018) before being classified as a TB contact were excluded. In this study, TB contacts who developed subsequent TB activation were included in the LTBI cohort. Information on age, sex, monthly income, and comorbidities was collected for analysis. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (VGHIRB No. 2015-04-004AC).
Comparison Group
For each newly classified TB contact, 4 age- and sex-matched subjects were randomly selected using the same index date. Comparison subjects were selected using computer software and incidence density sampling.10 Comparison subjects were excluded if they had received a diagnostic code for TB contact, preexisting TB disease, or cancer before inclusion in the study. TB contacts and the matched groups were followed until a diagnosis of cancer, death, withdrawal from the NHI system, or December 31, 2012.
Variables
Subject characteristics such as age, sex, and monthly income were retrieved and matched between groups. Covariates identified in previous studies as risk factors for cancer or as major comorbidities linked to TB were assessed in the analyses, including diabetes mellitus, coronary heart disease, heart failure, cerebrovascular disease, hypertension, systemic lupus erythematosus, rheumatoid arthritis, chronic kidney disease/end-stage renal disease, liver cirrhosis, asthma, chronic obstructive pulmonary disease, and nontuberculous mycobacterium.
Outcomes
The endpoint of the study was incident cancer. The Registry for Catastrophic Illness was used to identify patients who received a cancer diagnosis. Peer review of pathohistologic reports is required before a cancer diagnosis is reported to the Registry for Catastrophic Illness. All patients were followed from the date of enrollment until a first diagnosis of cancer, date of death, withdrawal from national health insurance, or December 31, 2012.
Validation
Identification of patients with an ICD-9-CM code for TB contact was validated by analysis of randomly selected samples (300 outpatients) from the claims database of Taipei Veterans General Hospital (a 2800-bed tertiary referral hospital in Taiwan) for 2011 to 2013. The content of this database is used for reimbursement and is similar to that of the NHIRD. Two pulmonologists (VY-FS and S-WP) independently reviewed the clinical and imaging data from the selected samples. All instances of disagreement were resolved by consensus. We defined a TB contact as someone who had close contact with a TB patient for longer than 8 hours. To assess interobserver agreement on identification of TB contacts, we calculated the Cohen kappa coefficient (κ) to express probability beyond chance (κ = 0.00–0.40, slight-to-fair agreement; κ = 0.40–0.60, moderate agreement; κ = 0.60–0.80, good agreement; κ = 0.80–1.00, excellent agreement).
Statistical Analysis
All data extraction, matching, and computation were done using the SAS 9.4 software package (SAS Institute, Cary, NC). Comparisons between 2 groups were made by the independent Student t test (for continuous variables) or Pearson χ2 test (for categorical variables), as appropriate. The Kaplan–Meier method was used for analysis of survival, with significance assessed by the log-rank test. We used univariate and multivariate conditional Cox proportional hazards models to identify predictors of cancer development among patients. Risk factors with a P value less than 0.1 were entered into the multivariate analysis. All statistical analyses were performed using IBM SPSS statistical software (version 19.0 for Windows; IBM Corp, New York, NY). All data are expressed as means ± standard deviation (SD) or percentages unless otherwise stated. A P value less than 0.05 was considered to indicate statistical significance.
RESULTS
Characteristics of the Study Population
We identified 14,654 individuals who had been classified as a TB contact during the period from January 1, 1996 through December 31, 2012. After excluding those younger than 20 years (n = 2748), those with antecedent cancer (n = 169), and those with antecedent TB disease (n = 215), 11,522 patients were included in the TB contact group. Another 46,088 subjects without TB contact were randomly selected for inclusion in the comparison group. For the period 1996 to 2012, we observed the TB contact and comparison cohorts for 34,558 and 155,347 person-years, respectively. Supplemental Figure 1 shows the process of enrollment and follow-up. The basic characteristics of the TB contact cohort and matched cohort are shown in Table 1. During the follow-up period, there were 191 (1.66%) cancer events in the TB contact cohort and 886 (1.92%) cancer events in the matched cohort (P = 0.066). Incidences in the TB contact cohort and control group were 55.27 and 57.03 persons per 10,000 person-years, respectively.
TABLE 1.
