Abstract
Activation of the mitogen-activated protein kinase (MAP kinase) pathways in cultured porcine aortic vascular smooth muscle cells (VSMCs) was determined following a 5-min stimulation with endothelin-1 (ET-1), phorbol 12-myristate 13-acetate (PMA), H2O2, or sodium arsenite. Extracellular signal-related kinase (ERK1/2), p38, and c-Jun N-terminal kinase (JNK1/2) MAP kinase activation was assessed using anti-phospho-MAPK kinase antibodies. The activation of these kinase cascades was also determined by resolving lysates on Mono Q using a fast protein liquid chromatography (FPLC) system and measuring the phosphorylation of specific substrates ERK1, c-Jun, and hsp27. The substrates were subsequently resolved from each other and the [γ-32P]ATP in the reaction mixture by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the incorporation of 32P was quantified by phosphor imaging. This technique revealed the presence of multiple peaks of activity phosphorylating ERK1 (5), c-Jun (7), and hsp27 (9). Differences in activation revealed by the chromatographic technique suggest that, although equivalent levels of activation may be detected by immunoblotting, the actual nature of the response differed depending upon the stimulus. Each stimulus that activated the MAP kinase cascades did not result in equivalent ‘profile’ of activation of kinase activities. These results suggest the presence of a mechanism of structural organization of the MAP kinase signaling molecules themselves resulting in the compartmentalization of responses with respect to the various cellular stimuli.
Keywords: MAP kinases, Vascular smooth muscle, Mitogens, Oxidative stress
1. Introduction
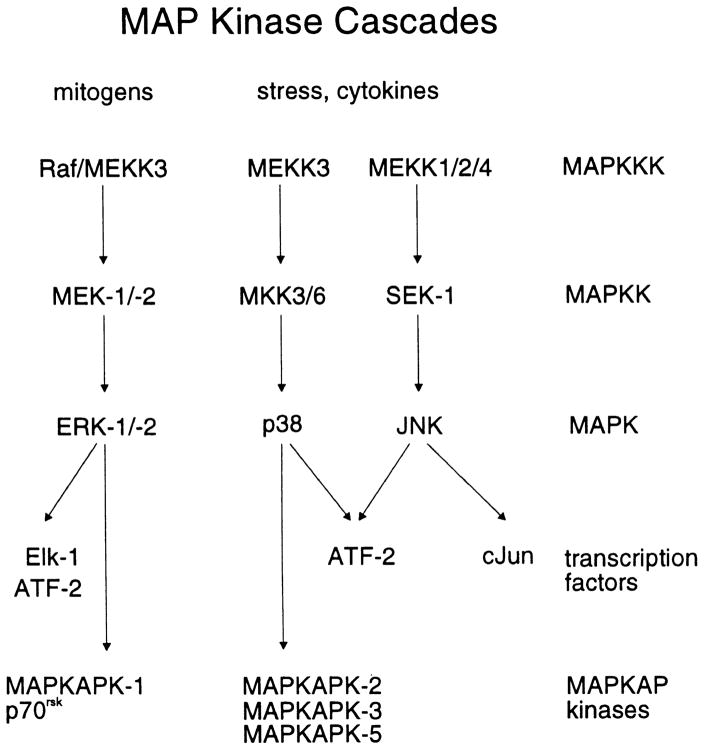
The three parallel well-characterized subfamilies of the mitogen-activated protein kinase (MAP kinase) family are the extracellular-signal regulated kinase (ERKs), the c-Jun N-terminal kinases (JNKs) that are also known as stress-activated protein kinases (SAPKs), and the p38 MAP kinases (Fig. 1). MAP kinases are proline-directed, serine/threonine protein kinases that play a central role in the intracellular signal transduction pathways in response to a variety of cellular stimuli. A MAP kinase cascade consists of three sequentially acting kinases, and this organization may function as a switch to provide a threshold-like input–output response to receptor activation (Ferrell, 1996; Huang & Ferrell, 1996). The last member of the cascade, MAPK, is activated by dual phosphorylation at tyrosine and threonine residues in a T–X–Y motif, where X represents the amino acids glutamic acid, glycine, and proline for ERK, p38, and JNK, respectively. In each case, the activating kinase is the second kinase in the cascade, MAPKK. MAPKK is, in turn, activated by phosphorylation at serine and threonine residues by the first member of the cascade, MAPKKK. These cascades of kinase reactions occur in the cytosol with the activated MAPK phosphorylating targets in both the cytosol and nucleus.
Fig. 1.
Scheme of the MAP kinase cascades.
Both ERK (Adams & Hathaway, 1993; Childs & Mak, 1993; Childs et al., 1992; Gerthoffer et al., 1996) and p38 (Hedges et al., 1998; Yamboliev, Hedges, Mutnick, Adam, & Gerthoffer, 2000) MAP kinases phosphorylate caldesmon, an actin-binding protein that inhibits actin-activated myosin ATPase activity in a phosphorylation-dependent manner (Ngai & Walsh, 1987). In addition, hsp27, a substrate for MAP kinase-activated protein kinase-2 (MAPKAP kinase-2) (Stokoe, Engel, Campbell, Cohen, & Gaestel, 1992), is an actin-capping protein that has been implicated in regulating smooth muscle contraction via interactions with the actin filaments (Bitar, Kaminski, Hailat, Cease, & Strahler, 1991). In both airway and colonic smooth muscle, muscarinic agonists induce activation of p38 MAP kinase and phosphorylation of hsp27 (Larsen, Yamboliev, Weber, & Gerthoffer, 1997). These effects are blocked by the specific inhibitor of p38 MAP kinase, SB203580 (Larsen et al., 1997). Inhibition of p38 (Yamboliev, Hedges, et al., 2000), but not ERK (Gorenne, Su, & Moreland, 1998; Yamboliev, Hedges, et al., 2000), reduces smooth muscle contraction, whereas both ERK and p38 activity are required for the regulation of chemotactic migration in colonic myocytes (Yamboliev, Wiesmann, Singer, Hedges, & Gerthoffer, 2000). Hence, the p38 and ERK MAP kinase cascades may play roles in regulating smooth muscle function.
We report here a means whereby the activation of the ERK, p38, and JNK MAP kinase cascades can be determined simultaneously. In addition, we demonstrate that different patterns of kinase activation, which were not apparent when the activation status of the MAP kinases were determined by their phosphorylation, may be observed using this methodology. These data suggest that, even within a given MAP kinase cascade, there exists a level of organization such that specific modules become activated in response to different agonists or forms of cellular stress.
