Abstract
Objective
To systematically analyze the effects of direct-to-consumer advertising (DTCA) on patient requests for medication and physician prescribing across psychiatry-relevant studies.
Data Sources
MEDLINE, PsychINFO, ISI Thompson's Web of Knowledge, and Google Scholar were searched from 1999 through 2014 using variations of the terms direct-to-consumer advertising and psychiatric. Reference lists and an online repository of DTCA manuscripts were also scrutinized.
Study Selection
English-language studies collecting data at the point of service, focusing on or including psychiatric medication, and assessing DTCA's effects on patient and/or physician behavior were included. Of 989 articles identified, 69 received full-text review. Four studies across five manuscripts met inclusion criteria.
Data Extraction
Data were extracted on participants, study design, methodological quality, and results. Methodological quality of individual studies was assessed using adapted criteria from the Effective Public Health Practice Project. Confidence in conclusions across studies was determined using principles from the well-established GRADE system.
Findings
Due to lack of replication across strong randomized controlled trials (RCTs), no conclusions merited high confidence. With moderate confidence, we concluded that DTCA requests: 1) are granted most of the time [1 RCT, 3 observational]; 2) prompt higher prescribing volume [1 RCT, 1 observational]; 3) promote greater adherence to minimally acceptable treatment guidelines for patients with depression [1 RCT], and 4) stimulate overprescribing among patients with an adjustment disorder [1 RCT].
Conclusions
Findings suggest that DTCA requests are typically accommodated, promote higher prescribing volume, and have competing effects on treatment quality. More methodologically strong studies are needed to increase confidence in conclusions.
Keywords: direct-to-consumer, advertising, psychiatric, medication, systematic review
Direct-to-consumer advertising (DTCA) of prescription medications has been extremely lucrative for the pharmaceutical industry in the United States. After the Food and Drug Administration (FDA) relaxed its guidelines for marketing pharmaceuticals in 1997, DTCA expenditures skyrocketed.1, 2 Spending on DTCA grew from under $800 million in 1996 to $2.5 billion in 2000, eventually peaking at $4.9 billion in 2007.2, 3 From 2007 through 2014, DTCA of pharmaceuticals remained a multi-billion dollar enterprise with annual expenditures between $3.5 and $4.5 billion.3, 4 Analyses of DTCA spending suggest that every $1 investment translates to $2.20–$4.20 of increased pharmaceutical sales.5, 6
Psychiatric medications are among the most heavily advertised prescriptions in the United States. Shortly after the revised FDA guidelines, psychiatric drugs comprised three of the five most advertised classes of medication and were among the first drugs to attain “blockbuster” status.7,8 For instance, Prozac sales rose 9% in 1997 to reach $2.56 billion by year end.9, 10 More recent data from 2014–2015 indicate that psychiatric medications comprise 20% of the 10 most advertised drugs and 10% of the 100 top-selling drugs.11 Several features of psychiatric medications make them attractive for DTCA from the pharmaceutical firm's perspective: the medications are relatively safe and target conditions that are highly prevalent, chronic, associated with significant impairment, and substantially under-treated.12, 13
The prominence of DTCA in the United States has led both researchers and policy makers to scrutinize advertising practices and analyze their effect on public health. Consequently, DTCA of pharmaceutical products has been the subject of numerous excellent review articles.6, 14–16 and special journal issues in BMJ, JAMA, Health Affairs, Journal of Health Communication, and Research in Social and Administrative Policy. Across this work, several common arguments about DTCA's advantages and disadvantages have emerged. DTCA proponents have asserted that it enhances patient awareness and education by providing legitimate information about conditions and treatment options.17–19 It has been further argued that DTCA promotes the diagnosis and treatment of under-treated conditions, by encouraging patients to more actively request prescriptions.20 Meanwhile, DTCA opponents have asserted that it provides inaccurate and biased information fundamentally favoring pharmaceutical companies,21 thereby promoting unnecessary prescribing.22–24
The ability of prior DTCA reviews to inform psychiatry practice has been limited by several factors. First, previous work has focused on DTCA in general without considering the unique benefits and challenges related to prescribing psychiatric medication.14–16, 25 Psychiatric conditions remain some of the most prevalent, stigmatized, and under-treated illnesses,26 making patients' treatment-seeking behaviors in response to DTCA especially important. Second, extant reviews have given equal attention to chart reviews, retrospective surveys, qualitative studies, and randomized trials,15, 25 despite significant differences in the scope and rigor of these approaches. Consideration of methodological quality is imperative to accurately determine the strength of evidentiary support for various arguments. Finally, the vast majority of prior reviews have not attempted to synthesize the effects of DTCA on patient and physician behavior in a systematic way.
To date, there has been one systematic review of the benefits and harms of a DTCA approach, conducted by Gilbody and colleagues.27 The investigators found evidence that DTCA was associated with increased physician prescribing. However, this review's relevance to psychiatric medication was questionable: of the 2853 citations identified, only four studies were included in the analysis and three were specifically focused on medications for non-psychiatric conditions (i.e., antihistamines, antihypertensives, acid-peptic disorder medications, benign prostatic hypertrophy medications, antilipemics, migraine medication, and toe-nail fungus medication). Furthermore, findings were published over a decade ago, which limits applicability to current practice. The paucity of psychiatry-relevant data highlights the need for a current and focused synthesis of the literature.
