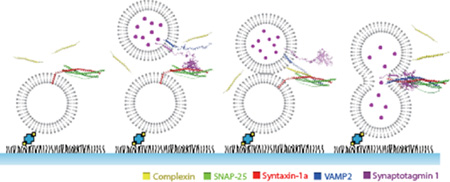
Abstract
Complexin (Cpx) is a major regulator for Ca2+-triggered fast neuroexocytosis which underlies neuronal communication. Many psychiatric and neurological disorders accompany changes in the Cpx expression level, suggesting that abnormal Cpx levels may elicit aberrant cognitive symptoms. To comprehend how the changes in the Cpx level might affect neuronal communication, we investigated Ca2+-triggered exocytosis at various Cpx concentrations. Ca2+-triggered content-mixing between a single proteoliposome of t-SNARE and another single proteoliposome of v-SNARE plus Ca2+-sensor synaptotagmin 1 was examined with total internal reflection microscopy. We find that Cpx enhances Ca2+-triggered vesicle fusion with the yield changing from approximately 10% to 70% upon increasing Cpx from 0 to 100 nM. Unexpectedly, however, the fusion efficiency becomes reduced when Cpx is increased further, dropping to 20% in the micromolar range, revealing a bell-shaped dose–response curve. Intriguingly, we find that the rate of vesicle fusion is nearly invariant through the entire range of Cpx concentrations studied, suggesting that a reevaluation of the current Cpx clamping mechanism is necessary. Thus, our results provide insights into how delicately Cpx fine-tunes neuronal communication.
Graphical abstract
Complexins (Cpx) are a family of small proteins that are specifically localized at the presynapse to regulate neurotransmitter release.1,2 Cpx is thought to modulate both spontaneous and evoked release in the neuron. There is strong evidence that the deletion of Cpx reduces evoked exocytosis significantly,3–8 although controversy surrounds the proposition that Cpx suppresses spontaneous release.4,6,7,9–12
The effect of Cpx on neuroexocytosis is of great interest because changes in Cpx could elicit the disruption of the exocytosis patterns, which could affect behavioral and cognitive activities. While the causal role is yet to be elucidated, indeed apparent changes in Cpx levels have been observed in schizophrenia as well as in neurodegenerative diseases such as Parkinson’s and Alzheimer’s.13
It is generally believed that synaptic vesicle fusion, required for the neurotransmitter release, is mediated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs).14 Additionally, a major Ca2+ sensor synaptotagmin 1 (Syt1)15,16 and the SNARE binding Cpx are considered to be two principal regulatory components that orchestrate fast Ca2+-triggered vesicle fusion.17–19
Toward our understanding of the roles that Cpx plays in neuroexocytosis, in vitro membrane fusion assays have made contributions.19,20 In these assays, SNARE proteins and Syt1 are appropriately reconstituted into the two populations of liposomes and fusion between two respective proteoliposomes is monitored spectroscopically. Of particular interest is the single-vesicle content-mixing assay which is capable of dissecting docking, lipid mixing, and content-mixing steps along the fusion pathway.21,22 The assay revealed that Cpx stimulates and synchronizes Ca2+-triggered vesicle fusion while inhibiting spontaneous fusion.23,24 Moreover, it was shown that Cpx accelerates the rate of vesicle docking.25
Although the results from single vesicle fusion assays have revealed important features of the Cpx function in exocytosis there are a few shortcomings. The assay requires high Ca2+ to produce appreciable yields of vesicle fusion,21 which is still not comparable to the highly efficient evoked vesicle fusion in the neuron.26,27 Moreover, it was shown that overexpression of Cpx reduces Ca2+-triggered exocytosis in cells,28,29 which is not explainable with the qualitative data accumulated so far. This raises some concerns whether the single-vesicle content-mixing assay, in its current form, is sufficiently robust to faithfully recapitulate the essential features of Cpx function in the neuroexocytosis.
In this work, we find that the pretreatment of t-SNARE with Cpx improves the efficiency of vesicle fusion dramatically and recovers the natural high Ca2+-sensitivity. Moreover, with this improved method, we discover that Cpx stimulates Ca2+-triggered exocytosis in a concentration-dependent manner below 100 nM. But the trend reverses its course above 200 nM and shows the dose-dependent decrease in the higher concentration range, resulting in a bell-shaped response curve. Thus, our results describe how the change in the Cpx level might affect the neurotransmitter release quantitatively.
MATERIALS AND METHODS
Plasmid Construct and Site-Directed Mutagenesis
DNA sequences encoding rat Syntaxin 1A (amino acids 1–288 with three native cysteines replaced by alanines), VAMP2 (amino acids 1–116 with C103 replaced by alanines), soluble VAMP2 (VpS, amino acids 1–94), SNAP-25 (amino acids 1–206 with four native cysteines replaced by alanines), rat complexin I (Cpx, amino acids 1–134), truncation mutant Cpx 27 (amino acids 27–134), and double mutant Cpx M5E/K6E were inserted into the pGEX-KG vector as N-terminal GST fusion proteins. Rat synaptotagmin 1 (Syt1, amino acids 50–421 with four native cysteines C74, C75, C77 and C79 replaced by alanines and another C82 replaced by serine) was inserted into pET-28b vector as C-terminal His-tagged proteins. DNA sequences were confirmed by the Iowa State University DNA Sequencing Facility.