2. Materials and methods
2.1. Materials
[γ-32P]ATP was from Amersham Pharmacia Biotech (Baie d’Urfé, Québec). Membrane grade (reduced) Triton X-100, leupeptin, and phenylmethylsulfonyl fluoride (PMSF) were from Roche Molecular Biochemicals (Laval, Québec). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) reagents, nitrocellulose, and Bradford protein assay reagents were from Bio-Rad Laboratories (Canada) (Mississauga, Ontario). Microcystin LR and phorbol 12-myristate 13-acetate (PMA) were from Calbiochem-Novabiochem (San Diego, CA). Endothelin-1 (ET-1) was from American Peptide (Sunnyvale, CA). Cyclic AMP-dependent protein kinase inhibitory peptide (PKI, amino acid sequence TTYADFIASGRTGRRNAIHD) was from the University of Calgary Peptide Synthesis Core Facility (Calgary, Alberta). Canine hsp27, cloned into the pET24a expression vector (Larsen, Gerthoffer, Hickey, & Weber, 1995), was from Dr. William Gerthoffer, Reno, NV. Glutathione S-transferase (GST)-ERK1 (K71A), GST-c-Jun (1–169), and antibodies raised against a synthetic peptide, CGGPFTFDMELDDLPKERLKELIFQETARFQPGAPEAP, which corresponds to residues 333–367 of rat ERK1 MAP kinase and recognizes both ERK1 and ERK2, were from Dr. Steven Pelech, Vancouver, BC. Anti-phospho-ERK1/2, p38, and JNK1/2 were from New England BioLabs (Mississauga, Ontario). Antibodies against p38 MAP kinase and JNK1 were from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were of analytical grade or best grade available.
2.2. Culture of vascular smooth muscle cells (VSMCs)
Cultured porcine aortic smooth muscle cells (passage 5) were seeded onto 100-mm Petri dishes and grown to 80% confluence in medium (DMEM, supplemented with 2 mM glutamine, amphotericin B and gentamycin) containing 10% calf serum and 10% fetal bovine serum as described previously (Hadrava, Tremblay, & Hamet, 1989). Cells were washed with phosphate-buffered saline (PBS) and placed in serum-free medium for 16 h. The medium was replaced with fresh serum-free medium 90 min before the cells were stimulated.
2.3. Stimulation of VSMCs and preparation of lysates
Cells were stimulated for 5 min at 37°C with 10 −7 M ET-1, 10 −7 M PMA, 2 mM H2O2, or 0.2 mM sodium arsenite. Agonists were prepared as 1000-fold stock solutions in dimethyl sulfoxide (DMSO) (PMA, ET-1) or dH20 and added directly to the medium while mixing. To terminate the incubation, cells were placed on ice and washed twice with ice-cold TBS (20 mM Tris, 150 mM NaCl, pH 7.5). Cells were drained and lysed in the presence of 1.0 ml of ice-cold lysis buffer, which comprised 50 mM Tris-HCl (pH 7.5 at 5°C), 20 mM β-glycerophosphate, 20 mM NaF, 5 mM EDTA, 10 mM EGTA, 1 mM Na3VO4, 10 mM benzamidine, 0.5 mM PMSF, 10 μg/ml leupeptin, 5 mM dithiothreitol (DTT), 1 μM microcystin LR, and 1% (v/v) Triton X-100 (reduced). Cells were scraped into lysis buffer, transferred to 1.5-ml microcentrifuge tubes, extracted by mixing for 15 min at 5°C using a clinical rotator, centrifuged at 13,000 ×g and 5°C for 15 min, and the soluble fraction retained. Protein concentrations were determined by the method of Bradford (1976) using bovine γ-globulin as standard.
2.4. Determination of phospho-MAP kinases
Phospho-ERK1/2, phospho-p38, and phospho-JNK1/2 were determined in lysates using anti-phosphoprotein antibodies following the procedure described by the manufacturer. Briefly, 100 μg of each lysate was resolved on 10% acrylamide mini-gels. Following SDS-PAGE, samples were transferred at 100 V and 5°C for 90 min onto 0.2-μm reinforced nitrocellulose membranes in 25 mM Tris base, 192 mM glycine, and 5% methanol. Membranes were blocked for 2 h in a solution of 5% (w/v) skimmed milk powder (Carnation) in 25 mM Tris (pH 7.5 at 20°C), 150 mM NaCl (TBS), and 0.05% (v/v) Tween-20 (TBST). Membranes were incubated with primary antibodies, diluted 1:1000 with 1% BSA plus 0.4% (w/v) sodium azide in TBST, for 16 h at 5°C. After washing with TBST, membranes were reblocked for 10 min with TBST containing 5% skimmed milk powder and then incubated in the presence of horseradish peroxidase-labeled anti-rabbit immunoglobulin, diluted 1:20,000 in blocking buffer, for 2 h at room temperature. Immune complexes were detected by the ECL Western blotting detection method (Renaissance Plus, NEN Life Sciences, Boston, MA) according to the manufacturer’s instructions and visualized using Kodak BioMax ML film. In order to reprobe the nitrocellulose membranes, they were stripped by incubating twice for 15 min at room temperature in 0.2 M NaOH with constant mixing, followed by a brief rinse with TBST (Suck & Krupinska, 1996; Wang et al., 1999). Membranes were subsequently blocked and incubated with primary antibodies as described above. Control experiments determined that this stripping method effectively removed the previous primary and secondary antibodies.
2.5. Fast protein liquid chromatography of myocyte lysates
In preparation for separation by fast protein liquid chromatography (FPLC), lysates were diluted to a protein concentration of 1.5 mg/ml with lysis buffer, recentrifuged (13,000 × g, 10 min, 5°C), and injected into a 1.0-ml sample loop. The chromatography system was maintained in a chromatography cabinet at 5°C. Separation was achieved using a Mono Q HR5/5 column equilibrated with 50 mM Tris–HCl (pH 7.4 at 5°C), 20 mM β-glycerophosphate, 2 mM EDTA, 2 mM EGTA, 5% (v/v) glycerol, 0.03% (v/v) Brij 35, 1 mM benzamidine, 1 μg/ml leupeptin, 1 mM Na3VO4, and 0.1% (v/v) β-mercaptoethanol. Following a 5-ml isocratic wash, proteins were eluted using a NaCl gradient (24 ml, 0–0.40 M NaCl; 0.1 ml, 0.40–1.0 M NaCl; 0.9 ml, 1.0 M NaCl) at a flow rate of 0.3 ml/min. Sixty fractions of 0.5 ml were collected.
