Supplemental Digital Content is available in the text
Abstract
The role of fruit and vegetable (FV) intake in relation to prostate health remains inconclusive. This 4-year longitudinal study aims to explore the association of FV intake and the development of lower urinary tract symptoms (LUTS, a cluster of chronic urinary symptoms occurring in bladder, prostate and urethra), incidence of symptomatic benign prostatic hyperplasia (BPH) and erectile dysfunction (ED) in Chinese elderly men.
Data were obtained from a 4 years longitudinal study (Mr OS Hong Kong, the largest prospective study on bone health in Chinese elderly). Two thousand Chinese men aged 65 years and older were recruited from the local community, of whom 1998 (99.9%) at baseline and 1564 (78.2%) at 4-year follow-up reported data on LUTS, which were evaluated by a validated International Prostate Symptoms Scale (IPSS). Erectile function was evaluated by the International Index of Erectile Dysfunction-5 (IIEF-5) questionnaires at 2- (n = 386) and 4-year (n = 475) follow-ups. Dietary intake was assessed using a validated food frequency questionnaire at baseline. Analysis was conducted using multivariate linear and logistic regression.
For total FV and most of their subclasses, moderate consumption had the lowest mean changes of LUTS; we thus applied the moderate levels as the reference in the regression models. The high levels of total FV intake (>350 g/1000 kcal/day) were significantly associated with reduced IPSS by scores of -1.174 ± 0.459 (or -17.3% of basal IPSS, P = 0.011) relative to the moderate groups (250–350 g/1000 kcal/day). FV consumption had no significant association with the score change of ED or the odds of sexual activities at 4-year (all P > 0.05). High intake of dark and leafy vegetables (>50 g/1000 kcal/day) significantly reduced the risk of LUTS progression by 37.2% [odds ratio (OR) (95% confidence interval, 95% CI): 0.628 (0.466∼0.848), P = 0.002] or risk of symptomatic BPH by 34.3% [OR (95% CI): 0.657 (0.442–0.976), P = 0.038] after 4 years compared with the moderate group (25–50 g/1000 kcal/day).
Adequate FV intakes, especially dark and leafy vegetables, were associated with improved LUTS among Chinese elderly men, but lack an association with ED and sexuality.
INTRODUCTION
Lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) are highly prevalent conditions among elderly men associated with an impaired quality of life and an increased risk of sexual dysfunction and mortality.1 LUTS represents a cluster of chronic urinary problems occurring in more than half of men in their 60s and increase with age.2 BPH is the principal underlying cause of LUTS.3 An estimated 612 million men will have BPH globally by 2018.4 Studies have shown a strong correlation between sexual dysfunction and the severity of LUTS.5 Pharmacological treatments are available for management of symptoms but can be expensive and may be associated with adverse events6; lifestyle modifications therefore become the primary treatment strategies for improvement of prostate health due to its noninvasive and modifiable properties.7
Fruits and vegetable (FV) contain high levels of antioxidants, polyphenols, vitamins, minerals, and fibers, and are essential components of a healthy diet and beneficial for a range of chronic conditions. Evidence from basic studies suggested that FV intake may beneficially influence the disorders underlying BPH via inhibiting inflammation and oxidative damage, altering the hormonal or growth-regulatory factors to inhibit cellular proliferation,8 or modulating sympathetic nervous system and subsequently affect prostate smooth muscle tone.9 Thus, FV consumption may offer feasible therapeutic targets to delay the disease onset, prevent the progression, or attenuate the severity of LUTS.
Although LUTS/BPH is a common condition in elderly men, dietary factors especially FV intake on prostate health, remain largely undefined and inconsistent. Most of previous studies were conducted among Caucasian populations and majority of reports have been based on cross-sectional or hospital-based case–control studies. Evidence from prospective cohort studies was limited. It is unclear whether various subclasses of FV may have a different impact on prostate health.
In this study, we used data from study by Mr OS,10 a longitudinal study among Chinese elderly men to examine whether dietary FV intake and its subclasses affect the development and progression of LUTS, risk of BPH, and erectile function. The potential mechanisms related to dietary vitamin C, fiber, and isoflavones were also examined. We hypothesize that dietary FV intakes improve prostate health of Chinese elderly men.
METHODS
Participants
This was a 4-year prospective cohort study (Mr Os Hong Kong) among 2000 Chinese men aged 65 years and older. The details of subjects’ recruitment have been described previously.10 In brief, participants who were able to walk independently were recruited on voluntary basis from local community of Hong Kong (South China) in a health survey between 2001 and 2003. Stratified sampling was adopted in order to have around 33% of subjects in each of the following age groups: 65 to 69; 70 to 74; and ≥75 years. Recruitment notices were placed in housing estates and community centers for the elderly. Subjects were invited to the research center for interviews and physical examination. The present study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving human subjects were approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong. Written informed consent was obtained from all subjects.
Data Collection
The cohort study initially collected information related to risk factors associated with osteoporosis (bone mineral density and fracture incidence) and later expanded to include measurements of LUTS and sexuality (the details please see the following information on Erectile Function and Sexual Activity). Subjects were interviewed using structured and standardized questionnaires or examination form covering the following aspects: socio-demographic characteristics (such as age, education, income, marital status, etc.), medical history [such as fracture, depression, cardiovascular diseases (CVDs), and cancers, etc.] and medications, lifestyle factors including cigarette smoking, alcohol consumption, dietary intake, and physical activity (for details please see the following paragraph of “Other covariates”).
Fruit and Vegetable Consumption
Dietary intake at baseline was assessed using a 289-item validated semi-quantitative food frequency questionnaire (FFQ).11 The food consumption and nutrients intake were calculated on the basis of food composition database from McCance and Widdowson12 and the Chinese Food Composition Table.13 Each subject was asked by a trained interviewer to report the frequency and the usual amount of consumption of each food item over the past year. A series of food photographs with individual food portions were provided to participants for estimation of portion sizes. The participants were asked to indicate how often, on average, they consumed each food, with 9 possible response categories ranging from “never” to “every day.” The FFQ consisted of 8 categories: bread/pasta/rice; vegetables; fruits; meat/fish/eggs; beverages; dimsum/snacks; soups; and oil/salt/sauces.
