Abstract
Despite widely published speculation regarding a potential potency advantage of short-wavelength (blue-appearing) light for Seasonal Affective Disorder (SAD) treatment, there have been few systematic studies. Those comparing short-wavelength to broad-wavelength (white) light under actual clinical conditions suggest equivalent effectiveness. This multicenter, parallel-group design trial was undertaken to compare the effects of light therapy on SAD using blue (~465nm) versus blue-free (595–612nm) LED lights. Fifty-six medication-free subjects aged 21–64 years who met DSM-IV-TR criteria for recurrent major depression with winter-type seasonal pattern were enrolled in this blinded study at 5 participating centers between January and March 2012. Thirty-five subjects met criteria for randomization to 30 minutes of either blue (~465nm) or blue-free (595–612nm) daily morning light therapy. Twenty-nine subjects completed the study; three subjects withdrew due to treatment-related adverse events, including migraines, and three withdrew for non-study-related reasons. The primary effectiveness variable was depression score (SIGH-ADS) after six weeks of daily light treatment. Secondary effectiveness variables included Quality of Life and suicidality ratings. Using an intent-to-treat analysis, mean depression scores were different at baseline for the blue group (29±5 vs. 26±5, p=0.05 blue vs. blue-free, respectively), and initial score was used as a covariate. Baseline scores were not significantly different between treatment groups among those who completed the study, and no significant differences in depression scores were observed after 6 weeks (mean ± s.d. scores at 6 weeks: 5.6±6.1 vs. 4.5±5.3, p=0.74, blue vs. blue-free, respectively). In addition, the proportion of subjects who met remission criteria, defined as a depression score ≤8, was not significantly different between the two groups (p=0.41); among the 29 subjects who completed the study, 76% of subjects experienced remission by the end of the trial, which coincided with the beginning of spring. Quality of Life and suicidality ratings were also significantly improved from pre- to post-treatment, with no significant difference between treatments. No subject experienced worsening or non-improved symptoms over the 6-week trial. The main finding of this study is that subjects treated with blue light did not improve more than subjects treated with blue-free light; both showed substantial improvement on multiple measures. Failure to find differences may have resulted from methodological constraints, including a small sample size. Recruitment began mid-winter during an unusually mild season, and the trial was terminated earlier than planned by the study sponsor due to a failure to detect difference. However, if confirmed in a larger randomized sample, these results suggest that blue wavelengths are not necessary for successful SAD treatment.
Keywords: Seasonal Affective Disorder, Light treatment, Wavelength
Introduction
Recurrent major depression with a fall/winter seasonal pattern, also known as Seasonal Affective Disorder (SAD), is a prevalent and disruptive disorder (Magnusson, 2000; Levitt & Boyle, 2002). Since the 1990s, cool-white fluorescent sources capable of yielding 10,000 lux polychromatic white light have been the treatment standard (Lam & Levitt, 1999; Golden, Gaynes et al., 2005). Although generally well-tolerated, some transient adverse effects of 10,000 lux white light have been reported clinically (Labbate, Lafer et al., 1994; Kogan & Guilford, 1998; Terman & Terman, 1999), such as agitation or feeling “wired,” insomnia, headache, eye or vision problems, nausea, sedation, and chest tightness. The more common of these complaints--headache, eye or vision problems, and insomnia--remit rapidly after discontinuation of light exposure (Oren, Brainard et al., 1991).
These findings have motivated an examination of alternative light sources. Prior to the availability of LED light, a few investigators probed potential wavelength differences in SAD treatment; contrasting green vs. red, and green vs. white, respectively (Oren, Brainard et al., 1991; Stewart, Gaddy et al., 1991).Light-emitting diode (LED) sources became available after the majority of published SAD clinical trials were conducted. For research purposes, LED lights have made it practical to use narrower bandwidth light. If certain wavelengths were more potent in antidepressant effectiveness than white light, it is possible they could be administered at lower intensities to reduce adverse effects and increase tolerability of the treatment (Glickman, Byrne et al., 2006). Desan et al.(Desan, Weinstein et al., 2007) found white LED light superior to placebo negative-ion treatment across a 4-week trial in SAD patients.
Advances in understanding the anatomy and physiology of the ocular system, including the discovery of intrinsically photosensitive retinal ganglion cells (ipRGCs) that express the photopigment melanopsin, revealed differences among specific wavelengths of light on certain non-visual effects (Lucas, Peirson et al., 2014). Studies on the effects of light exposure during the subjective night, including on the suppression of melatonin secretion and phase resetting of the circadian pacemaker, suggest that 446–477 nm wavelengths of light (the visible violet/blue range) are most potent (Brainard, Hanifin et al., 2001; Thapan, Arendt et al., 2001; Lockley, Brainard et al., 2003). In addition, exposure to short-wavelength light consistently enhances neurobehavioral performance and subjective alertness during both the subjective night and daytime hours (Lockley, Evans et al., 2006; Rahman, Flynn-Evans et al., 2014). Glickman et al. used LED sources to contrast blue vs red in SAD patients (Glickman, Byrne et al., 2006).
Previously, we conducted a small randomized 3-week outpatient clinical trial, which found 45 minutes/day of a ~98 lux blue-appearing (narrow-bandwidth 467nm) LED light exposure in the morning performed comparably to a ~700-lux white-appearing (400–700nm) light with respect to decreasing symptoms of depression among SAD patients (Anderson, Glod et al., 2009). Meesters et al. (Meesters, Dekker et al., 2011) compared high intensity (10,000 lux) fluorescent white to blue-enriched white light of lower intensity (750 lux) in SAD outpatients and found improvement after 22 days of treatment in both groups with no significant difference between the treatment groups.
