Abstract
This study contrasted the anthocyanins of investigational grape clusters that developed without light incidence (light-excluded), to those of control clusters that were shaded naturally beneath the vine canopy (control-shaded). Treatment grape clusters were light-excluded during ripening by opaque white polypropylene enclosures; temperature, vapor pressure deficit, and light intensity were measured continually. All 15 ‘Merlot’ grape anthocyanins accrued in both groups, indicating no accumulations were terminated from light-exclusion during ripening. Light-excluded clusters had an overall lower anthocyanin concentration (98.1 mg/100 g of berries) than that of control clusters (162.0 mg/100 g of berries), but it was not significantly different. Light-excluded clusters showed altered concentrations of nine individual anthocyanins that were significantly higher in control-shaded clusters. Although the changes in anthocyanin composition could not be attributed solely to the elimination of light, as there were also deviations in berry temperature and vapor pressure deficit concurrent with preventing light from reaching the treatment clusters.
Keywords: Analytical chemistry, Food science
1. Introduction
Anthocyanins are one of many grape metabolites important to winemaking, but they are also the quality-defining components for each red wine grape varietal and crucial to the style of every wine, as well reviewed by Cheynier (2005). Numerous studies have examined vineyard management’s ability to influence grape anthocyanin composition, and one method to effect quality is by regulating the amount of sunlight upon ripening clusters (DeBolt et al., 2008; Downey et al., 2004; Lee and Skinkis, 2013; Ristic et al., 2007). As microclimates were recognized to influence grape quality components, understanding the links between sun exposure and anthocyanin accumulation became an important area of vineyard management research, to be able to define microclimate regimes for targeted grape metabolite accumulations (Lee and Skinkis, 2013; Tarara et al., 2008).
Previous studies on how light-exclusion changed grape anthocyanin profiles only recorded measurements around the cluster for a short time period, or did not collect all necessary microclimate data during the experimental period. For example, Downey et al. (2004) conducted a ‘Shiraz’ shading experiment, but did not measure the microclimate inside the light-exclusion box for the entire duration of the experiment. While Cortell and Kennedy (2006) did not measure vapor pressure deficit (VPD) or photosynthetically active radiation (PAR), and provided little detail of how temperature was monitored. DeBolt et al. (2008) did without temperature measurements on the assumption that temperatures within the boxes (research conducted in Nuriootpa, South Australia) were similar to ambient, they based that decision on using the same exclusion boxes as Downey et al., (2004)(conducted in Willunga, South Australia), despite the two vineyards being 126 km apart and possibly different in climate zones.
Many techniques have been used for past cluster shading/light exposure research. Methods have included altering canopy management (e.g., less or more leaf removal around the grape cluster; Chorti et al., 2010; Lee and Skinkis, 2013), selecting clusters at different depths within the canopy (Tarara et al., 2008), with or without shade cloths (Caravia et al., 2016; Greer and Weedon, 2013; Koyama and Goto-Yamamoto, 2008), with or without plastic netting (Chorti et al., 2010), and with or without light exclusion boxes (Cortell and Kennedy, 2006; DeBolt et al., 2008; Downey et al., 2004; Ristic et al., 2007). Such different light management approaches add further complexity to already mixed findings. The objective of this work was to establish how the absolute removal of sunlight, under field conditions by utilizing a light exclusion box, influenced the production of ‘Merlot’ grape anthocyanins and other metabolites.
2. Materials and methods
2.1. Research vineyard setup and plant material
This study was conducted in 2009 at the Irrigated Agriculture Research and Extension Center (IAREC; Prosser, WA, USA; 46.30° N; 119.75° W), on nine-year-old own-rooted ‘Merlot’ (Vitis vinifera L.). Vineyard rows were oriented North-South and 2.1 m apart; with the vines spaced every 1.8 m. Vines were trained to bilateral cordons (1.1 m above ground) and spur-pruned annually. The vineyard was managed according to the region’s commercial convention for red wine grapes. Vine yield was not measured.