Characteristics of the TB Contact Cohort and Matched Controls
Incidence of cancer development did not significantly differ between the TB contact cohort and comparison cohort (log-rank test, P = 0.714, Figure 1). As compared with patients without cancer, the subgroup with incident cancer had a higher proportion of women (54.9%) and higher proportions of individuals with diabetes mellitus, coronary heart disease, heart failure, cerebrovascular disease, hypertension, systemic lupus erythematosus, rheumatoid arthritis, chronic kidney disease/end-stage renal disease, liver cirrhosis, asthma, and chronic obstructive pulmonary disease (Supplemental Table 1). After multivariate adjustment, TB contact was not associated with incident cancer (Supplemental Table 2).
FIGURE 1.
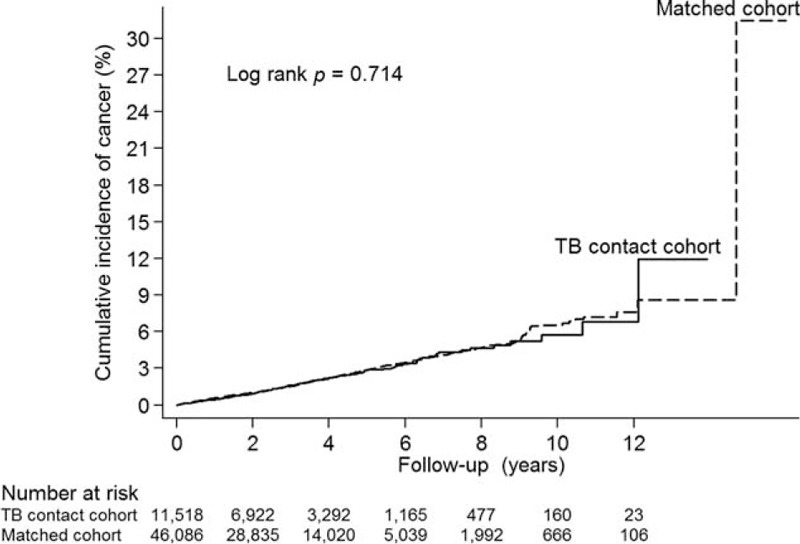
Cumulative incidence of cancer in tuberculosis (TB) contact cohort and the matched cohort.
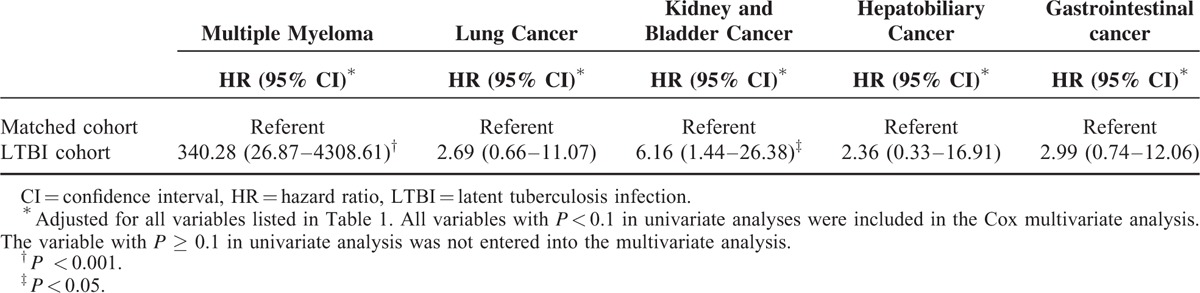
We analyzed the association between cancer development and TB contact with or without subsequent TB activation. Of the 130 LTBI patients (TB contact with subsequent TB activation), 11 (8.46%) developed cancer. The log-rank test showed a higher cumulative incidence of cancer in the LTBI group than in the TB contacts without subsequent TB activation during follow-up (P < 0.001; Figure 2). This suggests that the risk of subsequent cancer is higher for individuals with LTBI than for TB contacts without subsequent TB activation. Furthermore, analysis of the association between cancer development and isoniazid prophylaxis for TB contacts showed that none of the 136 TB contacts who received isoniazid prophylaxis developed cancer (P = 0.442; Figure 3). Also, none of the 9 LTBI patients with isoniazid prophylaxis developed cancer (P = 0.511; Supplemental Figure 2). Interestingly, after multivariate adjustment, the hazard ratio (HR) for the LTBI group was significantly higher (2.29; 95% confidence interval [CI], 1.26–4.17; P < 0.001) and the HR for TB contacts receiving isoniazid prophylaxis was 0.00 (P = 0.880; Table 2). The association between LTBI and risk of specific cancer types is summarized in Table 3. There was increase in cancer incidences for several specific cancer types, including multiple myeloma (HR 340.28, 95% CI 26.87–4308.61, P < 0.001), lung (HR 2.69, 95% CI 0.66–11.07, P = 0.170), kidney and bladder (HR 6.16, 95% CI 1.44–26.381, P = 0.014), hepatobiliary (HR 2.36, 95% CI 0.33–16.91, P = 0.393), and gastrointestinal (HR 2.99, 95% CI 0.74–12.06, P = 0.124) cancers.