In the present study, we aimed to contrast in SAD patients with no history of mania the efficacy and tolerability of circa 465nm LED light from a portable 11cm × 6cm device (goLITEPro™) with an identical device that emitted virtually no short-wavelength light (i.e., narrow-bandwidth “goLITE”) in order to address whether short-wavelength blue light is not only sufficient but also necessary for effective treatment. Important differentiating characteristics from our previous trial are as follows:
the present trial was extended to 6 weeks to establish the durability of the antidepressant effect.
we instructed subjects to use the light within 30 minutes of awakening in order to better standardize the “dose” of light.
It has long been suggested that the therapeutic response of SAD patients to light exposure involves circadian mechanisms such as phase shifting (e.g., a 30-minute phase advance means that physiologic events regulated by the suprachiasmatic nuclei occurring at 7 a.m. clock time pre-light would occur at 6:30 a.m. after the phase-advance shift), and it is known that the strength of a phase-shift response will depend upon the circadian phase of light administration (Lewy, Sack et al., 1987). The earlier in the morning light is presented, including blue light, the stronger the shift produced (Khalsa, Jewett et al., 2003; St Hilaire, Gooley et al., 2012; Rüger, St Hilaire et al., 2013). We anticipated that the administration of morning light treatment within the first half-hour of the patient’s habitual wake time could maximize the effectiveness of the light treatment, because any advance shifts in circadian phase would be larger (see Instructions to Subjects, Table 1). In this respect, our specific instructions to patients were predicted [based on (Kronauer, Forger et al., 1999; 2000; St Hilaire, Klerman et al., 2007)] to enhance response rates relative to our previous protocol, in which a wider range of morning times for light treatment was allowed.
Table 1.
Instructions to subjects.
| Light Therapy: Instructions for Home Use |
|
The instructions to all subjects in this successful treatment trial address timing of the light exposure relative to the patient’s day/night cycle, distance from the light (based on specific characteristics of the source), and duration of light exposures.
Finally, in order to more broadly characterize the consequences of treatment for SAD, we assessed not only symptoms of depression but also perceived quality of life (QoL). Previously, Michalak and colleagues (2007) reported (based on a multicenter, randomized 8-week clinical trial of 10,000 lux white fluorescent light plus placebo pill versus 20-mg fluoxetine plus placebo light [i.e., the CAN-SAD study (Lam, Levitt et al., 2006)]) that QoL ratings on the Quality of Life Enjoyment and Satisfaction Questionnaire [Q-LES-Q; (Endicott, Nee et al., 1993)] went from markedly impaired at week 1 to significantly improved at week 8 (Michalak, Murray et al., 2007). The QoL self-ratings changed in the same direction as the changes in Hamilton depression ratings, but only ~16% of the variance in QoL at baseline was accounted for by the linear relationship with depression scores.
The present study was designed to test two a priori clinical hypotheses: Depressed SAD patients will demonstrate greater antidepressant therapeutic benefit from a ~465nm (shorter-wavelength) source than from a non-465-nm-emitting source as reflected in (a) depression severity scores and percentage of patients meeting remission criteria based on clinician ratings and (b) self-rated quality of life (H1); depressed SAD patients will manifest no greater adverse effects during treatment with the ~465nm (shorter-wavelength) source than the non-465-nm-emitting source (H2).
Methods
Apparatus
The treatment devices (goLITE®, Philips Healthcare Solutions) emitted either blue-appearing short- (~465nm) or orange-appearing medium-wavelength light (~595nm) from an LED array. Prior to their use in the study, the spectrum of each device was measured by a colorimeter (PR-650 SpectraScan Colorimeter, Photo Research Inc., Chatsworth, CA, USA) by an independent study staff member under ordinary room light conditions (~90 lux, 4100K fluorescent lamps). The average spectrum of 5 devices from each group is shown in Figure 1, which illustrates the difference in “blue” light between the two sources.
Figure 1.
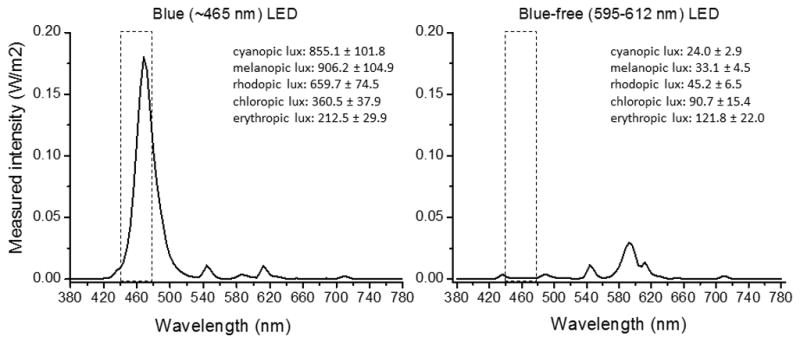
The spectral distribution (measured intensity in W/m2) of the blue (~465 nm) and blue-free (595–612nm) LED devices used in the present study. The box outlines the differences in the two LED devices in the blue component. We report 5 independent irradiance measures (S cone, cyanopic lux; melanopsin, melanopic lux; M cone, rod, rhodopic lux; chloropic lux; and L cone, eryhtropic lux) across the blue LED (n=5 devices) and blue-free LED (n=5 devices) using the toolbox described in Lucas et al. (26).
The total photons (3.4 × 1014 ± 3.7 × 1013 photons m−2 s−1 vs. 8.5 × 1013 ± 1.5 × 1013 photons m−2 s−1, ~465 nm device vs. ~595 nm device, respectively; p < 0.0001 by two-sample t-test) and the measured intensity in lux (149.2±12.1 lux vs. 119.6±21.3 lux, ~465 nm device vs. ~595 nm device, respectively; p = 0.03 by two-sample t-test) were higher from the short-wavelength LED (n=5 independent devices) compared with the medium-wavelength LED (n=5 independent devices) at a distance of 20 inches. Using the irradiance toolbox described in Lucas et al. (2014), we also compared 5 independent irradiance measures ([1] S cone, cyanopic lux; [2] melanopsin, melanopic lux; [3] M cone, rod, rhodopic lux; [4] chloropic lux; and [5] L cone, erythropic lux) across the short-wavelength LED (n=5 devices) and medium-wavelength LED (n=5 devices). The short-wavelength device produced higher irradiance levels for all 5 measures (Figure 1).