2.6. Assay of MAP kinase activities
MAP/ERK kinase (MEK), JNK, and MAPKAP kinase-2 activities were assayed using, respectively, GST-ERK1 (K72A), GST-c-Jun (1–169), and recombinant canine hsp27 (Stokoe et al., 1992) as substrates. The assay was for 60 min at 30°C in a final volume of 30 μl in the presence of 50 mM Tris–HCl (pH 7.5 at 30°C), 13 mM β-glycerophosphate, 1 μg hsp27, 1 μg GST-ERK1 (K72A), 1 μg GST-c-Jun (1–169), 10 mM MgCl2, 1.3 mM EDTA, 2 mM EGTA, 1.0 mM Na3VO4, 10 μM [γ-32P]ATP (3.3 Ci/mmol), 1 μM PKI, 10 μg/ml leupeptin, and 10 mM DTT. Reactions were initiated by the addition of 10 μl of 3 × assay media and terminated by the addition of 10 μl of 4 × Laemmli sample buffer for a final volume of 40 μl. Samples were heated to 70°C for 90 s and then 35 μl applied to SDS-PAGE gels. The phosphorylated substrates were resolved from one another and separated from [γ-32P]ATP by electrophoresis (see below).
2.7. Electrophoresis and immunoblotting
Protein samples were subjected to SDS-PAGE, using a discontinuous buffer system (Laemmli, 1970), in 10–20% acrylamide-gradient slab gels (1.5 mm thick) with a 5% acrylamide stacking gel. Gels were run for 3–4 h at 35 mA and 15°C until the dye front was 1 cm from the bottom of the gel, keeping the dye front containing the unincorporated [γ-32P]ATP on the gel. The dye front was cut off each gel and discarded as solid radioactive waste. This avoided the generation of 9 l of liquid radioactive waste with each experiment. Gels were stained in 45% (v/v) denatured ethanol, 10% (v/v) acetic acid containing 0.1% (w/v) Coomassie brilliant blue R-250 and diffusion destained in 20% (v/v) denatured ethanol containing 5% (v/v) acetic acid. Destained gels were dried between two sheets of cellophane (BioDesign) and exposed to Kodak BioMax MR film for 24 h at −80°C in cassettes fitted with Kodak TranScreen-HE intensifying screens. Following autoradiography, gels were exposed to Molecular Imaging screens for 48 h, and 32P incorporation was digitized and quantified by phosphor imaging (Bio-Rad GS 525 Molecular Analyzer).
2.8. Data quantification
Samples often contain endogenous proteins that become phosphorylated during the incubation with [γ-32P]ATP. To insure that the phosphorylation of the exogenously added kinase substrates was being quantified, the Coomassie-stained gels were overlaid with the autoradiograms for each experiment and used to identify the bands on the autoradiogram that corresponded to the exogenously added kinase subsrates (GST-ERK, GST-c-Jun, hsp27). This verification step is important as the phosphor imager does not generate a 1:1 image on the computer screen and thus aligning the phorphorylated proteins with the Coomassie-stained gel is difficult. Each lysate was eluted in 60 fractions, including the flow-through, and 57 fractions were assayed and separated on three 16 × 16 cm gels using Bio-Rad Protein II xi electrophoresis cells. All three gels were simultaneously exposed to the same phosphor imagining screen. The maximum pixel depth for the imaging screens is 64,000: the values obtained did not exceed 10,000. Quantification was performed using the software package Molecular Analyst (version 2.1.2) from Bio-Rad. Fraction 2, which preceded the elution of the flow-through fraction from the Mono Q column, was taken as background. The value, in pixel density × mm2 (PD × mm2) for fraction 2 was subtracted from the other 56 samples. The area integrated for each substrate was chosen based upon the area required to encompass the band corresponding to the peak phosphorylation for that substrate and kept constant for each of the five lysates. The data from the phosphor imager, in PD × mm2, was tabulated and normalized to the value corresponding to the highest phosphorylation for the substrate detected in the entire experiment, and expressed as ‘relative kinase activity.’ Data normalization, analysis, and plotting were performed using Prism 2.0 from Graph-Pad Software.
3. Results
We have examined the activation of the ERK, p38, and JNK MAP kinase cascades in cultured porcine aortic smooth muscle cells in response to mitogens and cellular stress. ET-1 and tumor-promoting phorbol esters are mitogens and activate the ERK pathway, whereas H2O2 and arsenite are activators of the stress-activated p38 and JNK MAP kinase pathways (Fig. 1). Kinase activities were determined both by using antisera for the dual-phosphorylated forms of ERK1/2, p38, and JNK1/2, and by chromatography of lysates from stimulated and control cells on Mono Q and assaying the fractions with GST-ERK (the substrate for MEK1/2), GST-c-Jun (the substrate for JNK1/2), and hsp27 (a substrate for MAPKAP kinases).
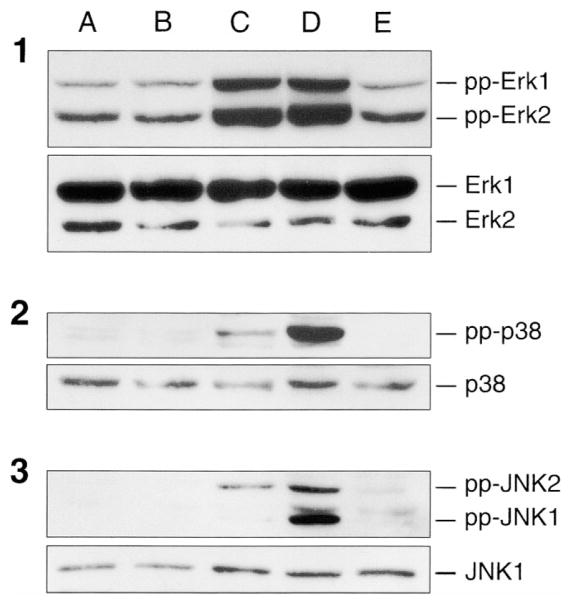
To stimulate the MAP kinase cascades, VSMCs were treated for 5 min with ET-1 (0.1 μM), PMA (0.1 μM), H2O2 (2.0 mM), or sodium arsenite (0.2 mM). The activation of ERK, p38 and JNK MAP kinases were determined by immunoblotting using antisera specific for the dual-phosphorylated, and therefore activated, forms of these enzymes. A basal level of ERK1/2 activity was detected and this activity was unaltered after a 5-min treatment with either ET-1 or arsenite (Fig. 2, panel 1). Both PMA and H2O2 induced strong increases in phosphorylation of both ERK1 (p44) and ERK2 (p42). Phospho-p38 specific antisera (Fig. 2, panel 2) revealed a weak activation of p38 MAP kinase after 5-min treatment with PMA, whereas H2O2 produced a strong activation. ET-1 and arsenite were ineffective in activating p38 MAP kinase. In addition, the data suggested the presence of more than one band of phospho-p38. Similarly, PMA induced an activation of JNK2 (p54) (Fig. 2, panel 3), whereas H2O2 activated both JNK1 (p46) and JNK2 (p54).
