Abstract
Background and objectives
Patients with CKD are advised to follow dietary recommendations that restrict individual nutrients. Emerging evidence indicates overall eating patterns may better predict clinical outcomes, however, current data on dietary patterns in kidney disease are limited.
Design, setting, participants, & measurements
This systematic review aimed to evaluate the association between dietary patterns and mortality or ESRD among adults with CKD. Medline, Embase, and reference lists were systematically searched up to November 24, 2015 by two independent review authors. Eligible studies were longitudinal cohort studies reporting the association of dietary patterns with mortality, cardiovascular events, or ESRD.
Results
A total of seven studies involving 15,285 participants were included. Healthy dietary patterns were generally higher in fruit and vegetables, fish, legumes, cereals, whole grains, and fiber, and lower in red meat, salt, and refined sugars. In six studies, healthy dietary patterns were consistently associated with lower mortality (3983 events; adjusted relative risk, 0.73; 95% confidence interval, 0.63 to 0.83; risk difference of 46 fewer (29–63 fewer) events per 1000 people over 5 years). There was no statistically significant association between healthy dietary patterns and risk of ESRD (1027 events; adjusted relative risk, 1.04; 95% confidence interval, 0.68 to 1.40).
Conclusions
Healthy dietary patterns are associated with lower mortality in people with kidney disease. Interventions to support adherence to increased fruit and vegetable, fish, legume, whole grain, and fiber intake, and reduced red meat, sodium, and refined sugar intake could be effective tools to lower mortality in people with kidney disease.
Keywords: Nutrition; chronic kidney disease; mortality; dialysis; diet quality; dietary patterns; Adult; Carbohydrates; Cohort Studies; Confidence Intervals; diet; Dietary Fiber; Edible Grain; Fabaceae; Fruit; Humans; Kidney Failure, Chronic; Longitudinal Studies; Red Meat; Risk; Sodium; Vegetables; Whole Grains
Introduction
CKD affects about 10%–13% of adults (1) and represents a public health challenge because of the substantially increased risks of death and cardiovascular disease among affected people (2,3). Patients who have CKD are advised to follow dietary recommendations that restrict individual nutrients, such as phosphorus, salt, potassium, and protein, to prevent short- and long-term clinical complications (4). Historically, dietary advice has been given on the basis of individual nutrients or food groups instead of whole eating patterns, despite being considered complex, challenging to adhere to, and an intense burden for some patients (5). In addition, there is limited evidence that restricting or supplementing specific nutrients or single food groups effectively prevents clinical complications, including kidney failure or death (6–9). Fluid and dietary restrictions remain frequently identified as priority areas of research by patients with kidney disease and health care providers (10).
Recent evidence has linked dietary patterns rich in fruit and vegetables, fish, legumes, cereals, and nuts with reduced cardiovascular events and death in healthy adults and those at high risk of cardiovascular disease (11–14). In parallel, there is an emerging trend toward the study of whole dietary patterns rather than single nutrient or food group restrictions among people with kidney disease (15–17). However, existing cohort studies of dietary patterns in people with kidney disease have small sample sizes, whereas existing randomized trials are insufficiently powered to establish the role of whole dietary patterns on mortality and kidney failure, limiting the impact of single studies to inform clinical practice and policy (18,19). Existing dietary guidelines lack robust evidence for effects on patient-centered outcomes (20).
The aim of this study was to conduct a meta-analysis of the evidentiary basis for the association of dietary patterns with mortality and cardiovascular end points, in order to establish the potential role of dietary patterns among people with CKD.
Materials and Methods
Our primary aim was to assess the association of healthy dietary patterns with the risk of mortality and ESRD in adults with CKD. This systematic review followed a prespecified review protocol, was prospectively registered in the International Prospective Register of Systematic Reviews (21) and was reported using the preferred reporting items for systematic reviews and meta-analysis (22).
Data Sources and Searches
We searched Medline, Embase, and reference lists of retrieved studies for prospective cohort studies available online reporting the association between dietary patterns and clinical outcomes among adults who have CKD on November 24, 2015. We did not have any language or date restriction for the search. The search terms are shown in Supplemental Table 1.