Protein Expression and Purification
VAMP2, SNAP-25, syntaxin 1a, VpS, Cpx, and Cpx mutants were expressed as GST fusion proteins. Escherichia coli BL21 Rosetta (DE3) pLysS (Novagen) was used to express the recombinant GST fusion proteins. The cells were grown in LB medium at 37 °C with ampicillin (100 µg/mL) until the ~0.6–0.8 absorbance at 600 nm. Isopropyl β-d-1-thiogalactopyranoside (0.3 mM final concentration) was then added to induce protein expression. The cells were grown for another 12 h at 16 °C. The cell pellets were then harvested via centrifugation at 6000g for 10 min. The pellets were resuspended in PBS at pH 7.4 containing 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 2 mM EDTA, and 2 mM dithiothreitol. Transmembrane proteins required 0.5% Triton X-100, 0.05% Tween 20, and 10% N-lauroylsarcosine additionally in the buffer. Cells were broken up via sonication immersed in an ice bath. The supernatant was collected by centrifugation at 15000g for 20 min. The glutathione-agarose beads in buffer were added and nutated at 4 °C for 2 h. The unbound proteins were then washed out, and the GST fusion proteins were cleaved off from the beads by thrombin (Sigma-Aldrich) at room temperature for 2 h. Thrombin cleavage buffer for membrane proteins contained 50 mM Tris-HCl, 150 mM NaCl, and, pH 8.0 and 1% n-octyl glucoside.
Syt1 (amino acids 51–421) was expressed with the C-terminal 6-histidine-tag in E. coli BL21 Rosetta (DE3) pLysS and purified with the aforementioned protocol except for using Ni-NTA beads (Qiagen). Elution was carried out with buffer of 25 mM HEPES, 400 mM KCl, 500 mM imidazole, and 0.8% OG. Purified proteins were examined with 15% SDS-PAGE, and the purity was at least 90% for all of the proteins.
Proteoliposome Reconstitution
We used the following lipid molecules to make proteoliposomes: 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS), 1-palmitoyl-2-oleoylsnglycero-3-phosphocholine (POPC), phosphatidylinositol-4,5-bisphosphate (PIP2, from porcine brain), and cholesterol. All lipids were obtained from Avanti Polar Lipids. In reconstituting the SNAREs into liposomes, molar ratios of lipids were 15:63:20:2:0.1 (DOPS: POPC: cholesterol: PIP2: biotin-DPPE) for the t-vesicles, and 5:75:20 (DOPS: POPC: cholesterol) for the v-vesicles, respectively. The lipids were mixed in the chloroform soluble state and dried in a glass tube with nitrogen gas and stored overnight in a desiccator under house vacuum. The t-vesicle lipid film was resuspended with HEPES buffer (25 mM HEPES, 100 mM KCl, pH 7.4), whereas v-vesicle lipid film was resuspended with HEPES buffer containing 20 mM SRB (Invitrogen). After 10 freeze–thaw cycles between hot water and liquid nitrogen, large unilamellar vesicles (~100 nm in diameter) were prepared by extrusion through the polycarbonate filter (Avanti Polar lipids). The t-SNAREs were mixed with liposomes (10 mM in total lipid concentration), while VAMP2 and Syt1 were reconstituted with SRB (20 mM)-containing liposomes for ~15 min. We used a 200:1 lipid/protein molar ratio for all reconstitution. The liposome/protein mixture was diluted 2 times with the HEPES buffer and then dialyzed in 2 L dialysis buffer at 4 °C overnight. For the v-vesicles, free SRB was removed using the PD-10 desalting column (GE healthcare) after dialysis.
Single Vesicle Content-mixing Assay and Docking assay
The imaging quartz surface (25 × 75 × 1.0 mm) was PEGylated with the PEG and PEG-biotin mixture with a 40:1 molar ratio (Laysan Bio). The imaging surface was divided to form 10 independent flow chambers. The flow chambers were incubated with streptavidin (0.2 mg/mL, Sigma-Aldrich) for 10 min followed by thorough washing. A mixture containing 125 µM t-vesicles with 100 nM Cpx in HEPES buffer was introduced into the flow chamber, and the t-vesicles were allowed to be immobilized on the PEG-coated surface while maintaining 100 nM Cpx concentration. After 15 min incubation, unbound t-vesicles were washed using HEPES buffer containing 100 µM Cpx. A mixture of 100 nM Cpx and v-vesicles (20 mM SRB) in HEPES buffer was injected, and the sample was incubated in the flow chamber for 10 min to allow vesicle–vesicle docking. The unbound v-vesicles were washed out using HEPES buffer containing 100 nM Cpx. The channels were then imaged and Ca2+ was injected (1.2 mL/1 min) into the flow chamber while recording via TIR microscope. A stepwise jump in the fluorescence intensity was detected as an indication of content-mixing, which was the result of the SRB dequenching. The details of TIR microscope imaging and single molecule data analysis have been reported in our previous work.24 The time when the stepwise increase was observed was recorded manually and plotted onto a histogram with the bin size of 1 s. The histogram was then fitted with a single exponential decay in order to obtain the first-order time constant.