Safety
At the irradiance levels emitted by commonly utilized light therapy devices, dermatologic safety concerns are minimal. Similarly, thermal damage to the cornea, lens or retina requires milliwatt-to-watt exposure (Sliney, 2006) (Sliney, personal communication), far in excess of that emitted from the therapeutic devices. Ocular safety for 10,000 lux white fluorescent sources has been assessed, and comprehensive ophthalmologic examinations of individuals with healthy eyes who used white-light therapy daily during the fall/winter months for up to 5 years did not reveal adverse effects (Gallin, Terman et al., 1995). Shorter wavelengths of light are of greater concern due to photo-keratitis of the cornea and cataract of the lens from 180–400nm ultraviolet light, and photochemical injury to the retina at 310–550 nm with a peak near 440 nm (Sliney, personal communication). Both LED sources were determined by a medical physicist to have an averaged radiance well below the 10 mW/cm2*sr safety limit for continuous viewing. Subjects received pretreatment ophthalmological evaluations to screen for a history of ophthalmic disorders or use of photosensitizing medications as well as posttreatment ophthalmological evaluations.
Subject Selection and Randomization
Subjects in this multicenter trial were recruited and treated at one of five participating sites between January and March 2012. The five study sites were the Brigham & Women’s Hospital and McLean Hospital (Boston MA 42°21′28s″N), Mayo Clinic (Rochester MN 44-01′18s″ N), University of Minnesota (Minneapolis MN 44-58′48s″ N), and Clinical Research (Cincinnati OH 39-09′42s″ N).
Consenting procedures were approved by the IRB for each site. In general, prospective subjects at each site met with an experienced clinical psychologist and/or psychiatrist trained to consider both cognitive and emotional factors that affect the ability to consent. Screening sessions were scheduled in advance; nothing in the circumstances of this outpatient protocol required a hasty decision on the part of the potential subject. Upon each outpatient visit for the physical screening and for study procedures, the subject’s mental status was reassessed. Due to the nature of SAD symptoms and the inclusion/exclusion criteria for the study, which excluded patients with a history of mania (Table 2), we did not enroll subjects whose ability to consent was transitory. Prospective subjects were informed that their participation in this study was entirely voluntary and that the decision to participate would in no way affect their further evaluation or therapy. Participants were given a complete and thorough oral explanation of the nature of the study in association with benefits, risks, or discomforts, in accordance with IRB approval. Prospective subjects were also asked to read along as the investigator reviewed the informed consent form, which described the study procedures, expectations, potential risks and benefits, and HIPAA requirement. Subjects were given time to ask questions. Written informed consent was obtained at the site prior to study participation. The subject was provided with a copy of the consent form to take home. Subjects were encouraged to inform their medical providers and therapists about their interest in participating in the study. Subsequently, subjects were followed if necessary until seasonal depression severity reached a Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS) (Williams & Terman, 2003) score of at least 20, which is a clinically-significant moderate level of depression. At that point, the subject was randomized to one of the two treatment groups. The subject was instructed on the appropriate use of the light device. The subject also received a wrist actigraph (Actiwatch Spectrum™, Philips Healthcare, Best, The Netherlands) and a sleep-wake and light treatment diary.
Table 2.
Inclusion and exclusion criteria.
| Inclusion Criteria |
|
| Exclusion Criteria |
|
DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision; GAF: Global Assessment of Functioning; LOCS3: Lens Opacities Classification System III; SCID-I: Structured Clinical Interview for DSM IV Axis I Disorders.
The original sample size calculation for this protocol was based on our previous randomized, double-blind trial which investigated 3-week 45 min/day outpatient treatment with blue-appearing (goLITE®) or blue-enriched white-appearing light in 18 moderately-depressed adults (Anderson, Glod et al., 2009). The difference in depression change between the two treatment groups in that study yielded an effect size of −0.66. Thus, a sample of 40 subjects per group would have been required to attain 80% power to test observed differences between the treatments with a significance level of 0.05. Based on those data, in the present study design, up to 50 subjects per group were planned to be studied over two consecutive winter seasons.
The study was reviewed and approved by the Institutional Review Board (IRB) at each site.
Study Procedures
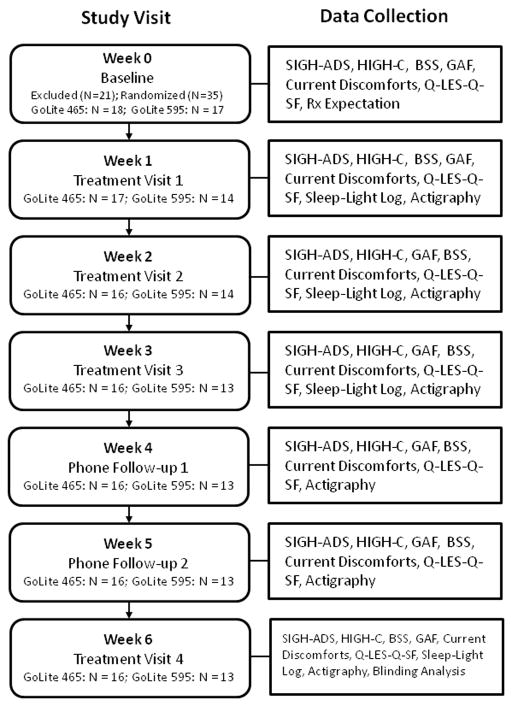
The study procedures are outlined in Figure 2. Symptom rater(s) blind to the treatment condition of the subjects administered the SIGH-ADS, Hypomania Interview Guide (Including Hyperthymic) Current (HIGH-C) (Williams, Terman et al. (1994,1997), and Beck Scale for Suicide Ideation (BSS) (Beck (1991) prior to and for each week during the 6-week trial, in addition to questionnaires covering potential adverse effects. The same rater followed the patient throughout the protocol. Details of the procedures for maintaining double-blind of subjects and clinicians are described in Appendix). Subjects used the light device and returned weekly for the first three weeks of the trial for symptom ratings and quality of life self-report on the Q-LES-Q. At each visit, each subject was seen first by a symptom rater blind to the treatment condition and then separately by a clinician blind to the treatment condition. The clinician collected the subject’s light/sleep diary information and reviewed the use of the light. The symptom rater conducted telephone symptom ratings after week 4 and after week 5. Subjects returned for the final treatment visit at week 6, at which time a blinding analysis was conducted, in which subjects were asked “Do you believe you received a light therapy device designed to improve Seasonal Affective Disorder?” Finally, study personnel met with the subject to discuss his or her response to the light treatment. The data collected each week are outlined in Figure 2.