Fruit intake was assessed by the inclusion of fresh fruit, cooked or canned fruit, dried fruit and fruit juices based on 28 fruit items, such as apple, apricot, banana, cherry, grape, raisins, lemon, lychee, logan, mango, melon, papaya, pear, plum, peach, pineapple, prunes, orange, persimmon, watermelon, kiwi, pomelo, strawberry, carambola. Citrus fruit intake in our study included intake of grape fruit, lemon, orange, and pomelo.
Total vegetable intakes were assessed by the inclusion of green vegetables, root vegetables, pulses, salad vegetables, and mixed-vegetable dishes based on 64 vegetable items, such as green leafy vegetables, corn, onion, cucumber, pepper, asparagus, egg-plant, bamboo Shoot, Chinese radish and chives, Chinese water chestnut, lotus root, celery, leeks, peppers, lily, pumpkin, taro, tomatoes, and legumes. Subclasses of vegetable were further assessed for cruciferous, dark and leafy vegetables, legumes, soy, and tomatoes. Cruciferous group in our study included broccoli, sauerkraut, coleslaw, cooked cabbage, cauliflower, Brussels sprouts, and kale.
Lower Urinary Tract Symptoms (LUTS) and Symptomatic BPH
The presence and severity of LUTS were assessed using a validated Chinese version of the International Prostatic Symptoms Scale (IPSS)14,15 at baseline and at the end of 4-year follow-up by an interviewer-administered mode. The IPSS is an 8-item questionnaire including 7 symptom questions (nocturia, frequency, urgency, intermittency, weak stream, incomplete emptying, and straining) and 1 global quality of life question. For the 7 symptoms during the last month, each has a score from 1 to 5 for a total of maximum 35 points. According to the IPSS,14 men were defined as severe LUTS if they scored ≥20; moderate LUTS if they scored 8∼19, and mild LUTS with a score ≤7. The overall and 2 components (voiding and storage symptoms) of the IPPS were reported separately as outcomes. Voiding symptoms included 4 items such as incomplete bladder emptying, intermittency, weak urinary stream, and hesitancy, while storage symptoms included 3 questions on urinary frequency, urgency, and nocturia.
The analyses of progression of LUTS were based on the absolute score change of IPSS at 4-year follow-up from baseline. The progression of overall LUTS was defined as IPSS increase≥3.16 For voiding and storage symptoms, the increase of score ≥2 indicated progression over time.17 Symptomatic BPH incidence was assessed over 4 years and was defined as either the new self-report of BPH or receiving pharmaceutical or surgical treatment on prostate or the second report of an IPSS of 15 or higher.18
Erectile Function and Sexual Activity
Erectile dysfunction (ED) was measured by an abridged 5-item version of the International Index of Erectile Function (IIEF-5) questionnaire.19 This is a validated and sensitive indicator of changes in ED and treatment outcomes and was use to evaluate symptoms at 2- and 4-year follow-up. The total IIEF-5 score was calculated by totaling the response to all 5 questions. For the assessment of sexual activity at 4 years, all subjects were asked whether they had sexual intercourse in the previous 6 months.
Other Covariates
Cigarette smoking and alcohol consumption were investigated on the basis of self-report using validated methods.20 Information on the duration and level of past and current use of cigarettes, cigars, and pipes was obtained. For current smokers, the number of cigarettes smoked per day over the previous 12 months was collected. For alcohol consumption, subjects were asked to report their daily frequency of intake of alcohol and other beverages in portion sizes. One standard drink was defined as 1 unit of alcohol, which is equal to 10 g of alcohol. For beer, 1 standard drink equals to 1 can of beer (330 mL), 1 glass of wine (100 mL), or 1 shot of spirit or liquor (30 mL).
Anthropometric measures were conducted at the baseline and 4-year follow-ups by standardized protocol. Body weight and height were measured using the Physician Balance Beam Scale (Healthometer, Alsip, IL, with an accuracy of 0.1 kg) and the Holtain Harpenden standiometer (Holtain, Crosswell, UK, with an accuracy of 0.1 cm), respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
A validated 12-item Physical Activity Scale of the Elderly (PASE)21 was used to assess the level of physical activity. The PASE is composed of self-reported occupational, household, and leisure items over a 1-week period. It uses frequency, duration, and intensity level of activity to determine the score, with higher scores indicating greater physical activity level. Depressive symptoms were assessed using a validated 15-item Chinese version of the Geriatric Depression Scale (GDS),22 with depression being defined as a score of 8 or more.
Posteriori Sample Size Estimation
The posteriori sample size is estimated on the basis of the primary outcome of our analysis—the 4-year score change of IPSS. With a conservative IPSS change of 1.0 (the current change of standard deviation of 6.25), assuming 80% power and a convention assumption of α level 0.05, the posteriori sample size in our study is 1228.
Statistical Analysis
Statistical analyses were performed using the statistical package SPSS 19.0 (SPSS Inc., Chicago, IL). Α level of 5% was used as the level of significance. We evaluated the associations of FV intakes and their subcategories in relation to the score change of IPSS and ED, the progression of LUTS, and the incidence of symptomatic BPH after 4 years by either General Linear Models (GLMs) or logistic regression models. Adjusted variables were age, education, cigarette smoking (no, current, ever), coffee (mL/day), alcohol (g/day), use of anti-hypertensive medication (yes or no), BMI, GDS score, medical history of fracture, hypertension, stroke, diabetes, heart attack and any kinds of cancers (yes or no), PASE total score, and dietary energy (kcal/day). Dietary fruit and/or vegetable intakes and subclasses were adjusted for total energy using multivariate nutrient density model23 and then categorized as low, moderate, and high levels on the basis of conventional cut-points,24–26 which were further confirmed by the curve estimation of polynomial regression models (data not shown). For total FV intakes and their subclasses, there were no significant differences between low and moderate consumption in the changes of IPSS or other outcomes. In addition, several comparisons indicated that compared with moderate group (not low consumption group), the high consumption group had a significant reduction of IPSS or reduced risk of progression of LUTS or incidence of symptomatic BPH. We thus applied the moderate level as the reference in regression models. The moderate levels (g/1000 kcal/day) of various FV were defined as 250 to 350 for total FV; 100 to 150 for total vegetables; 25 to 50 for dark and leafy vegetables; 15 to 30 for soy foods; 7.5 to 15 for crucifies, 2.5 to 10 for tomatoes; 100 to 150 for total fruits; and 15 to 30 for citrus fruits.
