Abstract
Background
Osteoprotegerin (OPG) is a member of the tumor necrosis factor superfamily. Reduced OPG levels are related to obesity, insulin resistance, and non-alcoholic fatty liver disease (NAFLD).
Objectives
The aim of this study was to evaluate the relationship between OPG levels, obesity, insulin resistance, and NAFLD in pediatric patients.
Methods
This was a prospective, cross-sectional, controlled study that was conducted in the department of pediatrics at Bagcilar training and research hospital in Istanbul, Turkey, between April and August 2015. The study was performed on 107 children with obesity and 37 controls aged 5 - 17 years. In the obese subset, 62 patients had NAFLD. Homeostatic model assessment-insulin resistance (HOMA-IR) was used to calculate insulin resistance. Insulin resistance was defined as a HOMA-IR value greater than 2.5. Plasma OPG levels were measured using enzyme-linked immunosorbent assays. NAFLD was diagnosed by hepatic ultrasound.
Results
The mean age was 11.25 ± 3.38 years in the patient group and 10.41 ± 3.15 years in the control group. The OPG level in the obese group with the mean of 55.20 ± 24.55 pg/mL (median = 48.81 pg/mL) was significantly lower than that in the control group with the mean of 70.78 ± 33.41 pg/mL (median = 64.57 pg/mL) (P = 0.0001). The optimal cut-off point (sensitivity, specificity) of the OPG level for the diagnosis of obesity was ≤ 46, 19 pg/mL. According to logistic regression analysis, fasting insulin (P = 0.036) and OPG (P = 0.01) levels were most affected by obesity. In the obese patients, who had HOMA-IR < 2.5, the mean level of OPG was 58.91 ± 6.88729 pg/mL (median = 49.55). In the obese patients, who had HOMA-IR ≥ 2.5, the mean level of OPG was 54.19 ± 22.21 pg/mL (median = 48.47). No significant correlations were found between OPG and HOMA-IR (P = 0.791). No statistically significant difference was observed in the mean OPG between patients with hepatosteatosis (mean = 54.55 ± 25.01 pg/mL) (median = 49.46) and those without the disease (56.30 ± 24.02 pg/mL) (mean = 48.34) (P = 0.089).
Conclusions
We confirmed that serum OPG concentrations reduce in obese children. However, no correlation was identified between OPG and insulin resistance. OPG levels are not meaningful in the diagnosis of NAFLD in children with obesity.
Keywords: Osteoprotegerin, Obesity, Insulin Resistance, Non-Alcoholic Fatty Liver Disease, Children
1. Background
Obesity is a serious public health threat that has rapidly increased in prevalence in recent years. In the last 30 - 40 years, obesity has become a serious problem during the periods of childhood and adolescence (1, 2). Insulin resistance and dyslipidemia are early metabolic complications of childhood and adolescent obesity (3). Cardiovascular diseases, type 2 diabetes, and other metabolic problems are expected to develop in individuals with obesity (4). Experimental evidence shows that the number of macrophages in adipose tissue has increased in individuals with obesity. It is clear that these macrophages represent a possible origin of proinflammatory factors affecting adipocyte biology and systemic insulin resistance (5). Various tissues, such as those in the heart, veins, arteries, lungs, kidneys, and bone, as well as immune system cells, generate Osteoprotegerin (OPG), a soluble glycoprotein which is a member of the tumor necrosis factor (TNF) receptor superfamily. OPG was initially found as an inhibitor of bone resorption, and the expression and production of OPG are regulated by different cytokines and hormones (6). Along with its vital importance in the control of bone metabolism, OPG has significant anti-inflammatory and anti-apoptotic impacts (7). Due to the biological links that exist between OPG and inflammation, it is suggested that special attention must be paid when evaluating the role that the OPG-receptor activator of nuclear factor kappa B-ligand (RANKL) system plays in metabolic diseases (8). As a result of a population-based study, it was determined that the increased serum OPG levels represent an independent risk factor in the advancement of atherosclerosis and vascular mortality (9). Non-alcoholic fatty liver disease is one of the major manifestations of obesity. Since the regulation of the anti-inflammatory and anti-apoptotic impacts of OPG is performed by the RANKL and TRAIL signaling pathways, the identification of the connection between OPG and NAFLD is concerned. A decrease in circulating OPG levels in NAFLD subjects has been reported in a number of epidemiologic studies (10, 11). However, this relationship has been inconsistent (12).
Studies evaluating adult populations have indicated a potential relationship between insulin resistance, metabolic syndrome, NAFLD, and concentrations of OPG (10, 13-16). These studies reported that serum OPG level could be used as a non-invasive marker of liver damage in NAFLD (13, 16). However, studies concerning OPG level and its relationship with insulin resistance in children are limited (17). Suliburska et al. (17) reported OPG level and its relationship with insulin resistance only on obese adolescents. There is no study related to the role of OPG in obese pediatric NAFLD cases.