Figure 2.
A flow chart showing subject progression through the study stages.
The research protocol was designed so that subjects were offered the opportunity to use a commercially-available narrow-spectrum LED device until the end of the fall/winter season and were followed monthly if they chose to do so. The duration and timing of light use were adjusted during this open phase in order to maximize ongoing effectiveness. Alternative treatment modalities also were discussed, and referrals for follow-up care were provided as indicated. All forms of SAD treatment ceased by June 1, and patients returned the light devices at a Termination Visit. However, the number of subjects who received such extended or alternative treatments was insufficient to allow for quantitative analyses.
Biostatistical Analyses
Two analysis populations were evaluated. An intent-to-treat (ITT) analysis included all subjects randomized in the trial and was used for primary effectiveness and safety analyses. For subjects who terminated before the end of the 6-week trial, values were imputed using a “last observation carried forward” approach. For subjects with missing data during one of the intermediate visits, the missing value was replaced with the average of the visit before and after the missing visit. A second effectiveness analysis was done on completed cases, i.e., those who had complete data throughout the treatment period.
Pearson Chi-square tests or Fisher’s exact tests were used to assess baseline differences between the two light therapy groups for categorical variables (gender, race/ethnicity, education level, marital status, study site); t-tests or analysis of variance (ANOVA) were used for continuous variables (age, baseline SIGH-ADS score, baseline Q-LES-Q score). Adherence with instructions for use of the treatment device (i.e., within 30 minutes of wake) was assessed by comparing the time of the self-reported wake time to the self-reported onset of light treatment. The average was computed across all 6 weeks within an individual, and these averages were compared between groups via t-test.
To test the hypothesis that depressed SAD patients would demonstrate greater antidepressant therapeutic benefit from the ~465nm (shorter wavelength) source compared with the ~595nm (longer wavelength) source, we conducted a repeated-measures ANOVA using PROC MIXED in SAS 9.3 with treatment (~465nm vs. ~595nm) as a between-subject factor and time (treatment visit 1, treatment visit 2, treatment visit 3, phone assessment 1, phone assessment 2, and treatment visit 4) as a within-subject factor. This analysis was conducted across all subjects who were randomized for treatment (intent to treat analysis) and all subjects who completed the study (effectiveness analysis). Clinical variables that were found to be significantly different between the two treatment groups at baseline were included as covariates in the mixed model.
The remission rate was computed as a two-level categorical level for each subject. Two definitions of remission were used: 1) at least 50% reduction in SIGH-ADS depression rating at treatment visit 4 compared with baseline; 2) absolute SIGH-ADS rating of 8 or less at treatment visit 4. A Fisher’s exact test was conducted to determine whether the remission rate was different for subjects receiving the ~465nm vs. ~595nm treatment.
As a secondary effectiveness endpoint, we conducted a repeated-measures ANOVA using PROC MIXED in SAS 9.3 with treatment (~465nm vs. ~595nm) as a between-subject factor and time (treatment visit 1, treatment visit 2, treatment visit 3, phone assessment 1, phone assessment 2, and treatment visit 4) as a within-subject factor to test the hypothesis that the Q-LES-Q rating would be significantly improved in patients who received the ~465nm light source compared with the ~595nm light source.
Results
Subject Characteristics
Fifty-six subjects (44 female) were recruited from 5 health care centers. All were between 21–64 years (mean ± SD age = 46.8 ± 11.8 years), medication free, and met criteria for DSM-IV-TR recurrent major depression with a winter-type seasonal pattern as determined by the SCID-I (First, Spitzer et al., 2002). Twenty-one failed to meet all the inclusion/exclusion criteria, including depression severity. Thirty-five subjects were randomized to receive either the ~465nm (short wavelength, n=18) or ~595nm (longer wavelength, n=17) treatment, and 29 subjects completed the trial. On average, subjects started treatment during the first week of February 2012 and ended the study during the second to third week of March 2012; earliest enrollment was from 1/7/2012 to 2/17/2012 and latest enrollment was from 2/18/2012 to 3/29/2012. Six subjects did not complete the study: 3 subjects dropped out of the study after experiencing adverse events (n=2 ~465nm device, n=1 ~595nm device), two of which were related to the study device (n=1 ~465nm device, n=1 ~595nm device); 1 subject dropped out due to a personal emergency unrelated to the study (~595nm device); 1 subject dropped out because he/she felt that the light treatment was not working and had other life stressors that prevented full participation (~595nm device); and 1 subject dropped out due to ongoing gastrointestinal issues and non-compliance with the protocol (~595nm device).