GLMs were used to compare the adjusted mean changes in scores of PSS and ED at 4-year follow-up across low, moderate, and high intakes of FV groups. Separate models were created for voiding and storage symptoms of LUTS, respectively. Subjects with bladder or prostate cancers, or any medical or surgical treatment for prostate were excluded for the analyses. A total of 1667 men at baseline and 1301 at 4-year follow-up were included in the analysis.
Binary logistic regression was performed to calculate odds ratios (ORs) and 95% confidence interval (95% CI) for dichotomous outcomes of progression of LUTS and incidence of symptomatic BPH across low, moderate, and high FV intakes after controlling for above confounders within 4 years. Subjects with bladder or prostate cancers, LUTS >15 at baseline, or no self-reported BPH were excluded for the analysis. Thus, 1388 and 1275 men were included in the analysis for LUTS progression and incidence of symptomatic BPH, respectively.
To investigate the possible mechanisms of dietary vitamin C, fiber, and isoflavones relating to the associations of FV and LUTS, we conducted exploratory analysis to test whether the association of score changes of LUTS and FV was modified by dietary levels of vitamin C, fiber, and isoflavones intake in univariate models.
RESULTS
Among the 2000 recruited elderly men, 1998 (99.9%) at baseline and 1564 (78.2%) at 4-year follow-up reported data on LUTS. Baseline characteristics are described in Table 1. The mean age of participants was 72.4 ± 5.0 years. Their daily FV intakes were 132.2 ± 87.9 and 118.4 ± 74.8 g/1000 kcal, respectively. Of 1998 men, 41.0% suffered from moderate to severe LUTS. The progression of LUTS occurred in 485 (31.0%) participants after 4 years. Among the subjects who attended the 4-year follow-up (n = 1564), 69.4% (n = 1086) had no sexual intercourse over the past 6 months and 475 men answered questions on IIEF-5.
TABLE 1.
Baseline and Follow-up Characteristics of Participants
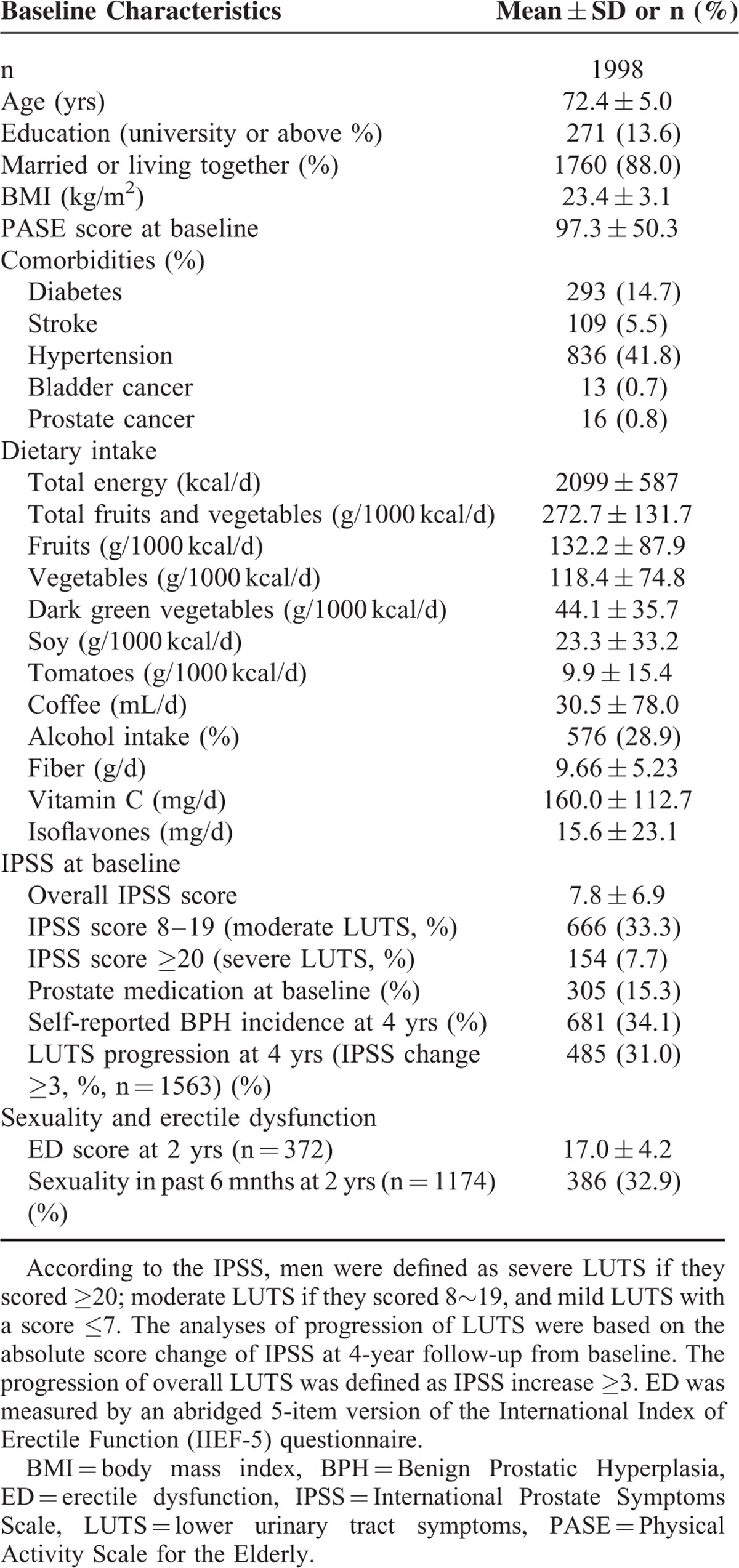
The adjusted mean changes in scores of overall, voiding, and storage symptoms by low, moderate, and high fruits or vegetables intake are indicated in Table 2 and Supplemental Figures 1 to 3 (see figures, Supplemental Figure 1–3, which demonstrates the adjusted mean changes in scores of overall, voiding, and storage symptoms by low, moderate, and high FV intakes). Compared with the moderate group, high levels of total FV intake (>350 g/1000 kcal/day) significantly reduced overall, storage, and voiding symptoms by scores of -1.174 ± 0.459 (-17.3% of basal PSS, P = 0.011), -0.448 ± 0.230 (P = 0.039), and -0.312 ± 0.190 (P = 0.042), respectively. High intake of fruit (>150 g/1000 kcal/day) and total vegetable (>150 g/1000 kcal/day), especially dark and leafy vegetable (>50 g/1000 kcal/day) and tomatoes (>10 g/1000 kcal/day), were significantly associated with decreased symptoms relative to moderate groups, with mean score changes for overall IPSS by -1.039 ± 0.451 (P = 0.021), -1.0 ± 0.479 (P = 0.037), -1.041 ± 0.375 (P = 0.006), and -0.944 ± 0.391 (P = 0.016), respectively. However, soy foods, cruciferous vegetables, and citrus fruits intake were not significantly associated with the changes of LUTS. Among 475 men who had sex in previous 6 months at 4-year follow-up, FV consumption and their botanical subgroups had no significant association with the score change of ED, as well as the odds of sexuality at 4 years (Table 3).