2. Objectives
The aims of the current study were to measure OPG concentrations in children with obesity and insulin resistance, and to evaluate the potential association of OPG level with NAFLD.
3. Methods
3.1. Study Population
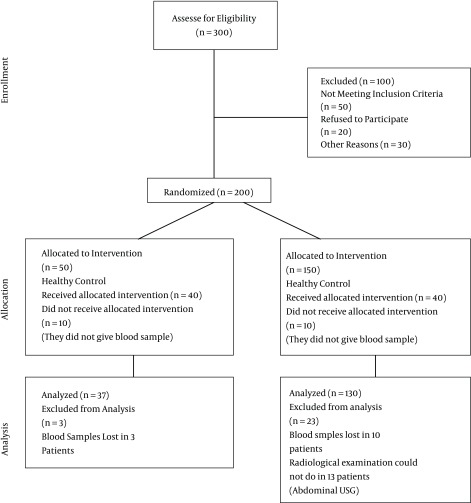
This single center, prospective, cross-sectional, controlled study was conducted in the department of pediatrics at Bagcilar training and research hospital in Istanbul, Turkey, between April and August 2015. Bagcilar is one of the most crowded districts of Istanbul. Our study population belonged only to the Bagcilar district. Bagcilar training and research hospital is a general tertiary referral governmental hospital. Department of pediatrics has 40 beds. Outpatient clinic and emergency service of hospital admit 2.000.000 patients a year. Between April and August 2015, 2000 children were admitted in the pediatric outpatient clinic. According to our inclusion and exclusion criteria, 144 out of 300 parcipitants (107 obese children and 37 normal controls aged 5-17 years) were included in the study. The consort flowchart of sample selection is shown in Figure 1.
Figure 1. Consort Flow Diagram.
The children who had any of infection, metabolic or endocrine diseases or used dietary supplementation and refused to give informed consent were excluded. Medical records were evaluated for age, gender, and physical examination findings.
The inclusion criteria for the study consisted of being obese and ages 5-17 years. Healthy children in the same age range, reffered to the pediatric polyclinic of the hospital for general medical examination, with BMI below the 95th percentile and without chronic diseases or infection symptoms included in the control group.
3.2. Anthropometric Measurements
Weights and heights of children were measured in the first examination in pediatric outpatient clinic. All anthropometric measurements were performed by the same pediatrician. Weights were measured with subjects in minimal (without shoes and with light clothing) underclothes, using a standard beam balance sensitive to 0.1 kg. Heights were determined to the nearest 1 mm using a portable Seca stadiometer. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters squared). Patients with a BMI greater than the 95th percentile for their age and gender were considered obese (18). The patients with the body mass index less than 95th percentile were accepted as non-obese.
3.3. Laboratory Measurements
After overnight fasting, blood samples were taken for determination of glucose, insulin, total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and alanine amino transferase (ALT) levels. All patients underwent a standardized clinical and laboratory evaluation. To measure OPG levels, peripheral venous blood samples were drawn after overnight fasting for 10 hours. These samples were centrifuged at 3000 rpm for 10 minutes, and the isolated plasma was stored at - 80°C until assayed. An enzyme linked immunosorbent assay (ELISA) was used to measure OPG (Affymetrix eBioscience, San diego ca 92121, USA, Human OPG kit, Cat no: BMS2021). Triglyceride, cholesterol, and ALT levels were studied using an enzymatic colorimetric method on a Roche Cobas 6000 device. The homeostasis model assessment of insulin resistance (HOMA-IR; fasting insulin × fasting glucose/22.5) was used as an index of insulin resistance. Insulin resistance was defined as a HOMA-IR value greater than 2.5 (19, 20).
The ultrasonographic (USG) examinations of all the children were performed using a 3.5 MHz convex transducer (TOSHIBA). All children were evaluated by the same radiologist in the department of radiology, Bagcilar training and research hospital. USG evidence of NAFLD was based on the bright hepatic echo pattern, increased echo attenuation, and loss of intrahepatic architecture (21).
The study protocol was approved by the research ethics committee of Bagcilar training and research hospital (approval number of 2015/365) in accordance with the declaration of Helsinki. informed consent was obtained from all study participants and/or their parents.