Control and treatment clusters were located within the west aspect of the canopy, and each was replicated three times (due to the limitation of vineyard space and equipment availability). Controls were ambient fruit shaded by the canopy. Treatment clusters were fruit from which light was excluded by opaque white enclosures (Fig. 1 and Table 1). The treatment was applied from the onset of véraison (first sign of one to two berries turning color; day of year [DOY] 227) until commercial ripeness, which was determined when a random composite sample of adjacent non-experimental fruit reached 23 Brix. All clusters were harvested on DOY 275, resulting in a treatment period of 48 days. Immediately after removal, clusters were placed within coolers (indirectly on dry ice) until return to the laboratory, where they were then stored at −80 °C until extraction and chemical component analysis.
Fig. 1.
Photos of the light exclusions boxes prior to harvesting at commercial-ripeness: closed (a) and opened (b). Box enclosures remained closed for the entire treatment duration.
Table 1.
Summary of the control and treatment (both n = 3) set up in the research block. Field equipment was installed when one or two berries had coloration (day of year [DOY] 227; August 15th 2009), microclimate monitoring started on DOY232 (August 20th 2009) until DOY265 (September 21st 2009). Clusters were harvested at DOY275 (October 1st 2009).
| Control − naturally shaded clusters | Light exclusion boxed clusters | |
|---|---|---|
| Canopy side | West | West |
| Description of the clusters selected for experiment | Well within the canopy with natural shading. | Due to shoot spacing, two clusters were covered with the box (see Fig. 1b), as with previously published works (Cortell and Kennedy, 2006; DeBolt et al., 2008; Downey et al., 2004; Ristic et al., 2007). |
The opaque white enclosures (Fig. 1) were identical to those originally used by Downey et al. (2004), rectangular boxes with a white exterior and a flat black interior. A slight modification (see Fig. 1) was made to the enclosures to allow the measurement of air and berry temperatures, PAR, and VPD. This modification can be seen in Fig. 1. The enclosures were suspended from the lowest wind wire of the trellis and fully contained individual clusters. As clusters were in very close proximity, each box enclosed a pair of clusters, with the more basal one being designated as the experimental cluster (Table 1). Experimental cluster temperatures were calculated from the mean temperature of four berries spread along a central vertical axis of the cluster. The berries for temperatures measurements were monitored with 0.13 mm diameter thermocouples (Type T), wired in parallel to comprise four junction thermopiles. Individual thermocouples were inserted just underneath the berry skin and secured with water-based adhesive, as described in Tarara et al. (2008). Temperature/humidity sensors (80 mm long × 12 mm diameter; model PC Series MINI, Rense Instruments, Danbury, CT, USA) monitored VPD and air temperature at each cluster. The sensors were positioned just north of clusters and had white tube-like enclosures to limit solar radiation error. Reference air temperature and VPD measurements were made at 2 m above ground level by a separate temperature/VPD sensor (Vaisala HMP-C, Campbell Scientific, Inc., Logan UT, USA). PAR was measured at the base of each cluster with quantum sensors (SQ-110, Apogee Instruments, Inc., Logan, UT, USA). The quantum sensors were mounted on vertical supports, which allowed the entire sensor to be positioned within the experimental cluster enclosures. Ambient clusters had the sensor head placed with the same distance and orientation to the cluster as those within the enclosures. Reference PAR was measured above the canopy, using the same type of sensor. All sensor signals were sampled at 5 s intervals and averaged every 12 min by a datalogger (CR-10X, Campbell Scientific, Inc). Location temperatures were recorded with a dedicated thermocouple multiplexer (AM-25T, Campbell Scientific, Inc).
2.2. Reagents, chemicals, and standards
All chemicals used in this study were HPLC grade and obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), unless specified otherwise. Malvidin-glucoside was purchased from Polyphenol AS (Sandnes, Norway). Free amino acid standards and derivatization materials were purchased from Agilent Technologies Inc. (Palo Alto, CA, USA).