TABLE 2.
Adjusted Mean of Absolute Changes of IPSS (Including Overall, Storage, and Voiding Symptoms) at 4-year Follow-up by Low, Moderate, and High Fruit and/or Vegetable Intake
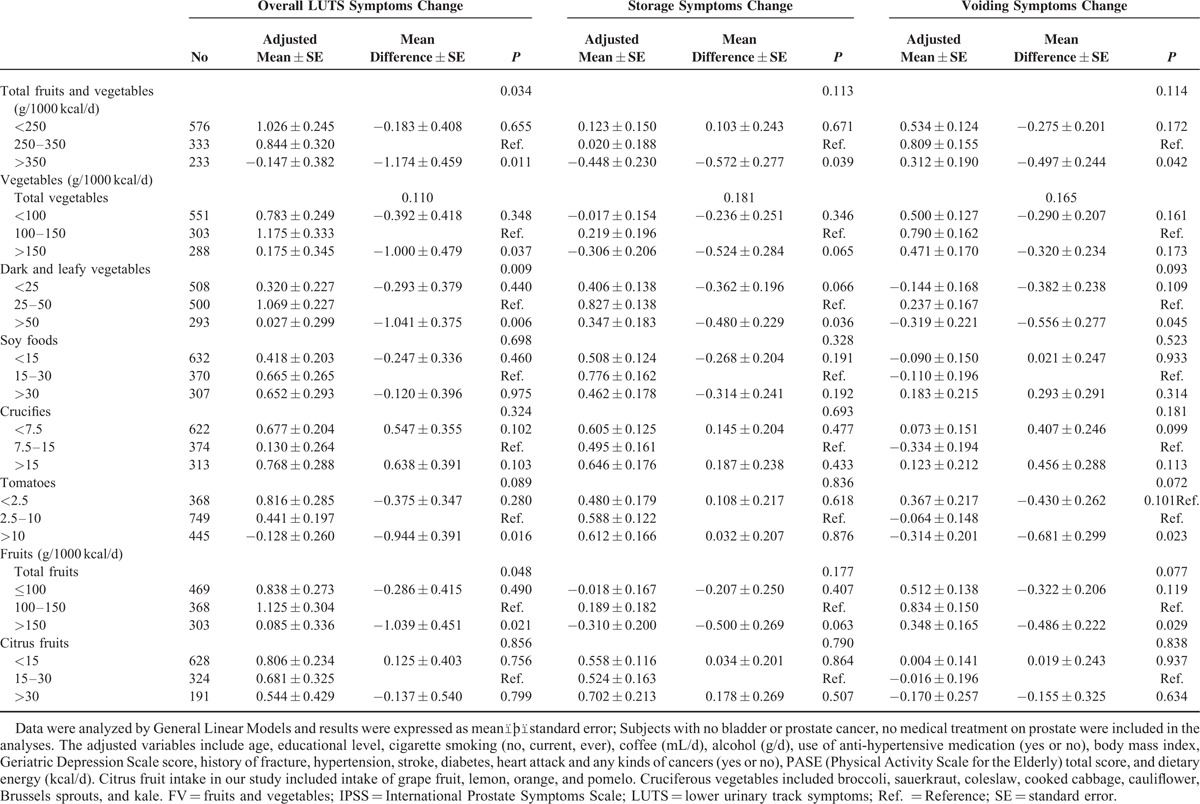
TABLE 3.
Adjusted Means of Score Change of Erectile Dysfunction and Odds Ratio (OR) of Sexuality at 4-year Follow-up by Low, Moderate, and High Levels of Fruit and/or Vegetable Intakes
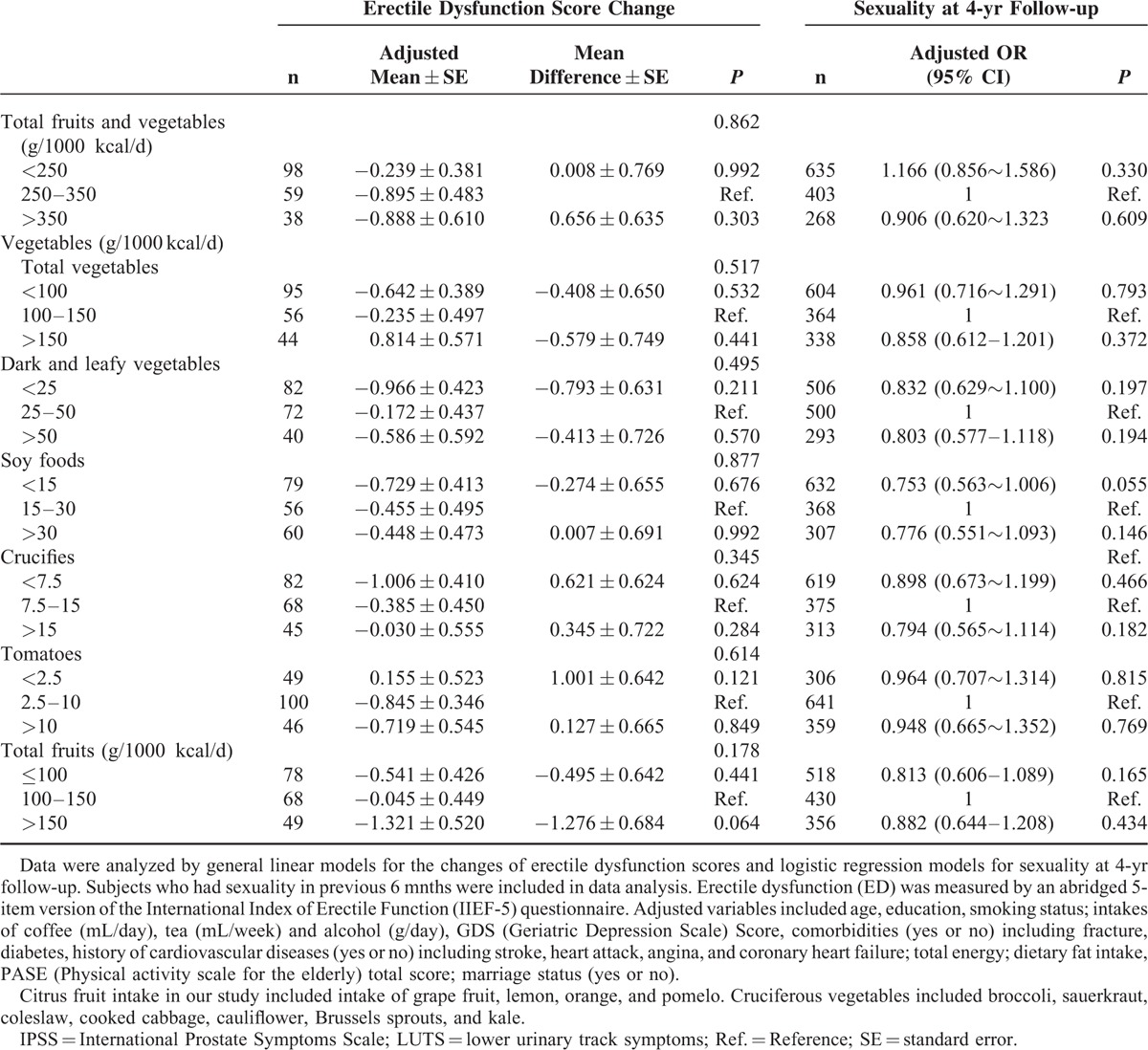
For the associations of FV intake and progression of LUTS, the multivariate logistic regression models (Table 4) indicated that compared with the moderate group, high intake of dark and leafy vegetables (>50 g/1000 kcal/day) significantly reduced the risk of LUTS progression by 37.2% [OR (95% CI): 0.628 (0.466∼0.848), P = 0.002] over 4 years. Total FV consumption