Abstract
Francisella tularensis is a highly virulent facultative intracellular bacterium and is considered a potential biological warfare agent. Inhalation tularemia can lead to the development of bronchopneumonia, which is frequently fatal without medical intervention. Treatment strategies that directly target the respiratory mucosa may extend the efficacy of therapy, particularly for the medical management of acute aerosol exposure. To this end, we describe an intranasal (i.n.) strategy for the treatment of pulmonary Francisella infection in mice that uses a combinatorial approach with the conventional antibiotic gentamicin and interleukin 12 (IL-12). The i.n. administration of IL-12 alone promoted bacterial clearance and extended the time to death but did not prevent mortality against lethal pulmonary challenge with Francisella tularensis subsp. novicida. However, i.n. treatment with gentamicin and IL-12 therapeutically at 8 and 24 h after challenge markedly enhanced the rate of survival (70 to 100%) against pulmonary infection compared to the rates of survival for animals treated with antibiotic alone (17%) or IL-12 alone (0%). A delay in combinatorial therapy over a span of 4 days progressively decreased the efficacy of this treatment regimen. This combinatorial treatment was shown to be highly dependent upon the induction of endogenous gamma interferon and may also involve the activation of natural killer cells. Together, these findings suggest that IL-12 may be a potent adjunct for chemotherapy to enhance drug effectiveness against pulmonary Francisella infection.
Francisella tularensis is an intracellular gram-negative bacterium that is the causative agent of tularemia (39). F. tularensis can be classified into several subspecies, including those most relevant to human disease: F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B) (11, 41). An additional subspecies, F. tularensis subsp. novicida, shares biochemical and genetic similarities to F. tularensis and is highly virulent in mice (15). F. tularensis has long been considered a potential biological weapon and has been actively studied as a potential germ warfare agent (5, 7, 18). Inhalation of as few as 10 organisms will cause disease that is frequently lethal and that therefore requires prompt medical management.
Treatment of tularemia in humans includes the use of aminoglycosides, particularly streptomycin and gentamicin (7, 12, 13, 25, 27). Treatment regimens with these antibiotics involve prolonged daily therapy, with relapses and failure rates up to 33% (12). Other classes of antibiotics, including the fluoroquinolones, such as ciprofloxacin (38), have demonstrated some efficacy after prolonged treatment in mice (35) and humans (21, 23). Aerosol studies with F. tularensis SCHU 4 in monkeys showed that the animals had to be treated within 24 h of exposure with 13 consecutive daily doses of tetracycline (200 mg intragastrically) to be effective (36). Delay of treatment resulted in febrile episodes and illness. In similar aerosol studies with human volunteers, continual daily treatment with tetracycline for approximately 2 weeks was shown to be required to clear the infection (36). The factors that contribute to the poor performance of conventional antibiotics against intracellular bacteria include reduced cellular uptake (22) and drug inactivation within subcellular compartments (28). A recent study by the Working Group on Civilian Biodefense (7) reported the need to develop a rapid postexposure means of protection against the illicit use of F. tularensis as an airborne bioweapon. Thus, any potential therapeutic application that can be safely administered at a reduced dosage and with a reduced length of treatment may provide a novel strategy that can be used to combat airborne pathogens.
The use of cytokines in combination with conventional antibiotics has shown promise against a variety of intracellular bacteria (34). Interleukin-12 (IL-12) is a pivotal regulatory cytokine that preferentially activates T-helper (Th1) and NK cells to induce the production of gamma interferon (IFN-γ) (42). Previous investigators (1-3, 24, 31) have provided convincing evidence for the use of soluble IL-12 delivered intranasally (i.n.) as a potent and safe (20) vaccine adjuvant for stimulating protective mucosal immunity. The ability of IL-12 to induce efficient Th1 immune responses has been shown to be important in combinatorial immunotherapy (37). IL-12 and antibiotics, such as clarithromycin and rifabutin, have been reported to promote the clearance of Mycobacterium avium (8). In addition, combination therapy with IL-12 and fluconazole was shown to be highly effective against cryptococcal infection (6).
In the present study, we demonstrate the synergistic effect of i.n. treatment with IL-12 and gentamicin in promoting the clearance of Francisella organisms. Our results show that this mode of treatment is highly dependent on IFN-γ and may be a viable strategy for the treatment of pulmonary Francisella infection without the need for prolonged antimicrobial therapy.
MATERIALS AND METHODS
Bacteria.
F. tularensis subsp. novicida was kindly provided by Francis Nano from the Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia, Canada. Bacteria were grown at 37°C in Typticase soy broth (TSB) supplemented with 0.1% cysteine. Aliquots of bacteria were stored at −70°C in TSB containing 80% glycerol.