Study Selection
Dietary patterns were defined as overall habitual food intake ascertained by healthy eating guidelines or a priori diet quality score, dietary pattern analysis, and/or consumption of whole food groups such as fruit and vegetables. We excluded single nutrient or food group–based modifications from this review, including isolated protein or sodium restriction. We required follow-up for at least 24 weeks to ensure sufficient follow-up of dietary patterns on patient-level outcomes, and explicit reporting of outcomes either as raw data or adjusted effect estimates with 95% confidence intervals (95% CIs). We used definitions of CKD according to international clinical practice guidelines (4).
Data Extraction and Quality Assessment
Two authors (J.T.K. and S.N.W.) independently reviewed all retrieved records for eligibility using reference management software. The two authors extracted data and adjudicated risk of bias, with differences resolved by discussion. We contacted authors for information that was missing or unclear from included studies. The risk of bias was assessed using the Newcastle–Ottawa tool (23). We then used the Grading of Recommendations Assessment, Development, and Evaluation methodology to rate the quality of the evidence for mortality as high, moderate, low, or very low (24). Observational studies began as low quality evidence, but could be rated upward to moderate or high quality evidence if they collectively demonstrated a large magnitude of effect, or a dose–response gradient. Outcomes were death, health-related quality of life, ESRD, major cardiovascular events, BP, serum cholesterol, and major adverse events.
Data Synthesis and Analysis
We carried out analyses according to a predefined protocol to compare healthy eating patterns (generally higher intake of fruit, vegetables, cereals, legumes, whole grains, fiber, and fish, and lower intake of red meat, salt, and refined sugar) with dietary intake less representative of these eating patterns. We then summarized adjusted risks (hazard ratio, odds ratio, or relative risk) provided in studies using random-effects inverse variance meta-analysis. A fixed-effect model was also used to ensure robustness of the model chosen and susceptibility to outliers. Estimated numbers of events incurred or avoided with dietary change were calculated as a risk difference on the basis of a 5-year risk of mortality reported in a systematic review of cohort studies (25). We used the I2 statistic to assess heterogeneity (the proportion of total variation observed in the association of dietary intake and outcome among studies beyond that expected by chance), with an I2 value <25% considered as low heterogeneity and >75% as high heterogeneity. We assessed for small study effects in analyses for mortality by visual evaluation of the funnel plot for symmetry.
Sensitivity analyses were done, excluding studies in which the same cohort of participants may have been represented more than once and those involving adults with ESRD. We planned subgroup analyses on the basis of sex, duration of follow-up, study quality, and geographical region. Analyses were performed using Stata 13, with 95% CIs, excluding a risk ratio of 1.0 used to denote statistical significance.
Results
Study Selection and Baseline Characteristics
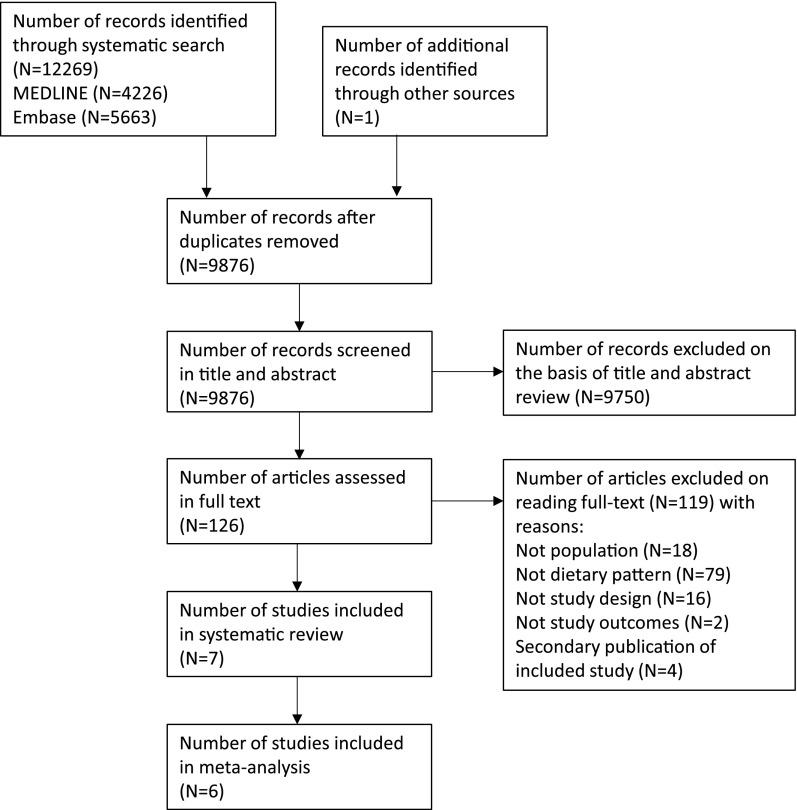