was not significantly associated with the overall LUTS progression, but marginal significance was observed in storage symptoms with high FV intake (P = 0.051) after adjusting for potential covariates.
TABLE 4.
Odds Ratio (OR) and 95% Confidence Interval (CI) of Consumption of Fruits and/or Vegetables With Progression of Overall Lower Urinary Track Symptoms, Voiding, and Storage Symptoms at 4-yr Follow-up by Multivariable Logistic Regression
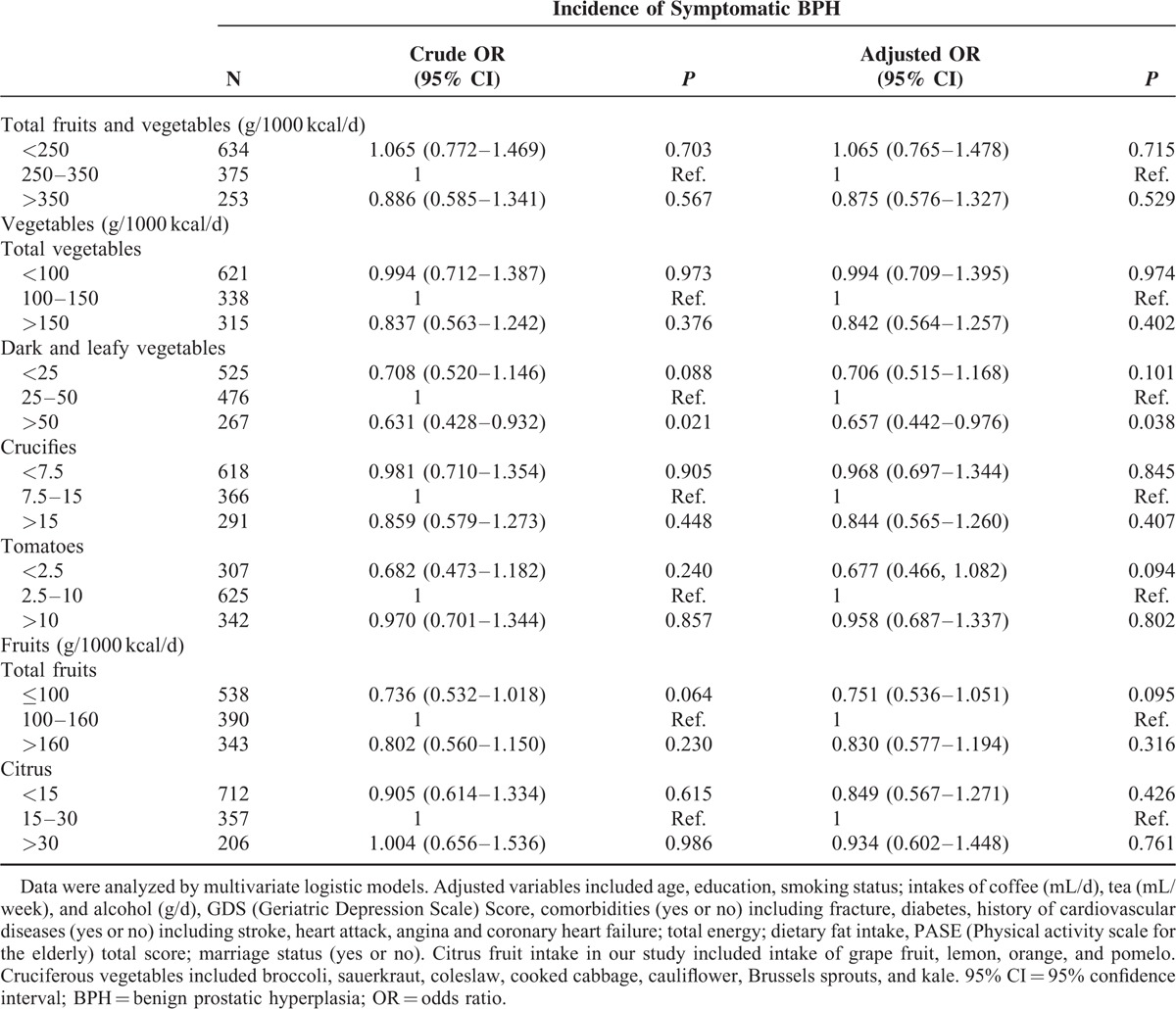
Table 5 summarizes the odds and 95% CI of incidence of symptomatic BPH at 4-year follow-up. After adjusting for potential covariates, the risk of BPH significantly decreased by 34.3% [OR (95% CI): 0.657 (0.442–0.976), P = 0.038] with high consumption of dark and leafy vegetables, respectively, compared with moderate group.