Mice.
Six- to 8-week-old female BALB/c mice were obtained from the National Cancer Institute (Bethesda, Md.). IFN-γ-deficient (IFN-γ−/−) and wild-type (IFN-γ+/+) BALB/c mice and NK-cell-deficient (C57BL/6J-Lystbg-j) and wild-type (C56BL/6J) mice were obtained from the Jackson Laboratories (Bar Harbor, Maine). The mice were housed in the animal facility at the University of Texas at San Antonio and were provided food and water ad libitum. All animal care and experimental procedures were performed in compliance with Institutional Animal Care and Use Committee guidelines.
Intranasal IL-12 treatment, bacterial load assessment, and survival studies.
For i.n. treatment, the mice were first anesthetized with 3% isoflurane with a rodent anesthesia system (Harvard Apparatus, Holliston, Mass.) (20). Anesthetized animals were treated i.n. on days −1 and 0 with 100 ng of IL-12 in phosphate-buffered saline (PBS) containing 1% normal BALB/c mouse serum (PBS-NMS); control mice were treated with PBS-NMS alone. The i.n. route of IL-12 delivery was chosen on the basis of the findings from preliminary experiments comparing intraperitoneal (i.p.) and i.n. treatments. The i.n. route was found to be the most efficacious, and no toxicity was observed. This is in agreement with previous findings (1-3) on the feasibility of the use of IL-12 as a mucosal adjuvant. All animals were then challenged i.n. with 100 CFU (10 50% lethal doses) of F. tularensis subsp. novicida 4 h after the final treatment. The actual concentration of the bacterial inoculum for each experiment was determined by serial dilution and plating on Trypticase soy agar (TSA) supplemented with 0.1% cysteine. The bacterial loads in groups of infected animals were assessed by removal of the target organs 24 and 72 h after pulmonary challenge. The organs were homogenized with an electric stirrer (Arrow Junior; Kimble/Kontes, Vineland, N.J.). The homogenates were serially diluted, plated on TSA supplemented with 0.1% cysteine, and incubated for 18 to 24 h at 37°C for bacterial enumeration. For the survival studies, the mice were treated either i.n. or i.p. with 100 ng of IL-12 in PBS-NMS on days −1 and 0 and were challenged 4 h later with 100 CFU of F. tularensis subsp. novicida. All animals were monitored daily for morbidity and mortality.
Pulmonary Francisella infection and IL-12-gentamicin treatment.
For i.n. challenge and treatment, the mice were first anesthetized as described above. The animals were immediately challenged i.n. with 1,000 CFU of F. tularensis subsp. novicida in 25 μl of sterile PBS. The larger challenge dose (1,000 CFU, or approximately 100 50% lethal doses) was selected for use in the treatment studies to better assess the efficacy of the combinatorial therapy. Animals were treated i.n. at 8 and 24 h after challenge with 100 μg of gentamicin (Invitrogen, Carlsbad, Calif.) or a combination of gentamicin and 100 ng of recombinant murine IL-12 (R&D Systems, Minneapolis, Minn.) in PBS-NMS. Some groups of mice were treated with IL-12 or PBS-NMS alone. In some experiments, we also examined the effects of delaying treatment against pneumonic tularemia. All mice were monitored daily.
Phagocytosis assay.
The effects of IL-12 and IFN-γ on the intracellular growth of Francisella was studied with murine macrophages (J774 cells; American Type Culture Collection, Manassas, Va.). J774 cells (105 cells/well) were incubated in microtiter plates and infected at a multiplicity of infection of 10:1 with F. tularensis subsp. novicida with or without 5 or 50 ng of recombinant IL-12 or IFN-γ (R&D Systems) per ml for 1 h. The cultures were then treated for an additional 1 h with medium containing gentamicin (10 μg/ml) to eliminate the extracellular bacteria. The cells were then treated for 24 h with or without IL-12 or IFN-γ. The cells were subsequently washed with Hanks balanced salt solution containing 0.1% gelatin, and the cell mixtures were lysed in 0.2% sodium deoxycholate (Sigma) and plated on TSA supplemented with 0.1% cysteine. Colonies were enumerated after 24 to 36 h of incubation at 37°C. Tumor necrosis factor alpha (TNF-α) secretion by activated macrophages was measured by enzyme-linked immunosorbent assay, as described previously (3).
Statistical analysis.
Survival data were analyzed by the Mann-Whitney rank sum test, and the bacterial loads and the results of the in vitro experiments were evaluated by Student's t test with the statistical software program SigmaStat. The data are presented as means ± standard deviations.
