Abstract
Background and Objective
Males and females who use methamphetamine (MA) differ in socio-demographics, MA diagnoses, co-morbidities, and brain activity. The objective of this study was to investigate sex differences in the characteristics of MA use and dependence in patients at a Thai substance treatment center.
Methods
Demographic, MA use, and diagnostic data for 782 MA users were obtained by using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), Thai version. Categorical comparisons of males (n=413, 53%) and females (n=369, 47%) were made by chi-square test. Factors significantly differentiating men and women with respect to MA dependence were identified by logistic regression analysis controlling for demographic, diagnostic, and MA use variables.
Results
Males admitted to residential drug treatment for MA use had an earlier age of onset for both MA use (17.7±4.1 vs. 19.7±6.2 years, t=-5.3, p<0.001) and dependence (20.4±5.2 vs. 22.2±6.4 years, t=-3.6, p<0.001). Females were more likely than males to be MA dependent (79% vs. 60%, χ21=33.7, p<0.001), and to experience MA withdrawal (65.3% vs. 48.9%, χ21=21.4, p<0.001), withdrawal related hypersomnia (77.2% vs. 64.8%, χ21=14.5, p<0.001), fatigue (77.5% vs. 70.3%, χ21=5.2, p=0.02) and psychomotor retardation (64.5% vs. 57.0%, χ21=4.5, p=0.03). Similarly, females had heavier (e.g., largest daily amount (χ21=12.4, p<0.001), more frequent (χ21 =5.1, p=0.02)) and greater lifetime episodes of MA use (χ21=24.1, p<0.001) than males. After controlling for such variables by logistic regression, being female remained a significant factor influencing the occurrence of MA dependence (OR=2.7, 95% CI=1.8-4.1, p<0.001). Shared associated factors (or comorbidities) for MA dependence in both sexes included nicotine dependence (in males; OR=4.1, 95% CI=2.4-7.0, p<0.001 and in females; OR=2.4, 95% CI=1.3-4.4, p=0.007), greater lifetime episodes of MA use (in males; OR=3.5, 95% CI=1.9-6.4, p<0.001 and in females; OR=5.9, 95% CI=3.1-11.4, p<0.001) and more frequent use (in males; OR=5.1, 95% CI=2.8-9.1, p<0.001 and in females; OR=3.6, 95% CI=1.9-6.9, p<0.001). Comorbid antisocial personality disorder predicted MA dependence in males only (OR=3.7, 95% CI=1.6-8.6, p=0.002).
Conclusions
The current study highlights both common (e.g., nicotine dependence and severity of MA use) and sex-specific differences (e.g., MA use/dependence characteristics and comorbidities), including sex itself, with respect to MA dependence in a Thai treatment cohort.
Keywords: Methamphetamine, dependence, associated factor, sex
Introduction
Methamphetamine (MA), the most common amphetamine-type stimulant used globally, is associated with a host of neuropsychiatric illnesses (e.g. psychosis (Cruickshank and Dyer, 2009, Grelotti et al., 2010), depression, and cognitive impairment (Scott et al., 2007, Cruickshank and Dyer, 2009)) and chronic physical conditions (e.g. cardiovascular disease (Kaye et al., 2007) and HIV infection (Montoya et al., 2013, Parsons et al., 2013)). Beginning in 2003, the Thai government enacted a national drug policy whose primary objective was to reduce the demand and supply of illicit drugs, especially MA (commonly known as speed or “yaba”). This policy included a drug user rehabilitation law, which required all identified illicit drug users to participate in a drug treatment program (Vongchak et al., 2005). Despite this policy, continued upward trends in illicit use were noted in 2008, with more than 80% of illicit-substance using Thais identifying MA as their primary drug of use (UNODC, 2011). Such trends were also associated with changing patterns of MA use, including a shift from primarily male laborers (who used the drug to work longer hours) to individuals doing so for purposes of recreation and performance enhancement (for reasons of curiosity, sexual activity, motorcycle racing, weight loss and school productivity), particularly among adolescent Thais of both sexes (Sherman et al., 2008, Sherman et al., 2009, Cohen, 2014).
Heritability for MA dependence is moderate (∼30%) (Tsuang et al., 1998, Kendler et al., 2000), and prior studies have weakly supported a host of candidate genes (i.e., BDNF, CYP2D6, GSTM1, SLC22A3, AKT1, GABRG2) as possibly associated with MA abuse or dependence (Bousman et al., 2009). In addition, antisocial characteristics (Herman-Stahl et al., 2007), recent drug (alcohol, nicotine, or cannabis) use, positive attitude toward MA use, and peer pressure toward MA use (Sattah et al., 2002) are associated with MA use disorders. Interestingly, male and female MA users are different with respect to socio-demographics, co-morbidities, pattern of use, and response to treatment (Supplementary table 1). In addition, brain structures and functional activities have also been reported to be different between sexes; male MA users had more hyperintensity of white matter, smaller corpus callosum and less perfusion of parietal and occipital areas compared to females (Bae et al., 2006, Dluzen and Liu, 2008).
There have been a few prior published studies about sex differences in MA use in Thailand, a country ranked among the top worldwide for lifetime prevalence of MA use in the past decade (UNODC, 2003). However, differences between the sexes have not been examined since the enactment of new drug law enforcement policies, affording an opportunity to evaluate potentially new and/or changing patterns of MA use between the sexes. While other studies have investigated sex differences in inpatient MA users in other countries, a number of variables have not been investigated, including, for instance, severity and symptoms of MA-dependence, MA withdrawal, and factors influencing the dependence trait by sex. Morever, the chosen Thai context differs in several important respects (Hser et al., 2005, He et al., 2013), including the criteria for hospitalization (since under the new policy, Thai treatment centers hospitalized all levels of MA use severity ranging from problematic use but no dependence to severe dependence). Therefore, in addition to analyses of sex differences in MA use and dependence, the current cohort also provided an opportunity for directly comparing individuals with and without MA dependence. Thus, the purpose of this study was to investigate sex differences in the characteristics of MA use and dependence and related factors in a population of inpatient MA users. The results from our study may provide information for development of assessment, treatment and prevention strategies in each sex.