The systematic search yielded seven cohort studies (Figure 1), involving 15,285 patients with CKD (Table 1) (17,26–31). The participants were followed for between 4 and 13 years on average, totaling approximately 91,000 person-years of follow-up. All but one study involved people with CKD defined as an eGFR<60–70 ml/min per 1.73 m2 body surface area or albuminuria (17,26–30). One study enrolled adults treated with dialysis (31). Studies involved people living in the United States (17,26,27,29,30), Sweden (28), and Japan (31). Healthy dietary patterns were reported as generally consistent with a higher intake of fruits and vegetables, legumes, cereals, whole grains, fiber, and fish, and lower intake of red meat and products containing sodium and refined sugars (Table 2). All studies were published between 2013 and 2015. There were 3983 deaths and 1027 ESRD events recorded during follow-up.
Figure 1.
Flow chart describing process of study selection.
Table 1.
Characteristics of included studies
| First Author | Dietary Pattern | Country | Study Name | No. of Participants | Yr of Follow-Up (Person-yr) | Definition of Kidney Disease | Age at Entry, (Mean or Median) | eGFR, Mean±SD, ml/min per 1.73 m2 | End Points (No. of Events) |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. 2016 (26) | Plant versus animal protein | United States | Third National Health and Nutrition Examination Survey (NHANES III) | 1065 men and women | 6.2 (6603) | eGFR<60 ml/min per 1.73 m2 | 20 yr or older (not reported) | 101±20 (quartile 1) | All-cause mortality (633) |
| Gutiérrez et al. 2014 (27) | Plant based | United States | Reasons for Geographic and Racial Differences in Stroke (REGARDS) study | 3972 men and women | 6.4 (25,421) | eGFR<60 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio >30 mg/g | 45 yr or older (67.1–69.8 yr) | 68.1 (SEM 0.8) (quartile 1) | All-cause mortality (816); ESRD (141) |
| Huang et al. 2013 (28) | Mediterranean diet | Sweden | Uppsala Longitudinal Study of Adult Men | 506 men | 9.9 (4648) | eGFR<60 ml/min per 1.73 m2 | Approximately 70 yr | 51.9 (median) (interquartile range, 46.3–56.6) | All- cause mortality (168) |
| Muntner et al. 2013 (29) | Diet score (fish, fruit/vegetables, sodium, sugar fiber, and carbohydrate) | United States | Reasons for Geographic and Racial Differences in Stroke (REGARDS) study | 3093 men and women | 4 (12,372) | eGFR<60 ml/min per 1.73 m2 | 45 yr or older (72.2 yr) | All-cause mortality (610); ESRD (160) | |
| Ricardo et al. 2015 (17) | American Heart Association | United States | Chronic Renal Insufficiency Cohort (CRIC) Study | 3006 men and women | 4 (12,024) | eGFR of 20–70 ml/min per 1.73 m2 | 21–74 yr (58 yr) | 43.39±13.34 (diet score 0) | All-cause mortality (437); CKD progression (50% decrease in eGFR or ESRD) (726); atherosclerotic events (355) |
| Ricardo et al. 2013 (30) | Healthy Eating Index based on Food Guide Pyramid | United States | Third National Health and Nutrition Examination Survey (NHANES III) | 2288 men and women | 13 (29,744) | eGFR<60 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio >30 mg/g | 20 yr or older (59 yr) | 88.4±1.7 (SEM) (healthy lifestyle score quartile 1) | All-cause mortality (1319) |
| Tsuruya et al. 2015 (31) | Meat, fish, and vegetable intake | Japan | Japan Dialysis Outcomes and Practice Patterns Study (JDOPPS) | 1355 men and women | Not reported | Hemodialysis | Not reported (61.4 yr) | Dialysis | All-cause mortality or hospitalization because of cardiovascular disease (not reported) |
Table 2.