Fig. 2.
Activation of MAP kinases upon stimulation of porcine aortic smooth muscle cells. Serum-starved porcine aortic smooth muscle cells were (A) untreated, or (B) exposed to 0.1 μM ET-1, (C) 0.1 μM PMA, (D) 2.0 mM H2O2, or (E) 0.2 mM sodium arsenite for 5 min at 37°C. For control cells, no addition was made; however, cells were removed from the incubator and subjected to gentle mixing as with the other conditions. After stimulation, cells were placed on ice, washed twice with ice-cold TBS, and lysed, as described in Materials and Methods. MAP kinase activation was assessed using antibodies that specifically recognize the dual phosphorylation state of ERK1/2 (panel 1, upper), p38 MAP kinase (panel 2, upper), and JNK1/2 (panel 3, upper). Equal protein loading was ascertained by stripping and reprobing with anti-ERK1/2 (panel 1, lower), p38 (panel 2, lower), or JNK1 (panel 3, lower) antibodies.
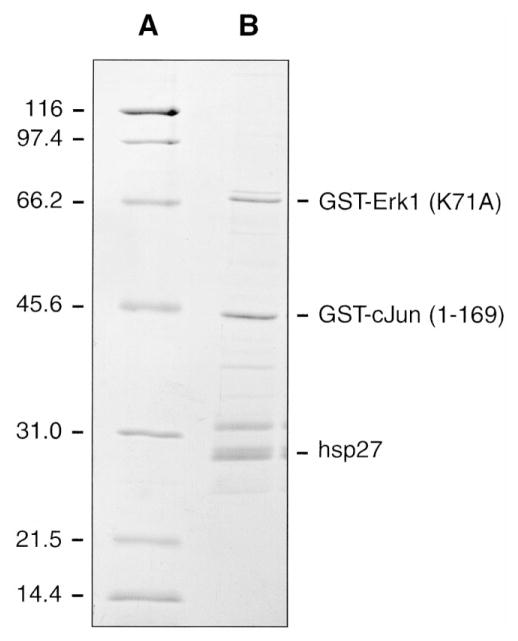
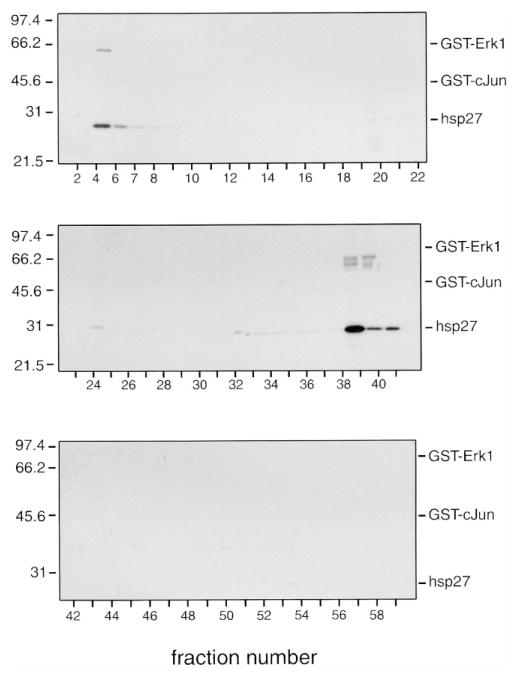
In order to study further the activation of each of the pathways, lysates (1.5 mg) from treated and control cells were chromatographed on Mono Q, and the fractions assayed with GST-ERK (K72A), GST-c-Jun (1–169), and hsp27. The mutated GST-ERK (K72A) form of ERK1 was employed, as it remains catalytically inactive when phosphorylated by MEK. These kinase substrates were employed as they allow assessment of the activation of each MAP kinase pathway. In addition, these proteins differ sufficiently in apparent molecular mass on SDS-PAGE (GST-ERK1, 67 kDa; GST-c-Jun, 45 kDa; hsp27, 27 kDa) that they are completely resolved, both from each other (Fig. 3) and from the dye front containing the unincorporated [γ-32P]ATP, by electrophoresis on 10–20% acrylamide-gradient SDS-PAGE. No signal is lost by moving up the ERK cascade and measuring MEK-catalyzed phosphorylation of ERK-1 instead of directly assaying ERK activity, as MEK and ERK proteins are present at roughly equal concentrations and in some cells MEK proteins are in excess (Ferrell, 1996). Control experiments in the absence of added substrates have indicated that there are no endogenous proteins being phosphorylated that co-migrate on the gels with the exogenously added substrates (not shown). Clear differences were noted in both the phosphorylation of endogenous proteins and the phosphorylation of the added substrates in Mono Q fractions from control (Fig. 4) vs. PMA-stimulated (Fig. 5) VSMCs. Phosphorylation data as shown in Figs. 4 and 5 have been digitized by phosphor imaging and presented in graphical form and summarized in tables (Fig. 6).
Fig. 3.
SDS-PAGE separation of GST-ERK1 (K71A), GST-c-Jun (1–169), and hsp27. (A) Broad-range SDS-PAGE standards and GST-ERK1 (K71A), GST-c-Jun (1–169) and (B) hsp27 (1 μg) were resolved by electrophoresis on 10–20% acrylamide-gradient SDS-PAGE at 35 mA for 4 h, and then visualized by staining with Coomassie brilliant blue as described in Materials and Methods. Numbers on the left indicate the molecular mass of the standard proteins (in kilodaltons).
Fig. 4.