RESULTS
Synergistic effect of i.n. IL-12 and antibiotic treatment against pulmonary Francisella infection.
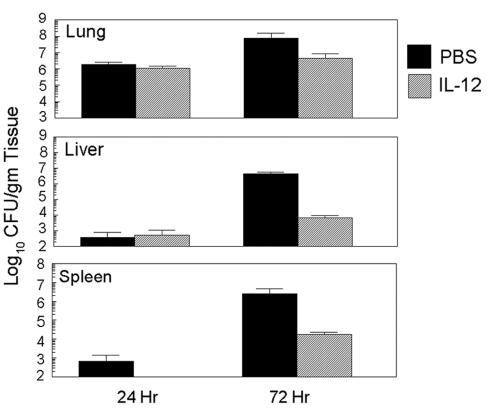
To directly assess whether IL-12 treatment can mediate resistance to pulmonary Francisella infection, BALB/c mice were pretreated (on days −1 and 0) with 100 ng of IL-12 or PBS-NMS i.n. and were subsequently challenged by the same route with 102 CFU of F. tularensis subsp. novicida. The animals were killed at 24 and 72 h after challenge, and the bacterial loads in various target organs were assessed. Within 24 h of pulmonary Francisella infection, similar levels of bacteria were recovered from the lungs and livers of both sets of animals (Fig. 1). However, no bacteria were recovered at 24 h from the spleens of the IL-12-treated animals, whereas they were recovered from the spleens of the controls. By 72 h after i.n. challenge, the IL-12-treated animals displayed a marked reduction of bacteria in the livers and spleens compared to the levels in the livers and spleens of the animals treated with PBS-NMS. To determine the effects of prophylactic treatment with IL-12 on survival, groups of mice were pretreated with IL-12 i.n. or i.p. and were subsequently challenged with 102 CFU of F. tularensis subsp. novicida. It was found that i.n. IL-12 treatment significantly increased the time to death compared to that for the animals that were pretreated i.p. with IL-12 or animals that received vehicle alone (Fig. 2). Therefore, although IL-12 administration clearly modulated the outcome of the bacterial load and the time of survival after infection, this treatment regimen did not reduce the overall rate of mortality among the animals.
FIG. 1.
Effects of intranasal IL-12 treatment on the course of pulmonary tularemia. BALB/c mice (age, 4 to 6 weeks; three mice per group) were pretreated i.n. with 100 ng of IL-12 in PBS-NMS or PBS-NMS only on days −1 and 0. All animals were challenged i.n. with 100 CFU of F. tularensis subsp. novicida 4 h after the last treatment. The animals were killed 24 and 72 h after infection; and the organs were removed, homogenized, and plated. Colonies were enumerated after 24 to 36 h of incubation at 37°C. Results are shown as the means ± standard deviations. The differences in the levels of bacteria in the livers of IL-12-treated and PBS-treated mice at 72 h were significant (P < 0.05).
FIG. 2.
Prophylactic IL-12 treatment enhances protection against pulmonary Francisella challenge. BALB/c mice (five mice per group) were pretreated i.n. (IN) or i.p. (IP) with 100 ng of IL-12 in PBS-NMS on days −1 and 0. As controls, some animals were treated i.n. with PBS-NMS. All animals were challenged i.n. with 100 CFU of F. tularensis subsp. novicida 4 h after the last treatment. The animals were monitored daily for survival. The differences in the times to death between i.n. IL-12-treated mice and PBS-treated mice were significant (P < 0.005).
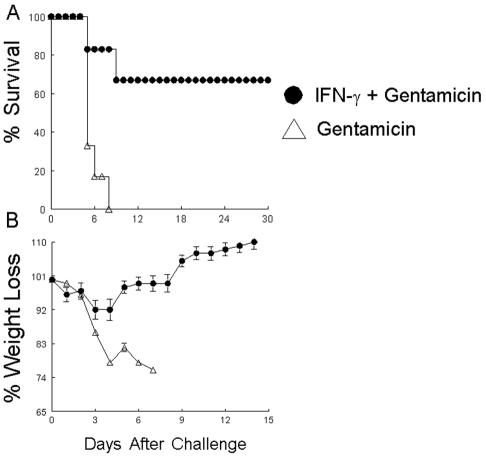
Combinatorial immunotherapy with antibiotics plus cytokines has been shown to be successful against several different organisms (6, 8, 37). To determine the therapeutic efficacy of this approach against Francisella, we used IL-12 and gentamicin, a conventional antibiotic that is used to treat individuals with acquired tularemia (7). BALB/c mice were challenged i.n. with 103 CFU of F. tularensis subsp. novicida. At 8 and 24 h after pulmonary challenge, the mice were treated with combinations of gentamicin (100 μg) and IL-12 (100 ng), gentamicin alone, IL-12 alone, or PBS-NMS. This therapeutic treatment regimen revealed that the combination of IL-12 and gentamicin was highly efficacious, resulting in about 70% survival among animals infected this highly infectious organism (Fig. 3A). This also was evident by the gradual increase in the body weights of the treated animals (Fig. 3B). In contrast to the combinatorial treatment, animals treated with gentamicin alone displayed only 17% survival, and animals receiving either IL-12 or PBS-NMS alone succumbed to the infection by day 6. The clearance of the bacterial infection was also evident by histological analyses, whereby the lungs of animals treated with combinatorial therapy 30 days after the challenge looked markedly healthy, with a minimal presence of organisms (data not shown).