TABLE 5.
Odds Ratio (OR) and 95% Confidence Interval (CI) for Incidence of Symptomatic Benign Prostatic Hyperplasia (BPH) by Low, Moderate, and High Fruit and Vegetable Intakes Using Multivariable Logistic Models Among Chinese Elderly Men
We further examined whether the associations of FV intake and the changes of LUTS or the odds of LUTS progression could be modified by dietary vitamin C, isoflavones, or fiber intakes. No statistically significant effect modification by these micronutrients was observed (P = 0.085∼0.527) in regression models (data not shown).
DISCUSSION
Summary and Implications
In this prospective cohort study, men who consumed adequate FV (>350 g/1000 kcal/day), especially dark and leafy vegetables (>50 g/1000 kcal/day), were more likely to have reduced symptoms or progression of LUTS over 4-year follow-up. To our knowledge, this is the first longitudinal study undertaken in Chinese elderly men to temporally assess the role of FV intake on prostate health. Because LUTS/BPH represents the most common urologic disease among elderly men, even the modest improvement of symptoms (-1.76 points or -17.3% basal score reduction of LUTS) may have important relevance on a public health level.
Age is the primary risk factor for LUTS and BPH. The Olmsted county study, which followed for 12 years in 2115 men aged 40 to 79 years, observed an average increase of IPSS by 0.18 point per year.27 Another 15-year longitudinal community-based study in Japanese men reported an annual change (standard deviation) of IPSS by 0.11 (0.40).28 Our findings in Chinese elderly men with FV intake above 350 g/1000 kcal/day reported an annual score reduction of 0.44 point (1.76/4), implying that adequate FV intake may overtly counteract the aging-exacerbated LUTS with effect size of 2 to 4-folds than age-related score increase.
The Chinese Dietary Guideline 2007 recommended daily consumption of 300 to 500 g vegetables and 200 to 400 g fruits.29 Our findings in Chinese elderly indicated that quite a portion of men (39.5%) had fruit intake less than 200 g/day, and around two-thirds (62.6%) had vegetable intake less than 300 g/day. FV are important components of a healthy diet. Our data suggested a room for improvement of FV consumption in Chinese elderly.
Comparison With Other Studies
We observed that men who consumed high amounts of FV, especially dark and leafy vegetables, were more likely to be associated with reduced symptoms or LUTS progression. Previous epidemiological studies examined the relationship of FV consumption and the risk of BPH reported conflicting findings and limited studies investigated the association with the changes of LUTS. Several small case–control studies30–32 and a cross-sectional study33 found that fruit or/and vegetable consumption was inversely associated with the risk of BPH. However, findings from prospective studies were inconsistent. An early cohort study34 among 6581 Japanese-American men in Hawaii reported no association of FV consumption and the incidence of BPH. A US cohort study reported an 11% reduction (OR = 0.89, 95% CI 0.80–0.99) in BPH prevalence when comparing the lowest and highest quintiles of vegetable intake,18 whereas fruit intake was not. The Prostate Cancer Prevention Trial that included 4770 placebo-arm participants reported a significantly lower risk (hazard ratio = 0.68) of BPH over 7 years among men who consumed at least 4 servings of vegetables daily.35 The discrepancies might be attributed to the differences in ethnicity of participants, study design, definition of BPH endpoints, duration of follow-up, dietary assessment methods, or unadjusted confounding factors.
Results Explanation
Our study indicated that a high intake of dark and leafy vegetables was associated with both reduced symptoms, risk of LUTS progression, and incidence of BPH. Dark and leafy vegetables are rich in beta-carotene, lutein, or vitamin C relative to general vegetables that may be beneficial to prostate health. However, the exploratory analysis in our results suggested that antioxidants or micronutrients such as vitamin C, isoflavones, and dietary fiber were not significantly associated with the absolute change or progression of LUTS or incidence of BPH. Our findings are consistent with previously reports18,31,36 and do not support an association of dietary antioxidant intake with BPH risk. It is possible that the observed favorable associations between FV intake and LUTS were not attributable to single micronutrients itself, but to some other food components in FV or the interactions of nutrients available in FV foods. In addition, as FV intakes are considered to be part of a healthy diet that improves general health and well-being, when adjusting for these factors in multivariate models, the associations with antioxidant nutrients may become nonsignificant.
We used the score change of LUTS to evaluate the temporal association of FV on LUTS in our study; the continuous outcome measures may increase the study power to find more significant associations than those of categorized outcomes (the progression of LUTS or incidence of BPH). The variations in our results when using different outcomes measures may also reflect a different constellation of underlying biologic factors. Our results suggested a nonlinear association between FV intake with LUTS and only adequate FV consumption (>350 g/1000 kcal/day) could significantly improve LUTS relative to those of low to moderate FV consumption. Epidemiological data reported that FV consumption is inversely associated with a range of chronic diseases, especially CVD risk; however, consistent with our findings, no evidence of a dose–response association was observed between increased FV intake and reduced risk of coronary heart disease,37,38 stroke,39 or other CVD.40
In this study, we observed that men with low FV intake had a trend of reduced LUTS over 4 years compared with men with moderate FV intake, although the mean differences were not statistically significant in most of the comparisons. It is possible that men with low FV intake at baseline may increase FV intake during follow-up due to increased health consciousness or regression to mean. Men with LUTS are likely to adopt healthy lifestyle that may increase FV intake or physical activity in order to reduce their symptoms. Although the prospective design of the study provides some confidence in the association being causal, we cannot dismiss the possibility that it may represent a partial reverse effect.