Materials and Methods
We evaluated demographic, diagnostic, and MA use variables collected using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA – Thai version) as part of ongoing studies of MA users (n=990) from across the country. Subjects agreed to be hospitalized for four months of residential (i.e., inpatient) drug treatment at the Princess Mother National Institute on Drug Abuse and Treatment (Thanyarak Institute) located in central Thailand (near Bangkok) during 2007-2011 (Kalayasiri et al., 2010, Intharachuti, 2012, Kalayasiri et al., 2014), a period immediately following implementation of the Thai government's new drug policies. The treatment program mainly used a modified therapeutic community program. Subjects and measures have been described elsewhere (Kalayasiri et al., 2014), but in brief, individuals aged 18 years or older with more than 10 episodes of lifetime MA (often called “yaba”) use were included without sampling; all available patients were invited for screening and informed consent, excluding those with primary psychotic (schizophrenia or bipolar affective disorder) or neurological (e.g., cerebrovascular disease, epilepsy) disorders. We also excluded participants with mood disorders due to small samples (five males and one female) or other primary illicit substance (opioid, cannabis, inhalants, kratom or Mitragyna speciosa) dependences (n = 208), resulting in a final study sample of 782 MA-using individuals. Written informed consent was obtained before research participation. The study was approved by the appropriate ethics committees at the Faculty of Medicine, Chulalongkorn University (Med Chula IRB), Thanyarak Institute for Drug Abuse, and Thailand Ministry of Public Health.
The SSADDA is a comprehensive interview used to diagnose various substance-related and other psychiatric disorders, including antisocial personality disorder (ASPD), based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (Pierucci-Lagha et al., 2007). The SSADDA was previously translated into Thai for the study of the genetics of opioid (Malison et al., 2011) and MA dependence (Kalayasiri et al., 2014) and been previously shown to have a high inter-rater/inter-instrument reliability (Malison et al., 2011, Kalayasiri et al., 2014). Rigorous quality control (including ten practice-interviews required for each beginning interviewer prior to data collection and regular cross-editing) for the SSADDA interview was applied based on established practice with the English version in the US (Kalayasiri et al., 2014). The instruments were administered as computerized versions and performed onsite at the treatment center by a team of six interviewers, each with a bachelor's degree in psychology or higher and specifically certified in the SSADDA protocol. Half of the interviewers were psychologists employed at the treatment center (and potentially involved in the care of patients if requested by attending clinicians). Sex was identified by using data from the national identity data with no gender match between interviewers and respondents.
Severity of MA dependence was defined by the number of DSM-IV criteria met for MA dependence (3-5 = moderate, 6-7 = severe) (Kalayasiri et al., 2006, Kalayasiri et al., 2010). For MA withdrawal, 9 symptoms were ascertained via the SSADDA, including feeling depressed, feeling restless, feeling fatigued, insomnia, hypersomnia, craving for MA, psychomotor retardation, increased appetite, and having nightmares that occurred most nights for two days or longer when MA use was stopped or reduced. Severity of MA withdrawal was classified into 3 levels including mild, moderate, and severe (1-3, 4-6, and 7-9 symptoms, respectively).
Statistical analysis
Data were assessed for normality both by visual inspection and by Kolmogorov–Smirnov testing. Non-normally distributed data were log transformed, and if still non-normal, categorized as non-continuous variables for further analysis. Males and females were compared with respect to demographics, diagnoses, MA-use variables, severity of MA dependence, and severity of MA withdrawal symptoms by using chi-square test or independent t-test when appropriate. Statistically significant variables in the initial analyses were then controlled in the adjusted model for sex difference by using logistic regression analysis. Demographic, diagnostic, and MA use variables were compared between individuals with and without MA dependence in both the total and sex specific groups by chi-square test or independent t-test. Effects of sex on MA dependence symptoms were tested by logistic regression analysis, controlling for variables identified as distinguishing men and women in the above analyses. Factors that achieved statistical significance (p<0.05) or were nearly significantly (p<0.10) associated with MA dependence in each sex were subsequently analyzed using logistic regression to identify factors that predicted the trait in males and females.
Results
Table 1 shows demographics and comorbid diagnoses for Thai and Thai-Chinese males (n = 413; 53%) and females (n = 369; 47%). Females were older (27.7±7.1 vs. 25.8±6.4, t=-3.9, p<0.001), less likely to be married (9.2% vs. 22.3%, χ22=30.9, p<0.001) and employed (9.8% vs. 47.5%, χ21=132.8, p<0.001) and had fewer years of education (7.6±2.8 vs. 8.5±2.8, t=4.5, p=0.03) than male MA-users. Regarding comorbidity, males were more likely to have ASPD than female participants (17.9% vs. 6.2%, χ21=24.5, p<0.001). However, history of conduct disorder, alcohol and nicotine dependence did not differ between the two groups.
Table 1. Demographic and comorbidity variables compared between Thai male and female methamphetamine users by chi-square test.