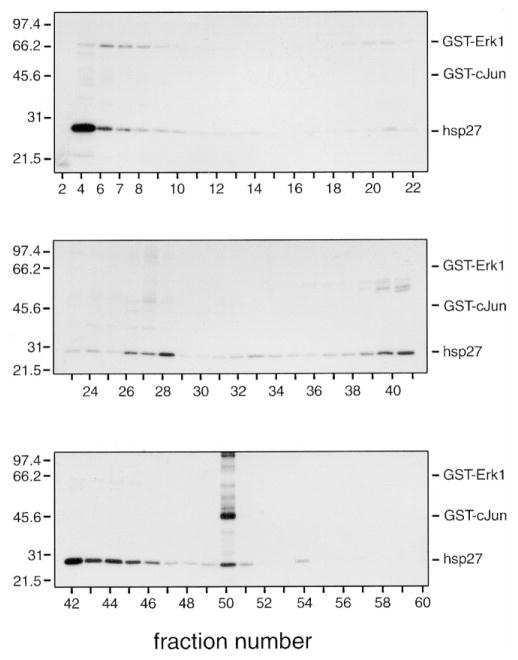
MonoQ chromatography of lysates from unstimulated VSMCs. Lysates from unstimulated porcine aortic smooth muscle cells (1.5 mg) were chromatographed on a Mono Q HR 5/5 column using an FPLC system, as described in Materials and Methods, and 60 fractions of 0.5 ml were collected. A 20-μl aliquot of the indicated fractions was assayed in the presence of GST-ERK1, GST-c-Jun, and hsp27. Reactions were terminated with 4 × SDS-PAGE sample buffer, and then the samples were subjected to electrophoresis on 10–20% acrylamide-gradient SDS-PAGE gels at 35 mA/gel and 15°C for 4 h. Gels were subsequently stained with Coomassie brilliant blue R250, dried between two sheets of cellophane, and subjected to autoradiography for 24 h at −80°C using Kodak BioMax MR film plus Kodak TranScreen-HE intensifying screens. Numbers on the left indicate the positions of the molecular mass marker proteins (in kilodaltons); numbers below indicate the column fractions. The relative positions of GST-ERK1, GST-c-Jun, and hsp27 are indicated on the right.
Fig. 5.
Mono Q chromatography of lysates from PMA-stimulated VSMCs. Lysates from PMA-stimulated (0.1 μM for 5 min at 37°C) porcine aortic smooth muscle cells (1.5 mg) were chromatographed on Mono Q, as described in Materials and Methods, and 60 fractions of 0.5 ml were collected. A 20-μl aliquot of the indicated fractions was assayed in the presence of GST-ERK1, GST-c-Jun, and hsp27. Reactions were terminated with 4 × SDS-PAGE sample buffer, and then the samples were subjected to electrophoresis on 10–20% acrylamide-gradient SDS-PAGE gels at 35 mA/gel and 15°C for 4 h. Gels were subsequently stained with Coomassie brilliant blue R250, dried between two sheets of cellophane, and subjected to autoradiography for 24 h at −80°C using Kodak BioMax MR film plus Kodak TranScreen-HE intensifying screens. Numbers on the left indicate the positions of the molecular mass marker proteins (in kilodaltons); numbers below indicate the column fractions. The relative positions of GST-ERK1, GST-c-Jun, and hsp27 are indicated on the right.
Fig. 6.
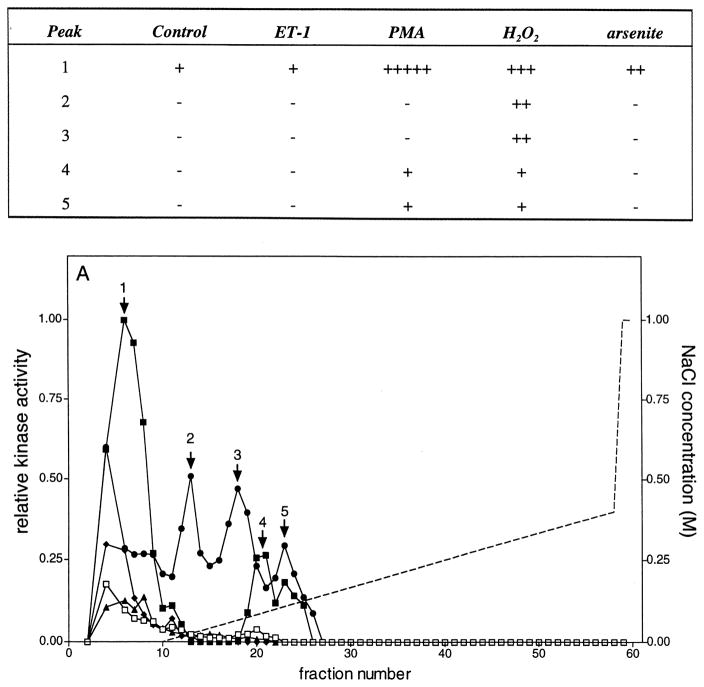
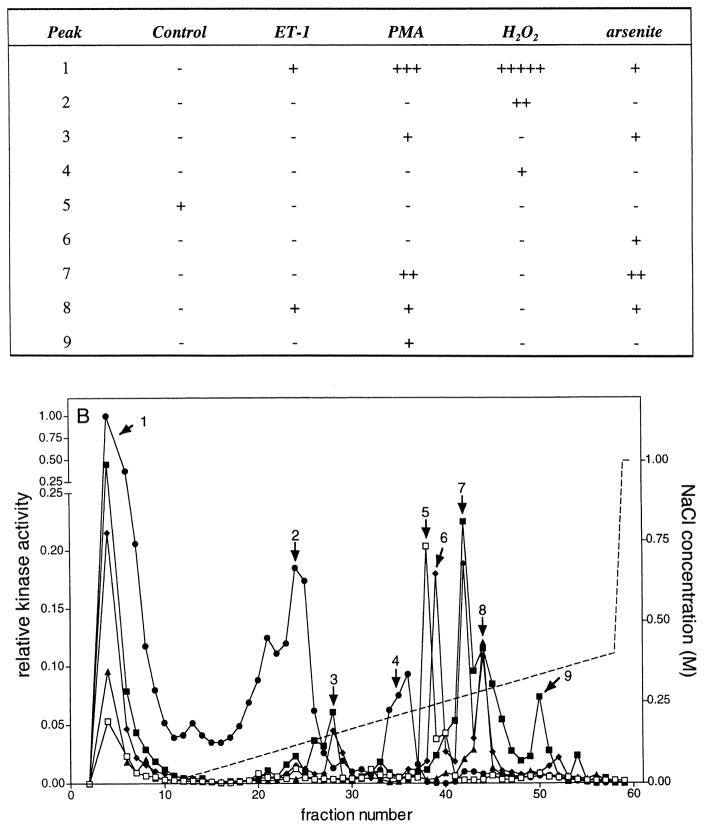
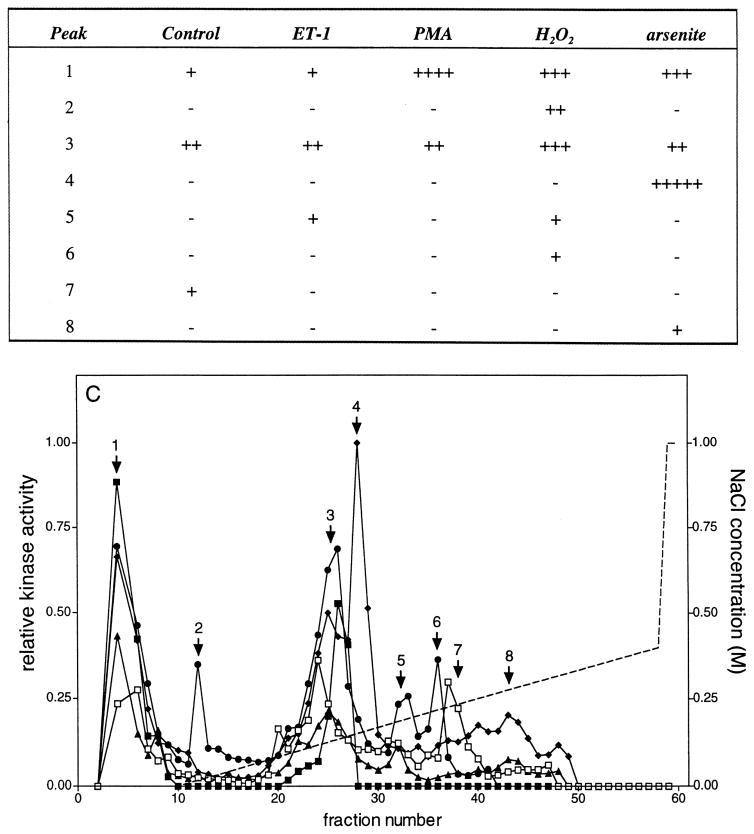
Mono Q chromatography of lysates from control and stimulated VSMCs. Porcine aortic smooth muscle cells were stimulated for 5 min at 37°C with no further additions (□), 0.1 μM ET-1 (▲), 0.1 μM PMA (■), 2.0 mM H2O2 (●), or 0.2 mM sodium arsenite (◆). Lysates were prepared, 1.5 mg chromatographed on a Mono Q HR 5/5 column, as described in Materials and Methods, and 60 fractions of 0.5 ml were collected. A 20-μl aliquot of the indicated fractions was assayed in the presence of GST-ERK1, GST-c-Jun, and hsp27. Reactions were terminated with 4 × SDS-PAGE sample buffer, and then the samples were subjected to electrophoresis on 10–20% acrylamide-gradient SDS-PAGE gels at 35 mA/gel and 15°C for 4 h. Gels were subsequently stained with Coomassie brilliant