The baseline characteristics of the two subject groups in the intent to treat analysis are reported in Table 3. The subjects in the ~465nm treatment group were significantly older than subjects in the ~595nm treatment group (49.9 ± 11.2 vs. 38.9 ± 11.4, respectively; p = 0.008, t-test), but there were no other demographic differences (i.e., gender, race, education level, marital status, or study site) between the two treatment groups. A significant difference was observed in the baseline SIGH-ADS scores such that the subjects who were randomized to receive the ~595nm treatment were significantly less depressed (mean ± standard deviation: 25.8 ± 5.1) than subjects who were randomized to receive the ~465nm treatment (29.4 ± 5.3)(p = 0.05, t-test). However, among the 29 subjects (N = 16 at ~465nm and N = 13 at ~595nm) who completed the study, there were no significant differences (p = 0.2) in baseline SIGH-ADS scores (28.9 ± 5.2 vs. 26.5 ± 5.0 for ~465nm vs. ~595nm, respectively). There were no significant differences in the baseline Q-LES-Q scores (42.5 ± 7.1 vs. 43.6 ± 6.4 for ~465nm vs. ~595nm, respectively) in the intent to treat analysis; however, 4 subjects (N = 2 ~465nm device, N=2 ~595nm device) had missing answers on their baseline Q-LES-Q and were not included in the analysis of the baseline score. Age and baseline SIGH-ADS scores were included as covariates in subsequent analyses, but did not have a significant impact on the results.
Table 3.
Subject characteristics by treatment group.
| Treatment Group
| |||
|---|---|---|---|
| Variable | goLite 465 | goLite 595 | p-value |
| Number of subjects | 18 | 17 | |
| Age, years | 49.9 ± 11.2 | 38.9 ± 11.4 | 0.008 |
| Gender, N (%) | 0.4 | ||
| Male | 6 (33.3) | 3 (17.6) | |
| Female | 12 (66.7) | 14 (82.4) | |
| Race, N (%) | 0.6 | ||
| Caucasian | 17 (94.4) | 15 (88.2) | |
| Other | 1 (5.6) | 2 (11.8) | |
| Education level | 0.3 | ||
| Technical or Some College | 4 (22.2) | 3 (17.6) | |
| 2-Year Degree | 4 (22.2) | 1 (5.8) | |
| 4-Year Degree | 7 (38.9) | 6 (35.3) | |
| Master’s or Doctoral | 3 (16.7) | 7 (41.2) | |
| Marital status | 1.0 | ||
| Single | 6 (33.3) | 6 (35.3) | |
| Married or Partnered | 8 (44.4) | 7 (41.2) | |
| Divorced or Widowed | 4 (22.2) | 4 (23.5) | |
| Study site, N (%) | 1.0 | ||
| BWH | 1 (5.6) | 2 (11.8) | |
| McLean | 1 (5.6) | 0 (0.0) | |
| Minnesota | 2 (11.1) | 2 (11.8) | |
| Mayo | 11 (61.1) | 11 (64.7) | |
| Comm Res | 3 (16.7) | 2 (11.8) | |
| Baseline SIGH-ADS | 29.4 ± 5.3 | 25.8 ± 5.1 | 0.05 |
| Baseline Q-LES-Q-SF | 42.5 ± 7.1 | 43.6 ± 6.4 | 0.5 |
Q-LES-Q-SF: Quality of Life Enjoyment and Satisfaction Questionnaire – short form; SIGH-ADS: Structured Interview Guide for the Hamilton Depression Rating Scale, Atypical Depression Symptoms.
Adherence
Sleep-wake and light diary and actigraphy data were available from 28 subjects (n=15 from the ~465nm treatment group and n=13 from the ~595 nm treatment group). Of these, subjects reported both a wake onset and a light treatment onset for a mean of 36.0 ± 6.9 days. Wake times varied widely both within and across participants. Average wake times across individuals ranged from 04:16 to 09:15 (accounting for daylight savings time); no significant differences in wake times were observed between the two groups (06:32 ± 0:57 vs. 06:38 ± 01:15, ~465nm vs. ~595 nm treatment group, respectively; p=0.82). The average onset of light treatment following awakening was 27.4 ± 18.1 minutes. Overall, subjects initiated treatment within 30 minutes of wake 74.3% (±28.1%) of the reported times. There was no significant difference in the onset of timing between the two treatment groups (p=0.4).
SIGH-ADS
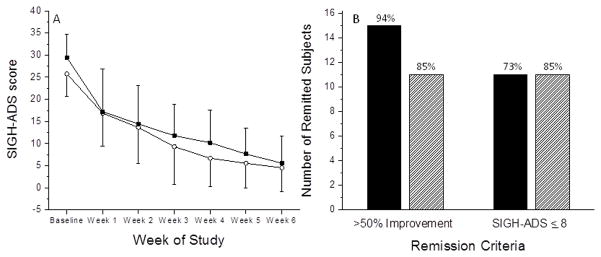
Figure 3A shows the average SIGH-ADS score in each treatment group across each week of the study. A repeated-measures ANOVA on the 35 subjects who were randomized to one of the two treatment conditions revealed that there was no significant difference (p=0.7) in the SIGH-ADS scores across the treatment weeks between the ~465nm and ~595nm treatment groups, and no interaction between the treatment group and the treatment week (p=0.9). There was a significant main effect of the treatment week (p<0.0001), and a post-hoc analysis revealed that subjects were significantly less depressed (p<0.0001) during the final treatment assessment (i.e., after 6 weeks of treatment) compared with the first treatment assessment (i.e., after 1 week of treatment). A repeated-measures ANOVA on the 29 subjects who completed the study revealed similar findings: no significant main effect of treatment condition (p=0.9) or interaction effect of treatment condition and week (p=0.7), but a significant effect of treatment week (p<0.0001). No significant differences in depression scores were observed after 6 weeks of treatment (mean ± s.d. scores at 6 weeks: 5.6 ± 6.1 vs. 4.5 ± 5.3, p=0.7, blue vs. blue-free, respectively).
Figure 3.
(A) The SIGH-ADS score by week for the ~465nm treatment group (filled squares) and the ~595nm treatment group (open circles) is shown. (B) The remission rates for the ~465 nm treatment group (filled bars) and the ~595 nm treatment group (hatched bars) are shown.