The current review aimed to systematically evaluate the effects of DTCA on patient and physician behavior in the United States. To ensure relevance to psychiatry, we restricted the review to studies focused specifically on psychiatric medication or encompassing a range of medications including psychiatric. Our review was guided by two key questions: 1) How does DTCA affect patient requests for advertised medication? 2) How does DTCA affect physician prescribing in response to patient requests? Across these questions, our objective was to synthesize the results of publicly available studies measuring behavior at the point-of-service in order to determine the strength of conclusions that can be made. Addressing these questions represents an important step toward understanding the effects of DTCA on patient requests for psychiatric medication and physician prescribing, which can inform policy around this controversial issue.
Methods
Study Selection
We conducted our systematic review and report our results in accordance with the latest PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses: http://www.prisma-statement.org) guidelines.28 Studies of the effects of DTCA on patients and physicians in the United States were selected according to these criteria: (a) presented quantitative data on patient prescription requests in response to DTCA and/or physician prescribing in response to patient requests; (b) gathered data directly from patients or physicians at the point-of-service (i.e., excluded aggregate-level data obtained from national databases or retrospective survey data); (c) measured the effects of DTCA for psychiatric medication specifically or for a range of medications including psychiatric (i.e., allowed studies based in generalist practices as long as physicians could prescribe psychiatric medication, but excluded studies focused on non-psychiatric medications or in non-psychiatric specialty settings).; (d) published in or after 1999 to reflect the finalization of the FDA's guidance on DTCA; (e) collected data in the United States; and (f) published in a peer-reviewed journal in English.
We restricted our search to studies that directly measured individuals' behavior either prospectively or in real-time at the point-of-service. We excluded studies using retrospective recall in order to minimize bias.29 The limitations of retrospective reports have been well-established30, 31 and researchers have recommended avoiding the use of retrospective data to test hypotheses that demand precision in estimating event occurrence.32. Because we were interested in patients' and physicians' actual behavior, studies of DTCA's effects on knowledge, awareness, impressions, behavioral tendencies, or expected behaviors were excluded. Multiple manuscripts from the same dataset were treated as one study and data were extracted accordingly.
Search Strategy
Studies meeting inclusion criteria were identified via a targeted search a search of Medline, PsychInfo, the aggregated Social Sciences database on ISI Thompson's Web of Knowledge, and Google Scholar from 1999 to February 2015. Search terms included combinations of the following keywords: “direct-to-consumer”, “DTC”, “DTC marketing', “DTC advertising”, and “psychotropic” or “mental health” or “psychiatric.” During our search, we identified an online repository of 449 DTCA studies published between 1983 and 2013 compiled by a non-profit, nonpartisan website called Prescription Drug Ads: Pros and Cons (see http://prescriptiondrugs.procon.org/), which we hand searched to identify additional articles. We also manually searched reference lists and conducted a Google Scholar search for articles citing identified work.
All identified articles were subject to two rounds of review. In the first round, two researchers (study co-authors) examined the abstracts and titles of potentially relevant studies and excluded those that were clearly not original studies, not focused on psychiatric medication, and not based in the United States. In the second round, full-length copies of the remaining studies were scrutinized to determine eligibility.
Data Extraction
Two researchers independently extracted data from studies meeting inclusion criteria. First, each article was examined to determine if it measured patient requests for prescriptions and/or physician prescribing behavior. Additional data extraction pertained to sample selection, data measurement and analysis, methodological quality, and study findings. A primary goal of this study was to determine the strength of conclusions that could be made from publically available evidence; hence, coders did not search for grey literature or contact study authors for unpublished data. Any disparities that emerged during coding were resolved through review by a third independent coder.
Assessment of Quality
Methodological quality of each study was assessed using adapted criteria from the Effective Public Health Practice Project (EPHPP)33 instrument. The EPHPP was developed to be suitable for evaluating a range of study designs including randomized clinical trials (RCTs) and observational studies. The instrument has been used in multiple systematic reviewssee 34–37 relevant to mental health treatment and has demonstrated content and construct validity.38, 39 This is the first systematic review to adapt the EPHPP to evaluate studies of DTCA. Consideration of study quality included four of the six EPHPP criteria: [1] selection bias – the extent to which the sample was representative of the target population; [2] study design – the degree to which the design isolated the effects of DTCA on patient and/or physician behavior; [3] blinding – whether patients and physicians (or session raters, if applicable) were aware of the study objectives; and [4] data collection – whether study measures were valid and reliable. Given our focus on DTCA, we added a fifth criterion to rate the specificity and replicability with which DTCA was operationalized. Using our adapted EPHPP grading scheme (see Table 1), we rated criteria as Strong, Moderate, or Weak. Studies with at least three criteria rated “Strong” and no criteria rated “Weak,” were designated Strong. Those studies with no more than one “Weak” rating were deemed Moderate and remaining studies were rated “Weak.”
Table 1.