FIG. 3.
(A) Efficacy of combinatorial treatment with gentamicin and IL-12 against pulmonary tularemia. BALB/c animals (six mice per group) were challenged i.n. with 103 CFU of F. tularensis subsp. novicida in 25 μl of sterile PBS. At 8 and 24 h after infection the mice were treated i.n. with 100 μg of gentamicin and 100 ng of soluble recombinant IL-12, 100 μg of gentamicin alone, 100 ng of IL-12 alone, or PBS alone. (B) The animals were weighed and monitored daily for survival. The differences in the rates of survival between mice treated with gentamicin and IL-12 and mice treated with gentamicin alone were significant (P < 0.001).
To determine the efficacy of this combined treatment delivered at later times during an ongoing infection, we delayed the combinatorial treatment regimen over a span of 4 days. As shown in Fig. 4A, delayed treatment resulted in a reduction of the survival rates that correlated with the length of time between challenge and the initiation of therapy. Animals treated at 8 and 24 h after challenge (103 CFU) were highly protected, as expected, with a minimal loss of body weight (Fig. 4B). When the treatment was delayed to 24 and 36 h postinfection, the survival rate dropped to 66%, but it was still statistically significantly (P < 0.05) higher than that for the untreated controls. Further delay of the treatment to 48 and 60 h postchallenge resulted in about 40% survival, whereas delay of treatment to 72 and 84 h after challenge resulted in the deaths of all of the animals.
FIG. 4.
(A) Efficacy of delayed combinatorial treatment against pulmonary tularemia. BALB/c animals (six mice per group) were challenged i.n. with 103 CFU of F. tularensis subsp. novicida. At various intervals after challenge, the mice were treated i.n. with a combination of 100 μg of gentamicin and 100 ng of soluble recombinant IL-12. As a control some animals were treated with PBS alone. (B) The animals were weighed and monitored daily for survival. The differences in the rates of survival between mice treated with gentamicin and IL-12 at 8 and 24 h, 24 and 36 h, and 48 and 60 h and mice treated with PBS alone were significant (P < 0.001).
The effects of combinatorial IL-12-gentamicin treatment are dependent on IFN-γ.
IL-12 administered i.n. or parenterally has profound regulatory effects on the immune system through its ability to preferentially activate Th1 and NK cells and induce IFN-γ production (42). To assess the contribution of IFN-γ in mediating the therapeutic effects of the combined treatment, IFN-γ−/− and corresponding wild-type mice were infected with F. tularensis subsp. novicida and treated 8 and 24 h later with gentamicin plus IL-12. All of the IFN-γ−/− animals succumbed to the infection by day 5, whereas 80% of the IFN-γ+/+ animals were protected through 30 days (data not shown). Since NK cells serve as a prime target for IL-12 and play an important role in innate immunity, we determined the role of these cells in mediating the protective effects of this treatment. Mice defective in NK cell activity (C57BL/6Jbg) and corresponding wild-type (C57BL/6J) animals were infected and treated as described above. Whereas 50% of NK-cell-deficient animals survived the disease, all the wild-type animals were fully protected (data not shown). Only a transient loss of weight was seen in treated wild-type animals, whereas the NK-cell-deficient mice displayed enhanced weight loss and only gradually regained their initial body weights.
Since the effect of IL-12 on drug-induced bacterial clearance is dependent on IFN-γ, we investigated whether therapeutic administration of IFN-γ would directly augment the effects of gentamicin. Mice infected i.n. with F. tularensis subsp. novicida were treated at 8 and 24 h after challenge with a combination of either 100 ng of murine IFN-γ and gentamicin or gentamicin alone. As shown in Fig. 5, IFN-γ effectively substituted for the activity of IL-12 and promoted survival against F. tularensis subsp. novicida. In contrast, i.n. treatment with gentamicin alone was ineffective in controlling the disease. These results suggest that the combinatorial effects of IL-12-gentamicin treatment are highly dependent on IFN-γ production.