| Variables | Total (N=782) |
Male (N=413) |
Female (N=369) |
χ2 | df | P-Value | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | % | n | % | n | % | ||||
|
| |||||||||
| Age (years) | |||||||||
| ≤ 20 | 165 | 21.1 | 108 | 26.2 | 57 | 15.4 | 14.6 | 2 | 0.001 |
| 21-30 | 412 | 52.6 | 210 | 50.8 | 202 | 54.7 | |||
| > 30 | 205 | 26.2 | 95 | 23.0 | 110 | 29.8 | |||
|
| |||||||||
| Level of education (years) (n=781) | |||||||||
| ≤ 9 | 631 | 80.8 | 323 | 78.4 | 308 | 83.5 | 3.2 | 1 | 0.072 |
| > 9 | 150 | 19.2 | 89 | 21.6 | 61 | 16.5 | |||
|
| |||||||||
| Marital status (n=781) | |||||||||
| Never married | 549 | 70.3 | 281 | 68.0 | 268 | 72.8 | 30.9 | 2 | <0.001 |
| Widow/divorced/separated | 106 | 13.6 | 40 | 9.7 | 66 | 17.9 | |||
| Married | 126 | 16.1 | 92 | 22.3 | 34 | 9.2 | |||
|
| |||||||||
| Employment | 232 | 29.7 | 196 | 47.5 | 36 | 9.8 | 132.8 | 1 | <0.001 |
|
| |||||||||
| Comorbidity | |||||||||
| Alcohol dependence | 144 | 18.4 | 81 | 19.6 | 63 | 17.1 | 0.8 | 1 | 0.36 |
| Nicotine dependence | 390 | 49.9 | 194 | 47.0 | 196 | 53.1 | 2.9 | 1 | 0.10 |
| Antisocial personality disorder | 97 | 12.4 | 74 | 17.9 | 23 | 6.2 | 24.5 | 1 | <0.001 |
| History of conduct disorder | 36 | 4.6 | 17 | 4.1 | 19 | 5.1 | 0.5 | 1 | 0.49 |
With respect to MA use and dependence variables, males had earlier onset of MA use -- about 17.7 years of age compared with 19.7 years in females (t=-5.3, p<0.001) -- and had an earlier onset of dependence at about 20.4 years compared with 22.2 years in females (t=-3.6, p<0.001). However, female users were more likely to be dependent on MA (χ21=33.7, p<0.001), to be more severely dependent (χ21=4.9, p=0.03), to use more MA (lifetime episodes; χ21=24.1, p<0.001) and with greater intensity (daily MA pills; χ21=12.4, p<0.001), and frequency (days per month of MA use; χ21=5.1, p=0.02) during periods of heaviest lifetime use. In contrast, duration of MA use, route of MA use, and the severity of MA withdrawal did not differ between groups (although sex-related differences in patterns of MA withdrawal symptoms were noted; see below) (p>0.05; Table 2). With respect to the adjusted model – adjusted for sex difference by controlling for demographic, diagnostic, and MA use variables -- females were more likely to be educated less than 9 years (OR=1.6, 95% CI=1.0-2.4, p=0.034), be widowed, divorced or separated (OR=2.2, 95% CI=1.1-4.3, p=0.019), and be unemployed (OR=8.0, 95% CI=5.2-12.4, p<0.001); and less likely to have ASPD (OR=3.7, 95% CI=2.2-6.4, p<0.001), compared to males. In addition, females were more likely to have initiated MA use later than males (age of onset > 18 years old; OR=2.7, 95% CI=1.7-4.2, p<0.001) and use MA with greater intensity during the heaviest period of use (daily MA pills ≥ 5; OR=1.5, 95% CI=1.0-2.3, p=0.045) (Table 3).
Table 2. Methamphetamine use characteristics and severity of methamphetamine dependence and withdrawal compared between Thai male and female users by chi-square test.
| Variables | Total (N=782) |
Male (N=413) |
Female (N=369) |
χ2 | df | P-Value | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | % | n | % | n | % | ||||
|
| |||||||||
| Age at first MA use onset (years) | |||||||||
| ≤15 | 213 | 27.2 | 120 | 29.1 | 93 | 25.2 | 27.8 | 2 | <0.001** |
| 16-18 | 298 | 38.1 | 184 | 44.6 | 114 | 30.9 | |||
| >18 | 271 | 34.7 | 109 | 26.4 | 162 | 43.9 | |||
|
| |||||||||
| Age at MA dependence onset (years) (n=538) | |||||||||
| < 20 | 261 | 48.5 | 139 | 56.5 | 122 | 41.8 | 11.6 | 1 | 0.001** |
| ≥ 20 | 277 | 51.5 | 107 | 43.5 | 170 | 58.2 | |||
|
| |||||||||
| Duration of MA use (years) (n=781) | |||||||||
| ≤ 5 | 324 | 41.5 | 175 | 42.4 | 149 | 40.5 | 3.9 | 2 | 0.14 |
| 6-10 | 225 | 28.8 | 107 | 25.9 | 118 | 32.1 | |||
| >10 | 232 | 29.7 | 131 | 31.7 | 101 | 27.4 | |||
|
| |||||||||
| Daily number of MA pills during the heaviest period of MA use | |||||||||
| 1-4 | 485 | 62.0 | 280 | 67.8 | 205 | 55.6 | 12.4 | 1 | <0.001** |
| ≥-5 | 297 | 38.0 | 133 | 33.2 | 164 | 44.4 | |||
|
| |||||||||
| Days per month of using MA during the heaviest period of MA use | |||||||||
| ≤ 15 | 280 | 35.8 | 163 | 39.5 | 117 | 31.7 | 5.1 | 1 | 0.02* |
| > 15 | 502 | 64.2 | 250 | 60.5 | 252 | 68.3 | |||
|
| |||||||||
| Number of episodes of MA use in lifetime (n=776) | |||||||||
| ≤ 1000 | 324 | 41.8 | 204 | 50.0 | 120 | 32.6 | 24.1 | 1 | <0.001** |
| > 1000 | 452 | 58.2 | 204 | 50.0 | 248 | 67.4 | |||
|
| |||||||||
| MA dependence | 537 | 68.7 | 246 | 59.6 | 291 | 78.9 | 33.7 | 1 | <0.001** |
|
| |||||||||
| Severity of MA dependence as measured by DSM-IV symptom count (n=536) | |||||||||
| Non-severe (3-5) | 224 | 41.8 | 115 | 46.9 | 109 | 37.5 | 4.9 | 1 | 0.03* |
| Severe (6-7) | 312 | 58.2 | 130 | 53.1 | 182 | 62.5 | |||
|
| |||||||||
| Severity of MA withdrawal: number of withdrawal symptoms (n=642) | |||||||||
| Mild (1-3) | 191 | 29.8 | 101 | 31.1 | 90 | 28.5 | 0.5 | 2 | 0.78 |
| Moderate (4-6) | 370 | 57.6 | 184 | 56.4 | 186 | 58.9 | |||
| Severe (7-9) | 81 | 12.6 | 41 | 12.6 | 40 | 12.7 | |||
|
| |||||||||
| Route of MA use | |||||||||
| Smoking | 726 | 92.8 | 381 | 92.3 | 345 | 93.5 | 0.454 | 1 | 0.501 |
| Orally | 132 | 16.9 | 75 | 18.2 | 57 | 15.4 | 1.022 | 1 | 0.312 |
| Snort | 41 | 5.2 | 27 | 6.5 | 14 | 3.8 | 2.952 | 1 | 0.086 |
| Injection | 10 | 1.28 | 5 | 1.2 | 5 | 1.4 | - | - | 1.000a |
=Fisher's Exact Test
p < 0.05, chi-square test, two-tail
p < 0.01, chi-square test, two-tail
Table 3. Adjusted model comparing females to males based on logistic regression analysis that controlled for different variables including socio-demographics (age, level of education, marital status, employment), antisocial personality disorder and MA-use characteristics.