A Fisher’s exact test among completed subjects was conducted to determine whether there was a significant difference in remission rate between the subjects receiving ~465nm and those receiving ~595nm. No difference in the number of subjects in which SIGH-ADS was reduced 50% from the baseline score at the final treatment visit was observed between the two groups (p=0.6) (Figure 3B). In addition, no difference in the number of subjects in which SIGH-ADS was 8 or less at the final treatment visit was observed between the two groups (p=0.4) (Figure 3B).
Quality of Life ratings
A repeated-measures ANOVA revealed that there was no significant difference (p=0.2) in the change in Q-LES-Q scores across the treatment weeks relative to baseline between the ~465nm and ~595nm treatment groups and no interaction between the treatment group and the treatment week (p=0.7), but a significant main effect of the treatment week (p<0.0001). Similar findings were observed among subjects who completed all 6 weeks of treatment. Mean Q-LES-Q scores after 6 weeks of treatment were 55.3 ± 9.9 and 57.0 ± 7.8 (~465nm and ~595nm treatment groups, respectively; p = 0.63), representing an ~13-point increase in mean Q-LES-Q scores from baseline in both groups (Table 3). In contrast, Michalak et al. (2007) reported an ~21-point increase in mean Q-LES-Q scores after 8 weeks of treatment.
Adverse Effects
No serious adverse events (AEs) associated with either the blue or blue-free treatments were reported. The most frequent AE reported was headache in 11 subjects (31.4%), of which 6 (17.1%) were deemed to be related to the light treatment. All 6 of these subjects were receiving treatment from the blue-free (~595nm) device. Five subjects (14.3%) also reported migraines, of which 3 (8.6%) were study-related (n=2 ~595 nm treatment, n=1 ~465 nm treatment). Other study-related AEs included nausea (n=1); insomnia (n=1); eye strain (n=1), eye sensitivity (n=1), muscle spasm (n=1), report of a white flashing light (n=1), and report of a “hot spot on the eye” (n=1). Two subjects dropped out due to study-related AEs: one subject receiving the ~465 nm treatment dropped out after experiencing a migraine and a “hot spot on the eye” and one subject receiving the ~595 nm treatment dropped out after experiencing multiple AEs related to the study treatment, including headache, migraine, eye strain, and the report of a white flashing light; a third subject, who was receiving the ~465 nm treatment, dropped out after experiencing migraines unrelated to the study device.
Suicidality
We assessed weekly responses on the BSS. Scores ranged from 0–7 at baseline (mean ± SD: 0.7±1.6), which reflect minimal levels of suicidal ideation. A repeated-measures ANOVA revealed that there was no significant difference in the BSS between groups (p=0.5). There was also no interaction between the treatment group and the treatment week (p=0.9). There was a significant main effect of the treatment week (p=0.008) such that BSS scores improved throughout the 6-week trial. Scores ranged from 0–3 at week 6 (mean±SD: 0.3 ± 0.8), which reflect minimal levels of suicide ideation.
Expectation
All randomized subjects were asked to rate their confidence in receiving an effective light therapy treatment based on the following question: “How confident are you, on a scale from 1 to 10, with 1 being the least confident and 10 being the most confident, that the light therapy treatment you receive in this study will help to improve your Seasonal Affective Disorder?” There was no significant difference in expectation at baseline between the two groups (6.7 ± 1.9 vs. 7.4 ± 1.6, ~465nm and ~595nm treatment groups, respectively; p = 0.30). After one week of treatment, subjects were asked “Do you believe you received a light therapy device designed to improve Seasonal Affective Disorder?” Of those who responded to this question, 16 out of 16 (100%) of the blue device group and 12 out of 13 (92%) of the blue-free device group reported “Yes” to this question. After 6 weeks of treatment, subjects were asked the same question; 15 out of 16 (94%) in the blue device group and 11 out of 13 (85%) of the blue-free device group reported “Yes” to this question.
Discussion
Many randomized clinical trials of bright white fluorescent light for SAD were conducted between 1980 and 2000. Based on the results, light treatment became established as the standard of care. White fluorescent light exposure has been evaluated relative to placebo treatment (Eastman, Young et al., 1998; Lam, Levitt et al., 2006), and meta-analyses have found bright white fluorescent light efficacious (Golden, Gaynes et al., 2005). White fluorescent light treatment has performed well relative to antidepressant medication such as SSRIs (Lam, Levitt et al., 2006). Scientific and technological advances have continued, including both the discovery of ipRGCs as key components of the non-visual effects of light and also the wide availability of LED as light sources. In addition, variations in the gene sequence of melanopsin, the photopigment associated with ipRGCs, and also retinal sensitivity (i.e., post-illumination pupil response) have been associated with seasonal affective disorder (Roecklein, Wong et al., 2013). Although these new developments have generated speculation about certain wavelengths of light (Holzman, 2010) and about use of LED sources, the clinical trial database remains inadequate to evaluate either.
More recent scientific evidence, obtained since the identification of melanopsin-containing retinal ganglion cells projecting to the endogenous circadian pacemaker in the hypothalamus (Güler, Ecker et al., 2008), demonstrate a complex and multifaceted physiology underlying circadian light perception. The functional importance of cone input has been elucidated in animal studies, and data show that blue-sensitive retinal ganglion cells do not act exclusively or in isolation (Walmsley & Brown, 2015). This knowledge influences how relative irradiance of different lights is calculated. Ours is one of the first SAD trials to report all 5 independent representations of irradiance (Lucas, Peirson et al., 2014) for our light sources.*
Earlier speculation that the short-wavelength-sensitive retinohypothalamic pathway might play a unique role clinically (Glickman, Byrne et al., 2006) has not been borne out by preferential antidepressant potency (Anderson, Glod et al., 2009; Meesters, Dekker et al., 2011; Gordijn, t Mannetje et al., 2012). Our current results are consistent with this trend, inasmuch as our clinical trial data do not support the hypothesis that short-wavelength (blue) light is required for improvement of depression in SAD patients. However, our results are in no way conclusive as regards efficacy of LED lights of any wavelength composition. The two devices compared in the current study were not matched on any of the five components of irradiance/intensity. Further clinical trials are needed in order to evaluate the efficacy of any LED sources for SAD treatment and it will be important to address irradiance/intensity equivalence across different sources.