Quality Assessment Component Definitions and Ratings Adapted from the EPHPP Instrument
| Component | Strong | Moderate | Weak |
|---|---|---|---|
| Selection Bias | Very likely to be representative of target population, participation > 80% | Somewhat likely to be representative of target population, participation > 60% | Unlikely to be representative of target population, participation < 60% or not described |
| Study design | Randomized controlled trial or controlled clinical trial | Comparative group design, cohort, case control, interrupted time series | Other designs or not reported |
| Blinding | Blinding of physicians and patients (and outcome assessors if applicable) to research question | Blinding of either physicians or patients | No blinding or not reported |
| Data collection | Tools have evidence of validity (content, construct, or discriminant) and reliability | Tools have evidence of validity, but reliability not described | No data on validity or reliability |
| DTCA definition | Definition clearly defined and replicable | Definition clearly defined but not easily replicable | No definition provided |
The criteria in this table are based upon the original EPHPP criteria published in Thomas et al., 2004.
Abbreviations: EPHPP = Effective Public Health Practice Project; DTCA = direct-to-consumer advertising
Individual study ratings were used to determine the confidence with which specific conclusions could be made across investigations. Using principles from the well-established GRADE system,40, 41 we rated the quality of evidence in support of specific conclusions as high, moderate, low, or very low/insufficient. The GRADE system is one of the most widely used strength of evidence assessment tools and was specifically designed to convey reviewers' confidence in the strength of a detected effect.41 Consistent with the GRADE handbook,42 we used the terms quality of evidence, strength of evidence, and confidence in evidence interchangeably in our synthesis of the literature; for simplicity, we consistently used the word conclusion when referencing a significant finding, outcome, or estimated effect. Because our goal was to determine confidence in conclusions and not to devise recommendations, we used the standard four-level quality of evidence rating scheme and not the binary classification of Strong or Weak used by guideline panels.see 41 Table 2 presents the rating criteria and definitions we used to evaluate confidence in conclusions across studies.
Table 2.
Confidence Rating Descriptions and Criteria Adapted from the GRADE System
| Rating | Description | Criteria | |
|---|---|---|---|
| High | Further research is very unlikely to change our confidence in the estimate of the effect | • | Several strong randomized controlled trials with consistently replicated results |
| • | In special cases, one large, strong quality multi-center trial | ||
|
| |||
| Moderate | Further research is likely to have an important effect on our confidence in the estimate of the effect and may change the estimate | • | One un-replicated methodologically strong study |
| • | Several studies with consistent results, each of which has methodological limitations | ||
|
| |||
| Low | Further research is very likely to have an important effect on our confidence in the estimate of the effect and will likely change the estimate | • | Several methodologically weak studies with consistently replicated results |
|
| |||
| Very low | Any estimate of the effect is very uncertain and our confidence is very low | • | Expert opinion, consensus guidelines, usual care, or case reports |
| • | No direct research evidence | ||
| • | One un-replicated methodologically weak study | ||
The criteria in this table are based on the definitions of ratings in Guyatt et al., 2008 and the description of evidence ratings in the GRADE handbook.
Abbreviations: GRADE = Grading of Recommendations, Assessment, Development and Evaluation
Results
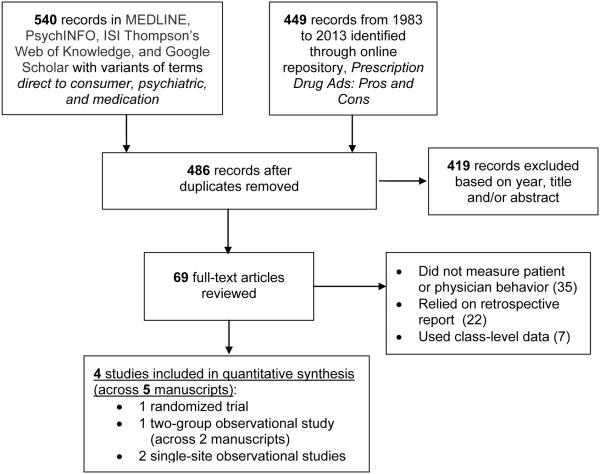
Search of the databases and online repository identified 989 articles potentially meeting inclusion criteria (Figure 1). After removing 503 duplicates, 486 articles remained. The first screening round excluded 419 articles, leaving 69 articles for full-text review. Of these, four studies (across five manuscripts) represented original, psychiatry-relevant research measuring the effect of DTCA on patient and/or physician behavior at the point-of-service. The most common reasons for exclusion were not including abehavioral outcome (e.g., measuring patient impressions, awareness, attitudes, or behavioral intentions), or not collecting data at the point-of-service (e.g., relying on retrospective reports).
Figure 1.
Flowchart of Study Selection
Due to the small number and heterogeneity of studies, we deemed a narrative synthesis of study characteristics and findings more appropriate than a meta-analysis.43 The following sections present the study designs, participants, methodological quality, and findings of the four studies (see Table 3 for an overview).
Table 3.