| Variables | Wald | df | Odds ratio | p-values | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
|
| ||||||
| Education ≤ 9 years | 4.5 | 1 | 1.6 | 0.034 | 1.0 | 2.4 |
| Widow/divorced/separated | 5.5 | 1 | 2.2 | 0.019 | 1.1 | 4.3 |
| Unemployment | 87.8 | 1 | 8.0 | <0.001 | 5.2 | 12.4 |
| No history of antisocial personality disorder | 22.7 | 1 | 3.7 | <0.001 | 2.2 | 6.4 |
| Age of onset > 18 years old | 18.4 | 1 | 2.7 | <0.001 | 1.7 | 4.2 |
| Using MA ≥ 5 pills/day during the heaviest period of use | 4.0 | 1 | 1.5 | 0.045 | 1.0 | 2.3 |
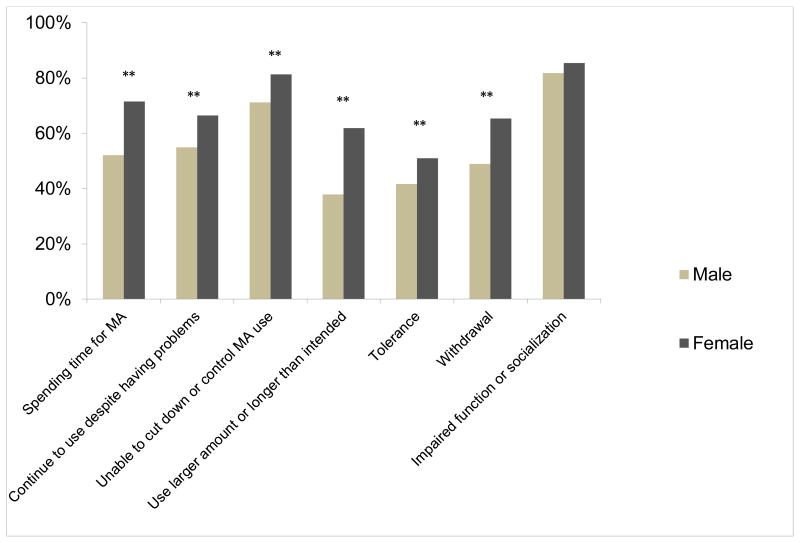
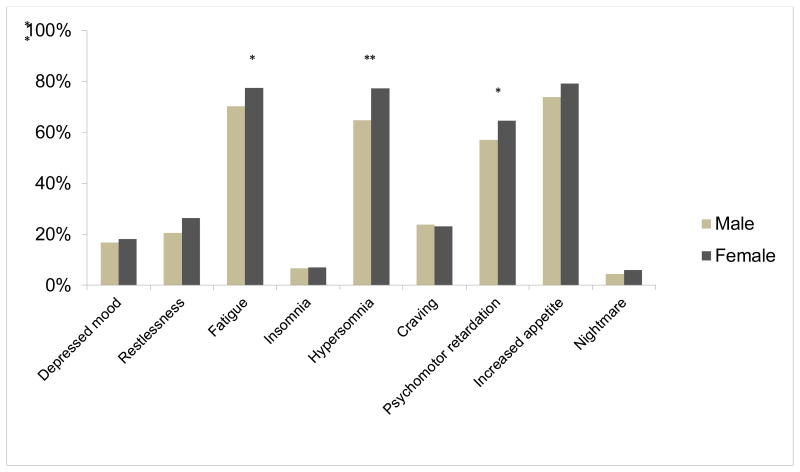
Regarding specific DSM-IV criteria for MA dependence, females more frequently endorsed 6 of the 7 items, including spending time on activities necessary to obtain the substance, use of the substance, or recovering from its effects (χ21=31.2, p<0.001), continuing to use despite knowledge of having a persistent physical or psychological problem (χ21=10.6, p=0.001), persistent desire or unsuccessful efforts to cut down or control substance use (χ21=10.9, p=0.001), using larger amounts or over a longer period than was intended (χ21=45.0, p<0.001), tolerance (χ21=6.8, p=0.009) and experiencing withdrawal symptoms (χ21=21.4, p<0.001) (Figure 1). The criterion “important social, occupational, or recreational activities are given up or reduced because of substance use” did not differ between the two groups. In term of withdrawal symptoms, larger numbers of females reported hypersomnia (χ21=14.5, p<0.001), fatigue (χ21=5.2, p=0.02) and psychomotor retardation (χ21=4.5, p=0.03) whereas other symptoms did not reach statistical significance (Figure 2).
Figure 1. Methamphetamine dependence symptoms based on DSM-IV criteria in males and females (n=782).
Females, compared to males, were more likely to have higher rate of most of the DSM IV symptom criteria for substance dependence, including spending time for MA, continuing to use despite knowledge of having a persistent problem, cannot cut down or control MA use, using larger amount or longer time of MA than intended, tolerance and withdrawal symptoms.
** p < 0.01, chi-square test, two-tail
Figure 2. Methamphetamine withdrawal symptoms in males and females (n=782).
Regarding MA withdrawal symptoms, females had higher proportion of fatigue, psychomotor retardation and hypersomnia symptom than males, whereas other symptoms did not reach statistical significance.