FIG. 5.
(A) Effects of substitution of IFN-γ for IL-12 in the combinatorial therapy against pulmonary tularemia. BALB/c animals (six mice per group) were challenged i.n. with 103 CFU of F. tularensis subsp. novicida. At 8 and 24 h after infection, the mice were treated i.n. with 100 μg of gentamicin and 100 ng of soluble recombinant IFN-γ or 100 μg of gentamicin alone. (B) The animals were weighed and monitored daily for survival. The differences in the rates of survival between mice treated with gentamicin and IFN-γ and mice treated with gentamicin alone were significant (P < 0.001).
IFN-γ treatment effectively inhibits intracellular replication of Francisella.
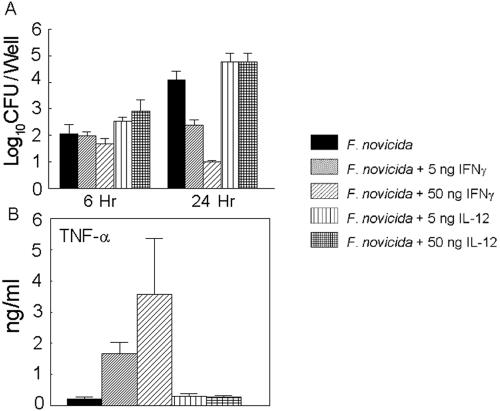
To determine the effects of IL-12-IFN-γ treatment on bacterial replication, J774 macrophages were infected with F. tularensis subsp. novicida in the presence or the absence of recombinant IL-12 or IFN-γ for 24 h. The cells were lysed, and the homogenates were plated to determine bacterial growth. As shown in Fig. 6A, F. tularensis subsp. novicida replicates significantly in macrophages over a span of 24 h. Treatment of cells with either 5 or 50 ng of IL-12 did not inhibit bacterial replication. However, macrophages treated with either 5 or 50 ng of IFN-γ during the 24-h period markedly inhibited replication of the bacteria in a concentration-dependent manner. Upon activation, murine macrophages are known to produce TNF-α, which plays a pivotal role in the antimicrobial effects of these cells (19). Whereas very little TNF-α was detected in macrophages exposed to bacteria with or without IL-12, cells incubated with bacteria in the presence of IFN-γ induced significant amounts of TNF-α (a 9-fold increase in the presence of 5 ng of IFN-γ and an 18-fold increase in the presence of 50 ng of IFN-γ) at 24 h (Fig. 6B). Therefore, enhanced TNF-α secretion by IFN-γ-stimulated cells may be involved in the significant inhibition of F. tularensis subsp. novicida replication.
FIG. 6.
(A) Effects of IL-12 or IFN-γ on intracellular growth of Francisella. J774 cells (105 cells/well) were infected at a multiplicity of infection of 10:1 with F. tularensis subsp. novicida with or without 5 or 50 ng of recombinant IL-12 or IFN-γ per ml for 1 h and were then treated for an additional 1 h with medium containing gentamicin. The cells were then treated for 24 h with or without IL-12 or IFN-γ. The cells were washed with Hanks balanced salt solution containing 0.1% gelatin, and the cell mixtures were lysed in 0.2% sodium deoxycholate and plated on TSA supplemented with 0.1% cysteine. The colonies were enumerated after 24 to 36 h of incubation at 37°C. (B) TNF-α levels in bacterial cultures treated with IL-12 or IFN-γ. The level of TNF-α production in culture supernatants after 24 h of treatment was measured by enzyme-linked immunosorbent assay. The results are shown as the means ± standard deviations. The differences in TNF-α levels between cells cultured with bacteria and IFN-γ and cells cultured with bacteria alone were significant (P < 0.005).
DISCUSSION
Management of intracellular bacterial infections with conventional antibiotic therapy is difficult. Antibiotic treatment of such infections often results in high treatment failure and relapse rates (9, 12). Moreover, many of the obligate and facultative intracellular bacteria have evolved defense mechanisms that allow replication within phagocytic cells, thereby allowing them to resist intracellular killing and avoid immune surveillance (14, 34, 46). The respiratory tract and the lungs are major portals of entry for inhalation and aerosol infections and serve as primary sites of infection before systemic spread. The delivery of bioweapons such as F. tularensis and Bacillus anthracis by these routes has an additional dimension because of the extreme infectivities of the organisms, the ease of transmission, and the high fatality rate from infections with these organisms. This was particularly evident with the illicit disseminations of inhalation B. anthracis and the resulting deaths from those incidents (4). All these obstacles pose significant challenges, particularly with intracellular bacteria, such as F. tularensis, that are highly infectious and that are the agents of biological warfare. Therefore, there is substantial interest from a public health management standpoint in developing prophylactic and therapeutic antimicrobial treatment strategies that can directly target the respiratory epithelium. Here we show the effectiveness of i.n. combinatorial treatment with gentamicin plus IL-12 for the therapeutic treatment of pulmonary tularemia. Such treatment protocols that enhance the antimicrobial activities of antibiotics without increasing the drug dosage or prolonging drug use are promising alternatives as therapies against F. tularensis infection.