Summary of Study Characteristics
| Author, Year | Setting/Focus | Design | Participants | DTCA Definition | Outcomes | Results | |
|---|---|---|---|---|---|---|---|
| Allison-Ottey et al., 2003 |
General practice clinics (n=8) across 5 states; Psychiatry specific?: No |
Observational point of service (POS); Doctors recorded patient behaviors after patient visits; Blinding: None |
N = 11 physicians, 1065 patients; Participation rate: not reported |
Doctors asked: Did the patient ask you about a specific medication that they saw advertised? | 1) | Patient requests for DTCA | DTCA requests: 9% of patients |
|
| |||||||
| Kravitz et al., 2005 |
Primary care clinics in 3 cities across 2 states; Psychiatry specific?: Yes, antidepressants |
RCT; Standardized patients (SPs) portray 2 conditions (depression/MDD, adjustment/ADJ) and make 3 requests (brand-specific/B, general/G, none/N); Blinding: Doctors and session raters |
N = 152 physicians, 298 visits; Participation rate: 53–61% across practices |
SPs used scripts to request a DTCA drug by name (B condition) or by class (G condition; antidepressant seen on TV) | 1) | Physician prescribing rates for MDD and ADJ | Reported consecutively for B, G, and N groups. MDD prescribing rates: 53%, 76%, 31%. Adjustment prescribing: 55%, 39%, 10%. Minimally acceptable care for MDD rates: 90%, 98%, 56%. |
| 2) | Physician meeting minimally acceptable guidelines for MDD | ||||||
|
| |||||||
| Mintzes et al., 2003 |
Primary care clinics across 2 cities in U.S. and Canada; Psychiatry-specific?: No |
Two-group observational POS; Doctors recorded patient behaviors after consecutive patient visits in 2 settings; Blinding: Patients |
N (U.S. only) = 38 physicians, 683 patients Participation rate: 61% physicians, 69% patients |
Doctors recorded if patient requested any prescription. Two raters coded drug as DTCA if it was among 50 most heavily advertised in past year. |
1) | Patient requests for DTCA |
DTCA requests: 7.2% of patients Prescribing rates: 78% of DTCA requests given prescription |
| 2) | Physician prescribing rates | ||||||
|
| |||||||
| Parnes et al., 2005 |
Primary care clinics (n=22) across one state; Psychiatry specific?: No |
Observational POS; Doctors recorded patient behaviors after consecutive patient visits; Blinding: Patients |
N = 168 doctors, 1647 patients; Participation rate: 22% of physician practices |
Doctors recorded if patient requested any prescription. Two coders rated if drug had been advertised recently. |
1) | Patient requests for DTCA |
DTCA requests: 2.6% of patients (3.5% made any request) Prescribing rates: 72% of all requests given prescription |
| 2) | Physician prescribing rates | ||||||
Abbreviations: DTCA = direct-to-consumer advertising
Study Overview and Designs
Studies meeting inclusion criteria were all published between 2002 and 2009. While the two questions guiding this review were intentionally broad in scope, the identified studies focused on two specific aspects of patient and physician behavior: rates of patients requesting DTCA prescriptions and rates of physicians granting DTCA requests. Of the four studies, only one by Kravitz and colleagues44 was an RCT focused specifically on psychiatric medication. This study used standardized patient (SP) actors to manipulate both the types of requests made for antidepressants and the patient's level of severity. Six assignments were made by crossing two conditions (major depression or adjustment disorder) with three different types of DTCA drug requests (brand-specific, general, or none). The other three studies measured patient requests for any medication including but not restricted to psychiatric.
The study by Mintzes and colleagues45 (also described in a second manuscript46) was a two-group observational point-of-service study comparing the behaviors of patients and physicians in a United States setting where DTCA is allowed to a Canadian setting where DTCA is prohibited. Consistent with our inclusion criteria, only the data from the United States site were extracted, though the overall design was considered when evaluating methodological quality. Data collection occurred on pre-determined days and a variety of potential confounders were controlled when comparing the two groups. The remaining two studies (Allison-Ottey et al.47 and Parnes et al.48) were observational point-of-service studies in which physicians recorded patient and physician behaviors on encounter forms after patient visits.
Sample Selection
All of the studies but Kravitz et al.44 used actual patients and all four used actual physicians. Focusing only on participants recruited in the United States, sample sizes ranged from 683 to 1,647 patients (total n = 3,395) and 11 to 162 physicians (total n = 369). All four studies were based in general practice settings, with physicians identifying their focuses as family practice, internal medicine, geriatrics, and/or women's health. Two projects recruited physicians from physician collectives or networks (n = 320 physicians)44, 48, one recruited from a medical directory of general practitioners (n = 38 physicians)45, and one recruited from eight medical sites (n = 11 physicians).47
Strategies used to select patients were heterogeneous. Two investigative teams recruited and consented patients in physician waiting rooms,45, 47 while the others solely recruited physicians.44, 48 Participation rates were reported in three of the four studies. Mintzes et al.45 reported participation rates of both physicians (n = 38, 60%) and patients (n = 683, 69%). Kravitz et al.44 enrolled 190 individual physicians with participation rates of 53–61% across settings (raw data not provided for analysis). Parnes et al.48 reported that 22 physician practices enrolled, which represented 28% of 78 invited practices.