Characteristics of dietary exposures used in meta-analyses
| Study | Dietary Pattern | Dietary Exposure | Exposure Category | Reference Category | Covariates Included in Risk Ratio |
|---|---|---|---|---|---|
| Chen et al. 2016 (26) | Plant versus animal protein | Plant protein ratio quartiles (grains, fruits, vegetables, legumes, nuts, and seeds) | Quartile 4 >43.5% plant-to-protein ratio | Quartile 1<25.3% plant-to-protein ratio | Total protein intake, age, sex, race, smoking, alcohol use, calorie intake, exercise, body mass index, hypertension, cancer, myocardial infarction, congestive heart failure, stroke, and diabetes |
| Gutiérrez et al. 2014 (27) | Plant based | Plant-based defined using principal component analysis (fruits, vegetables, fish) | Quartile 4 (highest) | Quartile 1 (lowest) | Age, sex, race, geographic region, energy intake, lifestyle factors (self-reported frequency of exercise, current smoking), comorbidities (heart disease, hypertension), educational achievement, family income, urinary albumin-to-creatinine ratio, eGFR |
| Huang et al. 2013 (28) | Mediterranean diet | Mediterranean diet score (polyunsaturated fats/saturated fatty acids >0.34; vegetables and legumes >69 d; fruit >115 g/d; cereals and potatoes >361 g/d; fish >25 g/d; meat and meat products <92 g/d; milk and milk products <328 g/d; moderate alcohol | High adherence (dietary score 6–8) | Low adherence (dietary score 1–2) | Body mass index, physical activity, smoking status, education, hypertension, hyperlipidemia, diabetes |
| Muntner et al. 2013 (29) | Diet score | Healthy diet score based on fish (≥servings/wk), fruit and vegetable consumption (≥4.5 cups/d), and sodium (<1500 mg/d), sugar (<450 kcal/wk), and fiber/carbohydrate ratio intake (>0.1) | Intermediate dietary score (2–3 components) | Poor dietary score (0–1 components) | Age, race, sex, geographic region, income, education, history of stroke and coronary heart disease |
| Ricardo et al. 2015 (17) | American Heart Association | Healthy diet score (American Heart Association: fruits/vegetables >2.8 cups/d; fish >1.3 oz/wk; whole grains >0.88 oz/d; 24-hr urine sodium excretion <152 mEq/d; sweets/sugar-sweetened beverages <571 ml/wk) | Ideal (healthy diet score 4–5) | Dietary score 0–3 | Clinical center, age, sex, race/ethnicity, education, diabetes, dyslipidemia, hypertension, any cardiovascular disease, angiotensin-converting enzyme/angiotensin receptor blocker use, eGFR, urine protein excretion |
| Ricardo et al. 2013 (30) | Healthy Eating Index based on Food Guide Pyramid | Healthy Eating Index based on ten dietary components (grains, vegetables, fruits, milk, meat, total fat, saturated fat, cholesterol, sodium, and dietary variety) | Healthy Eating Index score 73.1–100 | Healthy Eating Score <54.5 | Age, sex, race/ethnicity, annual household income, education, eGFR, microalbuminuria, diabetes, cardiovascular disease, cancer, systolic BP, serum cholesterol, use of statin, use of angiotensin-converting enzyme inhibitor |
| Tsuruya et al. 2015 (31) | Meat, fish, and vegetable intake | Consumption of approximately equal amounts of food from meat, fish, and vegetable groups. | Well-balanced | Unbalanced | Age, sex, dialysis duration, serum albumin, body mass index, energy intake, diabetes, coronary heart disease, cerebrovascular disease, peripheral vascular disease |
Risk of Bias and Evidence Quality
Risks of bias in the included studies is shown in Supplemental Figure 1. Overall, studies were considered at low risk of bias for characteristics considered important to the reliability of cohort studies. When the Grading of Recommendations Assessment, Development, and Evaluation (directness, precision, consistency, and study limitations) recommendations were considered, the evidence quality for all-cause mortality was considered low on the basis of the nonrandomized study design, without incurring further downgrades in evidence quality for indirectness, imprecise results, heterogeneity, or study reporting limitation.