3.4. Statistical Analysis
All statistical descriptions and hypothesis tests were analyzed using SPSS version 23 and MedCalc 16.2 software. The explanatory statistics are given as the mean ± standard deviation for the continuous variables and as the frequency and percentage for the discrete variables. The conformity of the continuous variables with the normal distribution was evaluated using the Kolmogorov-Smirnov test. Two independent sample t-tests were used to compare the variables that were in conformity with the normal distribution, while the Mann-Whitney U test was used to compare the variables that were not in conformity with the normal distribution. A ROC analysis was conducted to evaluate whether the serum OPG values of the patient group were distinct from those of the control group, and the area under the curve was assessed. Logistic regression analysis was performed to identify factors affecting obesity. The results were evaluated at a significance level of P < 0.05.
4. Results
The mean age was 11.25 ± 3.38 years in the patient group and 10.41 ± 3.15 years in the control group, while the male/female ratio was 64/43 in the patient group and 21/16 in the control group. No statistically significant difference was observed in the mean age and gender distribution between the obese children and control group (P > 0.05). The mean HDL in the obese group (52.15 ± 16.23 mg/dL) was significantly lower than that of the control group (59.73 ± 13.43 mg/dL) (P = 0.012), whereas the mean triglyceride level was significantly higher in the obese group (112.47 ± 51.74 mg/dL) compared to the control group (82.81 ± 36.51 mg/dL) (P = 0.002). The mean ALT level was significantly higher in the obese group (25.93 ± 14.67 U/L) compared to the control group (14.92 ± 4.77 U/L) (P = 0.0001). The mean fasting insulin level was significantly higher in the obese group (21.81 ± 16.65 mU/mL) compared to the control group (10.12 ± 9.54) (P = 0.0001). The mean HOMA-IR level was significantly higher in the obese group (4.94 ± 4.2) compared to the control group (2.29 ± 2.36) (P = 0.0001). These results are consistent with obesity (Table 1).
Table 1. Comparison of the Data in the Obese and Control Groupsa.
| Control Group 37 | Obese Group 107 | P | Power | |
|---|---|---|---|---|
| Age (year) | 10.41 ± 3.15 | 11.25 ± 3.38 | 0.184 | |
| Gender | ||||
| Female | 16 (43.24%) | 43 (40.19%) | 0.745 | |
| Male | 21 (56.76%) | 64 (59: 37%) | ||
| Cholesterol (mg/dL) | 164.08 ± 30.52 | 163.48 ± 25.63 | 0.908 | |
| LDL (mg/dL) | 87.76 ± 29.72 | 92.77 ± 26.24 | 0.337 | |
| HDL (mg/dL) | 59.73 ± 13.43 | 52.15 ± 16.23 | 0.012 | %99 |
| Triglycerides (mg/dL) | 82,81 ± 36,51 | 112,47 ± 51,74 | 0.002 | %99 |
| Fasting glucose (mU/mL) | 90.43 ± 8.34 | 90.4 ± 8.49 | 0.984 | |
| Fasting insulin (mU/mL) | 10.12 ± 9.54 | 21.81 ± 16.65 | 0.0001 | %99 |
| HOMA-IR | 2.29 ± 2.36 | 4.94 ± 4.2 | 0.0001 | %99 |
| ALT (U/L) | 14.92 ± 4.77 | 25.93 ± 14.67 | 0.0001 | %99 |
| OPG (pg/mL) | ||||
| Mean ± SD | 70.78 ± 33.41 | 55.20 ± 24.55 | ||
| Median (IQR) | 64.57 (51.63 - 74.50) | 48.81 (40.91 - 66.26) | 0.0001 | %99 |
Abbreviations: OPG, Osteoprotegerin; LDL, Low density lipoprotein; HDL, High density lipoprotein; HOMA-IR, Homeostasis model assessment-insulin resistance; ALT, Alanine aminotransferase.
aData are given as mean ± SD or n (%), data are given as mean ± SD and median for Osteoprotegerin.
The strength of the study was performed only for statistically significant comparisons. 80% strength is the minimum acceptable strength value (Table 1).
4.1. Osteoprotegerin Level in the Obese and Control Groups
The Mann-Whitney U test was employed to determine whether there was a difference between two groups in terms of Osteoprotegerin level. According to this test, the mean OPG was 55.207 ± 24.55 pg/mL in the obese group and 70.782 ± 33.41 pg/mL in the control group. Moreover, the median IQR value was 48.806 pg/mL in the obese group and 64.577 pg/mL in the control group, that showed a statistically significant difference between the groups in terms of the median values of Osteoprotegerin level (P < 0.001) (Table 1).