2.3. Extraction, sample preparation, and analysis
Berry weights were determined from the mean mass of 50 random berries. All extraction, sample preparation, and analyses did not differ from previously reported methods published by our group (Lee and Rennaker, 2011; Lee and Finn, 2007). Briefly, pH, % soluble solids, and titratable acidity was determined as previously described (Lee and Skinkis, 2013), individual sugars, organic acids, free amino acids, and anthocyanins (expressed in mg of malvidin-glucoside/100 g of berries) were determined by Agilent HPLC 1100, ammonia and total tannins (methylcellulose precipitation method; expressed in mg epicatechin/100 g of berries) were determined by spectrophotometric methods (Lee and Rennaker, 2011; and references therein). Ammonia and primary free amino acids were summed for yeast assimilable nitrogen content (YAN; expressed in mg of N/100 g of berries) as described in (Lee and Schreiner, 2010). All chemical analysis was conducted in duplicates. For conciseness, 100 g of berries were abbreviated to 100 g throughout the following text.
2.4. Statistical analysis
Statistical analysis was conducted using Statistica for Windows version 7.2 (StatSoft Inc., Tulsa, OK, USA). Mean and standard deviations were calculated. Two sample t-test was performed for comparing control-shaded and light-excluded (treatment) grape metabolites, weekly berry and air temperatures, experimental duration berry and air temperatures, weekly PAR, and weekly VPD at α = 0.05.
3. Results and discussion
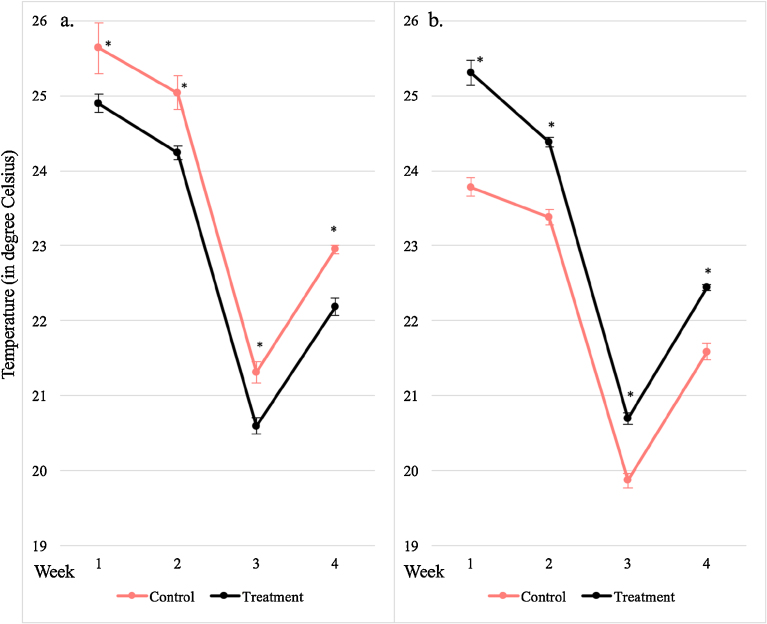
Although these light exclusion boxes were designed to eliminate temperature and humidity differences to unboxed clusters (Downey et al., 2004), there were measured differences in our work. When compared to controls, light exclusion boxes delivered significantly lower weekly berry temperatures, higher air temperatures, and they had lower PAR (as expected); somewhat different from what Downey et al. (2004) reported. The enclosures also significantly lowered weekly berry VPD, (ranging from 1.47 to 2.19 kPa) compared to control clusters (ranging from 1.61 to 2.41 kPa). The diffused PAR received by control clusters deep in the canopy did exceed the PAR values within enclosures (mean weekly PAR was 9.64 to 19.14 mol·m−2·day−1 versus −excluded clusters mean weekly PAR was 0.01 to 0.02 mol·m−2·day−1). Control clusters had more than double the total hours above 35 °C (control-shaded: 88; light-excluded: 38) and 40 °C (control-shaded: 6; light-excluded: 0); none of the light-excluded clusters reached above 40 °C. All weekly berry and air temperatures were significantly different between control-shaded and light-excluded (Fig. 2). It was also interesting to note that light-excluded clusters had berry temperatures significantly different to that of their surrounding air for weeks 1, 3, and 4 of the experimental period, while control-shaded clusters berry temperatures significantly differed from adjacent air temperatures for all 4 weeks. Thermal time (degree-days [DD], base 10 °C) for control-shaded clusters was 384, and 370 for light-excluded clusters.