The single vesicle docking experiment was performed identical to the content-mixing assay prior to Ca2+ injection. Once the v-vesicles, with VAMP2 and Syt1, and t-vesicles were incubated and washed with buffer containing the appropriate Cpx concentration, we took multiple images and the immobilized spots were counted and plotted on a histogram. To ensure quality control, the full range of Cpx concentrations was performed on a single PEG slide, with multiple replicates.
RESULTS
Single-Vesicle Content-Mixing Assay
Previously, the effect of Cpx on Ca2+-triggered exocytosis has been studied with the single-vesicle content-mixing assay, initially in our group24 and later, extensively in Brunger’s group.23 Although the experiments have recapitulated some essential features of the Cpx function, there have been two apparent shortcomings. First, the assay requires unusually high Ca2+; several hundreds of µM instead of biologically relevant tens of µM. Second, the outcomes do not explain why the release decreases when Cpx is overexpressed in cells.28,29
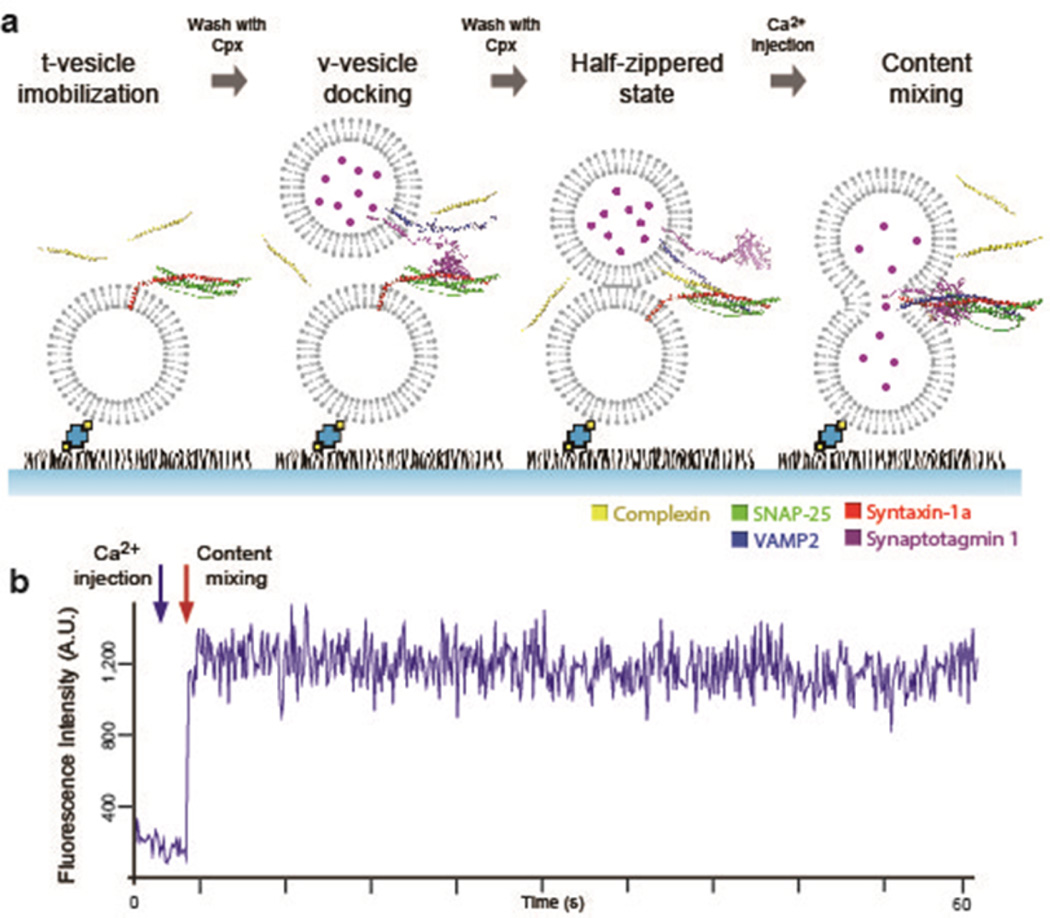
In the single-vesicle content-mixing assay based on total internal reflection (TIR) microscopy, the t-SNARE-carrying liposomes (t-vesicles) were tethered to the imaging surface followed by the docking of v-SNARE plus Syt1-carrying liposomes (v-vesicles) onto the t-vesicles (Figure 1a). In all previous experiments, Cpx was premixed with v-vesicles, and the mixture was flown into the flow cell while t-vesicles were not pretreated with Cpx. This time, however, realizing that Cpx might interact with t-SNAREs,30 we pretreated t-vesicles with Cpx before injecting v-vesicles which were also premixed with Cpx. All subsequent washing of untethered excess vesicles were conducted in the presence of Cpx such that the Cpx concentration would remain constant throughout the experiment. The v-vesicles were encapsulated with ~20 mM sulforhodamine B (SRB) for the fluorescence detection of content-mixing.22 Subsequent Ca2+ injection into the flow chamber promotes vesicle–vesicle fusion that results in content-mixing. Content-mixing induces a step-like sudden rise of the fluorescence intensity (Figure 1b) as a result of dequenching of the SRB fluorescence due to the fusion-induced dilution.