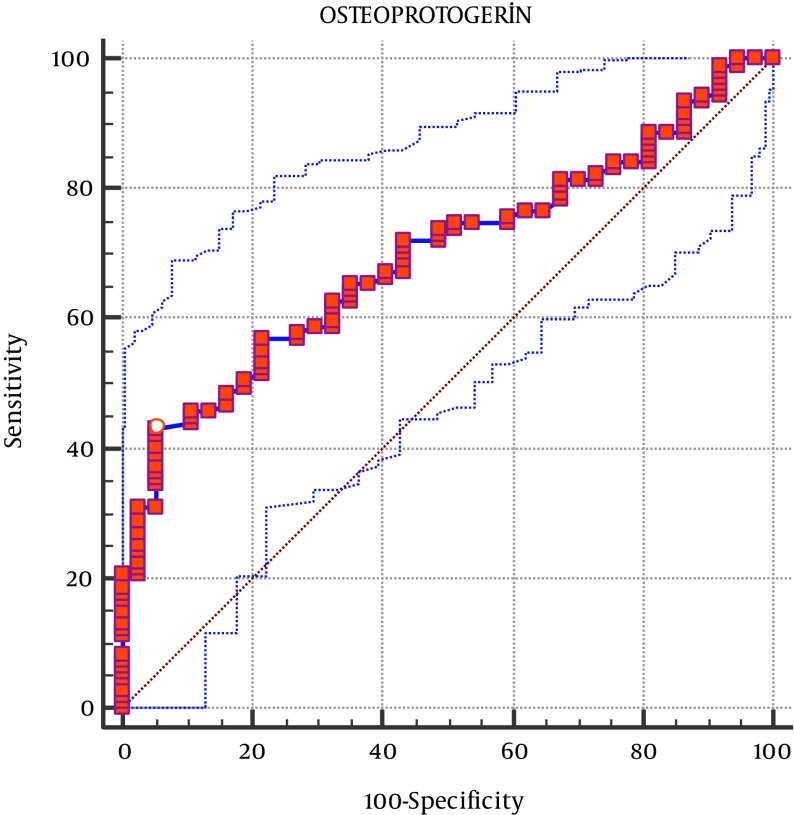
For the definitive diagnosis of obesity, the area under the ROC curve was calculated as 0.696, that within 95% confidence interval (CI) (0.613 - 0.796), was significant for OPG (P < 0.0001). This led to a cutoff OPG value of ≤ 46.19 pg/mL. For this OPG cut-off point, Youden index J was 0.3759, 95% CI was ≤ 42.9 - ≤ 50.77, the sensitivity was 42.99, and the specificity was 94.59 (Figure 2).
Figure 2. Receiver Operator Curves of Osteoprotegerin for Distinguishing Between Obese Children and Healthy Controls.
The area under curve was 0.696.
To determine the factors most affected by obesity, logistic regression analysis was performed using HDL, triglyceride, fasting insulin, HOMA-IR, and OPG levels as variables. Therefore, fasting insulin (P = 0.036) and OPG (P = 0.01) levels were determined as the factors most affected by obesity (Table 2).
Table 2. Logistic Regression Analysis of Factors Associated with Obesity.
| B | S.E. | P | OR | OR %95 CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| HDL | - 0.02 | 0.02 | 0.291 | 0.98 | 0.95 | 1.01 |
| Triglycerides | 0.01 | 0.01 | 0.435 | 1.01 | 0.99 | 1.02 |
| Fasting insulin | 0.35 | 0.17 | 0.036 | 1.42 | 1.02 | 1.98 |
| HOMA-IR | - 1.06 | 0.67 | 0.113 | 0.35 | 0.09 | 1.29 |
| OPG | - 0.02 | 0.01 | 0.01 | 0.98 | 0.96 | 1.00 |
Abbreviations: OPG, Osteoprotegerin; HDL, High density lipoprotein; HOMA-IR, Homeostasis model assessment-insulin resistance.
4.2. Relationship of Osteoprotegerin and HOMA-IR
Since HOMA-IR and osteoprotegerin data did not follow the normal distribution, the Mann-Whitney U test was used. Those with HOMA-IR ≥ 2.5 in the obese group were consistent with insulin resistance. The mean of OPG level in those with HOMA-IR ≥ 2.5 in the obese group was 54.19 ± 22.21. No significant relationship was found between the level of HOMA-IR (below or above 2.5) and OPG level (P = 0.791) (Table 3).
Table 3. Comparison of the Serum OPG Levels of the Obese Group According to HOMA-IR Level.
| Osteoprotegerin (pg/mL) | HOMA-IR < 2.5, n = 22 | HOMA-IR ≥ 2.5, n = 85 | P Value |
|---|---|---|---|
| Mean ± SD | 58.91 ± 33.13 | 54.19 ± 22.21 | |
| Median (IQR) | 49.55 (44.63-73.20) | 48.47 (49.45-58.92) | 0.791 |
4.3. Relationship of Osteoprotegerin and Hepatosteatosis in Obese Group
According to the results of the Mann-Whitney U test in the obese group, the mean Osteoprotegerin level in those with hepatosteatosis was 54.55 ± 25.01 pg/mL (median 49.4602 pg/mL), while the mean in those without hepatosteatosis was 56.30 ± 24.02 pg/mL (median 48.3433 pg/mL), which showed no statistically significant difference between these groups in terms of the median values (P = 0.098) (ROC analysis P > 0.05). Hence, osteoprotegerin cannot be used as an indicator in the determination of hepatosteatosis.