blue R250, dried between two sheets of cellophane, and subjected to autoradiography for 24 h at −80°C using Kodak BioMax MR film plus Kodak TranScreen-HE intensifying screens. Gels were exposed to phosphor imaging screens for 48 h, and then the phosphorylation of (A, lower panel) GST-ERK1, (B, lower panel) hsp27, and (C, lower panel) GST-c-Jun (1–169) were quantified as described in Materials and Methods. These data have been summarized and presented in tabular form in the upper panels of (A)–(C). Kinase activity was normalized to the highest activity detected for each substrate within an experiment and expressed as relative kinase activity. In the experiment shown here, peak activities before normalization were 2958, 96,183, and 1282 PD × mm2 for GST-ERK1 (K71A), hsp27, and GST-c-Jun (1–169) phosphorylation, respectively. Similarly, the integration areas were 7.8 × 3.8, 8.3 × 5.6, and 7.8 × 3.8 mm for GST-ERK1 (K71A), hsp27, and GST-c-Jun (1–169) phosphorylation, respectively. The broken line indicates the NaCl gradient.
Five separate peaks of ERK-phosphorylating activity ‘1–5’ were eluted from Mono Q (Fig. 6A). MEK1-antibodies immunoprecipitated the ERK-phosphorylating activity in peaks 1 and 2 (not shown). Other kinase activities present in these fractions remained in the supernatant of the immunoprecipitation. Distinct differences were observed in activation of these peaks of activity. The first peak was eluted in the flow-through fractions and activated to the highest extent by PMA. Arsenite and H2O2 also increased this activity, but to a lesser extent. Peaks 2 and 3 were activated exclusively by H2O2. Peak 4 was activated by PMA, whereas either H2O2 or PMA activated peak 5. Hence, although both PMA and H2O2 produced strongly positive signals for the activation of the ERK kinase cascade when determined by immunoblotting (Fig. 2), the data presented in Fig. 6 demonstrate clear differences in the manner in which this cascade was activated in response to these two stimuli. In each case both ERK1 and ERK2 were activated; however, the nature of the ERK-phosphorylating activities was markedly different. In addition, no increase in phospho-ERK1/2 was observed following treatment with arsenite (Fig. 2), whereas a weak activation of peak ‘1’ was observed in Fig. 6A.
Nine separate peaks of hsp-27-phosphorylating activity were detected eluting from Mono Q (Fig. 6B). The highest level of activation was of peak ‘1’ in the flow-through fractions and resulted from treatment with H2O2. This peak of activity was affected to varying degrees by all treatments examined. Peaks 2 and 4 were selectively activated by H2O2, whereas peaks 5, 6, and 9 were activated only in unstimulated, arsenite-, or PMA-treated cells, respectively. Other peaks of activity responded to more than one stimulus. Peak 3 showed slight responses to either PMA or arsenite. Either PMA or H2O2 activated peak 7. Peak 8 was activated equally by PMA, ET-1, or arsenite, but unaffected by H2O2.