The types of sample characteristics reported also varied. The three observational point-of-service studies all provided some descriptive information about both patients and physicians, while the Kravitz et al. study44 (which used SPs) only gave information about physicians. Across the three observational reports45, 47, 48, 65% of the 3,395 patients were female. Only two of the three studies provided information about patient race/ethnicity47, 48 and cumulatively 70% of the 2,712 patients were minority group members. Very little data were provided about physicians beyond descriptions of their medical specialties. Only Allison et al.47 reported on physician race/ethnicity (n = 11 physicians, 100% African-American) and only Mintzes et al.45 reported on physician gender (n = 48 physicians, 79% male).
Outcomes
Across studies, the primary outcomes of interest were patient requests for DTCA medication and physician prescribing. Measurement of patient requests for DTCA medication occurred in the three observational point-of-service studies45, 47, 48 and varied depending on how “DTCA drugs” were operationalized. Two of three studies45, 48 measured patient requests for any prescription and then had the investigative team classify which medications were DTCA; Mintzes and colleagues45 classified a drug as DTCA if it was among the 50 products with the highest DTCA budgets during data collection, whereas Parnes and colleagues48 had two authors classify drugs as DTCA if they had been advertised in the last few years. Allison-Ottey et al.47 simply asked physicians a yes/no question, “Did the patient ask you about a specific medication that they saw advertised during this visit?”
Measurement of physician prescribing was more homogeneous and was the proportion of patients requesting DTCA medication(s) who were granted the medication. The only exception was Parnes et al.,48 which reported the prescribing rate for any requested medication, and did not disaggregate DTCA prescribing. Kravitz et al.44 also measured physician adherence to minimally acceptable care guidelines for major depression treatment, defined as offering any combination of antidepressant, mental health referral, or follow-up within two weeks.
Quality Assessment
Quality ratings of the four studies are provided in Table 4. Kravitz et al.44 was deemed strong due to its RCT design, blinding of both physicians and independent evaluators, and use of collateral data to verify physician prescribing. Mintzes et al.45 was rated moderate due to its use of a comparative two-group design, modest participation rates, blinding of patients, and strong DTCA operationalization. Remaining studies were rated weak.
Table 4.
Methodological Quality Ratings of Studies Meeting Inclusion Criteria
| Author, Year | Selection Bias | Study Design | Blinding | Data Collection | DTC Definition | Overall |
|---|---|---|---|---|---|---|
| Allison-Ottey et al., 2003 | Weak | Weak | Weak | Moderate | Moderate | Weak |
| Kravitz et al., 2005 | Moderate | Strong | Strong | Strong | Strong | Strong |
| Mintzes et al., 2003 | Moderate | Moderate | Moderate | Weak | Strong | Moderate |
| Parnes et al., 2009 | Weak | Weak | Moderate | Moderate | Moderate | Weak |
Specific areas of concern across studies included selection bias (driven by low or non-reported participation) and study design (driven by observational methods with limited ability to isolate DTCA effects). Most studies received strong or moderate quality ratings for blinding, since at least patients (and physicians in Kravitz et al.44) were not aware that their behavior was recorded. Some investigators provided sufficient detail to confirm construct or content validity of measures (thereby garnering data collection ratings of moderate), but reliability was rarely reported. DTCA operationalization also varied in quality; two studies provided definitions that could be replicated,44, 45 one studied relied on physician impressions of whether the patient requested a DTCA drug,47 and one studied relied on coders' impressions of whether the drug had been advertised (without clarifying how these impressions were determined).48
Study Results
In the three observational point-of-service studies,45, 47, 48 the proportion of patients requesting DTCA medication ranged from 2.6% to 9%. The Mintzes et al.45 study of moderate quality found that 7.2% of patients at the United States site requested DTCA medications versus 3.3% of patients at the Canada site (significant difference). The Allison-Ottey et al.47 and Parnes et al.48 studies of weak quality found that 9% and 2.6% of patients requested DTCA medication, respectively. Two of these studies45, 48 tested factors predicting DTCA requests and identified six significant predictors: patient seen in private practice (versus community health center), patient on three or more chronic medications, patient self-reported exposure to advertising, patient self-reported reliance on advertising, patient had condition(s) potentially treatable by medication, and physician was female.
All four studies measured physician prescribing in response to DTCA requests. The Kravitz et al.44 study of strong quality found that for SPs with depression, prescribing rates were 53%, 76%, and 31%, for brand-specific, general, and no requests, respectively. Rates of physicians meeting minimally acceptable depression guidelines across these conditions were 90%, 98%, and 56%. For SPs with adjustment disorder, prescribing rates were 55%, 39%, and 10% respectively. Comparisons across conditions indicated that prescribing rates were significantly higher in the brand-specific and general request conditions than the no request condition. Of clinical importance, minimally acceptable depression treatment guidelines were met significantly more often in the brand-specific and general request conditions. There was also a significant interaction between type of request and condition, such that brand-specific requests had a more pronounced effect on prescribing for adjustment disorder than depression. Based on these data, the investigators concluded that DTCA requests (both brand-specific and general) had the following effects: 1) higher rates of physician prescribing, 2) higher rates of physicians meeting minimally acceptable treatment guidelines among patients with depression, and 3) overprescribing among patients with adjustment disorder.