The mean fasting insulin level was significantly higher in those with hepatosteatosis (25.08 ± 17.94 mU/mL) than those without hepatosteatosis (17.29 ± 13.61 mU/mL) (P = 0.016). The mean HOMA-IR level was significantly higher in those with hepatosteatosis (5.74 ± 4.56) than those without hepatosteatosis (3.82 ± 3.38) (P = 0.019). In addition, ALT level was significantly higher in those with hepatosteatosis (28.91 ± 17.13 U/L) than those without hepatosteatosis (21.83 ± 9.04 U/L) (P = 0.013) (Table 4).
Table 4. Characteristics of the Obese Patients with Hepatosteatosis and Without Hepatosteatosisa.
| Hepatosteatosis (-), n = 45 | Hepatosteatosis (+), n = 62 | P | |
|---|---|---|---|
| Cholesterol (mg/dL) | 166.42 ± 25.7 | 161.35 ± 25.58 | 0.315 |
| LDL (mg/dL) | 99.05 ± 25,9 | 88.34 ± 25.77 | 0.04 |
| HDL (mg/dL) | 53.55 ± 16.18 | 51.16 ± 16.32 | 0.464 |
| Triglycerides (mg/dL) | 100.8 ± 45,3 | 120.95 ± 54.76 | 0.046 |
| Fasting glucose (mU/mL) | 89.5 ± 6.48 | 91.04 ± 9.69 | 0.362 |
| Fasting insulin (mU/mL) | 17.29 ± 13.61 | 25.08 ± 17.94 | 0.016 |
| HOMA-IR | 3.82 ± 3.38 | 5.74 ± 4.56 | 0.019 |
| ALT (U/L) | 21.83 ± 9.04 | 28.91 ± 17.13 | 0.013 |
| OPG (pg/mL) | |||
| Mean ± SD | 56.30 ± 24.02 | 54.55 ± 25.01 | |
| Median (IQR) | 48.34 (41.20 - 74.31) | 49.46 (39.27 - 60.71) | 0.089 |
Abbreviations: OPG, Osteoprotegerin; LDL, Low density lipoprotein; HDL, High density lipoprotein; HOMA-IR, Homeostasis model assessment-insulin resistance; ALT, Alanine aminotransferase.
aData are given as mean ± SD or n (%), (%), data are given as mean ± SD and median for osteoprotegerin.
When the correlation analysis was performed in all individuals, a correlation was found only between osteoprotegerin and age (r = - 0.221) (P < 0.05).
5. Discussion
The main objective of this study was to determine whether a relationship exists between OPG level and insulin resistance in children with obesity. An additional objective was to determine whether OPG is a significant indicator of hepatosteatosis in children with obesity, as there is a paucity of published studies regarding OPG levels in children with NAFDL and obesity. Our main finding is that individuals with obesity have lower circulating OPG levels than their normal-weight counterparts. Previous studies have reported that decreased insulin sensitivity leads to increased insulin production, causing a predisposition to several metabolic disorders, including early atherosclerosis, progressive obesity, acanthosis nigricans, skin tags, hypertension, dyslipidemia, and fatty liver (22). In the present study, as expected, fasting insulin and HOMA-IR values were high in children with obesity, which led to insulin sensitivity. Furthermore, in these patients, HDL levels were low, triglyceride levels were high, deteriorated lipid profiles were observed, ALT levels were high, and hepatosteatosis was identified on abdominal ultrasonography. These findings are consistent with NAFLD. Furthermore, the children with obesity had significantly lower OPG levels compared to the control group. It is not known what role obesity plays in the regulation of circulating OPG. A decrease in OPG levels in subjects with obesity in comparison with lean controls has been reported in a number of studies (13, 23), whereas no relationship has been determined between OPG and BMI in other studies (24, 25). Obesity is accompanied by a number of metabolic changes, including increased insulin resistance. Ugur-Altan et al. (13) classified young patients with obesity into 3 groups based on HOMA-IR index. The OPG levels in their patients with obesity were found to be significantly lower when compared to a control group. Additionally, they reported that the lowest OPG levels were found in the group with the highest HOMA-IR values, and there were negative correlations between serum OPG level and HOMA-IR, fasting insulin, and glucose. In this present study, although the OPG level was determined to be low in the obese group, neither a negative nor a positive correlation was found between OPG level and HOMA-IR values. The number of studies that have been conducted on OPG in children and adolescents is low. It has been reported that PRG and RANKL levels are high in the stage of infancy, decrease to normal in the childhood and adolescence, and start to increase again after the age of 45 (26). In our study a negative correlation was detected between age and OPG level. Our result was in agreement with literature. In a study carried out by Suliburska et al. (17), adolescents with obesity were found to have higher OPG levels compared to a control group, and a significant positive correlation was found between OPG level and insulin resistance. Additionally, a negative correlation was found between insulin resistance and OPG level. Studies in which a positive relationship was determined between these two parameters have been conducted on patients known to have very high insulin resistance, such as individuals with type 2 diabetes or gestational diabetes (24, 27). In studies carried out on healthy individuals with obesity, negative correlations between OPG level and insulin resistance were found, and the OPG levels in these individuals were low (13, 28, 29). As mentioned above, OPG serum concentrations are used as a marker of cardiovascular disease and type 2 diabetes (15). A study by Suliburska et al. (17) conducted on a similar group of adolescents indicated that there is a risk for diabetes and cardiovascular disease in adolescents with obesity, especially due to significantly increased levels of glucose and insulin in these individuals when compared to people with normal weight.