Figure 1.
In vitro single-vesicle content-mixing assay with Cpx. (a) Schematics of the In vitro single-vesicle content-mixing assay with Cpx. The flow chamber maintains constant Cpx concentration throughout the experiment by pretreating t-vesicles with Cpx prior to immobilization to the imaging surface. After the t-vesicles are immobilized on the imaging surface, unbound t-vesicles are washed out with buffer containing the designated Cpx concentration. Subsequent docking and washing of unbound v-vesicles are also performed in the presence of Cpx. Once the v-vesicles and t-vesicles are docked in the presence of Cpx, we inject Ca2+ into the flow chamber to evoke content-mixing which is detected by a sudden stepwise increase of fluorescent intensity. (b) A representative fluorescent intensity time trace is shown. The blue arrow indicates the time of Ca2+ injection and the red arrow depicts content-mixing.
Bell-Shaped Response of Ca2+-Triggered Vesicle Fusion to Cpx
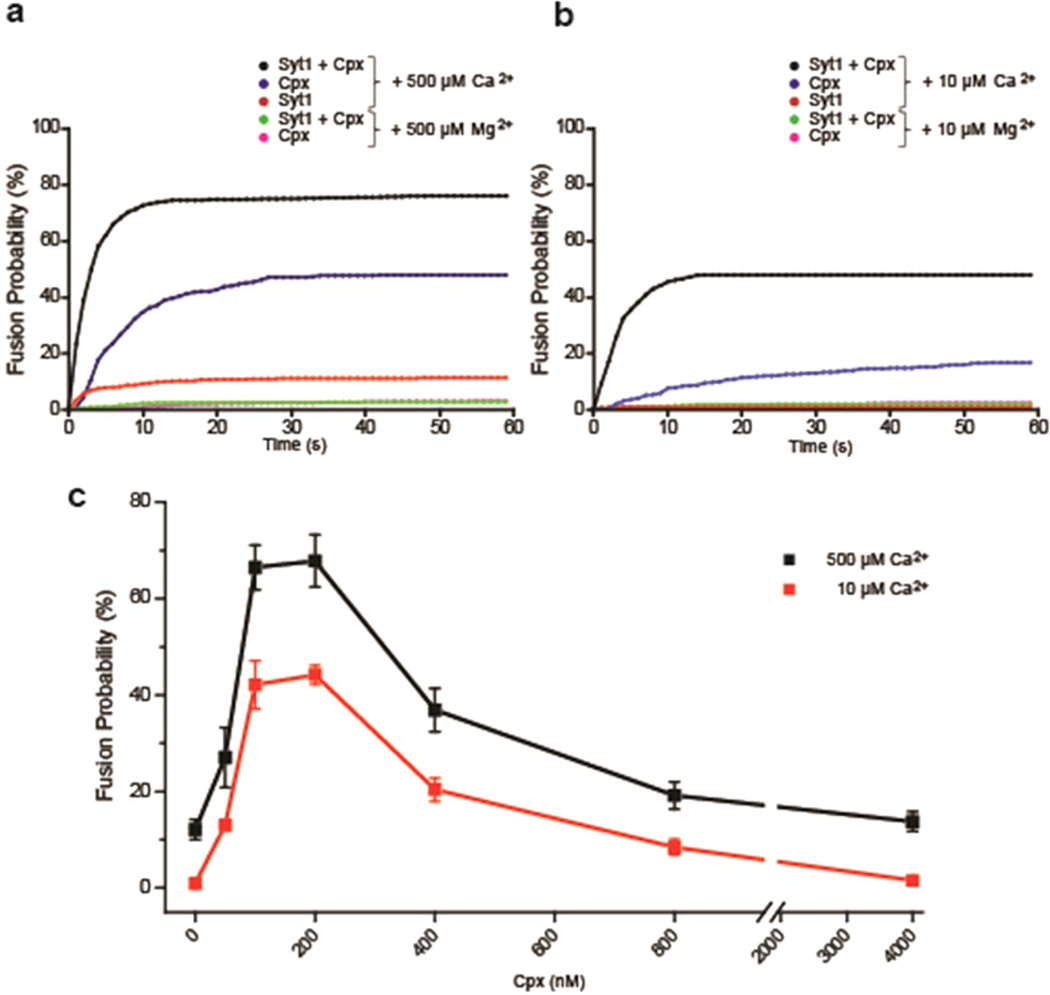
In the absence of Cpx, a mere ~12% of the docked vesicles exhibited content-mixing when triggered by 500 µM Ca2+, which has been seen consistently in previous studies.23,24 This is apparently short of reproducing in vivo synaptic vesicle fusion in which almost all vesicles in the readily releasable pool fuses with the plasma membrane when stimulated with only ~10 µM Ca2+.26,27 However, when we introduced 100 nM Cpx into our system, we observed a significant increase in content-mixing population. Over two-thirds of the docked vesicle pairs exhibited content-mixing at 500 µM Ca2+ (Figure 2a). In a control experiment, Mg2+ was not capable of promoting content mixing at all, indicating that Ca2+ and the Ca2+-sensor Syt1 played roles in triggering the membrane fusion reaction. On the other hand, while we observed a slight decrease at 4 µM Cpx, there was no obvious change in vesicle docking probability 0–800 nM Cpx, indicating that Cpx may be specifically involved in the fusion pore opening step (Figure S1).