Seven separate peaks of c-Jun-phosphorylating activity ‘1–7’ were detected eluting from Mono Q (Fig. 6C). Peak 1 eluted in the flow-through and was activated to varying extents by all treatments. A low level of activity was also present in unstimulated cells. Peaks 2, 5, and 6 were activated in response to H2O2. A small increase in the activity of peak 5 was also observed following treatment with ET-1. Peak 3 was activated to varying extents by all treatments and likely represents two different activities eluting in fractions 24 and 26, respectively. Peak 4 activated in response to arsenite. Peak 7 was only observed in unstimulated cells.
Of the three activities determined in the present study, hsp27 was phosphorylated to the highest level with a maximum phosphorylation value of 96,183 PD × mm2 in peak 1, whereas c-Jun phosphorylation was the lowest with a maximum phosphorylation of 1282 PD × mm2 in peak 4.
4. Discussion
We have examined the activation of the ERK, p38, and JNK MAP kinase cascades in cultured porcine aortic smooth muscle cells in response to mitogens and cellular stress. Weak responses were observed following treatment with arsenite and this could be due to the short treatment times employed in the present study. Arsenite is a potent activator of p38 MAP kinase and MAPKAP kinase-2 activity (Rouse et al., 1994); however, activation of p38 MAP kinase by arsenite is maximal at 60–120 min and ERK activation peaks after 3 h (Ludwig et al., 1998).
The phosphorylation of a small heat shock protein, hsp27, was used to quantify activation of the p38 MAP kinase cascade. There are currently five known kinases that catalyze the phosphorylation of hsp27: the cyclic AMP-dependent protein kinase (PKA) (Gaestel et al., 1991), protein kinase C (PKC) (Gaestel et al., 1991; Maizels et al., 1998), MAPKAP kinase-2 (Stokoe et al., 1992), MAPKAP kinase-3 (Clifton, Young, & Cohen, 1996), and MAPKAP kinase-5/PRAK (New et al., 1998; Ni, Wang, Diener, & Yao, 1998). Phosphorylation by PKC is lipid-dependent, and the experiments conducted herein were performed without lipid and in the presence of EGTA plus the inhibitory peptide of PKA (PKI, 1 μM); hence, PKA and PKC would not be active. Thus, under the conditions employed herein, hsp27 phosphorylation is a measure of the activation of the MAPKAP kinases 2, 3, and 5/PRAK. Each of these enzymes is activated by p38 MAP kinase. Four isoforms of p38 MAP kinase have been cloned and sequenced (Han, Richter, Li, Kravchenko, & Ulevitch, 1995; Jiang et al., 1996, 1997; Li, Jian, Ulevitch, & Han, 1996) and all four p38 family members are activated by MAPKK3 or MAPKK6 via phosphorylation of a TGY motif (Derijard et al., 1995). The large number of members in the p38 and MAPKAP kinase families likely explains, in part, the numerous peaks of hsp27-phosphorylating activity detected.
In many cases, with each substrate employed, unique peaks of activity were produced by the various stimuli. These differences represent more than just changes in the magnitude of a given response as they were eluted from Mono Q as distinct peaks at different NaCl concentrations. Each stimulus that activated a MAP kinase cascade did not result in equivalent ‘profile’ of activation of kinase activities and thus the responses themselves were not equivalent. This could result from the presence of multiple kinases at a given level of a cascade (e.g., MEK1/2, ERK1/2, MAPKAP kinase 2, 3, 5). However, the specificity in the responses suggest the presence of one or more mechanisms of structural organization of the MAP kinase signaling molecules themselves within a given cascade resulting in the segregation or compartmentalization of responses with respect to the various cellular stimuli. This may be achieved by the incorporation of a kinase into different complexes or scaffolds. For example, IB1/JIP-1 has been shown to regulate the activation of JNK (Bonny et al., 2000; Dickens et al., 1997; Whitmarsh, Cavanagh, Tournier, Yasuda, & Davis, 1998), whereas KSR-1 and MP1 interact with MEK and ERK to regulate signaling through the ERK cascade (Joneson et al., 1998; Schaeffer et al., 1998; Sugimoto, Stewart, Han, & Guan, 1998). Scaffold proteins have previously been proposed to prevent cross-talk between signaling pathways and to provide a preformed, multi-molecular complex that can rapidly become activated in incoming signals. In addition, the formation of scaffolds may serve to regulate the threshold levels for activation of generic signaling molecules in a cell-specific manner (Levchenko, Bruck, & Sternberg, 2000). Regulation of scaffold protein function and/or expression levels may serve as another mechanism of regulating the activation of signaling molecules or pathways.
In summary, we have described a means whereby the activation of the ERK, p38, and JNK MAP kinase cascades can be determined simultaneously. In addition, we have demonstrated that different patterns of kinase activation may be observed using this methodology, which are not apparent when the activation status of the MAP kinases are determined by their dual phosphorylation. These data suggest that, even within a given MAP kinase cascade, there exists a level of organization wherein specific modules become activated in response to different agonists or forms of cellular stress.
Acknowledgments
This paper is supported by grants from the Medical Research Council of Canada (MT-14725), the Quebec Heart and Stroke Foundation, the Fonds de la Recherche en Santé du Québec (FRSQ), and the Montreal Heart Institute Research Center. BGA and ET are currently research scholars of the Heart and Stroke Foundation of Canada.