Studies assessing the role of diet on ED are limited. FV consumption may have a protective effect against ED.41 In this study, we did not observe a significant association of FV intakes with ED and sexuality. This could be due to the large proportion of men who were not sexually active in this cohort within the previous 6 months and the small sample size may have limited statistical power to conclude a significant association. In addition, ED is often associated with chronic conditions such as hypertension, dyslipidemia, and diabetes42; thus, the adjustment for these comorbidities may attenuate the association of FV and ED.
MECHANISMS
It was hypothesized that oxidative damage might contribute to the disorder of BPH.43 De De Nunzio et al44 suggested that the prostate may be particularly vulnerable to oxidative stress, especially in the setting of chronic intraprostatic inflammation. FV contain high levels of antioxidants such as beta-carotene, anthocyanins, flavonoids, lutein, lycopene, selenium, vitamin C, A, and E, etc that may play important roles in altering inflammatory pathways and influencing cell growth and differentiation associated with the pathogenesis of BPH. In addition, dietary factors can affect both steroid hormone concentrations and the sympathetic nervous system.45,46 It is possible that the physiologic effects of FV moderate both the hormonally regulated prostate growth and heightened smooth muscle tone that cause BPH.
STRENGTHS
The strengths of our study include its large, prospective design, use of validated instruments for measuring dietary intake and urinary symptoms, and availability of data on many potential confounding variables. Furthermore, the survey assessed symptoms rather than diagnosed conditions, thereby capturing the broader spectrum of the population who may suffer and diagnostic bias was avoided.
LIMITATIONS
The study has several limitations. First, as in all observational studies of diet and disease, reversal confounding effects could exist despite prospective design. Second, FV intake and other dietary factors were self-reported and not assessed by objective biomarkers. Potential biases due to the misclassification of dietary assessment, exposure categories, and the reference category cannot be excluded. However, any bias from the measurement errors would probably tend to attenuate the associations.
Third, in our study, assessment of diagnosed BPH was not conducted. The variability in the case definition of BPH (histological, radiological, symptomatic, or surgical BPH) renders it a problematic endpoint to compare across different study populations. The diagnostic instruments for BPH would be more time-consuming and invasive and impractical in a large cohort like ours. In addition, the measurement errors or misclassification on BPH can only reduce the chances of detecting significant associations.
Fourth, LUTS is a common problem in most elderly men, but remission in symptoms may occur in some individuals.47 The operational definition of progression did not distinguish between men who had symptom remission from men who had stable IPSS scores. However, in sensitivity analysis, we excluded 302 men with remission (IPSS change ≤ -3); the significant findings on high FV intake and LUTS change remained (data not shown). Also, the measurement of IPSS change over time as a primary outcome could reduce the impact of these fluctuations in our study.
In addition, the study sample consisted of all volunteers who were of a higher educational level and more likely to be married than the general Hong Kong population in the same sex and age groups.48 Thus, the results may not be entirely generalizable. However, the selection bias would not affect the estimates of exposure–outcome associations.49 Finally, the baseline data were collected more than 10 years ago (2001∼2003); thus, new evidence is needed to confirm our findings.
CONCLUSION
This 4-year cohort study among Chinese elderly men indicated that adequate FV intake, especially dark and leafy vegetables, was associated with improved LUTS among Chinese elderly men, although there was a lack of significant association with ED and sexuality. Clinical trials examining this directly and replication in other ethnic groups are warranted.
Supplementary Material
Acknowledgment
We wish to thank all participants for their participation and Dr Edith Lau for her contribution in setting up the cohort.
Footnotes
Abbreviations: BPH = Benign prostatic hyperplasia, ED = Erectile dysfunction, FFQ = Food frequency questionnaire, FV = Fruit and vegetable, GLM = General Linear Model, IIEF-5 = International Index of Erectile Function, IPSS = International Prostatic Symptoms Scale, LUTS = Lower urinary tract symptoms, OR = Odds ratio, PASE = Physical Activity Scale of the Elderly.
The work was supported by grants from the Research Grants Council of Hong Kong (CUHK 4101/02M); the Hong Kong Jockey Club Charities Trust; the S.H. Ho Centre for Gerontology and Geriatric; and the Centre for Nutritional Studies, The Chinese University of Hong Kong. The funders play no role in study design, data collection and analysis, results interpretation, and report preparation.
ZML, SYW, and CW conceived the study. ZML analyzed the data and drafted the manuscript. DC, BY, and LAT helped in results explanation and statistical consultation. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
All the authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Cornu JN, Lukacs B. Lower urinary tract symptoms in men: refocus on your patients. Eur Urol 2015; 67:1110–1111. [DOI] [PubMed] [Google Scholar]
- 2.Sarma AV, Wei JT. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med 2012; 367:248–257. [DOI] [PubMed] [Google Scholar]
- 3.Warren K, Burden H, Abrams P. Lower urinary tract symptom: still too much focus on the prostate? Curr Opin Urol 2014; 24:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Kopp ZS, Agatep B, et al. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011; 108:1132–1138. [DOI] [PubMed] [Google Scholar]
- 5.Braun MH, Sommer F, Haupt G, et al. Lower urinary tract symptoms and erectile dysfunction: co-morbidity or typical “Aging Male” symptoms? Results of the “Cologne Male Survey”. Eur Urol 2003; 44:588–594. [DOI] [PubMed] [Google Scholar]
- 6.Ilic D. Lycopene for the prevention and treatment of prostate disease. Recent Results Cancer Res 2014; 202:109–114. [DOI] [PubMed] [Google Scholar]
- 7.Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol 2011; 21:1–4. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Imp Res 2008; 20 Suppl 3:S11–S18. [DOI] [PubMed] [Google Scholar]