Fig. 2.
All pairs of weekly berry (a- Tberry) and air (b- Tair) temperatures were significantly different from each other between control-naturally shaded (Control) and light exclusion boxed (Treatment) clusters as indicated by * (p ≤ 0.05; n = 3). In general, control-shaded Tberry was higher than light-excluded Tberry and control Tair was lower than light-excluded Tair. Error bars indicate standard deviations.
Past work conducted using the identical light exclusion boxes (Cortell and Kennedy, 2006; DeBolt et al., 2008; Downey et al., 2004; Ristic et al., 2007) will be the focus of comparison and discussion here. The majority of quality components (other than individual anthocyanins, discussed later) analyzed did not differ significantly between control-shaded and light-excluded clusters. Fruit maturity indices (% soluble solids, pH, and titratable acidity) were not significantly different between control-shaded and light-excluded clusters (Table 2). Mean berry weight, yeast assimilable nitrogen (YAN), glucose, fructose, tartaric acid, and malic acid were also not significantly different between control-shaded and light-excluded clusters (Table 2). None of the 23 free amino acids (from HPLC results) were significantly different between control-shaded and light-excluded clusters (data not shown, used for YAN calculation; see Table 2). Control-shaded clusters had 35.9 mg of N/100 g of total free amino acids, and light-excluded had 33.9 mg of N/100 g of total free amino acids. In these ‘Merlot’ grapes, the chief free amino acid was arginine (control-shaded: 12.8 mg of N/100 g; light-excluded: 14.6 mg of N/100 g), followed by proline (control-shaded: 10.8 mg of N/100 g; light-excluded: 8.2 mg of N/100 g).
Table 2.
Fruit maturity indices from harvest samples. There was no significant difference (p > 0.05) within the pair of samples (both n = 3) for all listed measurements. Values in parenthesis are standard deviations.
| Control − naturally shaded clusters | Light exclusion boxed clusters | |
|---|---|---|
| Berry weight (g) | 1.10 (0) | 1.00 (0.4) |
| % soluble solids | 26.4 (0.80) | 23.2 (6.55) |
| pH | 3.73 (0.06) | 3.60 (0.10) |
| Titratable acidity (g of tartaric acid/100 g of berries) | 0.59 (0.04) | 0.72 (0.18) |
| YAN (Yeast Assimilable Nitrogen; mg of N/100 g) | 26.2 (12.0) | 27.4 (12.9) |
| Glucose (g/100 g) | 11.2 (0.5) | 9.2 (3.8) |
| Fructose (g/100 g) | 11.1 (0.4) | 9.2 (3.3) |
| Tartaric acid (g/100 g) | 0.65 (0.02) | 0.71 (0.09) |
| Malic acid (g/100 g) | 0.14 (0.03) | 0.16 (0.04) |
Complete light exclusion did not terminate production for any of ‘Merlot’ grape’s 15 individual anthocyanins (listed in Table 3). Malvidin-glucoside was the main anthocyanin, as previously reported (Tarara et al., 2008). Total anthocyanins were not significantly different between control-shaded (162.0 mg/100 g) and light-excluded clusters (98.1 mg/100 g), although control-shaded clusters were 60% higher in concentration (Table 3). There were significant differences for nine individual anthocyanin concentrations; where delphinidin-glucoside cyanidin-glucoside, petunidin-glucoside, delphinidin-acetyl-glucoside, cyanidin-acetyl-glucoside, malvidin-caffeoyl-glycoside, cyanidin-coumaroyl-glucoside, petunidin-coumaroyl-glucoside, and peonidin-coumaroyl-glucoside were each higher in control-shaded clusters. There were significant differences for two individual anthocyanins when expressed as proportion of the total (listed in Table 3); cyanidin-glucoside and malvidin-acetyl-glucoside. Other researchers reported shading to have no significant difference in total anthocyanins to either ‘Pinot noir’ (in mg/berry; Cortell and Kennedy, 2006), or to ‘Shiraz’ (in mg/g berry; Ristic et al., 2007). Downey et al. (2004) found no significant differences in ‘Shiraz’ anthocyanins for two of three growing seasons, but the third season’s control-shaded clusters did have higher levels of anthocyanins than those of light-excluded clusters. Ristic et al. (2007) made wines with their light exclusion experimental ‘Shiraz’ samples, and all light-excluded wines were significantly lower in anthocyanins compared to control, at each of the three sample time points (bottling, 8 months of aging, and 3 years of aging).