Outcomes
All-Cause Mortality.
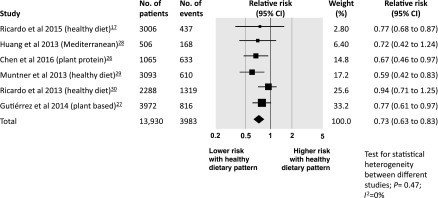
When compared with other dietary patterns, a dietary pattern richer in vegetables, fruit, fish, cereals, whole grains, fiber, legumes, and nuts and seeds, and lower in red meat, sodium, and refined sugars was associated with a lower risk of death. In six studies among 13,930 participants followed for between 4 and 13 years, the relative risk of all-cause mortality was 0.73 (95% CI, 0.63 to 0.83) (Figure 2). There was no heterogeneity between studies (I2=0%) and no evidence of small study effects (Supplemental Figure 2). On the basis of an estimated 5-year mortality of 17% in people with CKD (25), the risk difference with a healthy dietary pattern compared with other dietary patterns was 46 fewer deaths per 1000 people (29–63 fewer) over 5 years.
Figure 2.
Risk of all-cause mortality associated with healthy dietary patterns among adults with CKD. 95% CI, 95% confidence interval.
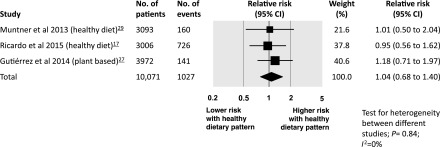
ESRD.
There was no evidence of an association between a healthy dietary pattern and risk of ESRD in three studies (n=10,071 people), with follow-up ranging from 4 to 6.4 years. The risk of ESRD among people with CKD was 1.04 (95% CI, 0.68 to 1.40) with no evidence of statistical heterogeneity between studies (Figure 3).
Figure 3.
Risk of ESRD associated with healthy dietary patterns among adults with CKD. 95% CI, 95% confidence interval.
Major Cardiovascular Events, Health-Related Quality of Life, Adverse Events, and BP.
There were insufficient numbers of studies to conduct meta-analysis for risks of major cardiovascular events. In one study involving 3006 people, a healthy diet score was not associated with risk of atherosclerotic events (adjusted relative risk, 1.01; 95% CI, 0.47 to 2.18) (17). In a single study among 1355 dialysis patients, an “unbalanced dietary pattern” of high sodium and vegetable intake and lower fish and meat intake was associated with a higher risk of a composite of hospitalization because of cardiovascular disease or death because of any cause (31).
There was no reporting of health-related quality of life, cardiovascular-related death, adverse events, or hyperkaliemia as individual end points. There was no information about the effects of healthy dietary patterns on BP or serum cholesterol levels during follow-up.
Sensitivity Analyses
Results were similar when single studies were removed to exclude the possibility that participants had been included in analyses more than once (Supplemental Table 2). There was no evidence that results in meta-analyses for mortality were different on the basis of country of origin, age, duration of follow-up time, or quality of studies (Supplemental Table 3).
Discussion
This meta-analysis, comprised of approximately 90,000 person-years of follow-up and including 3983 mortality events, showed that dietary patterns rich in vegetables and fruits, legumes, whole grains, and fiber together with lower consumption of red meat, sodium, and refined sugars were consistently associated with lower mortality in people with CKD. Existing cohort studies provide evidence of the association of healthy dietary patterns with risk of ESRD, major cardiovascular complications, and health-related quality of life, although there is considerable uncertainty in the results for these outcomes because of wide confidence intervals from meta-analyses that included few studies. To our knowledge, this is the first cumulative assessment of whole dietary patterns and their association with mortality and clinical complications in people with CKD.