* p < 0.05, chi-square test, two-tail
** p < 0.01, chi-square test, two-tail
With respect to categorical comparisons of MA dependent vs. non-dependent individuals (Supplementary table 2), those with MA dependence in the total sample were less likely to be married (p=0.004) and employed (p<0.001), more likely to be dependent on alcohol (p<0.001) and nicotine (p<0.001), have ASPD (p<0.001), have earlier onset of MA use (p=0.001), have longer history of MA use (p=0.001), use more MA on a daily (p<0.001) and monthly basis (p<0.001) during their period of heaviest use, as well as have more lifetime episodes of MA use (p<0.001). Likewise, being currently unmarried (p=0.039), unemployed (p=0.006), having comorbid alcohol dependence (p<0.001), nicotine dependence (p<0.001) and ASPD (p<0.001), having earlier onset of MA use (p=0.003), and having longer history of MA use (p<0.001) were associated with MA dependence in males, while alcohol (p=0.032) and nicotine dependence (p<0.001) were associated with MA dependence in female MA users. In both sexes, individuals with MA dependence were more likely to have greater daily (p<0.001) and monthly MA use (p<0.001) during the periods of their heaviest use as well as having more lifetime episodes of MA use (p<0.001). With respect to logistic regression analysis, nicotine dependence (in males; OR=4.1, 95% CI=2.4-7.0, p<0.001 and in females; OR=2.4, 95% CI=1.3-4.4, p=0.007), severe MA-use (e.g., > 1000 episodes of use in their lifetime; in males; OR=3.5, 95% CI=1.9-6.4, p<0.001 and in females; OR=5.9, 95% CI=3.1-11.4, p<0.001), using MA 15 or more days per month during the heaviest use period (in males; OR=5.1, 95% CI=2.8-9.1, p<0.001 and in females; OR=3.6, 95% CI=1.9-6.9, p<0.001)) were predictors of MA dependence in both sexes. However, comorbid ASPD predicted MA-dependence in males only (OR=3.7, 95% CI=1.6-8.6, p=0.002) (Table 4).
Table 4. Effect of sex and factors associated with methamphetamine dependence in overall sample, male and female methamphetamine users, identified by logistic regression analysis.
| Variables | Wald | df | Odds ratio | ∼p-values | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
|
| ||||||
| Total sample (n=782) | ||||||
| Sex-female | 30.0 | 1 | 2.7 | <0.001 | 1.8 | 4.1 |
| Nicotine dependence | 33.2 | 1 | 3.2 | <0.001 | 2.2 | 4.7 |
| Antisocial personality disorder | 6.5 | 1 | 2.7 | 0.011 | 1.3 | 5.7 |
| Using > 1000 times in lifetime | 34.0 | 1 | 3.8 | <0.001 | 2.4 | 6.0 |
| Using ≥-5 pills/day during the heaviest use | 8.1 | 1 | 2.1 | 0.004 | 1.3 | 3.6 |
| Using ≥ 15 days/month during the heaviest use | 34.7 | 1 | 3.6 | <0.001 | 2.4 | 5.5 |
| Male (n=413) | ||||||
| Nicotine dependence | 27.7 | 1 | 4.1 | <0.001 | 2.4 | 7.0 |
| Antisocial personality disorder | 9.6 | 1 | 3.7 | 0.002 | 1.6 | 8.6 |
| Using > 1000 times in lifetime | 16.5 | 1 | 3.5 | <0.001 | 1.9 | 6.4 |
| Using ≥ 15 days/month during the heaviest use | 28.7 | 1 | 5.1 | <0.001 | 2.8 | 9.1 |
| Female (n=369) | ||||||
| Nicotine dependence | 7.3 | 1 | 2.4 | 0.007 | 1.3 | 4.4 |
| Using > 1000 times in lifetime | 28.5 | 1 | 5.9 | <0.001 | 3.1 | 11.4 |
| Using ≥ 15 days/month during the heaviest use | 15.6 | 1 | 3.6 | <0.001 | 1.9 | 6.9 |
All models were analysed based on logistic regression that controlled for age, level of education, antisocial personality disorder, history of conduct disorder, other substance dependence and pattern of MA use.
To determine whether sex itself was as an independent predictor for MA dependence (Table 4), we used logistic regression analysis, controlling for the above-identified, MA-dependence associated factors. Accordingly, the result showed a remaining influence of being female on the occurrence of MA dependence (OR=2.7, 95% CI=1.8-4.1, p<0.001).
Discussion
It is first important to note that our study did not employ systematic population-based sampling – our study population consisted of patients being treated at a specific treatment center. As such, we cannot draw conclusions regarding populations other than the one under study. Results based on this kind of sampling must reflect various well-known ascertainment biases. With that major caveat in mind, the four main findings of our study with respect to this sample are: 1) onset of MA use and dependence was earlier in males as compared to females 2) females were more likely to experience MA withdrawal, (especially hypersomnia), 3) females were more likely to be MA dependent and to use MA more heavily than males, and 4) nicotine dependence is an associated factor shared by MA dependent men and women, whereas ASPD is more characteristic of males.
The onset of substance use disorders most often occurs during the late teenage to early adult years, or roughly 18-27 years of age with mean age at 19.9 years according to epidemiologic surveys in the United States (US) (Kessler et al., 2005, Compton et al., 2007). Results from the 2012 US National Survey on Drug Use and Health reported that 19.7 years was the average age of onset for new US MA users (SAMHSA, 2013), which is similar to that for other illicit drugs. Since that report considered a population-based sample and ours a clinical sample of sufficient severity to require hospitalization, it is not surprising that age of onset in our sample tended to be earlier, although other factors could explain the difference-- for instance, the shifting trends of MA use in adolescent Thais. Prior studies of MA using populations have suggested potential differences between men and women. For example, although Brecht et al. (Brecht et al., 2004) found no sex differences in overall residential and outpatient US populations with respect to age of first MA use, they noted that females progressed more quickly to becoming regular users of the drug than males. In a study of Taiwanese MA users in a detention center, Lin et al. (Lin et al., 2004) found that females were significantly younger at the time of first use, a finding the authors suggest might have been explained by military service requirements for men (a duty hypothesized to protect them from illegal drug exposure in late teenage to early adulthood). In contrast to these, we found evidence for an earlier onset of MA in Thai males and comparable rates of progression from use to dependence among males and females. While reasons for these differences are unclear, we hypothesize that our findings may be related to risky behaviors, including sexual behavior as suggested by prior work in adolescent males in Thailand (Ruangkanchanasetr et al., 2005, Liu et al., 2006, Assanangkornchai et al., 2007), the effect of law enforcement focusing on males which could recruit male MA-users into the treatment program earlier than females, and the societal difference in the response to substance use among males compared to females in terms of attitude toward males as having more severe and problematic substance use and negative consequence than females. It is worth noting, however, that despite an earlier age of MA use onset in males, females more commonly met criteria for MA dependence. The dissociation is remarkable, insofar as early age of onset has often been suggested to associate with increased chances of dependence. We speculate that differences between males and females in our treatment cohort may account for our finding. For example, females in our cohort were older at the time of recruitment, which might associate with later MA onset compared to males. In addition, we speculate that age of onset and sex difference might contribute to severity of MA dependence independently. Nevertheless, earlier age of onset is still associated with the occurrence of MA dependence in the total group.