- 9.Lambert GW, Straznicky NE, Lambert EA, et al. Sympathetic nervous activation in obesity and the metabolic syndrome - causes, consequences and therapeutic implications. Pharmacol Ther 2010; 126:159–172. [DOI] [PubMed] [Google Scholar]
- 10.Wong SY, Leung JC, Woo J. A prospective study on the association between lower urinary tract symptoms (LUTS) and erectile dysfunction: results from a large study in elderly Chinese in Southern China. J Sex Med 2009; 6:2024–2031. [DOI] [PubMed] [Google Scholar]
- 11.Woo J, Leung SSF, Ho SC, et al. A food frequency questionnaire for use in the Chinese population in Hong Kong: description and examination of validity. Nutr Res 1997; 17:1633–1641. [Google Scholar]
- 12.Paul AASD. McCance & Widdowson's: the composition of foods. London: HMSO; 1978. [Google Scholar]
- 13.Yang YWG, Pan X. China Food Composition 2002. Peking: University Medical; 2002. [Google Scholar]
- 14.Shiri R, Hakkinen J, Koskimaki J, et al. Erectile dysfunction influences the subsequent incidence of lower urinary tract symptoms and bother. Int J Imp Res 2007; 19:317–320. [DOI] [PubMed] [Google Scholar]
- 15.Choi EP, Lam CL, Chin WY. Validation of the International Prostate Symptom Score in Chinese males and females with lower urinary tract symptoms. Health Qual Life Outcomes 2014; 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1557.discussion 1564. [DOI] [PubMed] [Google Scholar]
- 17.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774. [DOI] [PubMed] [Google Scholar]
- 18.Rohrmann S, Giovannucci E, Willett WC, et al. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 2007; 85:523–529. [DOI] [PubMed] [Google Scholar]
- 19.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49:822–830. [DOI] [PubMed] [Google Scholar]
- 20.Lau EM, Chan HH, Woo J, et al. Normal ranges for vertebral height ratios and prevalence of vertebral fracture in Hong Kong Chinese: a comparison with American Caucasians. J Bone Mineral Res 1996; 11:1364–1368. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993; 46:153–162. [DOI] [PubMed] [Google Scholar]
- 22.Nyunt MS, Fones C, Niti M, et al. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Mental Health 2009; 13:376–382. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65 4 Suppl:1220S–1228S.discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 24.Agudo A. Measuring Intake of Fruit and Vegetables. Background paper for the joint FAO/WHO workshop on fruit and vegetables for health. 2005. Available at: http://www.who.int/dietphysicalactivity/publications/f&v_intake_measurement.pdf; accessed November 7, 2015. [Google Scholar]
- 25.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000; 102:2284–2299. [DOI] [PubMed] [Google Scholar]
- 26.Society CN. Chinese Dietary Guideline 2007. 2007. Available at: http://www.cnsoc.org/content/details_208_3722.html; accessed November 11, 2015. [Google Scholar]
- 27.Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int 2008; 101 Suppl 3:17–21. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int 2006; 97 Suppl 2:3–6.discussion 21-22. [DOI] [PubMed] [Google Scholar]
- 29.Chinese Nutrition Society. Chinese Dietary Guideline 2007. Beijing, China; 2007. [Google Scholar]
- 30.Araki H, Watanabe H, Mishina T, et al. High-risk group for benign prostatic hypertrophy. Prostate 1983; 4:253–264. [DOI] [PubMed] [Google Scholar]
- 31.Lagiou P, Wuu J, Trichopoulou A, et al. Diet and benign prostatic hyperplasia: a study in Greece. Urology 1999; 54:284–290. [DOI] [PubMed] [Google Scholar]
- 32.Bravi F, Bosetti C, Dal Maso L, et al. Food groups and risk of benign prostatic hyperplasia. Urology 2006; 67:73–79. [DOI] [PubMed] [Google Scholar]
- 33.Koskimaki J, Hakama M, Huhtala H, et al. Association of dietary elements and lower urinary tract symptoms. Scand J Urol Nephrol 2000; 34:46–50. [DOI] [PubMed] [Google Scholar]
- 34.Chyou PH, Nomura AM, Stemmermann GN, et al. A prospective study of alcohol, diet, and other lifestyle factors in relation to obstructive uropathy. Prostate 1993; 22:253–264. [DOI] [PubMed] [Google Scholar]
- 35.Poon KS, McVary KT. Dietary patterns, supplement use, and the risk of benign prostatic hyperplasia. Curr Urol Rep 2009; 10:279–286. [DOI] [PubMed] [Google Scholar]
- 36.Kristal AR, Arnold KB, Schenk JM, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 2008; 167:925–934. [DOI] [PubMed] [Google Scholar]
- 37.He FJ, Nowson CA, Lucas M, et al. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens 2007; 21:717–728. [DOI] [PubMed] [Google Scholar]
- 38.Dauchet L, Amouyel P, Hercberg S, et al. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 2006; 136:2588–2593. [DOI] [PubMed] [Google Scholar]
- 39.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet (London, England) 2006; 367:320–326. [DOI] [PubMed] [Google Scholar]
- 40.McEvoy CT, Wallace IR, Hamill LL, et al. Increasing fruit and vegetable intake has no dose-response effect on conventional cardiovascular risk factors in overweight adults at high risk of developing cardiovascular disease. J Nutr 2015; 145:1464–1471. [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Dai S, Wang M, et al. Erectile dysfunction and fruit/vegetable consumption among diabetic Canadian men. Urology 2013; 82:1330–1335. [DOI] [PubMed] [Google Scholar]
- 42.Hodges LD, Kirby M, Solanki J, et al. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract 2007; 61:2019–2025. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki S, Platz EA, Kawachi I, et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr 2002; 75:689–697. [DOI] [PubMed] [Google Scholar]
- 44.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 2011; 60:106–117. [DOI] [PubMed] [Google Scholar]
- 45.Dorgan JF, Judd JT, Longcope C, et al. Effects of dietary fat and fiber on plasma and urine androgens and estrogens in men: a controlled feeding study. Am J Clin Nutr 1996; 64:850–855. [DOI] [PubMed] [Google Scholar]
- 46.Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol 2006; 26:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poyhonen A, Hakkinen JT, Koskimaki J, et al. Natural course of lower urinary tract symptoms in men not requiring treatment: a 5-year longitudinal population-based study. Urology 2014; 83:411–415. [DOI] [PubMed] [Google Scholar]
- 48.Wong SY, Woo J, Leung JC, et al. Depressive symptoms and lifestyle factors as risk factors of lower urinary tract symptoms in Southern Chinese men: a prospective study. Aging Male 2010; 13:113–119. [DOI] [PubMed] [Google Scholar]
- 49.Mealing NM, Banks E, Jorm LR, et al. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol 2010; 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