Table 3.
Concentration of total and individual anthocyanins (listed in order of HPLC elution), and total tannin results from control-shaded clusters (naturally shaded, n = 3) and light-excluded clusters (light exclusion boxed, n = 3). Anthocyanins expressed in malvidin-glucoside equivalents (mg/100 g of berries). Tannins expressed in epicatechin equivalents (mg/100 g of berries). The significant difference (p ≤ 0.05) within the pair of samples was indicated as * for concentration (mg of/100 g of berries) and ** for proportion of the total. Values in italicized font are the proportion of the total anthocyanins. Values in parenthesis are standard deviations.
| Control − naturally shaded clusters | Light exclusion boxed clusters | |||
|---|---|---|---|---|
| Total anthocyanin | 162.0 (12.0) | 100 | 98.1 (42.2) | 100 |
| Delphinidin-glucoside * | 12.0 (1.9) | 7.4 (1.3) | 4.2 (3.0) | 3.8 (1.9) |
| Cyanidin-glucoside *, ** | 2.5 (0.5) | 1.5 (0.3) | 0.9 (0.6) | 0.8 (0.3) |
| Petunidin-glucoside * | 11.0 (1.4) | 6.8 (0.8) | 4.6 (3.1) | 4.2 (1.9) |
| Peonidin-glucoside | 10.8 (1.8) | 6.7 (0.8) | 6.4 (2.5) | 6.6 (0.4) |
| Malvidin-glucoside | 67.7 (8.2) | 41.7 (1.9) | 49.4 (19.4) | 40.2 (3.3) |
| Delphinidin-acetyl-glucoside * | 2.8 (0.3) | 1.8 (0.3) | 1.22 (0.9) | 1.1 (0.6) |
| Cyanidin-acetyl-glucoside * | 0.6 (0.1) | 0.4 (0.1) | 0.3 (0.2) | 0.3 (0.1) |
| Petunidin-acetyl-glucoside | 3.0 (0.2) | 1.9 (0.2) | 1.4 (1.0) | 1.3 (0.7) |
| Peonidin-acetyl-glucoside | 4.1 (0.3) | 2.5 (0.1) | 3.0 (1.1) | 3.2 (0.4) |
| Malvidin-acetyl-glucoside ** | 24.9 (3.0) | 15.4 (1.8) | 18.7 (7.7) | 19.2 (0.6) |
| Malvidin-caffeoyl-glucoside * | 0.2 (0) | 0.1 (0) | 0.1 (0) | 0.1 (0) |
| Cyanidin-coumaroyl-glucoside * | 0.8 (0.1) | 0.5 (0.1) | 0.3 (0.2) | 0.3 (0.1) |
| Petunidin-coumaroyl-glucoside * | 1.7 (0.1) | 1.0 (0.1) | 0.9 (0.4) | 0.9 (0.1) |
| Peonidin-coumaroyl-glucoside * | 4.5 (0.4) | 2.8 (0.4) | 3.1 (0.6) | 3.5 (1.4) |
| Malvidin-coumaroyl-glucoside | 15.2 (1.7) | 9.4 (1.1) | 12.6 (1.6) | 14.6 (6.2) |
| Total tannins | 732 (28) | 902 (255) | ||
When summed based on anthocyanidins, the delphinidin-, cyanidin-, and petunidin-based anthocyanins (mg/100 g) were significantly higher in control-shaded clusters. Summed by whether anthocyanins were acylated or not, acylated anthocyanins and non-acylated anthocyanins (mg/100 g) were not significantly different between control-shaded and light-excluded, as reported by Ristic et al. (2007). When looking at dihydroxylated- and trihydroxylated-based anthocyanins (summed based on anthocyanin biosynthetic pathway; mg/100 g), the dihydroxylated-based anthocyanins were significantly higher in control-shaded compared to light-excluded, though there was no difference seen with the trihydroxylated-based anthocyanins. There was also no significant difference between control-shaded and light-excluded clusters when comparing the ratio of non-methoxylated (sum of cyanidin and delphinidin based) to methoxylated (sum of petunidin, peonidin, and malvidin based) anthocyanins (mg/100 g). In past work conducted in the same vineyard (Tarara et al., 2008), sun-exposed clusters were compared to natural-shaded clusters (naturally shaded within the canopy), using the previously established cooler side of the canopy (east-side). When heated air was used during the ripening period to maintain natural-shaded clusters at sun-exposed temperatures, delphinidin-, cyanidin-, petunidin-, peonidin-, non-acylated, dihydroxylated-based anthocyanin, and total anthocyanins (mg/100 g) from berry skin samples from sun-exposed clusters were significantly higher than those from the natural-shaded-heated clusters. Conversely, when sun-exposed clusters were kept at natural-shaded cluster temperatures during the ripening period by cooled air, malvidin-based and acylated anthocyanins (mg/100 g) in berry skins were also significantly different; those anthocyanins were higher in natural-shaded clusters compared to sun-exposed-cooled clusters.