FIGURE 2.
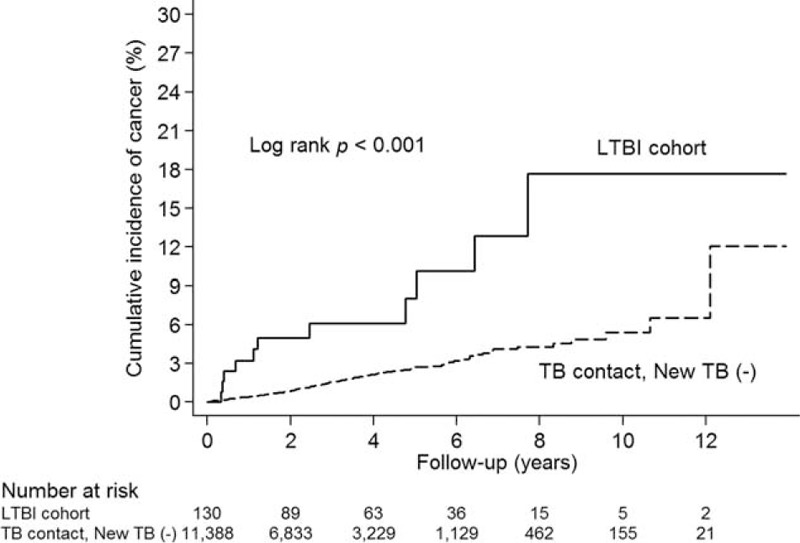
Cumulative incidence of cancer in latent tuberculosis infection (LTBI) cohort and tuberculosis contacts without subsequent tuberculosis activation [TB contact, New TB (−)].
FIGURE 3.
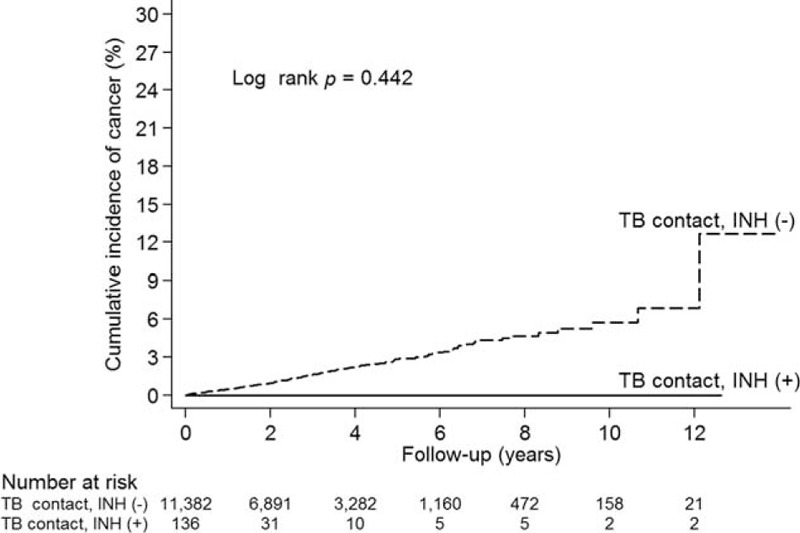
Cumulative incidence of cancer in tuberculosis (TB) contacts with and without isoniazid (INH) prophylaxis.