Combinatorial therapy with cytokines plus antibiotics has been proven to be successful for the treatment of persistent intracellular pathogens. The combination of IFN-γ and either vancomycin or gentamicin has been reported to improve therapy against drug-resistant Enterococcus faecalis infection in mice (32, 33). Doherty and Sher (8) showed that low doses of IL-12 together with either clarithromycin or rifabutin decreased the splenic loads of M. avium to a greater extent than treatment with the cytokine or drugs alone. Similar strategies have been described for combined treatments for fungal infections that are extremely difficult to treat with conventional drugs (37). The synergistic effects of these different cytokine and drug combinations have been attributed to the enhanced uptake of the drug (40) and to activation of phagocytic cells to increase the levels of oxidative burst and killing (44). Using another alternative strategy for the treatment of pulmonary tularemia, Wong et al. (45) demonstrated that aerosol administration of liposome-encapsulated ciprofloxacin prolonged the retention of the drug in the lower respiratory tract and provided enhanced protection against lethal challenge compared to the protection offered by the free drug alone.
Using an i.n. treatment approach to target the respiratory compartment directly, we have shown the effectiveness of combinatorial therapy with gentamicin plus IL-12 for the treatment of pulmonary tularemia. This treatment regimen administered twice, i.e., at 8 and 24 h, after challenge significantly protected the animals from pulmonary tularemia compared with the protection that animals treated either with the drug alone or with IL-12 alone received. A delay of the combinatorial therapy to 24 and 36 h was still highly effective against the advanced form of the pulmonary disease, as seen by the 67% rate of survival, while further delay of this therapy to 48 and 60 h resulted in 33% survival. Although combinatorial therapy needs be initiated by at least 60 h after infection, a shorter delay may be necessary when dealing with the more virulent subspecies of the pathogen in humans. Thus, the combined therapy is most likely useful for the treatment of a known exposure to the pathogen rather than following an unexpected bioterrorist attack, when individuals would likely present at clinics with nondiagnostic symptoms several days after exposure and when the window of treatment may have expired.
Our results also show that the effects of the combinatorial approach for the treatment of pulmonary tularemia are primarily mediated by IFN-γ and that NK cells may contribute to the clearance of this pulmonary infection. IFN-γ has a variety of immunoregulatory functions, which include the induction of Th1 cell differentiation and the activation of NK cells (42). After IL-12 treatment the greater reduction of bacterial loads in the liver and spleen compared to that in the lungs, the primary site of infection, may be attributed to the increased numbers of NK cells and NK T cells in these organs that can be directly activated by IL-12 to enhance cytolytic activity and the killing of infected cells (26). IFN-γ has also been shown to directly activate macrophage activity and killing (30). The failure of the combinatorial therapy in infected IFN-γ-deficient animals supports the pivotal role of IFN-γ in activating innate defenses. In addition, IFN-γ-activated macrophages very efficiently mediated the intracellular killing of Francisella in vitro compared to the level of killing mediated by cells treated with IL-12. This enhanced killing may be related to the augmented induction of TNF-α seen after IFN-γ treatment of the macrophages. These results are in agreement with those of Fortier et al. (16), who have shown that TNF-α may act as an autocrine signal to amplify IFN-γ-induced production of NO to inhibit the growth of Francisella. Although IL-12 plays a major role in regulating the IFN-γ-mediated clearance of Francisella infection, mechanisms independent of IFN-γ that involve the IL-12 p40 subunit (10) alone or in combination with cytokines such as IL-23 (43) may be involved.
Our results show that direct administration of IFN-γ acts in synergy with gentamicin to promote bacterial clearance. However, IL-12 may be the more appropriate cytokine for combinatorial therapy for several reasons. Foremost, IL-12 has a longer sustainable half-life than IFN-γ in vivo, is produced upstream of IFN-γ, and should induce amounts of IFN-γ greater than those provided by the injected dose (17). Additionally, i.n. treatment with IL-12 is associated with very little toxicity, as demonstrated by a recent report (20) that shows that IL-12 delivered i.n. induces less systemic IFN-γ production and fewer pathological changes. We are aware that i.n. pulmonary challenges tend to deposit in the respiratory mucosa, whereas aerosol delivery results in dispersion of the infectious inoculum into the alveoli (29). Thus, while the combinatorial therapy is highly effective against F. tularensis subsp. novicida, it would be important to determine if the same treatment regimen is also successful against aerosol challenges with F. tularensis subsp. tularensis, which is highly virulent for humans and mice.