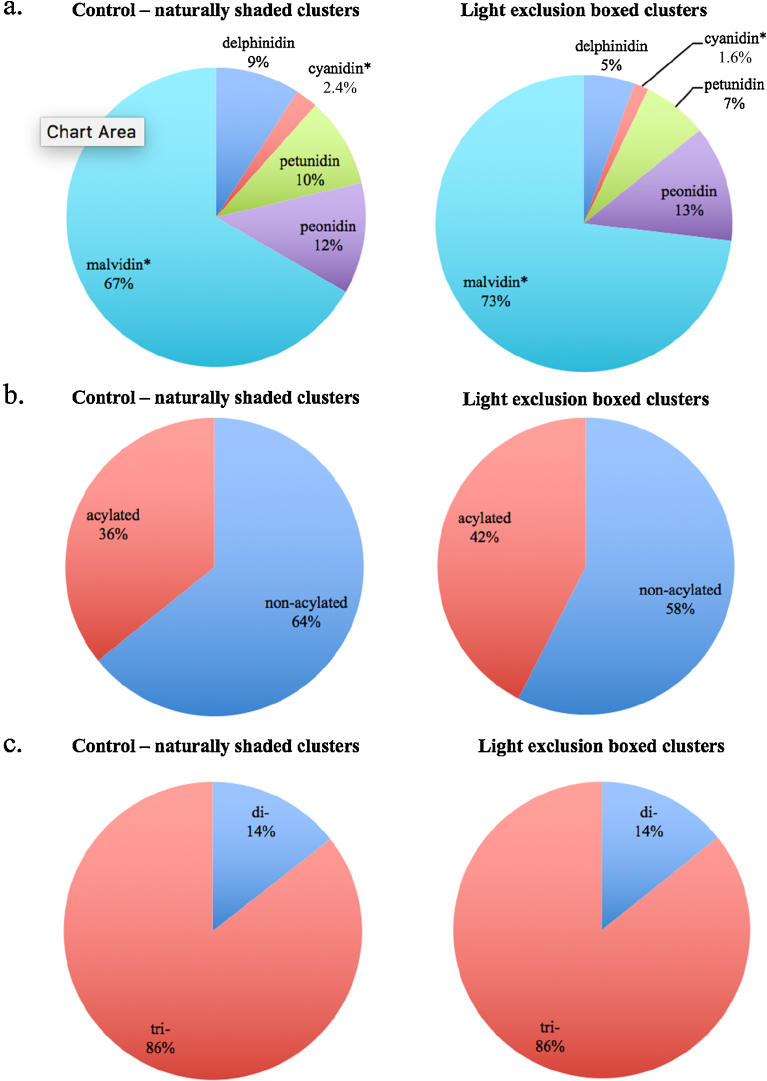
Throughout the anthocyanin proportion comparisons (Fig. 3), only cyanidin-based and malvidin-based anthocyanins were significantly different between control-shaded and light-excluded clusters (no significant difference in delphinidin-, petunidin-, peonidin-, acylation versus non-acylation, dihydroxylated versus trihydroxylated, and non-methoxylated versus methoxylated). Proportionally, malvidin-based anthocyanins were higher, and cyanidin-based anthocyanin lower, in light-excluded clusters compared to control-shaded clusters (Fig. 3a). The opposite of this trend has been seen in ‘Pinot noir’ anthocyanins from light-excluded clusters, when a reduction in the proportion of malvidin-based anthocyanins was observed (Cortell and Kennedy, 2006). Dihydroxylated and trihydroxylated proportions were identical between the control and treatment (Fig. 3b). In light-excluded ‘Shiraz’, Ristic et al. (2007) found significantly higher proportions of dihydroxylated and lower proportions of trihydroxylated anthocyanins. Downey et al. (2004) found proportion of cyanidin- and peonidin-based anthocyanins were increased in light-excluded clusters compared to non-light-excluded clusters, but that was not the case in this work (Fig. 3).