TABLE 2.
Association Between Latent Tuberculosis Infection (LTBI) and Risk of Incident Cancer
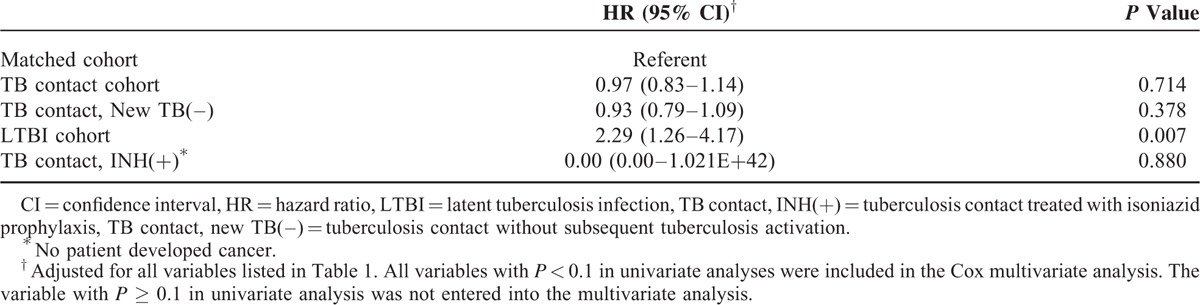
TABLE 3.
Association Between Latent Tuberculosis Infection (LTBI) and Risk of Specific Cancer Types
Validation
During the period from January 1, 2011 through December 31, 2013, 1737 outpatients had claims data indicating exposure to tuberculosis, from which 300 patients were randomly selected for validation. Among the 300 patients, the diagnosis of TB exposure was confirmed for 295 subjects and unconfirmed for 5 subjects (sensitivity, 98.3%). Interobserver agreement was excellent (κ = 0.91; 95% CI, 0.89–0.93).
DISCUSSION
To date, this has been the largest cohort study of cancer risk among TB contacts and the LTBI cohort. The incidence of cancer development was increased in the LTBI cohort (TB contacts with subsequent TB activation). As compared with the general population, the LTBI cohort had an HR of 2.29 for cancer risk. However, TB contact was not associated with increased cancer risk. Interestingly, none of the 136 TB contacts who received isoniazid prophylaxis developed cancer.
The relationship between pulmonary TB and subsequent lung cancer development has been investigated for several decades. The causal relation between TB and carcinoma had been discussed in case reports, case-control studies, and cohort studies. A systematic review3 of studies published between January 1966 and December 2008 investigated the association between TB and the risk of subsequent lung cancer. A total of 47 articles were included. Preexisting TB significantly increased lung cancer risk (relative risk, 1.74; 95% CI, 1.48–2.03). The increase in lung cancer risk appeared to be highest within the first 5 years after active TB infection. However, the risk remained elevated for more than 20 years after a TB diagnosis. A meta-analysis4 of studies published during the period from January 1960 to August 2010 confirmed the results of the review: previous pulmonary TB was associated with an increased risk of lung cancer.
In contrast to Western countries, TB remains endemic in the Asia-Pacific and Africa.2 Three cohort studies from the Asia-Pacific5–7 and 1 cohort study from Europe11 investigated lung cancer risk among TB patients. Wu et al5 and Yu et al6 used data from the Taiwan NHIRD to conduct a nationwide population-based study of lung cancer risk after pulmonary TB infection. The results of these 2 cohort studies5,6 from Taiwan are consistent with those from the previous meta-analyses.3,4 Wu et al5 enrolled 5657 TB patients and 23,485 age- and sex-matched controls for the period 1997 to 2008, who were followed until 2008. As compared with the controls, the HR for lung cancer in the TB cohort was 1.64 (95% CI, 1.24–2.15). Yu et al6 recruited 712,392 subjects without TB and 4480 patients with newly diagnosed TB during 1998 to 2000 and followed them until 2007. As compared with the controls, the incidence of lung cancer was 11-fold higher in patients with TB, and the HR for the TB cohort was 4.37. A population-based cohort study7 from Korea reported similar results. These cohort studies from Asia indicate that the risk of lung cancer is 2-fold to 4-fold higher among TB patients. Using Danish nationwide databases, Simonsen et al11 conducted a population-based cohort study comparing cancer risk in TB patients and the general population. The standardized incidence ratio for lung cancer was 3.40 (95% CI, 3.09–3.74). In addition, TB infection is reported to be associated with increased risk of nonpulmonary malignancies. A population-based study of the Taiwan NHIRD found that the risks of several nonpulmonary cancers were higher after a tuberculosis diagnosis.8 The standardized incidence ratio for multiple myeloma was 2.43 (95% CI, 0.66–6.21). In the Danish population-based cohort study11 the standardized incidence ratio for nonpulmonary cancer was 1.29 (95% CI, 1.22–1.36). However, no patient developed multiple myeloma in Danish study. One important finding of this study is that multiple myeloma may be more likely to occur in the LTBI patients.