The association of healthy dietary patterns with lower mortality in people with CKD is in contrast to the lack of association of restrictions of individual dietary components for food groups including serum phosphorus (7,32,33), sodium (6), and protein (34) intake with mortality, although individual studies addressing these questions have had small sample sizes and low power to discern the relative association of nutritional modifications on clinical outcomes. The findings of the current meta-analysis are consistent with accruing large-scale evidence of consistent mortality benefits with adherence to a plant-based dietary pattern among people without existing chronic disease (35), although in a large randomized controlled trial on Mediterranean diet, a primarily plant-based diet including extra virgin olive oil or nuts, there was no statistical evidence of lower mortality alone in people at high risk of cardiovascular events, while a Mediterranean dietary pattern lowered the risk of a composite of nonfatal and fatal cardiovascular events (11). To date, randomized trials testing the effects of dietary patterns rich in fruits and vegetables or a Mediterranean diet in adults with kidney disease are preliminary and have not examined mortality as an end point (18,36,37). As in our study, there is limited evidence for the association of eating patterns with risk of ESRD in the literature, although cohort studies suggest dietary patterns rich in fruit and vegetables may lower risk of progression to CKD and decrease albuminuria and BP (38–42).
Recent research in CKD has seen a shift from the decades-long focus on assessing and modifying single nutrient components of diet among people with CKD, reflected in practice guidelines (4), to an increasing analysis of whole dietary patterns. As a result, this study shows accumulating evidence over the last 5 years of analyses that consider all food groups thought to be important for health. While existing single nutrient approaches have had limited impact on health in people with kidney disease, this study on the building evidence for healthy dietary patterns on mortality risk suggests that this shift to wider dietary approaches across several food groups is appropriate and aligns with existing patient priorities (10). Given the prevalence of CKD in the community, data supporting specific dietary patterns potentially has an important public health impact, and warrants the prioritization of additional resources to support a randomized trial of dietary intake in this population. Highly efficient trial design, embedded within registries or electronic health records, might increase the feasibility and reduce the costs of an adequately powered dietary trial in the wider population with kidney disease. This is particularly relevant given the progressive shift toward more Western dietary patterns (43) and the relative lack of treatments proven to lower the burden of premature death and kidney failure among people with kidney disease. A recent additional cohort study showing a dose–dependent association between red meat intake and risk of ESRD, and lower risks when other sources of protein are substituted, further adds weight to the need to understand the association of whole food dietary patterns with clinical outcomes in the setting of kidney disease (44).
Although this study was prospectively planned and conducted independently by two authors, providing highly consistent findings among studies and precise risk estimates for the mortality end point, some limitations of this study can be identified. First, the healthy dietary patterns we identified were not standardized and represent a heterogeneous range of dietary intake. For example, some dietary patterns included milk products as healthy food groups (27), whereas others defined milk and milk product intake as less desirable (26,28,30). However, the key elements of greater fruit and vegetable intake were present in all studies. Second, these studies were on the basis of dietary self-recalls via differing methods (food frequency questionnaires versus food records), although the results among all studies were consistent, and not apparently influenced by this factor. Third, included patients had a range of kidney function, although all had an eGFR<60–70 ml/min per 1.73 m2 or albuminuria. Fourth, all of the studies included in meta-analysis for mortality were conducted in the United States or Sweden, and thus the results may not be generalizable to other global regions, including lower resourced regions. Fifth, we did not find any association of dietary change with ESRD. ESRD is a rarer complication of CKD because of the competing risk of death; accumulated studies evaluating the associations of diet with this outcome had relatively few recorded events, as would be expected, even when linked to dialysis census databases. Sixth, it was not possible to assess for evidence of publication bias. Finally, this study is based on nonrandomized data, leading to the potential for the findings to be partly explained by residual confounding and leading to lower quality evidence. The results are hypothesis-generating and represent an important indication for a future randomized trial and public policy, particularly as dietary and lifestyle interventions are highly ranked research priorities by patients and clinicians.