The Mintzes et al.45 study of moderate quality found similar prescribing rates to Kravitz et al.,44 with physicians granting DTCA requests in 78% of encounters in the United States and 72% in Canada (non-significant difference). This study also found that patients requesting one or more DTCA drugs had significantly higher odds of receiving a new prescription than patients not requesting DTCA drugs.
The two studies of weak quality by Allison-Ottey et al.47 and Parnes et al.48 found more modest prescribing rates of 33% and 54%, respectively. As noted previously, the prescribing rate reported by Parnes et al.48 was cumulative and did not specifically isolate requests for DTCA medications.
Confidence in Findings
Based on principles from the GRADE system, we determined that no conclusions could be made with high confidence due to lack of replication across methodologically strong randomized controlled trials (RCTs). Four conclusions were made with moderate confidence based on data from one methodologically strong RCT (and in some cases replication in observational studies). Specifically, we concluded that DTCA requests: 1) are granted in the majority (i.e.>50%) of encounters [1 RCT, 3 observational]; 2) prompt higher prescribing volume [1 RCT, 1 observational study]; 3) promote greater adherence to minimally acceptable treatment guidelines for patients with depression [1 RCT]; and 4) stimulate overprescribing among patients with an adjustment disorder [1 RCT]. Based on data from three methodologically weaker studies, we made two additional conclusions with weak confidence: 1) DTCA medications are requested in a minority (i.e.<10%) of clinical encounters [3 observational studies]; and 2) patient, physician, and practice setting attributes are associated with higher rates of requests for DTCA medication. There was very low/insufficient evidence from this review to make conclusions about specific variables that predicted higher rates of requests for DTCA medication, as tests of specific variables were not replicated across studies.
Discussion
This was the first psychiatry-relevant systematic review to analyze patient and physician behavior in response to DTCA for medication. Our comprehensive search of almost 1000 articles identified only four studies that measured patient and physician behavior in real-time as opposed to relying on registry data, reports of past behavior, or reports of intended behavior. Of these four studies, only one focused specifically on psychiatric medication (antidepressants), while the others focused on patient requests for medication (both psychiatric and non-psychiatric) in general practice settings. An analysis of methodological quality revealed several areas of improvement for future DTCA evaluations, most notably in the areas of study design and selection bias.
Despite the lack of methodologically strong trials in this review, our synthesis indicates that patient requests for DTCA medication are granted in the majority (i.e., more than 50%) of encounters and result in higher physician prescribing rates. These conclusions are consistent with those of Gilbody et al.27 that DTCA results in increased prescribing volume. However, our review does not provide definitive evidence as to whether these prescribing rates are beneficial for patients. With moderate confidence, we can conclude that DTCA requests result in both better adherence to minimally acceptable care guidelines for patients with depression and overprescribing among patients with an adjustment disorder, suggesting that DTCA has competing effects on quality.
One conclusion (albeit supported by weak evidence) that is unique to this review is that DTCA requests consistently occurred in less than 10% of clinical encounters, a modest proportion compared to the rates that have been reported in retrospective patient and physician surveys (i.e. rates from 22–72%49–51). The discrepancy between the conservative rates found here and those in other published surveys may reflect our reliance on the measurement of patient behavior in real-time as opposed to retrospective self-report, which may produce biased estimates of actual behavior.29 Because the evidence in support of this conclusion is weak, more methodologically strong studies are needed to replicate the conservative rates of DTCA requests found in this review.
The conclusions of our review are limited not only by the small number of methodologically strong studies, but also by our search criteria and the characteristics of the included studies. Our focus on studies that collected data at the point-of-service was intended to reduce bias, but significantly reduced the number of articles available for analysis. The final pool of studies also focused on primary care settings in which a range of medications (including psychiatric) could be requested, suggesting that the results may not pertain to specialty psychiatry settings. Finally, the studies collected data across multiple regions and/or states, but none of the studies collected data nationally, suggesting that the findings might not be representative of patient and physician behaviors in all regions of the United States.
For researchers and physicians interested in the effects of DTCA of psychiatric medication on patient and physician behavior, there are significant opportunities for further research. Although some researchers have referred to DTCA as a “huge, uncontrolled public health experiment,” Kravitz and colleagues showed that controlled evaluations of DTCA can be done. Additional designs such as case-control, cohort, and interrupted time-series also hold great promise for rigorous tests of DTCA. At a minimum, our review suggests that more studies conducted at the point-of-service focused on the effects of DTCA for psychiatric medication would be of significant value, given the limited data from methodologically strong studies available in this area. Future research evaluating the effects of DTCA in specialty psychiatry settings would also be beneficial due to the predominant focus on primary care settings in extant investigations.
Clinical Points.
Medications for psychiatric conditions are heavily advertised, but the effects of direct-to- consumer advertising (DTCA) on patient prescription requests and physician prescribing are not well understood.
A systematic search identified only four studies relevant to psychiatry that measured the effects of DTCA on patient and/or physician behavior at the point-of-service.
DTCA requests appear to be accommodated in the majority of encounters, promote higher prescribing volume, and have competing effects on treatment quality, though more methodologically strong studies are needed to increase confidence in conclusions.