Regarding MA withdrawal symptoms, our result showed that females were more likely to experience MA withdrawal symptoms (but not greater withdrawal severity), a finding that is partly consistent with another prior MA inpatient study (He et al., 2013). The difference between studies were that ours found the withdrawal symptoms more common in females were hypersomnia, fatigue and psychomotor retardation, whereas He's study found chilly feeling and sweating were more common in females. However, these differences may be accounted for by the differences in symptoms ascertained. Ours used criteria from the SSADDA, which are more specific to MA withdrawal (e.g. depressed mood, hypersomnia, increased appetite, psychomotor retardation); the other study's criteria did not include all of these symptoms (He et al., 2013). Instead, they used 16 general criteria for substance withdrawal such as chilly feeling, sweating, runny nose, palpitation or insomnia. Although the biological mechanisms underlying sex differences in MA withdrawal are unknown, the higher rate of MA-withdrawal in females may be related to the heavier MA use observed in dependent females.
Our findings showed that females in this sample were more likely to be MA dependent and to use MA more heavily than males, and this is consistent with prior studies (Kim and Fendrich, 2002, Dluzen and Liu, 2008, Gonzales et al., 2008). Reasons for this difference are unknown, but biological factors may well be involved. For example, females have been shown to be more vulnerable to the reinforcing effect of psychostimulants (Lynch et al., 2002, Roth et al., 2004), and experience MA as more euphorigenic than males during the follicular, estrogen-related phase of their menstrual cycle (Lynch et al., 2002). Females also have less neuronal toxicity as measured by the severity of brain white matter hyperintensities (Bae et al., 2006) and have less dopamine release in response to psychostimulant administration (Munro et al., 2006). Environmental and/or psychosocial factors are also very likely to be important - specifically, since our study focused on a hospitalized MA sample, factors that differentially influence men and women with respect to entering into inpatient drug-treatment. For example, sex differences in responsibilities for the care of children and/or family might differentially influence decisions to electively pursue such a prolonged, residential rehabilitation program, or males are possibly focused by law enforcement and thus more likely to enter treatment program at a lower dependence threshold than females. Some studies (Green, 2006, Greenfield et al., 2007) have noted that female substance users reported being less likely to enter a treatment program as compared to males over their lifetime as a result of pregnancy and childcare responsibilities. In contrast, however, Senjo (2005) reported a greater willingness to enter a treatment program among female as compared to male MA dealers (albeit a group that comprised primarily of heavy users). Lin et al. (2004) found that MA female users in Taiwan were more likely to seek treatment than males, a finding they interpret as relating to the greater perceived need for treatment associated with mood disorder (especially depression, which is more common in females). Major depressive episode was excluded from analysis in our study due to the small number of individuals affected.
In addition to the above, we also identified other factors associated with the occurrence of MA dependence that were shared by men and women including nicotine dependence. Nicotine is a legal drug believed to serve as a gateway for subsequent illegal drug use via both biological and psychosocial mechanisms (Senjo, 2005, Green, 2006, Alegria et al., 2013). ASPD, a more common disorder in males than in females in general (Swanson et al., 1994, Moran, 1999, Grant et al., 2004, Compton et al., 2005, Alegria et al., 2013) and in our sample in specific, has been widely associated with various substance use disorders, including that for MA (Kleinman et al., 1990, Goldstein et al., 2007, Glasner-Edwards et al., 2010, Howard et al., 2010, Yang et al., 2014), especially in institutional settings (Regier et al., 1990). Moreover, substance use in individuals with ASPD was also found to correlate with greater substance-related problems compared to those without ASPD (Westermeyer and Thuras, 2005, Goldstein et al., 2007). Thus, our findings that male MA users had higher prevalence rates of ASPD than female users (17.9% vs. 6.2%) is consistent with the broader illicit substance use literature in general and with other studies of ASPD and MA specifically (Lin et al., 2004).
Among the strengths of our study is the larger number of the MA subjects included in the analysis compared to other studies to date. To our knowledge, our study is also the first to examine the severity and symptoms of MA-dependence, as well as MA-dependence associated factors according to sex. These findings may be important implications for sex-specific approaches to the treatment of MA users in Thai substance treatment centers, and perhaps in other populations as well. For example, if replicated, particular attention to the symptomatic management of MA withdrawal in females may be warranted or, in terms of policy, the government should provide a strategy to carefully monitor female MA-users based on the results showing that females were more likely to be MA dependent and to use MA more heavily than males. In addition, greater research into the shared association of, nicotine dependence, in both sexes, seems warranted. Certainly, there are some limitations that need to be considered in the context of the current study, including the non-systematic sampling approach alluded to above as well as the relevant secular trends inasmuch as data collection was affected by the context of a national “war on drugs,” shifts in MA use, and shifts in those receiving treatment. First, the data are cross-sectional, and thus, the causal relationships between associated factors and MA dependence cannot be determined. Second, our study investigated only inpatients, which are likely to have more severe MA use than outpatients (although this may be mitigated by policies permitting anyone to voluntarily receive treatment). Third, given the location of the treatment center in central Thailand, our results may not be representative of all MA users in the country. Although the treatment center in our study is the largest substance use disorder treatment center in Thailand and accepts patient referrals from all regions of the country, the relevance of our findings for other treatment centers in other parts of Thailand remain to be established. Further research examining sex differences associated wtih MA use and dependence in outpatient, multicenter and randomly sampled population-based studies are recommended to confirm the effect of sex on various MA-related characteristics.