We hypothesize that chronic inflammation and sustained infection are important in the pathogenesis of cancer in patients with LTBI. Several studies have found that chronic infection and inflammation,12 such as viral infections,13,14 autoimmune diseases,15 and tuberculosis,3,4 are associated with cancer. Individuals infected with Mycobacterium tuberculosis have only a 10% chance of converting to active TB infection during their lifetime. Most infected individuals develop LTBI, which is defined as M tuberculosis infection without clinical signs or symptoms of disease and a normal chest radiograph. LTBI represents a state of equilibrium in which the host is able to control M tuberculosis infection but not completely eradicate the bacteria. Chronic infection results in sustained tissue damage, cellular proliferation, and tissue repair. Chronic sustained infection in damaged tissue results in metaplasia or dysplasia.16 Chronic sustained M tuberculosis infection may also contribute to carcinogenesis.
This is the first study to address the relationship of LTBI and subsequent cancer development. Our research design, which included an unbiased subject selection, a strict definition of TB exposure, and age-, sex-, and index date–matched comparison subjects, increases the validity of our findings. In Taiwan, TB has for decades been the most commonly reported infectious disease, and strength of this study is the rigorous TB reporting system in Taiwan. Every case of active TB must be reported to Taiwan Centers for Disease Control within 7 days after diagnosis. TB contacts are referred for pulmonologist follow-up assessments and routine chest radiography. Nonetheless, we examined the internal validity of TB contact classification using the same ICD-9-CM coding and confirmed that interobserver agreement and accuracy in identifying TB contact were excellent. In addition, patients with a certificate of catastrophic illness are free from related medical costs, especially hospital and medication costs. Pathologic confirmation of cancer is necessary to receive a catastrophic illness certificate of cancer, and all cancer diagnoses are confirmed by peer review. Because participation in the NHI is mandatory, and all Taiwanese residents can utilize health care with low copayments, follow-up of individuals is complete, with very low referral bias. Therefore, classification of TB contact and cancer diagnoses are reliable and thorough.
The increased incidence of cancer in LTBI patients is likely caused by the presence of chronic inflammation.17 This study is the largest study of its kind to date and the first cohort study to evaluate cancer risk among LTBI patients. The power of a study is the largest and can increase the statistical power. A particular strength of this study is its nationwide, population-based study design, which allowed us to identify nearly all cases of TB contact and cancer in Taiwan and thus minimize referral bias, as all medical care is covered by the national insurance system. Additionally, the large sample size was powered to detect even very small differences between the LTBI cohort and general population.