A correlation between OPG level and insulin resistance has not been clearly delineated. In the study conducted by Gannage-Yared MH et al. (25), a positive correlation was determined between OPG and HOMA-IR. There are significant beneficial impacts of OPG on vascular tissues since it associates with endothelial dysfunction and insulin resistance. In a previous study in which vascular calcification caused by the treatment with warfarin and supraphysiological doses of vitamin D in rats was prevented by OPG applied parenterally, the protective effects of OPG on vasculature were clearly observed (30). In contrast, recent studies have reported an increase in OPG levels in subjects with type 2 diabetes and coronary artery disease (9, 31). It was put forward that an increase in serum OPG levels can be defined as an inadequate compensatory self-defense reaction for the purpose of inhibition of progressing bone loss and the advancement of atherosclerosis (9, 31-33). In recent times, Schoppet et al. (32) identified apoptotic cells surrounding calcified regions in arteries affected by Monckeberg’s sclerosis and atherosclerosis, with the simultaneous identification of OPG and TRAIL in the apoptotic regions. Vascular endothelial dysfunction during obesity can be enlightened by the association between serum OPG levels and HOMA-IR values.
In the present study, serum OPG levels were low in the children with obesity, an observation that is concordant with previous studies. Serum OPG levels have been previously reported to be negatively correlated with HOMA-IR values. In our study, the serum OPG concentrations in the children with obesity decreased, while their HOMA-IR values increased, but no significant differences were detected. Less severe complications and metabolic injuries may be related to early obesity in children.
In the present study, no difference was found in OPG levels between children with obesity who also presented with hepatosteatosis and those who did not. With a sudden increase in obesity observed in children for a period of the previous three decades, it has been detected that the prevalence of NAFLD has increased as well. The risk factors for NAFLD are obesity, hyperlipidemia, insulin resistance, and diabetes (34, 35). In this study, LDL cholesterol, triglyceride, fasting insulin, and HOMA-IR levels were significantly higher in the children with obesity and fatty liver compared to the children with obesity who did not have fatty liver. OPG is associated with insulin resistance (13, 29). Furthermore, it has been stated that OPG regulates bone mineral density in subjects having chronic liver disease (36). OPG performs a function of a decoy receptor for TRAIL and at the same time, its biological impacts are neutralized by OPG (37). Additionally, TRAIL activates apoptosis in hepatocytes (38). A decrease in serum levels of OPG in NAFLD can lead to defects in the mechanisms that act to protect hepatocytes from apoptosis (39). Of much interest and importance, OPG accumulation has been shown to correlate with decreased apoptosis in several cell types (37). In the present study, the obese children with NAFLD had higher ALT levels than those without NAFLD. This finding supports the suggestion that high ALT levels are an initial finding in NAFLD (40). As a result of the above mentioned observations, a general protective impact of OPG has been detected against the pathophysiologic changes that play a role in NAFLD via minimum two independent mechanisms. The first mechanism involves insulin resistance and the second one involves protection of hepatic cells from apoptosis. Nevertheless, a more detailed examination is required for the specific mechanisms causing a decrease in OPG in subjects having NAFLD. Because neither the cellular origin of serum OPG nor the mechanisms of its secretion are clearly known (37), it is not understood whether the important reductions in serum levels of OPG observed in subjects with NAFLD reflect a lack of production or increase in consumption of this molecule.