Acknowledgment of Assistance
The authors would like to thank Anthony Spirito, Ph.D., Professor (Research), Department of Psychiatry and Human Behavior, Brown University Medical School, for his critical review of the first draft of this manuscript.
Sources of Financial Support:
This work was supported by the National Institute on Drug Abuse under award K23DA031743 to Dr. Sara Becker. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse
Footnotes
Conflict of Interest / Disclosure Statement:
The authors report no financial or other relationship relevant to the subject of this article.
Dr. Spirito does not have any conflicts of interest to disclose.
References
- 1.Cole CL. Viagra Gets Out of the Game. J Sport & Social Issues. 2007;31:101–2. [Google Scholar]
- 2.Rosenthal MB, Berndt ER, Donohue JM, Frank RG, Epstein AM. Promotion of prescription drugs to consumers. N Engl J Med. 2002;346:498–505. doi: 10.1056/NEJMsa012075. [DOI] [PubMed] [Google Scholar]
- 3.IMS . Total US Promotional Spend by Type. IMS Health website; [Accessed June 17, 2015]. 2010. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/StaticFile/Top_Line_Data/2010_Promotional_Data.pdf. [Google Scholar]
- 4.Staton T. Pharma's ad spending vaults to $4.5B, with big spender Pfizer leading the way. Fierce Pharma Marketing website; [Accessed June 17, 2015]. http://www.fiercepharmamarketing.com/story/pharmas-ad-spend-vaults-45b-big-spender-pfizer-leading-way/2015-03-25. Updated March 25, 2015. [Google Scholar]
- 5.Anonymous . Impact of Direct-to-Consumer Advertising on Prescription Drug Spending. The Henry J. Kaiser Family Foundation; 2003. [Google Scholar]
- 6.Ventola CL. Direct-to-Consumer Pharmaceutical Advertising: Therapeutic or Toxic? Pharma Therapeut. 2011;36:669–84. [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health Care Management . Prescription drugs and mass media advertising, 2000. National Institute for Health Care Management Research and Educational Foundation; Washington, DC: 2001. [Google Scholar]
- 8.Bhanji NH, Baron DA, Lacy BW, et al. Direct-to-consumer marketing: An attitude survey of psychiatric physicians. Prim Psychiatry. 2008;15:67–71. [Google Scholar]
- 9.Prozac Print Campaign [Accessed March 12, 2015];Marketing Campaign Case Studies website. http://marketing-casestudies.blogspot.com/2008/10/prozac-print-campaign.html. Updated October 25, 2008.
- 10.Eli Lilly and Company . Lilly Announces Fourth-Quarter Financial Results and 1997 Financial Results. PR Newswire; [Accessed October 12, 2015]. http://www.prnewswire.com/news-releases/lilly-announces-fourth-quarter-financial-results-and-1997-financial-results-76476212.html. Updated January 29, 1998. [Google Scholar]
- 11.Brooks M. [Accessed October 10, 2015];100 Best-selling, Most Prescribed Branded Drugs Through June. Medscape. http://www.medscape.com/viewarticle/849457. Updated August 13, 2015.
- 12.McDevitt C. [Accessed April 1, 2015];The Big Money: Depression and the Recession. The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/08/28/AR2009082804069.html. Updated August 30, 2009.
- 13.Donohue JM, Berndt ER. Effects of Direct-to-Consumer Advertising on Medication Choice: The Case of Antidepressants. J Public Policy Mark. 2004;23:115–27. [Google Scholar]
- 14.Gellad ZF, Lyles KW. Direct-to-Consumer Advertising of Pharmaceuticals. Am J Med. 2007;120:475–80. doi: 10.1016/j.amjmed.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frosch DL, Grande D, Tarn DM, Kravitz RL. A Decade of Controversy: Balancing Policy with Evidence in the Regulation of Prescription Drug Advertising. Am J Public Health. 2010;100:24–32. doi: 10.2105/AJPH.2008.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter DM. Direct-to-Consumer (DTC) Pharmaceutical Marketing: Impacts and Policy Implications. SPNHA Review. 2011;7:5. [Google Scholar]
- 17.Holmer AF. Direct to consumer prescription drug advertising builds bridges between patients and physicians. J Am Med Assoc. 1999;281:380–2. doi: 10.1001/jama.281.4.380. [DOI] [PubMed] [Google Scholar]
- 18.Berndt ER. To Inform or Persuade? Direct-to-Consumer Advertising of Prescription Drugs. N Engl J Med. 2005;352:325–8. doi: 10.1056/NEJMp048357. [DOI] [PubMed] [Google Scholar]
- 19.Holmer AF. Direct-to-consumer advertising--strengthening our health care system. N Engl J Med. 2002;346:526–8. doi: 10.1056/NEJM200202143460714. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Gupta S. The impact of direct-to-consumer advertising of prescription drugs on physician visits and drug requests: Empirical findings and public policy implications. Int J Res Mark. 2011;28:205–17. [Google Scholar]
- 21.Davis JJ. Riskier than we think? The relationship between risk statement completeness and perceptions of direct to consumer advertised prescription drugs. J Health Commun. 2000;5:349–69. doi: 10.1080/10810730050199141. [DOI] [PubMed] [Google Scholar]