Fig. 3.
Anthocyanins in proportions of total grouped by anthocyanidin-based (a), acylation versus non-acylation (b), and dihydroxylated (abbreviated as di-; sum of cyanidin- and peonidin-based) versus trihydroxylated (abbreviated as tri-; sum of delphinidin-, petunidin-, and malvidin-based) anthocyanin based (c). The symbol * indicate significant difference (p ≤ 0.05) within the pair of samples (left side presented are control-naturally shaded cluster results and right side presented are light exclusion boxed cluster results).
Total tannins were not significantly different between the two groups, with concentration for control being 731 mg/100 g and treatment at 902 mg/100 g (Table 3). Downey et al. (2004) also found no difference in ‘Shiraz’ tannin levels (whether expressed as per g or per berry) between light-excluded versus non-light-excluded, while Ristic et al. (2007) found significantly higher tannin levels (mg/g berry) in light-excluded ‘Shiraz’. Cortell and Kennedy (2006) found decreased levels of ‘Pinot noir’ skin proanthocyanidins (mg/berry) due to light exclusion.
There are mixed results in the current literature regarding the influence of shading and wine grape metabolites. There are many reasons for these reported differences, including the regional climates of research vineyards, variations in growing seasons, disparity in environment and experimental equipment, timing of treatment, cultivar, extraction technique, analytical method, etc. (Cortell and Kennedy, 2006; DeBolt et al., 2008; Downey et al., 2004; Ristic et al., 2007). While there are studies on how individual anthocyanins provide distinct color properties due to particular glycosylations, acylations, ratios, and pH (Ahmadiani et al., 2014; Giusti et al., 1999; Stintzing et al., 2002), additional work is still needed to accurately discern how a specific proportion of individual anthocyanins within the fruit alters color, and how that ultimately equates to wine color. Although there has been a study on how anthocyanin ratios change flower (Phlox drummondii) color (Hopkins and Rausher, 2011), there remains much to be done in the area of wine grapes and wine color. Additionally, all of the environmental factors measured here likely influence metabolomics, connecting the steps of primary and secondary metabolite anabolism and catabolism to genomics (Rienth et al., 2016), but this was outside the scope of this investigation.
4. Conclusion
Light exclusion significantly modified individual anthocyanin concentrations, but not total concentrations. Other primary metabolites (simple sugars, organic acids, and free amino acids) and secondary metabolites (total anthocyanins and total tannins) were not significantly changed by light exclusion. Despite total anthocyanin concentrations not being significantly different, the individual anthocyanin amounts were altered by complete sunlight exclusion, and it also reduced berry temperatures and vapor pressure deficit (VPD) compared to naturally shaded clusters. Results from light exclusion box experiments attempting to separate light and temperature, as used here, make it difficult to determine if some grape component changes (and lack of changes) are specifically due to thermal time, exposure duration to certain temperatures, etc. Although complete light exclusion is not realistic in the commercial setting, this research was conducted to demonstrate that inferences made from light exclusion also requires data from factors (e.g., berry temperature or VPD measurements) that were altered in preventing light incidence to the fruit, for the entire experimental duration. This work contributes to our growing body of knowledge on the links between environmental factors and anthocyanin development in fruit.