Several limitations in our study must be considered. First, our sample size was limited. Due to lack of evidence for significant differences across treatments after the first winter of the study, the study sponsor discontinued this trial. As a result we were not able to recruit and enroll subjects in the autumn; the majority of subjects began treatment in the late winter months of an uncharacteristically mild season, such that overall improvement rates were likely influenced by gradually increasing day length. Our 6-week protocol resulted in the treatment extending later into the start of the spring season than would have occurred with a 3-week trial. As a result, we cannot differentiate the stability of the antidepressant effect of light treatment across 6 weeks from seasonal changes as causal factors in our observation of sustained remission of depressive symptoms beyond the first 3 weeks of the trial. Finally, our research design was not technically double-blind in that the treatment devices emitted different-appearing light. Our protocol included extensive procedures to simulate a double-blind design, which are detailed in the Appendix.* Notwithstanding these limitations, however, there is nothing in the results to suggest that a powerful and exclusive clinical effect of blue light has been obscured. In addition, the larger question of relative potency of different light sources remains to be directly empirically evaluated.
The “dose” of a light exposure is known from basic research studies of humans to be determined by multiple factors (Dewan, Benloucif et al., 2011; Eastman, 2011; Lucas, Peirson et al., 2014). Wavelength is one such factor, but our data suggest that short wavelengths are not crucial for SAD treatment. Past research on “dose” in SAD treatment has shown that the irradiance (i.e., intensity) of light (specifically considering white fluorescent sources) (Rosenthal, Sack et al., 1985; Terman, Terman et al., 1990a; b), time in the circadian cycle at which exposure occurs (Lewy, Sack et al., 1985; Lewy, Sack et al., 1987; Sack, Lewy et al., 1990; Wirz-Justice, Graw et al., 1993; Lewy, Lefler et al., 2006), and duration of the exposure (Terman, Terman et al., 1989) affect the rate of success in reducing symptoms of depression, just as these factors (irradiance, circadian phase, duration) are known to systematically affect specific phase-resetting, endocrine, and performance-enhancing effects of light exposure in healthy humans and other animals (Zeitzer, Dijk et al., 2000; Khalsa, Jewett et al., 2003; Lockley, Brainard et al., 2003; Lockley, Evans et al., 2006; Gooley, Rajaratnam et al., 2010; Chang, Santhi et al., 2012; St Hilaire, Gooley et al., 2012; Rüger, St Hilaire et al., 2013). Our instructions to subjects (see Table 1) were designed to address these aspects of dose.
In the current study focused on comparing blue- vs. non-blue LED light, we observed in both treatment groups a decline in depression severity, including suicidal ideation, and improved self-reported quality of life across 6 weeks of treatment. Data from wrist actigraphy and patient diaries indicate that our subjects were relatively compliant with the instructions. Based on the light stimuli themselves and the timing of light administration and sleep/wake schedules of our subjects, current mathematical models of human circadian modulation by light suggest that our two treatment conditions would have had relatively equivalent advancing effects on circadian phase, although the short-wavelength light is predicted to have had greater direct alerting affects (see St. Hilaire et al., 2013). These results suggest that further work to evaluate the efficacy of LED light in treatment of SAD should not be confined to short-wavelength sources. Moreover, rather than highlighting blue light in instructions to patients and clinicians, it is important to provide broader advice regarding “dose” which includes the timing, distance from the light source, and duration of the exposure.
Supplementary Material
Footnotes
Previously presented as:
Glod CA, Auger RR, Crow SJ, Rivera AN, Fuentes Salgado SM, Pullen SJ, Lynch AJ, Kaufman TK, Wolfe DJ, Anderson JL. Is short-wavelength “blue” light necessary for treatment of Seasonal Affective Disorder? American Psychiatric Association Annual Meeting May 18–22, 2013 San Francisco, CA, Abstract Number: 2969.
DECLARATION OF INTERESTS: The project was funded by Philips HealthCare Solutions and NIH 1 UL1 RR025758-04 to the Harvard Clinical and Translational Science Center from the National Center for Research Resources. MSH was supported by a National Heart, Lung and Blood Institute fellowship in the program of training in Sleep, Circadian and Respiratory Neurobiology at Brigham and Women’s Hospital (T32 HL079010). Dan Adams was instrumental in design and initiation of this research. All authors declare that they have no conflicts of interest to report.