In summary, this meta-analysis shows that adherence to dietary patterns rich in fruit and vegetables, fish, legumes, cereals, whole grains, and fiber, and lower in red meat and products containing sodium and refined sugars is associated with lower mortality in people with CKD. This finding represents a shift in evidence from management of single nutrient or food groups in the care of kidney disease, and aligns with the experiences of patients who describe nutritional advice as frequently complex and difficult to follow. This evidence might prompt the prioritization of randomized trials of dietary patterns among people with kidney disease, as well as the re-evaluation of dietary advice as a public health tool to lower mortality in people with kidney disease.
Disclosures
G.F.M.S. has received an honorarium from Servier (Suresnes, France), G.F.M.S. has done consultancy for Danone (Paris, France); K.L.C. and J.-J.C. are members of the Kidney Disease: Improving Disease Outcomes Clinical Practice Guidelines for Nutrition in CKD, cosponsored by the US Academy of Nutrition and Dietetics and the US National Kidney Foundation. K.L.C. is on the Kidney Health Australia Caring for Australasians with Renal Impairment guideline committee for guidelines on Autosomal Polycystic Kidney Disease, including a role as section chair for “Diet and Lifestyle Management.” K.L.C. reports personal fees from Shire Australia. J.-J.C. reports being council member of the International Society of Renal Nutrition and Metabolism, and general secretary of the European Renal Nutrition working group of the European Renal Association-European Dialysis and Transplantation Association. J.-J.C. also reports receiving honoraria during 2015 for lecturing at scientific symposia organized by Abbott Nutrition (Lake Bluff, IL) and Baxter Healthcare (Deerfield, IL) on topics related to this study. J.T.K., S.C.P., S.N.W., and M.R. have no relationships with companies or nonfinancial relationships that might be relevant to the submitted work.
Supplementary Material
Acknowledgments
J.T.K. is supported by an Australian Post Graduate Award scholarship through Bond University. J.-J.C. acknowledges funding from Stockholm County Council and the Swedish Heart and Lung Foundation. S.C.P. receives a Rutherford Discovery Fellowship from the Royal Society of New Zealand.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06190616/-/DCSupplemental.
References
- 1.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 5.Palmer SC, Hanson CS, Craig JC, Strippoli GF, Ruospo M, Campbell K, Johnson DW, Tong A: Dietary and fluid restrictions in CKD: A thematic synthesis of patient views from qualitative studies. Am J Kidney Dis 65: 559–573, 2015 [DOI] [PubMed] [Google Scholar]
- 6.McMahon EJ, Campbell KL, Bauer JD, Mudge DW: Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev (2): CD010070, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Su G, Guo X, Wu Y, Liu X, Zou C, Zhang L, Yang Q, Xu Y, Ma W: Dietary interventions for mineral and bone disorder in people with chronic kidney disease. Cochrane Database Syst Rev (9): CD010350, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouque D, Laville M: Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev (3): CD001892, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B: Omega 3 fatty acids and cardiovascular outcomes: Systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 5: 808–818, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Tong A, Chando S, Crowe S, Manns B, Winkelmayer WC, Hemmelgarn B, Craig JC: Research priority setting in kidney disease: A systematic review. Am J Kidney Dis 65: 674–683, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA; PREDIMED Study Investigators : Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368: 1279–1290, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S: ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev (8): CD009825, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A: Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 337: a1344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trichopoulou A, Bamia C, Trichopoulos D: Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 338: b2337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Fung TT, Hu FB, Curhan GC: Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis 57: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor EN, Fung TT, Curhan GC: DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 20: 2253–2259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, Fink JC, Frydrych A, Jensvold NG, Lustigova E, Nessel LC, Porter AC, Rahman M, Wright Nunes JA, Daviglus ML, Lash JP; CRIC Study Investigators : Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 412–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekki K, Bouzidi-bekada N, Kaddous A, Bouchenak M: Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct 1: 