In summary, the findings of the present study indicate a feasible alternative treatment approach for pulmonary tularemia. The i.n. delivery of combinatorial therapy with IL-12 and gentamicin directly targets the respiratory tract and promotes clearance of the organism without requiring increased doses of the drug alone. This combinatorial cytokine-drug therapy may also be generally applicable to other biological warfare agents that primarily infect the host via the respiratory tract.
Acknowledgments
We thank Jacqueline Coalson (UTHSCSA) and Ashlesh Murthy (UTSA) for histology consultations and gratefully acknowledge Neal Guentzel (UTSA) for critical review of the manuscript.
This work was supported by a PREF award from UTHSCSA and was partially supported by National Institutes of Health grants AR048973-02, SO6 GM008194-24, and WRCE U54 AI057156.
REFERENCES
- 1.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., R. H. Raeder, J. G. Nedrud, D. J. Bucher, J. Le, and D. W. Metzger. 2001. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166:226-231. [DOI] [PubMed] [Google Scholar]
- 4.Brookmeyer, R., and N. Blades. 2002. Prevention of inhalational anthrax in the U.S. outbreak. Science 295:1861. [DOI] [PubMed] [Google Scholar]
- 5.Christopher, G. W., T. J. Cieslak, J. A. Pavlin, and E. M. Eitzen, Jr. 1997. Biological warfare. A historical perspective. JAMA 278:412-417. [PubMed] [Google Scholar]
- 6.Clemons, K. V., E. Brummer, and D. A. Stevens. 1994. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob. Agents Chemother. 38:460-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, T. M., and A. Sher. 1998. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J. Immunol. 160:5428-5435. [PubMed] [Google Scholar]
- 9.Donowitz, G. R. 1994. Tissue-directed antibiotics and intracellular parasites: complex interaction of phagocytes, pathogens, and drugs. Clin. Infect. Dis. 19:926-930. [DOI] [PubMed] [Google Scholar]
- 10.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enderlin, G., L. Morales, R. F. Jacobs, and J. T. Cross. 1994. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin. Infect. Dis. 19:42-47. [DOI] [PubMed] [Google Scholar]
- 13.Evans, M. E., D. W. Gregory, W. Schaffner, and Z. A. McGee. 1985. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 64:251-269. [PubMed] [Google Scholar]
- 14.Fan, P., F. Dong, Y. Huang, and G. Zhong. 2002. Chlamydia pneumoniae secretion of a protease-like activity factor for degrading host cell transcription factors required for major histocompatibility complex antigen expression. Infect. Immun. 70:345-349. (Erratum, 70:1664.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsman, M., G. Sandstrom, and A. Sjostedt. 1994. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int. J. Syst. Bacteriol. 44:38-46. [DOI] [PubMed] [Google Scholar]
- 16.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gately, M. K., U. Gubler, M. J. Brunda, R. R. Nadeau, T. D. Anderson, J. M. Lipman, and U. Sarmiento. 1994. Interleukin-12: a cytokine with therapeutic potential in oncology and infectious diseases. Ther. Immunol. 1:187-196. [PubMed] [Google Scholar]
- 18.Harris, S. 1992. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann. N. Y. Acad. Sci. 666:21-52. [DOI] [PubMed] [Google Scholar]
- 19.Havell, E. A. 1992. Role of TNF in resistance to bacteria. Immunol. Ser. 56:341-363. [PubMed] [Google Scholar]
- 20.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, A., L. Berglund, L. Gothefors, A. Sjostedt, and A. Tarnvik. 2000. Ciprofloxacin for treatment of tularemia in children. Pediatr. Infect. Dis. J. 19:449-453. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. D., W. L. Hand, J. B. Francis, N. King-Thompson, and R. W. Corwin. 1980. Antibiotic uptake by alveolar macrophages. J. Lab. Clin. Med. 95:429-439. [PubMed] [Google Scholar]
- 23.Limaye, A. P., and C. J. Hooper. 1999. Treatment of tularemia with fluoroquinolones: two cases and review. Clin. Infect. Dis. 29:922-924. [DOI] [PubMed] [Google Scholar]
- 24.Marinaro, M., P. N. Boyaka, R. J. Jackson, F. D. Finkelman, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. Use of intranasal IL-12 to target predominantly Th1 responses to nasal and Th2 responses to oral vaccines given with cholera toxin. J. Immunol. 162:114-121. [PubMed] [Google Scholar]