Figure 2.
Bell-shaped response of Ca2+-triggered SNARE-dependent vesicle fusion in vitro to Cpx. (a) Cumulative fusion-probability with 500 µM Ca2+ in the presence (black line) and absence (red line) of 100 nM Cpx. The blue line is with 100 nM Cpx only, without Syt1. Controls using 500 µM Mg2+ in the presence of Cpx with and without Syt1 are shown in green and magenta (overlapped with green), respectively. (b) Cumulative fusion-probability triggered with either 10 µM Ca2+ or 10 µM Mg2+. (c) Total content-mixing percentage among docked vesicle pairs over 60 s period in the presence of 0, 50, 100, 200, 400, 800, and 4000 nM Cpx triggered by 500 (black line) and 10 µM Ca2+ (red line) respectively. Error bars are standard deviations (S.D.) obtained from five independent data acquisitions with independently prepared samples.
Having such high content-mixing percentage in the presence of Cpx, we ask if physiologically relevant Ca2+ conditions (10 µM) could trigger appreciable content-mixing which had not been previously achieved.23,24 As expected from previously reported results, content-mixing was hardly observable in the absence of Cpx at 10 µM Ca2+. However, in the presence of 100 nM Cpx, we observe ~45% content-mixing among docked vesicles (Figure 2b). Our results show that not only does Cpx significantly increase the probability of vesicle fusion but also dramatically improves the Ca2+ sensitivity in our in vitro assay. Such an improvement was observed only when t-vesicles were pretreated with Cpx.
Previously, it was shown that Cpx alone, even in the absence of a major Ca2+-sensor Syt1, could trigger SNARE-mediated lipid mixing in response to Ca2+.30 Similarly, we found that Cpx alone was able to trigger content mixing with Ca2+ (Figure 1a,b). However, membrane fusion with Cpx alone was less efficient and slower than it was when both Cpx and Syt1 were present: Particularly, under physiological 10 µM Ca2+, the fusion efficiency and time scale was approximately 3 times less and 6 times slower, respectively.
As we increase the Cpx concentration from 0 up to 100 nM, we are able to observe a steep enhancement of Ca2+-triggered content-mixing. Specifically, with 10 µM Ca2+, the yield of content-mixing increased from ~1% to ~45% as we increased the Cpx concentration from 0 to 100 nM.
Surprisingly, however, as we further increase the Cpx concentration above 200 nM the stimulating effect gradually diminishes in a concentration dependent manner. At 200, 400, 800, and 4000 nM Cpx, we observed approximately 44%, 20%, 8%, and 1% content-mixing, respectively. Thus, our results demonstrate that Cpx elicits a bell-shaped response on Ca2+-triggered vesicle fusion, an ascending trend under low concentrations (below 100 nM) but descending trend under high concentrations (above 200 nM). We also observed a similar bell-shaped curve for 500 µM Ca2+ with slight increase in yields over the entire Cpx concentrations. The overall slight lift of the response curve for 500 µM Ca2+ compared to 10 µM Ca2+ was sort of expected and in fact is quite consistent with the in vivo observation that the fusion efficiency is effectively saturated with only a small increase above 10 µM Ca2+.26
Cpx Contributes Little to the Synchronization of Ca2+-Triggered Vesicle Fusion
In neurons, when Cpx was deleted by knockout the amplitude of excitatory postsynaptic potential (EPSC) decreases significantly, but the time scale of EPSC changes little in cultured mammalian neurons.3,4,31 In the previous experiments by Brunger and co-workers, approximately a factor of 2–4 enhancement in the time scale of synchronization was observed for Cpx.23 This appears to be inconsistent with the observations in mammalian neurons.