110–115, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Stachowska E, Wesołowska T, Olszewska M, Safranow K, Millo B, Domański L, Jakubowska K, Ciechanowski K, Chlubek D: Elements of Mediterranean diet improve oxidative status in blood of kidney graft recipients. Br J Nutr 93: 345–352, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Reidlinger DP, Darzi J, Hall WL, Seed PT, Chowienczyk PJ, Sanders TA; Cardiovascular disease risk REduction Study (CRESSIDA) investigators : How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle-aged and older men and women? A randomized controlled trial. Am J Clin Nutr 101: 922–930, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Kelly J, Wai SN, Palmer S, Ruospo M, Carerro J-J, Strippoli G, Campbell K: Association of dietary patterns quality with mortality and quality of life and clinical outcomes in adults with chronic kidney disease: Systematic review and meta-analysis of cohort studies. PROSPERO 2015. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015029486. Accessed August 20, 2016
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339: b2535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed November 8, 2016
- 24.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wei G, Jalili T, Metos J, Giri A, Cho ME, Boucher R, Greene T, Beddhu S: The associations of plant protein intake with all-cause mortality in CKD. Am J Kidney Dis 67: 423–430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, Judd SE: Dietary patterns and risk of death and progression to ESRD in individuals with CKD: A cohort study. Am J Kidney Dis 64: 204–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Jiménez-Moleón JJ, Lindholm B, Cederholm T, Arnlöv J, Risérus U, Sjögren P, Carrero JJ: Mediterranean diet, kidney function, and mortality in men with CKD. Clin J Am Soc Nephrol 8: 1548–1555, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muntner P, Judd SE, Gao L, Gutiérrez OM, Rizk DV, McClellan W, Cushman M, Warnock DG: Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol 24: 1159–1165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricardo AC, Madero M, Yang W, Anderson C, Menezes M, Fischer MJ, Turyk M, Daviglus ML, Lash JP: Adherence to a healthy lifestyle and all-cause mortality in CKD. Clin J Am Soc Nephrol 8: 602–609, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuruya K, Fukuma S, Wakita T, Ninomiya T, Nagata M, Yoshida H, Fujimi S, Kiyohara Y, Kitazono T, Uchida K, Shirota T, Akizawa T, Akiba T, Saito A, Fukuhara S: Dietary patterns and clinical outcomes in hemodialysis patients in Japan: A cohort study. PLoS One 10: e0116677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selamet U, Tighiouart H, Sarnak MJ, Beck G, Levey AS, Block G, Ix JH: Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3-5: The modification of diet in renal disease study. Kidney Int 89: 176–184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S: Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrol Dial Transplant 27: 990–996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson L, Waugh N, Robertson A: Protein restriction for diabetic renal disease. Cochrane Database Syst Rev (4): CD002181, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofi F, Abbate R, Gensini GF, Casini A: Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr 92: 1189–1196, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 38.De Lorenzo A, Noce A, Bigioni M, Calabrese V, Della Rocca DG, Di Daniele N, Tozzo C, Di Renzo L: The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr Pharm Des 16: 814–824, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Jacobs DR Jr, Gross MD, Steffen L, Steffes MW, Yu X, Svetkey LP, Appel LJ, Vollmer WM, Bray GA, Moore T, Conlin PR, Sacks F: The effects of dietary patterns on urinary albumin excretion: Results of the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Kidney Dis 53: 638–646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L: Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis 24: 1253–1261, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, Clase CM, Mann JF, Yusuf S, Oberbauer R; ONTARGET Investigators : Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern Med 173: 1682–1692, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS: The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol 9: 1868–1875, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sofi F, Vecchio S, Giuliani G, Martinelli F, Marcucci R, Gori AM, Fedi S, Casini A, Surrenti C, Abbate R, Gensini GF: Dietary habits, lifestyle and cardiovascular risk factors in a clinically healthy Italian population: The ‘Florence’ diet is not Mediterranean. Eur J Clin Nutr 59: 584–591, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, Koh WP: Red meat intake and risk of ESRD [published online ahead of print July 14, 2016]. J Am Soc Nephrol https://dx.doi.org/10.1681/ASN.2016030248doi: 10.1681/ASN.2016030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.