Conclusion
Male and female MA users admitted to an inpatient drug-treatment facility in Thailand differed with respect to characteristics of their MA use and dependence and with respect to factors influencing the occurrence of MA dependence traits. Reasons for such differences are currently unknown, although both biological and psychosocial factors are likely to be involved. The observed differences could also reflect ascertainment bias, but we hypothesize that this bias does not account for our findings entirely. Future research aimed at replicating these differences in population-based samples will be important. Understanding such differences may shed light not only on important differences in the risk for and phenotypic expression of the disorder in men and women, but also point to important sex-specific approaches to treatment and prevention strategies.
Supplementary Material
Acknowledgments
We would like to thank Assist. Prof. Chaichana Nimnuan for statistic consultation and Assist Prof. Kuakarun Krusong and Prof. Henry R. Kranzler for valuable comments on the manuscript. We also acknowledge Mr. Wuthichai Hasook for data collection. The study was supported by Chulalongkorn University (Ratchadapiseksompotch Fund, Budget Year 2010), the Thailand Research Fund (TRF; co-funded by the Office of the Higher Education Commission of Thailand and Chulalongkorn University) (RMU5380025, MRG5080249), the Faculty of Medicine, Chulalongkorn University (Ratchadapiseksompotch Fund; RA056/50, RA005/51, RA/54) and supported by a US–Thai training grant (D43TW009087; J.G. & R.T.M.) co-funded by the Fogarty International Center (FIC), National Institute on Drug Abuse (NIDA) and National Human Genome Research Institute (NHGRI), and a NIDA career award (K24 017899; R.T.M.)
Footnotes
The authors have no conflict of interest to declare.
References
- Alegria AA, Blanco C, Petry NM, Skodol AE, Liu SM, Grant B, et al. Sex differences in antisocial personality disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions. Personality disorders. 2013;4(3):214–22. doi: 10.1037/a0031681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assanangkornchai S, Pattanasattayawong U, Samangsri N, Mukthong A. Substance use among high-school students in southern Thailand: Trends over 3 years (2002–2004) Drug and Alcohol Dependence. 2007;86(2–3):167–74. doi: 10.1016/j.drugalcdep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon S-J, et al. Increased white matter hyperintensities in male methamphetamine abusers. Drug and Alcohol Dependence. 2006;81(1):83–8. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Glatt SJ, Everall IP, Tsuang MT. Genetic association studies of methamphetamine use disorders: A systematic review and synthesis. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B(8):1025–49. doi: 10.1002/ajmg.b.30936. [DOI] [PubMed] [Google Scholar]
- Brecht M-L, O'Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addictive Behaviors. 2004;29(1):89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Cohen A. Crazy for Ya Ba: Methamphetamine use among northern Thai youth. International Journal of Drug Policy. 2014;25(4):776–82. doi: 10.1016/j.drugpo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005 doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of general psychiatry. 2007;64(5):566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: A review. Gender Medicine. 2008;5(1):24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug and alcohol review. 2010;29(1):12–20. doi: 10.1111/j.1465-3362.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Compton WM, Pulay AJ, Ruan WJ, Pickering RP, Stinson FS, et al. Antisocial behavioral syndromes and DSM-IV drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;90(2-3):145–58. doi: 10.1016/j.drugalcdep.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Saha TD, Ruan WJ, Compton WM, Grant BF. Antisocial behavioral syndromes and DSM-IV alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcoholism, clinical and experimental research. 2007;31(5):814–28. doi: 10.1111/j.1530-0277.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Ang A, McCann MJ, Rawson RA. An emerging problem: methamphetamine abuse among treatment seeking youth. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2008;29(2):71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan W, et al. Prevalence, correlates, and disability of personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2004 doi: 10.4088/jcp.v65n0711. [DOI] [PubMed] [Google Scholar]
- Green CA. Gender and use of substance abuse treatment services. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Kanayama G, Pope HG., Jr Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. The American journal of psychiatry. 2010;167(1):17–23. doi: 10.1176/appi.ajp.2009.08111695. [DOI] [PubMed] [Google Scholar]
- He J, Xie Y, Tao J, Su H, Wu W, Zou S, et al. Gender differences in socio-demographic and clinical characteristics of methamphetamine inpatients in a Chinese population. Drug Alcohol Depend. 2013;130(1-3):94–100. doi: 10.1016/j.drugalcdep.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Herman-Stahl MA, Krebs CP, Kroutil LA, Heller DC. Risk and protective factors for methamphetamine use and nonmedical use of prescription stimulants among young adults aged 18 to 25. Addictive behaviors. 2007;32(5):1003–15. doi: 10.1016/j.addbeh.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Howard MO, Perron BE, Vaughn MG, Bender KA, Garland E. Inhalant use, inhalant-use disorders, and antisocial behavior: findings from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Journal of studies on alcohol and drugs. 2010;71(2):201–9. doi: 10.15288/jsad.2010.71.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Evans E, Huang Y-C. Treatment outcomes among women and men methamphetamine abusers in California. Journal of substance abuse treatment. 2005;28(1):77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Intharachuti W. Polymorphism of COMT Val158Met is associated with inhalant use and dependence: a Thai substance dependence treatment cohort. Asian Biomed. 2012;6(4) [Google Scholar]
- Kalayasiri R, Kranzler HR, Weiss R, Brady K, Gueorguieva R, Panhuysen C, et al. Risk factors for cocaine-induced paranoia in cocaine-dependent sibling pairs. Drug Alcohol Depend. 2006;84(1):77–84. doi: 10.1016/j.drugalcdep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Mutirangura A, Verachai V, Gelernter J, Malison RT. Risk factors for methamphetamine-induced paranoia and latency of symptom onset in a Thai drug treatment cohort. Asian Biomed. 2010;3(6):635–43. [Google Scholar]