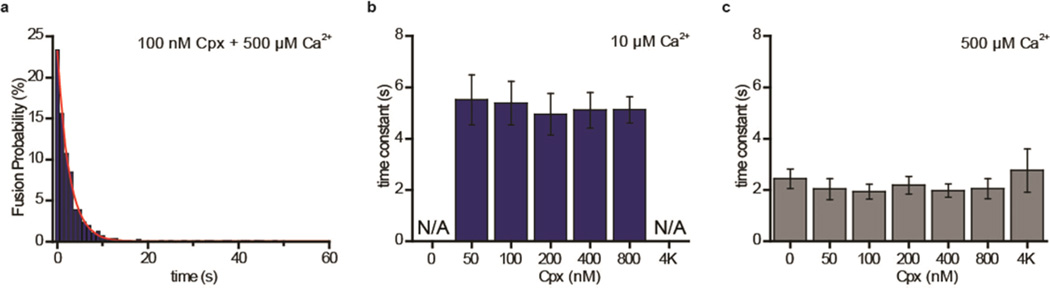
Here, we revisit the time scale changes with Cpx using the newly improved assay (Figure 3a). When the time scales of synchronization are examined as a function of Cpx we observed little variation over the entire range of Cpx concentrations studied (Figure 3b,c). The results suggest that Cpx is not involved in the synchronization of Ca2+-triggered vesicle fusion. While there is still controversy, our results are more in favor of the proposition that Cpx may not be involved in clamping and synchronization of vesicle fusion in mammalian neurons.3,4,31 However, we do note that an approximately 2 fold increase in synchronization was observed with 500 µM when compared to 10 µM Ca2+.
Figure 3.
First order time-constant for content-mixing exhibits little change with various Cpx concentrations. (a) Representative plot of content-mixing events triggered by 500 µM Ca2+ versus time in the presence of 100 nM Cpx (blue bars). The data were fitted by the first order kinetics with the time constant of ~2.6 s (red line). (b) Histogram of the first order time constant for content-mixing with 0, 50, 200, 400, 800, and 4000 nM Cpx triggered by 10 µM Ca2+. The time constants for 0 and 4000 nM Cpx are not determined due to insufficient fusion events. (c) Histogram of the first order time constant for content-mixing with 0, 50, 200, 400, 800, and 4000 nM Cpx triggered by 500 µM Ca2+. Error bars and standard deviations (S.D.) are obtained from three independent data acquisitions with independently prepared samples.
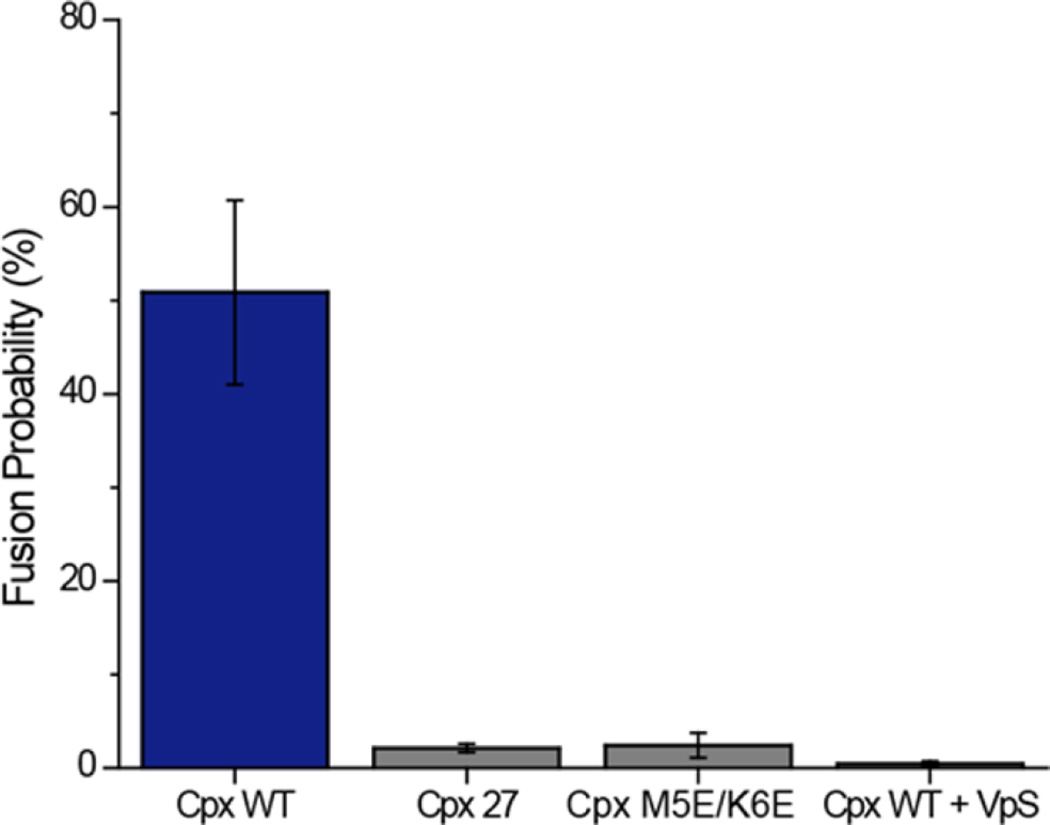
The N-Terminal of Cpx Is Essential for the Enhancement of the Fusion Probability
It was previously reported that the first 26 residues of Cpx facilitate the enhancement of fusion probability in mouse neurons. Moreover, the efficacy of Cpx was completely lost when Met5 and Lys6, which are considered to contribute in forming the N-terminal α helix, was mutated to glutamate.31,32
We prepared two Cpx mutants, Cpx 27 in which N-terminal 26 amino acids were deleted and a double point mutant Cpx M5E/K6E, in order to verify the role of the N-terminal region with our newly improved single vesicle fusion assay (Figure 4). The content-mixing assay was performed identically except for using the mutants instead of the wild-type at each step. In coherence with the in vivo results, Cpx 27 and Cpx M5E/K6E failed to promote vesicle fusion recapitulating the critical role of the N-terminal region of Cpx in stimulating Ca2+-triggered exocytosis.