- 22.Frosch DL, Krueger PM, Hornik RC, Cronholm PF, Barg F, K Creating Demand for Prescription Drugs: A Content Analysis of Television Direct-to-Consumer Advertising. Ann Fam Med. 2007;5:6–13. doi: 10.1370/afm.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollon MF. Direct-to-consumer marketing of prescription drugs: Creating consumer demand. J Am Med Assoc. 1999;281:382–4. doi: 10.1001/jama.281.4.382. [DOI] [PubMed] [Google Scholar]
- 24.Mintzes B. Direct to Consumer Advertising of Prescription Drugs. BMJ. 2008;337:526–7. doi: 10.1136/bmj.a985. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee A, Limbu Y, Wanasika I. A review of research on direct-to-consumer advertising of prescription drugs. IJPHM. 2013;7:226–43. [Google Scholar]
- 26.Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. (NSDUH Series H-48). HHS Publication No (SMA) 14-4863. [Google Scholar]
- 27.Gilbody S, Wilson P, Watt I. Benefits and harms of direct to consumer advertising: a systematic review. Qual Saf Health Care. 2005;14:246–50. doi: 10.1136/qshc.2004.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan E. Recall Bias can be a Threat to Retrospective and Prospective Research Designs. Internet J Epi. 2005;3:1–7. [Google Scholar]
- 30.Coughlin SS. Recall bias in epidemiologic studies. J Clinic Epi. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- 31.Hess DR. Retrospective Studies and Chart Reviews. Respir Care. 2004;49:1171–4. [PubMed] [Google Scholar]
- 32.Henry B, Moffitt TE, Caspi A, Langley J, Silva PA. On the “remembrance of things past”: A longitudinal evaluation of the retrospective method. Psychol Assess. 1994;6:92–101. [Google Scholar]
- 33.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 34.Pandor A, Kaltenthaler E, Higgins A, et al. Sexual health risk reduction interventions for people with severe mental illness: a systematic review. BMC Public Health. 2015;15:138. doi: 10.1186/s12889-015-1448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leamy M, Bird V, Le Boutillier C, Williams J, Slade M. Conceptual framework for personal recovery in mental health: systematic review and narrative synthesis. Brit J Psychiat. 2011;199:445–52. doi: 10.1192/bjp.bp.110.083733. [DOI] [PubMed] [Google Scholar]
- 36.Chong C, Tsunaka M, Tsang HWH, Chan EP, Cheung WM. Effects of Yoga on Stress Management in Healthy Adults: A Systematic Review. Altern Ther Health Med. 2011;17:32–8. [PubMed] [Google Scholar]
- 37.Jones L, Hughes K, Atkinson AM, Bellis MA. Reducing harm in drinking environments: A systematic review of effective approaches. Health Place. 2011;17:508–18. doi: 10.1016/j.healthplace.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 39.Jackson N, Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promot Intl. 2005;20:367–74. doi: 10.1093/heapro/dai022. [DOI] [PubMed] [Google Scholar]
- 40.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook for Quality of Evidence and Strength of Recommendations. The GRADE Working Group; 2013. Updated October 2013. Available from www.guidelinedevelopment.org/handbook. [Google Scholar]
- 43.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 44.Kravitz RL, Epstein RM, Feldman MD, et al. Influence of patients' requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. J Am Med Assoc. 2005;293:1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? A survey in primary care environments with and without legal DTCA. Can Med Assoc J. 2003;169:405–12. [PMC free article] [PubMed] [Google Scholar]
- 46.Mintzes B, Barer ML, Kravitz RL, et al. Influence of direct to consumer pharmaceutical advertising and patients' requests on prescribing decisions: two site cross sectional survey. BMJ. 2002;324:278–9. doi: 10.1136/bmj.324.7332.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allison-Ottey S, Ruffin K, Allison K, Ottey CC. Assessing the impact of direct-to-consumer advertisements on the AA patient: a multisite survey of patients during the office visit. J Natl Med Assoc. 2003;95:120–31. [PMC free article] [PubMed] [Google Scholar]
- 48.Parnes B, Smith PC, Gilroy C, et al. Lack of Impact of Direct-to-Consumer Advertising on the Physician-Patient Encounter in Primary Care: A SNOCAP Report. Ann Fam Med. 2009;7:41–6. doi: 10.1370/afm.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allison-Ottey S, Ruffin K, Allison KB. “To do no harm” survey of NMA physicians regarding perceptions on DTC advertisements. National Medical Association. J Natl Med Associ. 2002;94:194–202. [PMC free article] [PubMed] [Google Scholar]
- 50.Ball JG, Manika D, Stout P. Consumers young and old: segmenting the target markets for direct-to-consumer prescription drug advertising. Health Mark Q. 2011;28:337–53. doi: 10.1080/07359683.2011.623112. [DOI] [PubMed] [Google Scholar]
- 51.Datti B, Carter MW. The effect of direct-to-consumer advertising on prescription drug use by older adults. Drugs Aging. 2006;23:71–81. doi: 10.2165/00002512-200623010-00007. [DOI] [PubMed] [Google Scholar]