- 25.Mason, W. L., H. T. Eigelsbach, S. F. Little, and J. H. Bates. 1980. Treatment of tularemia, including pulmonary tularemia, with gentamicin. Am. Rev. Respir. Dis. 121:39-45. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita, T., K. Ando, K. Kimura, H. Ohnishi, M. Imawari, Y. Muto, and H. Moriwaki. 1999. IL-12 induces specific cytotoxicity against regenerating hepatocytes in vivo. Int. Immunol. 11:657-665. [DOI] [PubMed] [Google Scholar]
- 27.Maurin, M., N. F. Mersali, and D. Raoult. 2000. Bactericidal activities of antibiotics against intracellular Francisella tularensis. Antimicrob. Agents Chemother. 44:3428-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurin, M., and D. Raoult. 1996. Optimum treatment of intracellular infection. Drugs 52:45-59. [DOI] [PubMed] [Google Scholar]
- 29.McMurray, D. N. 2001. Disease model: pulmonary tuberculosis. Trends Mol. Med. 7:135-137. [DOI] [PubMed] [Google Scholar]
- 30.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada, E., S. Sasaki, N. Ishii, I. Aoki, T. Yasuda, K. Nishioka, J. Fukushima, J. Miyazaki, B. Wahren, and K. Okuda. 1997. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. Immunol. 159:3638-3647. [PubMed] [Google Scholar]
- 32.Onyeji, C. O., K. Q. Bui, D. P. Nicolau, C. H. Nightingale, L. Bow, and R. Quintiliani. 1999. Influence of adjunctive interferon-gamma on treatment of gentamicin- and vancomycin-resistant Enterococcus faecalis infection in mice. Int. J. Antimicrob. Agents 12:301-309. [DOI] [PubMed] [Google Scholar]
- 33.Onyeji, C. O., D. P. Nicolau, C. H. Nightingale, and L. Bow. 1999. Interferon-gamma effects on activities of gentamicin and vancomycin against Enterococcus faecalis resistant to the drugs: an in vitro study with human neutrophils. Int. J. Antimicrob. Agents 11:31-37. [DOI] [PubMed] [Google Scholar]
- 34.Ouadrhiri, Y., and Y. Sibille. 2000. Phagocytosis and killing of intracellular pathogens: interaction between cytokines and antibiotics. Curr. Opin. Infect. Dis. 13:233-240. [DOI] [PubMed] [Google Scholar]
- 35.Russell, P., S. M. Eley, M. J. Fulop, D. L. Bell, and R. W. Titball. 1998. The efficacy of ciprofloxacin and doxycycline against experimental tularaemia. J. Antimicrob. Chemother. 41:461-465. [DOI] [PubMed] [Google Scholar]
- 36.Sawyer, W. D., H. G. Dangerfield, A. L. Hogge, and D. Crozier. 1966. Antibiotic prophylaxis and therapy of airborne tularemia. Bacteriol. Rev. 30:542-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, D. A. 1998. Combination immunotherapy and antifungal chemotherapy. Clin. Infect. Dis. 26:1266-1269. [DOI] [PubMed] [Google Scholar]
- 38.Syrjala, H., R. Schildt, and S. Raisainen. 1991. In vitro susceptibility of Francisella tularensis to fluoroquinolones and treatment of tularemia with norfloxacin and ciprofloxacin. Eur. J. Clin. Microbiol. Infect. Dis. 10:68-70. [DOI] [PubMed] [Google Scholar]
- 39.Tarnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 40.Taylor, A. P., and H. W. Murray. 1997. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J. Exp. Med. 185:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titball, R. W., A. Johansson, and M. Forsman. 2003. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 11:118-123. [DOI] [PubMed] [Google Scholar]
- 42.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 43.van de Vosse, E., E. G. Lichtenauer-Kaligis, J. T. van Dissel, and T. H. Ottenhoff. 2003. Genetic variations in the interleukin-12/interleukin-23 receptor (beta1) chain, and implications for IL-12 and IL-23 receptor structure and function. Immunogenetics 54:817-829. [DOI] [PubMed] [Google Scholar]
- 44.Wolf, J. E., and S. E. Massof. 1990. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect. Immun. 58:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong, J. P., H. Yang, K. L. Blasetti, G. Schnell, J. Conley, and L. N. Schofield. 2003. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J. Control. Release 92:265-273. [DOI] [PubMed] [Google Scholar]
- 46.Zhong, G., T. Fan, and L. Liu. 1999. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 189:1931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]