In a study reported by Yilmaz et al. (10), two groups were formed from the adult patients with obesity, including a serious NAFLD group and a mild NAFLD group. The OPG levels in the serious NAFLD group were determined to be lower when compared to the mild NAFLD group and the control group. In a study conducted by Yang et al. (11), it was stated that OPG level decreased as NAFLD severity increased, implying a negative correlation between them. Liver biopsies were performed in both studies, and the results indicated that OPG could be used as a noninvasive marker of the damage caused by NAFLD in obesity. In another study conducted by Niu et al. (16), low serum OPG levels were determined in subjects with type 2 diabetes who also had NAFLD.
The above-referenced studies examining the relationship between OPG and NAFLD were carried out on groups of adults. In contrast, the patients in the current study were children, and their liver damage was likely not as severe as that observed in adults. This may explain why no significant difference was found in OPG levels between our subjects who did and did not present with hepatosteatosis.
There are some limitations of the current study. First, there is a known relationship between OPG levels and high HOMA-IR values in individuals with obesity. Additionally, vascular endothelial function and carotid artery intima thickness are affected in patients with NAFLD. Thus, in these patients, the carotid artery intima thickness could not be measured. Second, NAFLD was not confirmed by liver biopsy in our cohort for medical and ethical reasons. Also, our sample size was small. These are weak points of our study. This is the first study that investigates the level of OPG in obese children with insulin resistance and NAFLD. This is the strength and novelty of our study.
5.1. Conclusion
Children with obesity who had high fasting insulin levels and high HOMA-IR values had significantly low OPG levels. Although our patients with hepatosteatosis had lower OPG levels than those without hepatosteatosis, no statistically significant differences were found. Therefore, we were unable to reach a clear conclusion on whether OPG could be used as a noninvasive biomarker of NAFLD in childhood obesity and hence, we call for more studies.
Acknowledgments
We thank Bagcilar training and research hospital. This study was reviewed and approved by the review board of Bagcilar training and research hospital.
Footnotes
Authors’ Contribution:Study concept and design: Meltem Erol; acquisition of data: Meltem Erol and Ozlem Bostan Gayret; analysis and interpretation of data: Ozlem Bostan Gayret, Hikmet Tekin Nacaroglu, and Mehmet Tasdemir; drafting of the manuscript: Meltem Erol; critical revision of the manuscript for important intellectual content: Ozgul Yigit, Hikmet Tekin Nacaroglu and Ozlem Bostan Gayret; radiological material support: Mehmet Salih Akkurt; biochemical analysis: Oguzhan Zengi; study supervision: Meltem Erol.
Financial Disclosure:We declare that we have no commercial, financial, or any other relationship in any way related to the subject of this article that might create any potential conflict of interest.
Funding/Support:This study was supported by the educational planning coordination committee of Bagcilar training and research hospital.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolopoulou A, Kadoglou NP. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther. 2012;10(7):933–9. doi: 10.1586/erc.12.74. [DOI] [PubMed] [Google Scholar]
- 4.Kelishadi R, Azizi-Soleiman F. Controlling childhood obesity: A systematic review on strategies and challenges. J Res Med Sci. 2014;19(10):993–1008. [PMC free article] [PubMed] [Google Scholar]
- 5.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–13. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 6.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–46. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dovio A, Data V, Angeli A. Circulating osteoprotegerin and soluble RANKL: do they have a future in clinical practice? J Endocrinol Invest. 2005;28(10 Suppl):14–22. [PubMed] [Google Scholar]
- 8.Chang YH, Lin KD, He SR, Hsieh MC, Hsiao JY, Shin SJ. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism. 2011;60(8):1064–9. doi: 10.1016/j.metabol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109(18):2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz Y, Yonal O, Kurt R, Oral AY, Eren F, Ozdogan O, et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2010;70(8):541–6. doi: 10.3109/00365513.2010.524933. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Xu D, Liu Y, Guo X, Li W, Guo C, et al. Combined Serum Biomarkers in Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis. PLoS One. 2015;10(6):0131664. doi: 10.1371/journal.pone.0131664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayaz T, Kirbas A, Durakoglugil T, Durakoglugil ME, Sahin SB, Sahin OZ, et al. The relation between carotid intima media thickness and serum osteoprotegerin levels in nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2014;12(5):283–9. doi: 10.1089/met.2013.0151. [DOI] [PubMed] [Google Scholar]