Figure 4.
The N-terminal region of Cpx is necessary for the enhancement of content-mixing. Histogram of the content-mixing percentage among docked vesicle pairs triggered by 10 µM Ca2+ in the presence of 100 nM Cpx wild-type, Cpx 27 and Cpx M5E/K6E. Content-mixing with 100 nM Cpx and 20 µM VpS are also shown as a control. Error bars are standard deviations (S.D.) obtained from five independent data acquisitions with independently prepared samples.
As a control, we tested if membrane fusion was SNARE-dependent using soluble VAMP2 lacking the transmembrane domain (VpS, amino acids 1–94). VpS has been frequently used to verify the SNARE-dependency of the fusion reaction. We incubated VpS (20 µM) along with the v-vesicles and Cpx wild-type (Figure 4). In the presence of VpS, content-mixing was hardly observed, confirming that the fusion reaction was indeed SNARE-dependent.
DISCUSSION
Although the role of Cpx in synaptic membrane fusion has been highly controversial, it is generally agreed that Cpx stimulates evoked exocytosis.12 The results from our improved single-vesicle content-mixing assay are fully consistent with this notion.
What is new and interesting though is that the stimulatory effect of Cpx reverses its course after cresting at ~150 nM, thus showing a bell-shaped dose response curve. Previously, it has been somewhat mysterious why overexpression of Cpx in cells results in reduced evoked exocytosis despite its established positive role.28,29 Our results demonstrate, in a well-defined environment, that there is indeed a dose-dependent decrease of Ca2+-triggered vesicle fusion at high concentrations above 200 nM. Thus, if overexpression changed the Cpx level in the regime of 200 nM, a few µM one would observe the reduction of evoked exocytosis. Thus, the bell-shaped dose response for Cpx reconciles seemingly paradoxical results that both knockout and overexpression studies show the reduction of evoked exocytosis.
Our improved single-vesicle content-mixing assay made it possible to obtain the dose response curve for Cpx in Ca2+-triggered vesicle fusion. The dramatic improvement of the fusion efficiency over the previous work is apparent in our results. The Ca2+-sensitivity was increased to the natural level, and thus, the assay can now operate at physiological-relevant 10 µM Ca2+. We point out that the only tweak, compared to the previous studies, was the pretreatment of t-vesicles with Cpx prior to vesicle docking. Why would the pretreatment of t-SNARE with Cpx affect so much the fusion outcomes? There might be two possible scenarios. Scenario one is that when Cpx is delivered during or after docking the SNARE complex is not freely accessible by Cpx any more due to the steric crowding at the fusion site. This would in turn reduce the effectiveness of Cpx in regulating the SNARE function. Scenario two is that Cpx may have the capacity to prime t-SNAREs by a yet unknown mechanism. For instance, it is possible that Cpx might play a role in converting the inactive 2:1 complex to the active 1:1 complex.33 However, these postulations are purely speculative, warranting further experiments.
Intriguingly, we observe little change in the synchronization kinetics of vesicle fusion over the entire Cpx concentration rage of 0–4 µM. Our results are quite consistent with those from the whole cell patch clamp conducted with cultured mammalian neurons.3,4,31 The results suggest that Cpx may not be involved in the clamping and synchronization of exocytosis. However, the caveat of our experiments is that the time scale is still 3 orders of magnitude slower than what is normally observed in vivo. Thus, it is possible that our assay does not faithfully reproduce the synchronization kinetics of vesicle fusion. We note that some slower kinetics was observed in the absence of Cpx in Drosophila34 suggesting the variation of the Cpx function among different organisms.
Our results show that Cpx alone, in the absence of Ca2+-sensor Syt1, can trigger SNARE-mediated content mixing in response to Ca2+. Intriguingly, Cpx does not have an apparent Ca2+-sensing module or domain. However, it was previously shown that Cpx binds the membrane in the presence of Ca2+.35 We wonder if this Ca2+-mediated Cpx binding to the membrane is relevant to the Ca2+-sensing capacity in our in vitro membrane fusion assay. We note however that the biological relevance of the Ca2+-sensing activity of Cpx has not been established, warranting further investigation.
In conclusion, we have vastly improved the single-vesicle fusion assay and show that Cpx modulates evoked exocytosis with an unusual bell-shaped response curve. This quantitative description, which is not easily obtainable with knockout or overexpression studies in cellular environments, not only helps to understand the Cpx function in neuroexocytosis but also to understand the relationship between changes in Cpx and mental diseases associated with aberrant neurotransmitter release.
Supplementary Material
Acknowledgments
Funding
This work was supported by the U.S. National Institutes of Health grant (R01 GM051290) to Y.-K.S.