Our study has several limitations. First, information on some potential risk factors, as well as the behavior risk factors, including smoking, alcohol use, environmental exposure, and family history of malignancy, was not available. Nonetheless, some smoking-related health consequences could be partly reflected in the presentation of comorbidities such as hypertension and chronic obstructive pulmonary disease and coronary heart disease, which were included in the analysis. In addition, alcohol use could be reflected in the presentation of liver cirrhosis. Second, diagnoses of TB contact and cancer that is dependent on ICD-9-CM codes may be less accurate than those made in a prospective clinical setting. Nonetheless, diagnosis of TB contact by ICD-9-CM coding was validated in this study, indicating that the accuracy is excellent. Furthermore, diagnosis of TB contacts by ICD-9-CM has been widely used in epidemiologic studies.18,19 However, we did not validate LTBI cohort in this analysis. Patients with LTBI may be misclassified into comparison group and patients without LTBI may be misclassified into LTBI cohort and resulted in a misclassification bias. However, the nondifferential misclassification bias was a bias toward the null. In other words, if there is an association, it tends to minimize it regardless of whether it is a positive or a negative association. Third, the mean follow-up period was 3.30 years, which might be too short to detect cancer development. If so, a longer follow-up period would likely reveal a larger actual effect. Furthermore, the follow-up period might be not long enough to overcome the latency period for cancer and detection bias for occult cancer may occur. The comparison cohort had a longer follow-up period than TB contacts. In other words, the actual effect in TB contacts may be minimized. However, we used conditional Cox proportional hazards models to identify predictors of cancer development among patients. Follow-up time has been adjusted in these models. Finally, the external validity of our findings may be a concern because almost all our enrollees were Taiwanese. Taiwan remains a TB-endemic region. The generalizability of our results to low incidence countries requires further verification. However, our findings suggest new avenues for future research.
CONCLUSION
Cancer risk was higher in LTBI patients with TB activation (HR, 2.29) than in the general population. Interestingly, none of TB contacts who received isoniazid prophylaxis developed cancer. More comprehensive studies are needed to confirm our findings and identify the potential underlying mechanisms of cancer development in LTBI patients.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases 9th Revision Clinical Modification, LHID = Longitudinal Health Insurance Database, LTBI = latent tuberculosis infection, M tuberculosis = Mycobacterium tuberculosis, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, SD = standard deviation, TB = tuberculosis.
VY-FS and Y-FY contributed equally to this study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Dirlikov E, Raviglione M, Scano F. Global tuberculosis control: toward the 2015 targets and beyond. Ann Intern Med 2015; 163:52–58. [DOI] [PubMed] [Google Scholar]
- 3.Liang HY, Li XL, Yu XS, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer 2009; 125:2936–2944. [DOI] [PubMed] [Google Scholar]
- 4.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One 2011; 6:e17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CY, Hu HY, Pu CY, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer 2011; 117:618–624. [DOI] [PubMed] [Google Scholar]
- 6.Yu YH, Liao CC, Hsu WH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol 2011; 6:32–37. [DOI] [PubMed] [Google Scholar]
- 7.Bae JM, Li ZM, Shin MH, et al. Pulmonary tuberculosis and lung cancer risk in current smokers: the Seoul Male Cancer Cohort Study. J Korean Med Sci 2013; 28:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo SC, Hu YW, Liu CJ, et al. Association between tuberculosis infections and non-pulmonary malignancies: a nationwide population-based study. Br J Cancer 2013; 109:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaumont JJ, Steenland K, Minton A, et al. A computer program for incidence density sampling of controls in case-control studies nested within occupational cohort studies. Am J Epidemiol 1989; 129:212–219. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen DF, Farkas DK, Sogaard M, et al. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tuberc Lung Dis 2014; 18:1211–1219. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res 2006; 4:221–233. [DOI] [PubMed] [Google Scholar]
- 13.Uchida T, Takahashi K, Tatsuno K, et al. Inhibition of hepatitis-B-virus core promoter by p53: implications for carcinogenesis in hepatocytes. Int J Cancer 1996; 67:892–897. [DOI] [PubMed] [Google Scholar]
- 14.de-The G. The Epstein-Barr virus (EBV): a Rosetta Stone for understanding the role of viruses in immunopathological disorders and in human carcinogenesis. Biomed Pharmacother 1985; 39:49–51. [PubMed] [Google Scholar]
- 15.Suciu-Foca N, Berloco P, Cortesini R. Tolerogenic dendritic cells in cancer, transplantation, and autoimmune diseases. Hum Immunol 2009; 70:277–280. [DOI] [PubMed] [Google Scholar]
- 16.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med 1999; 190:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng JY, Chen SC, Lee MC, et al. Is 1-year follow-up adequate for adult tuberculosis contacts? Eur Respir J 2015; 45:1501–1504. [DOI] [PubMed] [Google Scholar]
- 19.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol 2012; 176:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