- Kalayasiri R, Verachai V, Gelernter J, Mutirangura A, Malison RT. Clinical features of methamphetamine-induced paranoia and preliminary genetic association with DBH-1021C-->T in a Thai treatment cohort. Addiction. 2014 doi: 10.1111/add.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye S, McKetin R, Duflou J, Darke S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction. 2007;102(8):1204–11. doi: 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of general psychiatry. 2000;57(3):261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim JYS, Fendrich M. Gender differences in juvenile arrestees' drug use, self-reported dependence, and perceived need for treatment. Psychiatric Services. 2002;53(1):70–5. doi: 10.1176/appi.ps.53.1.70. [DOI] [PubMed] [Google Scholar]
- Kleinman PH, Miller AB, Millman RB, Woody GE, Todd T, Kemp J, et al. Psychopathology among cocaine abusers entering treatment. The Journal of nervous and mental disease. 1990;178(7):442–7. doi: 10.1097/00005053-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Lin S-K, Ball D, Hsiao C-C, Chiang Y-L, Ree S-C, Chen C-K. Psychiatric comorbidity and gender differences of persons incarcerated for methamphetamine abuse in Taiwan. Psychiatry and Clinical Neurosciences. 2004;58(2):206–12. doi: 10.1111/j.1440-1819.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- Liu A, Kilmarx P, Jenkins RA, Manopaiboon C, Mock PA, Jeeyapunt S, et al. Sexual initiation, substance use, and sexual behavior and knowledge among vocational students in northern Thailand. International family planning perspectives. 2006:126–35. doi: 10.1363/3212606. [DOI] [PubMed] [Google Scholar]
- Lynch W, Roth M, Carroll M. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Malison RT, Kalayasiri R, Sanichwankul K, Sughondhabirom A, Mutirangura A, Pittman B, et al. Inter-rater reliability and concurrent validity of DSM-IV opioid dependence in a Hmong isolate using the Thai version of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) Addictive Behaviors. 2011;36(1):156–60. doi: 10.1016/j.addbeh.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Umlauf A, Abramson I, Badiee J, Woods SP, Atkinson JH, et al. Dynamic indices of methamphetamine dependence and HIV infection predict fluctuations in affective distress: a five-year longitudinal analysis. Journal of affective disorders. 2013;151(2):728–37. doi: 10.1016/j.jad.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P. The epidemiology of antisocial personality disorder. Social psychiatry and psychiatric epidemiology. 1999;34(5):231–42. doi: 10.1007/s001270050138. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex Differences in Striatal Dopamine Release in Healthy Adults. Biological psychiatry. 2006;59(10):966–74. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Kowalczyk WJ, Botsko M, Tomassilli J, Golub SA. Aggregate versus day level association between methamphetamine use and HIV medication non-adherence among gay and bisexual men. AIDS and behavior. 2013;17(4):1478–87. doi: 10.1007/s10461-013-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, et al. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug and Alcohol Dependence. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (eca) study. JAMA. 1990;264(19):2511–8. [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neuroscience & Biobehavioral Reviews. 2004;28(6):533–46. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ruangkanchanasetr S, Plitponkarnpim A, Hetrakul P, Kongsakon R. Youth risk behavior survey: Bangkok, Thailand. Journal of Adolescent Health. 2005;36(3):227–35. doi: 10.1016/j.jadohealth.2004.01.013. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [2015 18th August]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUHnationalfindingresults2012/NSDUHnationalfindingresults2012/NSDUHresults2012.htm. [Google Scholar]
- Sattah MV, Supawitkul S, Dondero TJ, Kilmarx PH, Young NL, Mastro TD, et al. Prevalence of and risk factors for methamphetamine use in northern Thai youth: results of an audio-computer-assisted self-interviewing survey with urine testing. Addiction. 2002;97(7):801–8. doi: 10.1046/j.1360-0443.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods S, Matt G, Meyer R, Heaton R, Atkinson JH, et al. Neurocognitive Effects of Methamphetamine: A Critical Review and Meta-analysis. Neuropsychol Rev. 2007;17(3):275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Senjo SR. Trafficking in meth: an analysis of the differences between male and female dealers. Journal of drug education. 2005;35(1):59–77. doi: 10.2190/966Q-R6Y3-7G08-DTP8. [DOI] [PubMed] [Google Scholar]
- Sherman SG, German D, Sirirojn B, Thompson N, Aramrattana A, Celentano DD. Initiation of Methamphetamine Use Among Young Thai Drug Users: A Qualitative Study. Journal of Adolescent Health. 2008;42(1):36–42. doi: 10.1016/j.jadohealth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Sutcliffe CG, German D, Sirirojn B, Aramrattana A, Celentano DD. Patterns of risky behaviors associated with methamphetamine use among young Thai adults: a latent class analysis. Journal of Adolescent Health. 2009;44(2):169–75. doi: 10.1016/j.jadohealth.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M, Bland R, Newman S. Antisocial personality disorders. Acta Psychiatrica Scandinavica. 1994;89(s376):63–70. [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Archives of general psychiatry. 1998;55(11):967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- UNODC. Global Illicit Drug Trends 2003. Austria: United Nations Office on Drugs and Crime; 2003. [Google Scholar]
- UNODC. Amphetamines and Ecstasy : 2011 Global ATS Assessment. Austria: United Nations Office on Drugs and Crime; 2011. Section LaS. [Google Scholar]
- Vongchak T, Kawichai S, Sherman S, Celentano DD, Sirisanthana T, Latkin C, et al. The influence of Thailand's 2003 ‘war on drugs’ policy on self-reported drug use among injection drug users in Chiang Mai, Thailand. International Journal of Drug Policy. 2005;16(2):115–21. [Google Scholar]
- Westermeyer J, Thuras P. Association of antisocial personality disorder and substance disorder morbidity in a clinical sample. The American journal of drug and alcohol abuse. 2005;31(1):93–110. [PubMed] [Google Scholar]
- Yang M, Mamy J, Zhou L, Liao YH, Wang Q, Seewoobudul V, et al. Gender differences in prevalence and correlates of antisocial personality disorder among heroin dependent users in compulsory isolation treatment in China. Addict Behav. 2014;39(3):573–9. doi: 10.1016/j.addbeh.2013.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.