- 13.Ugur-Altun B, Altun A, Gerenli M, Tugrul A. The relationship between insulin resistance assessed by HOMA-IR and serum osteoprotegerin levels in obesity. Diabetes Res Clin Pract. 2005;68(3):217–22. doi: 10.1016/j.diabres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen GM, Vind B, Nybo M, Rasmussen LM, Hojlund K. Acute hyperinsulinemia decreases plasma osteoprotegerin with diminished effect in type 2 diabetes and obesity. Eur J Endocrinol. 2009;161(1):95–101. doi: 10.1530/EJE-09-0141. [DOI] [PubMed] [Google Scholar]
- 15.Yaturu S, Rains J, Jain SK. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-alpha levels in men with type 2 diabetes. Cytokine. 2008;44(1):168–71. doi: 10.1016/j.cyto.2008.07.471. [DOI] [PubMed] [Google Scholar]
- 16.Niu Y, Zhang W, Yang Z, Li X, Fang W, Zhang H, et al. Plasma osteoprotegerin levels are inversely associated with nonalcoholic fatty liver disease in patients with type 2 diabetes: A case–control study in China. Metabolism. 2016;65(4):475–81. doi: 10.1016/j.metabol.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Suliburska J, Bogdanski P, Gajewska E, Kalmus G, Sobieska M, Samborski W. The association of insulin resistance with serum osteoprotegerin in obese adolescents. J Physiol Biochem. 2013;69(4):847–53. doi: 10.1007/s13105-013-0261-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 19.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27(2):314–9. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 20.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 21.Rumack C, Wilson S, Charboneau JW, Johnson JA. Diagnostic ultrasound. . Third ed. St Louis: Mosby; 2005. [Google Scholar]
- 22.Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89(6):2526–39. doi: 10.1210/jc.2004-0276. [DOI] [PubMed] [Google Scholar]
- 23.Holecki M, Zahorska-Markiewicz B, Janowska J, Nieszporek T, Wojaczynska-Stanek K, Zak-Golab A, et al. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity (Silver Spring). 2007;15(8):1925–9. doi: 10.1038/oby.2007.229. [DOI] [PubMed] [Google Scholar]
- 24.Gannagé-Yared MH, Yaghi C, Habre B, Khalife S, Noun R, Germanos-Haddad M, et al. Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Euro J Endocrinol. 2008;158(3):353–9. doi: 10.1530/EJE-07-0797. [DOI] [PubMed] [Google Scholar]
- 25.Gannage-Yared MH, Fares F, Semaan M, Khalife S, Jambart S. Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol (Oxf). 2006;64(6):652–8. doi: 10.1111/j.1365-2265.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 26.Buzi F, Maccarinelli G, Guaragni B, Ruggeri F, Radetti G, Meini A, et al. Serum osteoprotegerin and receptor activator of nuclear factors kB (RANKL) concentrations in normal children and in children with pubertal precocity, Turner's syndrome and rheumatoid arthritis. Clin Endocrinol (Oxf). 2004;60(1):87–91. doi: 10.1111/j.1365-2265.2004.01951.x. [DOI] [PubMed] [Google Scholar]
- 27.Akinci B, Celtik A, Yuksel F, Genc S, Yener S, Secil M, et al. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res Clin Pract. 2011;91(1):26–31. doi: 10.1016/j.diabres.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Ashley DT, O'Sullivan EP, Davenport C, Devlin N, Crowley RK, McCaffrey N, et al. Similar to adiponectin, serum levels of osteoprotegerin are associated with obesity in healthy subjects. Metabolism. 2011;60(7):994–1000. doi: 10.1016/j.metabol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Ugur-Altun B, Altun A. Circulating leptin and osteoprotegerin levels affect insulin resistance in healthy premenopausal obese women. Arch Med Res. 2007;38(8):891–6. doi: 10.1016/j.arcmed.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21(10):1610–6. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 31.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106(10):1192–4. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 32.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88(3):1024–8. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 33.Sattler AM, Schoppet M, Schaefer JR, Hofbauer LC. Novel aspects on RANK ligand and osteoprotegerin in osteoporosis and vascular disease. Calcif Tissue Int. 2004;74(1):103–6. doi: 10.1007/s00223-003-0011-y. [DOI] [PubMed] [Google Scholar]
- 34.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63(4):423–7. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 35.Sert A, Pirgon O, Aypar E, Yilmaz H, Dundar B. Relationship between aspartate aminotransferase-to-platelet ratio index and carotid intima-media thickness in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2013;5(3):182–8. doi: 10.4274/Jcrpe.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschen AR, Kaser A, Stadlmann S, Millonig G, Kaser S, Muhllechner P, et al. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005;43(6):973–83. doi: 10.1016/j.jhep.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 37.Reid P, Holen I. Pathophysiological roles of osteoprotegerin (OPG). Eur J Cell Biol. 2009;88(1):1–17. doi: 10.1016/j.ejcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Yin XM, Ding WX. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med. 2003;3(6):491–508. doi: 10.2174/1566524033479555. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18 - the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30(11-12):1103–9. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 40.Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50 Suppl:S412–6. doi: 10.1194/jlr.R800089-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]