References
- Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009:1–10. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Scale for Suicide Ideation. San Antonio, TX: Psych Corp. Pearson Education, Inc; 1991. [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. Human responses to bright light of different durations. J Physiol. 2012;590:3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desan PH, Weinstein AJ, Michalak EE, Tam EM, Meesters Y, Ruiter MJ, Horn E, Telner J, Iskandar H, Boivin DB, Lam RW. A controlled trial of the Litebook light-emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD) BMC Psychiatry. 2007;7:38. doi: 10.1186/1471-244X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI. How to get a bigger dose of bright light. Sleep. 2011;34:559–560. doi: 10.1093/sleep/34.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression. Archives of General Psychiatry. 1998;55:883–889. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. (SCID-I/P) [Google Scholar]
- Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. American Journal of Ophthalmology. 1995;119:202–210. doi: 10.1016/s0002-9394(14)73874-7. [DOI] [PubMed] [Google Scholar]
- Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light Therapy for Seasonal Affective Disorder with Blue Narrow-Band Light-Emitting Diodes (LEDs) Biol Psychiatry. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordijn MC, t Mannetje D, Meesters Y. The effects of blue-enriched light treatment compared to standard light treatment in Seasonal Affective Disorder. Journal of Affective Disorders. 2012;136:72–80. doi: 10.1016/j.jad.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman DC. What’s in a color? The unique human health effects of blue light. Environmental Health Perspectives. 2010;118:A23–A27. doi: 10.1289/ehp.118-a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. Journal of Physiology (London) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan AO, Guilford PM. Side effects of short-term 10,000-lux light therapy. The American journal of psychiatry. 1998;155:293–294. doi: 10.1176/ajp.155.2.293. [DOI] [PubMed] [Google Scholar]
- Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. JBiol Rhythms. 1999;14:500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- Kronauer RE, Forger DB, Jewett ME. Errata Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. JBiol Rhythms. 2000;15:184–186. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- Labbate LA, Lafer B, Thibault A, Sachs GS. Side effects induced by bright light treatment for seasonal affective disorder. The Journal of clinical psychiatry. 1994;55:189–191. [PubMed] [Google Scholar]
- Lam RW, Levitt AJ. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. 1999. [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, Tam EM. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. The American journal of psychiatry. 2006;163:805–812. doi: 10.1176/ajp.2006.163.5.805. [DOI] [PubMed] [Google Scholar]
- Levitt AJ, Boyle MH. The impact of latitude on the prevalence of seasonal depression. Can J Psychiatry. 2002;47:361–367. doi: 10.1177/070674370204700407. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Singer CM. Treating phase typed chronobiologic sleep and mood disorders using appropriately timed bright artificial light. Psychopharmacology Bulletin. 1985;21(3):368–372. [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatrica Scandinavica. 2000;101:176–184. [PubMed] [Google Scholar]
- Meesters Y, Dekker V, Schlangen LJ, Bos EH, Ruiter MJ. Low-intensity blue-enriched white light (750 lux) and standard bright light (10,000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry. 2011;11:17. doi: 10.1186/1471-244X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EE, Murray G, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Tam EM, Cheung A, Lam RW. Quality of life as an outcome indicator in patients with seasonal affective disorder: results from the Can-SAD study. Psychological Medicine. 2007;37:727–736. doi: 10.1017/S0033291706009378. [DOI] [PubMed] [Google Scholar]
- Oren DA, Brainard GC, Johnston SH, Joseph-Vanderpool JR, Sorek E, Rosenthal NE. Treatment of seasonal affective disorder with green light and red light. American Journal of Psychiatry. 1991;148:509–511. doi: 10.1176/ajp.148.4.509. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neuroscience and Biobehavioral Reviews. 2013;37:229–239. doi: 10.1016/j.neubiorev.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Carpenter CJ, Parry BL, Mendelson WB, Wehr TA. Antidepressant effects of light in seasonal affective disorder. American Journal of Psychiatry. 1985;142:163–170. doi: 10.1176/ajp.142.2.163. [DOI] [PubMed] [Google Scholar]
- Rüger M, St Hilaire MA, Brainard GC, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a single 6.5h pulse of short-wavelength light. J Physiol. 2013;591(Pt 1):353–363. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, White DM, Singer CM, Fireman MJ, Vandiver R. Morning vs evening light treatment for winter depression. Evidence that the therapeutic effects of light are mediated by circadian phase shifts. Archives of General Psychiatry. 1990;47:343–351. doi: 10.1001/archpsyc.1990.01810160043008. [DOI] [PubMed] [Google Scholar]
- Sliney DH. Risks of occupational exposure to optical radiation. Med Lav. 2006;97:215–220. [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–3045. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Klerman EB, Khalsa SBS, Wright KP, Jr, Czeisler CA, Kronauer RE. Addition of a non-photic component to a light-based mathematical model of the human circadian pacemaker. J Theoretical Biol. 2007;247:583–599. doi: 10.1016/j.jtbi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Klerman EB, Anderson JL. Modeling the morning light effects of 465nm vs. 595nm suggests a direct alerting mechanism in seasonal affective disorder (SAD) patients. Sleep. 2013;36:A316. [Google Scholar]
- Stewart KT, Gaddy JR, Byrne B, Miller S, Brainard GC. Effects of green or white light for treatment of seasonal depression. Psychiatry Research. 1991;38:261–270. doi: 10.1016/0165-1781(91)90016-i. [DOI] [PubMed] [Google Scholar]
- Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, Gallin PF, Quitkin FM. Efficacy of brief, intense light exposure for treatment of winter depression. Psychopharmacology Bulletin. 1990a;26:3–11. [PubMed] [Google Scholar]
- Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, Gallin PF, Quitkin FM. Identification, assessment, and treatment of seasonality in mood disorders. Psychopharmacology Bulletin. 1990b;26 [PubMed] [Google Scholar]
- Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. Journal of Clinical Psychiatry. 1999;60:799–808. [PubMed] [Google Scholar]
- Terman M, Terman JS, Quitkin FM, McGrath PJ, Stewart JW, Rafferty B. Light therapy for seasonal affective disorder: a review of efficacy. Neuropsychopharmacology. 1989 doi: 10.1016/0893-133x(89)90002-x. in press-in press. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley L, Brown TM. Eye-specific visual processing in the mouse suprachiasmatic nuclei. J Physiol. 2015;593:1731–1743. doi: 10.1113/jphysiol.2014.288225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JBW, Terman M, Link MJ, Amira L, Rosenthal NE. Clinical Assessment Tools Packet. Norwood, NJ: Center for Environmental Therapeutics; 1994. Hypomania Interview Guide (Including Hyperthymia). Current assessment Version (HIGH-C) [Google Scholar]
- Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS) New York: New York State Psychiatric Institute; 2003. [Google Scholar]
- Wirz-Justice A, Graw P, Krauchi K, Gisin B, Jochum A, Arendt J, Fisch HU, Buddeberg C, Poldinger W. Light therapy in seasonal affective disorder is independent of time of day or circadian phase. Archives of General Psychiatry. 1993;50:929–937. doi: 10.1001/archpsyc.1993.01820240013001. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.