Abbreviations
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- ERK
extracellular signal-related kinase
- ET-1
endothelin-1
- FPLC
fast protein liquid chromatography
- GST
glutathione S-transferase
- MAP kinase
mitogen-activated protein kinase
- MAPKAP kinase-2
MAP kinase-activated protein kinase-2
- MEK
MAP/ERK kinase
- PKC
protein kinase C
- PKI
cyclic AMP-dependent protein kinase inhibitory peptide
- PAGE
polyacrylamide gel electrophoresis
- PMA
phorbol 12-myristate 13-acetate
- PMSF
phenylmethylsulfonyl fluoride
- VSMCs
vascular smooth muscle cells
References
- Adams LP, Hathaway DR. Identification of mitogen-activated protein kinase phosphorylation sequences in mammalian h-caldesmon. FEBS Letters. 1993;322:56–60. doi: 10.1016/0014-5793(93)81110-l. [DOI] [PubMed] [Google Scholar]
- Bitar KN, Kaminski MS, Hailat N, Cease KB, Strahler JR. Hsp27 is a mediator of sustained smooth muscle contraction in response to bombesin. Biochemical and Biophysical Research Communications. 1991;181:1192–1200. doi: 10.1016/0006-291x(91)92065-r. [DOI] [PubMed] [Google Scholar]
- Bonny C, Oberson A, Steinmann M, Schorderet DF, Nicod P, Waeber G. IB1 reduces cytokine-induced apoptosis of insulin-secreting cells. Journal of Biological Chemistry. 2000;275:16466–16472. doi: 10.1074/jbc.M908297199. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Childs TJ, Mak AS. Smooth-muscle mitogen-activated protein (MAP) kinase: purification and characterization, and the phosphorylation of caldesmon. Biochemical Journal. 1993;296:745–751. doi: 10.1042/bj2960745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs TJ, Watson MH, Sanghera JS, Campbell DL, Pelech SL, Mak AS. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. Journal of Biological Chemistry. 1992;267:22853–22859. [PubMed] [Google Scholar]
- Clifton AD, Young PR, Cohen P. A comparison of the substrate specificity of MAPKAP kinase-2 and MAPKAP kinase-3 and their activation by cytokines and cellular stress. FEBS Letters. 1996;302:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu I, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends in Biological Sciences. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- Gaestel M, Schröder W, Benndorf R, Lippmann C, Buchner K, Hucho F, Erdmann VA, Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. Journal of Biological Chemistry. 1991;266:14721–14724. [PubMed] [Google Scholar]
- Gerthoffer WT, Yamboliev IA, Shearer M, Pohl J, Haynes R, Dang S, Sato K, Sellers JR. Activation of MAP kinases and phosphorylation of caldesmon in canine colonic smooth muscle. Journal of Physiology (London) 1996;495:597–609. doi: 10.1113/jphysiol.1996.sp021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinases does not alter smooth muscle contraction in swine carotid artery. American Journal of Physiology. 1998;275:H131–H138. doi: 10.1152/ajpheart.1998.275.1.H131. [DOI] [PubMed] [Google Scholar]
- Hadrava V, Tremblay J, Hamet P. Abnormalities in growth characteristics of aortic smooth muscle cells in spontaneously hypertensive rats. Hypertension. 1989;13:589–597. doi: 10.1161/01.hyp.13.6.589. [DOI] [PubMed] [Google Scholar]
- Han J, Richter B, Li Z, Kravchenko V, Ulevitch RJ. Molecular cloning of human p38 MAP kinase. Biochimica et Biophysica Acta. 1995;1265:224–227. doi: 10.1016/0167-4889(95)00002-a. [DOI] [PubMed] [Google Scholar]
- Hedges JC, Yamboliev IA, Ngo M, Horowitz B, Adam LP, Gerthoffer WT. p38 Mitogen-activated protein kinase expression and activation in smooth muscle. American Journal of Physiology. 1998;275:C512–C534. doi: 10.1152/ajpcell.1998.275.2.C527. [DOI] [PubMed] [Google Scholar]
- Huang CF, Ferrell JE., Jr Ultrasensitivity in the mitogen-activated protein kinase cascade. Proceedings of the National Academy of Sciences USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) Journal of Biological Chemistry. 1996;1996:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J. Characterization of the structure of the fourth member of p38 group mitogen-activated protein kinases, p38δ. Journal of Biological Chemistry. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- Joneson T, Fulton JA, Volle DJ, Chaika OV, Bar-Sagi D, Lewis RE. Kinase suppressor of ras inhibits the activation of extracellular ligand-regulated (ERK) mitogen-activated protein (MAP) kinase by growth factors, activated ras, and ras effectors. Journal of Biological Chemistry. 1998;273:7743–7748. doi: 10.1074/jbc.273.13.7743. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Gerthoffer WT, Hickey E, Weber LA. Cloning and sequencing of a cDNA encoding the canine hsp27 protein. Gene. 1995;161:305–306. doi: 10.1016/0378-1119(95)00290-m. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. American Journal of Physiology. 1997;273:L930–L940. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- Levchenko A, Bruck J, Sternberg PW. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proceedings of the National Academy of Sciences USA. 2000;97:5818–5823. doi: 10.1073/pnas.97.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jian Y, Ulevitch RJ, Han J. The primary structure of p38γ: a new member of the p38 group of MAP kinases. Biochemical and Biophysical Research Communications. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Häfner H, Neufeld J, Han J, Rapp UR. The stress inducer arsenite activates mitogen-activated protein kinases extracellular signal-regulated kinases 1 and 2 via a MAPK kinase 6/p38-dependent pathway. Journal of Biological Chemistry. 1998;273:1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Peters CA, Kline M, Cutler RE, Jr, Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the δ isoform of protein kinase C. Biochemical Journal. 1998;332:703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood LJ, Kato Y, Parry GCN, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO Journal. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai PK, Walsh MP. The effect of phosphorylation of smooth-muscle caldesmon. Biochemical Journal. 1987;244:417–425. doi: 10.1042/bj2440417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Wang XS, Diener K, Yao Z. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochemical and Biophysical Research Communications. 1998;243:492–496. doi: 10.1006/bbrc.1998.8135. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP-1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Letters. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Suck RW, Krupinska K. Repeated probings of Western blots obtained from Coomassie brilliant blue-stained or unstained polyacrylamide gels. BioTechniques. 1996;21:418–422. doi: 10.2144/96213bm17. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Stewart S, Han M, Guan KL. The kinase suppressor of ras (KSR) modulates growth factor and ras signaling by uncoupling Elk-1 phosphorylation from MAP kinase activation. EMBO Journal. 1998;17:1717–1727. doi: 10.1093/emboj/17.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bruderer S, Rafi Z, Xue J, Milburn PJ, Krämer A, Robinson RJ. Phosphorylation of splicing factor SF1 on Ser20 by cGMP-dependent protein kinase regulates spliceosome assembly. EMBO Journal. 1999;18:4549–4559. doi: 10.1093/emboj/18.16.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- Yamboliev IA, Hedges JC, Mutnick JLM, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 MAP kinase/hsp27 pathway. American Journal of Physiology. 2000;278:H1899–H1907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- Yamboliev IA, Wiesmann KM, Singer CA, Hedges JC, Gerthoffer WT. Phosphatidylinositol 3-kinases regulate ERK and p38 MAP kinases in canine colonic smooth muscle. American Journal of Physiology. 2000;279:C352–C360. doi: 10.1152/ajpcell.2000.279.2.C352. [DOI] [PubMed] [Google Scholar]