ABBREVIATIONS
- TIR
total internal reflection
- Cpx
complexin I
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- EPSC
excitatory postsynaptic potential
- SRB
sulforhodamine B
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Vesicle docking measurement data showing no significant variation of vesicle docking in the presence of complexin I in the concentration range of 0–4 µM (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Ishizuka T, Saisu H, Odani S, Abe T. Synaphin: a protein associated with the docking/fusion complex in presynaptic terminals. Biochem. Biophys. Res. Commun. 1995;213:1107–1114. doi: 10.1006/bbrc.1995.2241. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Missler M, Li C, Sudhof TC. Complexins - Cytosolic Proteins That Regulate SNAP Receptor Function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 3.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 4.Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin Controls the Force Transfer from SNARE Complexes to Membranes in Fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin maintains vesicles in the primed state in C. elegans. Curr. Biol. 2011;21:106–113. doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol. 2011;21:97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sorensen JB, Brose N, Chow RH. Complexin II plays positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19538–19543. doi: 10.1073/pnas.0810232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 10.Trimbuch T, Xu J, Flaherty D, Tomchick DR, Rizo J, Rosenmund C. Re-examining how Complexin Inhibits Neurotransmitter Release: SNARE complex Insertion or Electrostatic Hindrance? eLife. 2014:3. doi: 10.7554/eLife.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Cao P, Sudhof TC. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20777–20782. doi: 10.1073/pnas.1321367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trimbuch T, Rosenmund C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 2016;17:118–125. doi: 10.1038/nrn.2015.16. [DOI] [PubMed] [Google Scholar]
- 13.Brose N. Altered complexin expression in psychiatric and neurological disorders: cause or consequence? Mol. Cells. 2008;25:7–19. [PubMed] [Google Scholar]
- 14.Sudhof TC, Rothman JE. Membrane Fusion: Grappling with SNARE and SM Proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 16.Chapman ER. Annu. Rev. Biochem. Palo Alto: 2008. How does synaptotagmin trigger neurotransmitter release? pp. 615–641. Annual Reviews. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Maximov A, Shin O-H, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 19.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat. Struct. Mol. Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 20.Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM, Sollner TH. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2001–2006. doi: 10.1073/pnas.0812813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao J, Ishitsuka Y, Lee H, Joo C, Su Z, Syed S, Shin YK, Yoon TY, Ha T. A single vesicle-vesicle fusion assay for in vitro studies of SNAREs and accessory proteins. Nat. Protoc. 2012;7:921–934. doi: 10.1038/nprot.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyoung M, Zhang Y, Diao J, Chu S, Brunger AT. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat. Protoc. 2012;8:1–16. doi: 10.1038/nprot.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Y, Diao J, Cipriano DJ, Zhang Y, Pfuetzner RA, Padolina MS, Brunger AT. Complexin inhibits spontaneous release and synchronizes Ca2+-triggered synaptic vesicle fusion by distinct mechanisms. eLife. 2014;3 doi: 10.7554/eLife.03756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Y, Diao J, Liu Y, Ishitsuka Y, Su Z, Schulten K, Ha T, Shin Y-K. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1333–1338. doi: 10.1073/pnas.1218818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao J, Cipriano DJ, Zhao M, Zhang Y, Shah S, Padolina MS, Pfuetzner RA, Brunger AT. Complexin-1 enhances the on-rate of vesicle docking via simultaneous SNARE and membrane interactions. J. Am. Chem. Soc. 2013;135:15274–15277. doi: 10.1021/ja407392n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 27.Burgalossi A, Jung S, Man KN, Nair R, Jockusch WJ, Wojcik SM, Brose N, Rhee JS. Analysis of neurotransmitter release mechanisms by photolysis of caged Ca(2)(+) in an autaptic neuron culture system. Nat. Protoc. 2012;7:1351–1365. doi: 10.1038/nprot.2012.074. [DOI] [PubMed] [Google Scholar]
- 28.Itakura M, Misawa H, Sekiguchi M, Takahashi S, Takahashi M. Transfection analysis of functional roles of complexin I and II in the exocytosis of two different types of secretory vesicles. Biochem. Biophys. Res. Commun. 1999;265:691–696. doi: 10.1006/bbrc.1999.1756. [DOI] [PubMed] [Google Scholar]
- 29.Archer DA, Graham ME, Burgoyne RD. Complexin regulates the closure of the fusion pore during regulated vesicle exocytosis. J. Biol. Chem. 2002;277:18249–18252. doi: 10.1074/jbc.C200166200. [DOI] [PubMed] [Google Scholar]
- 30.Yoon TY, Lu X, Diao J, Lee SM, Ha T, Shin YK. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 2008;15:707–713. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue M, Craig TK, Xu J, Chao H-T, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat. Struct. Mol. Biol. 2010;17:U568–U565. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, Brose N, Rosenmund C. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 34.Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J. Neurosci. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seiler F, Malsam J, Krause JM, Sollner TH. A role of complexin-lipid interactions in membrane fusion. FEBS Lett. 2009;583:2343–2348. doi: 10.1016/j.febslet.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.