Declarations
Author contribution statement
Jungmin Lee: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by USDA-Agricultural Research Service (ARS) CRIS project number 2072-21000-047-00D and Northwest Center for Small Fruits Research (NCSFR, Corvallis, OR, USA).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
I thank Chris Rennaker (USDA-ARS) for assistance in the chemical component analysis, Julie Tarara, Cole Provence, and John Ferguson (all formerly USDA-ARS) for light exclusion box setup, collecting field measurements, and discussions, Bernardo Chaves (WSU) for initial statistical analysis, and our anonymous donor for the light exclusion boxes. I acknowledge Washington State University (WSU) for research vineyard access.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- Ahmadiani N., Robbins R.J., Collins T.M., Giusti M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014;62:7524–7531. doi: 10.1021/jf501991q. [DOI] [PubMed] [Google Scholar]
- Caravia L., Collins C., Petrie P.R., Tyerman S.D. Application of shade treatments during Shiraz berry ripening to reduce the impact of high temperature. Aust. J. Grape Wine Res. 2016;22:422–437. [Google Scholar]
- Cortell J.M., Kennedy J.A. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot noir fruit and extraction in a model system. J. Agric. Food Chem. 2006;54:8510–8520. doi: 10.1021/jf0616560. [DOI] [PubMed] [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005;81:223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Chorti E., Guidoni S., Ferrandino A., Novello V. Effect of different cluster sunlight exposure levels on ripening anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010;61:23–30. [Google Scholar]
- DeBolt S., Ristic R., Iland P.G., Ford C.M. Altered light interception reduces grape berry weight and modulated organic acid biosynthesis during development. HortScience. 2008;43:957–961. [Google Scholar]
- Downey M.O., Harvey J.S., Robinson S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004;10:55–73. [Google Scholar]
- Giusti M.M., Rodriguez-Saona L.E., Wrolstad R.E. Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin based anthocyanins. J. Agric. Food Chem. 1999;47:4631–4637. doi: 10.1021/jf981271k. [DOI] [PubMed] [Google Scholar]
- Greer D.H., Weedon M.M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013;4:491. doi: 10.3389/fpls.2013.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R., Rausher M.D. Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii. Nature. 2011;469:411–414. doi: 10.1038/nature09641. [DOI] [PubMed] [Google Scholar]
- Koyama K., Goto-Yamamoto N. Bunch shading during different developmental stages affects the phenolic biosynthesis in berry skins of ‘Cabernet Sauvignon’ grapes. J. Am. Soc. Hortic. Sci. 2008;133:743–753. [Google Scholar]
- Lee J., Finn C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agr. 2007;87:2665–2675. doi: 10.1002/jsfa.3029. [DOI] [PubMed] [Google Scholar]
- Lee J., Schreiner R.P. Free amino acid profiles from ‘Pinot noir’ grapes are influenced by vine N-status and sample preparation method. Food Chem. 2010;119:484–489. [Google Scholar]
- Lee J., Rennaker C. Influence of extraction methodology on grape composition values. Food Chem. 2011;126:295–300. [Google Scholar]
- Lee J., Skinkis P.A. Oregon ‘Pinot noir’ grape anthocyanin enhancement by early leaf removal. Food Chem. 2013;139:893–901. doi: 10.1016/j.foodchem.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Rienth M., Torregrosa L., Sarah G., Ardisson M., Brillouet J., Romieu C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016;16:164. doi: 10.1186/s12870-016-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic R., Downey M.O., Iland P.G., Bindon K., Franicis I.L., Herderich M., Robinson S.P. Exclusion of sunlight from Shiraz grapes alters wine colour, tannin and sensory properties. Aust. J. Grape Wine Res. 2007;13:53–65. [Google Scholar]
- Tarara J.M., Lee J., Spayd S.E., Scagel C.F. Berry temperature and solar radiation alter acylation, proportion: and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008;59:235–247. [Google Scholar]
- Stintzing F.C., Stintzing A.S., Carle R., Frei B., Wrolstad R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002;50:6172–6181. doi: 10.1021/jf0204811. [DOI] [PubMed] [Google Scholar]