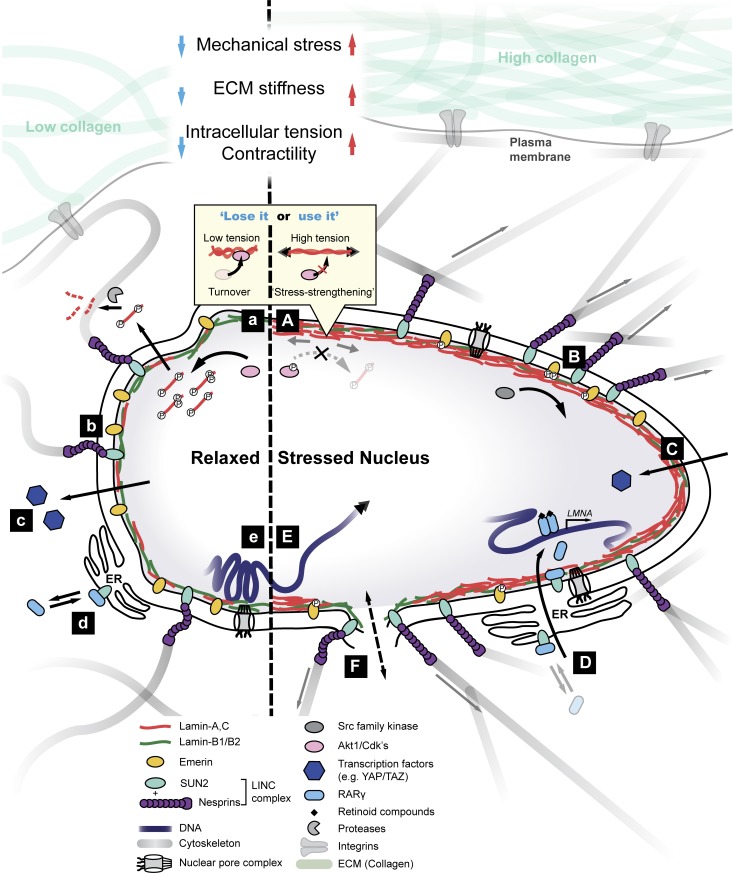
Cho, Irianto, and Discher review emerging mechanisms of nuclear mechanosensing and propose through meta-analyses of published data the universality of mechanosensing pathways.
Abstract
The nucleus is linked mechanically to the extracellular matrix via multiple polymers that transmit forces to the nuclear envelope and into the nuclear interior. Here, we review some of the emerging mechanisms of nuclear mechanosensing, which range from changes in protein conformation and transcription factor localization to chromosome reorganization and membrane dilation up to rupture. Nuclear mechanosensing encompasses biophysically complex pathways that often converge on the main structural proteins of the nucleus, the lamins. We also perform meta-analyses of public transcriptomics and proteomics data, which indicate that some of the mechanosensing pathways relaying signals from the collagen matrix to the nucleus apply to a broad range of species, tissues, and diseases.
Introduction
Tissue physical properties depend on the cells that make the tissue and also seem to be affected by tissue use. For example, muscle, cartilage, and bone, when suitably exercised, generate or resist mechanical forces that can be many times the weights of these tissues. It is therefore understandable that these tissues and their cells require some stiffness or rigidity to maintain their form under high stress. Brain and marrow, in contrast, are protected from external stress by bone, and so perhaps one reason they are soft is that they simply do not need to be stiff to resist stress. It is now reasonably well established that cells have the ability to sense and respond to mechanical forces of varying magnitude, direction, and frequency (Discher et al., 2005; Ingber, 2006). Furthermore, because the largest organelle of a cell is its nucleus, it is also plausible that the nucleus has a similar ability to mechanosense the tissue microenvironment. Forces and resistance external to nuclei are increasingly understood to affect processes ranging from protein conformation and assembly to localization of transcription factors, chromosome organization, and nuclear envelope dilation to rupture—all of which might affect gene expression (Fig. 1).
Tissue stiffness is molecularly determined by the most abundant proteins in vertebrates, the highly helical fibrillar collagens of the ECM. Cells interact physically with the ECM as the cytoskeleton exerts stress on the ECM via adhesions, and this stress is sufficient to alter the morphologies of cells (Marganski et al., 2003; Discher et al., 2005) and their nuclei (Dahl et al., 2008; Khatau et al., 2009; Versaevel et al., 2012; Kim et al., 2014a, 2015). With soft ECM, most normal cell types down-regulate their actin–myosin contractile machinery and exert much less tension than with stiff ECM. Importantly, cytoskeleton-induced stresses on matrix outside of the cell put an equal-but-opposite cytoskeletal stress on the nucleus inside (Chancellor et al., 2010; Lovett et al., 2013; Swift et al., 2013; Alam et al., 2015); it is as if the nucleus is just a spheroidal inclusion of ECM anchored within the cell by factors and assemblies that are functionally analogous to focal adhesions (which are well known to be mechanosensitive). Indeed, much like the plasma membrane and cortex at the cell–ECM boundary, the nuclear envelope is a dynamic, force-sensitive interface between the cytoplasm and the chromatin.
The nuclear envelope’s main structural “cortex” is the lamina, composed of the helix-rich fibrillar lamin proteins (Goldman et al., 2002) that assemble just below the inner nuclear membrane (INM; Gruenbaum et al., 2005). Lamins are A-type (lamins A and C from the LMNA gene) or B-type (lamins B1 and B2) and tether the nucleus to the cytoskeleton via the linker of nucleoskeleton and cytoskeleton complex (Crisp et al., 2006), referred to as LINC proteins. The nuclear envelope harbors many other proteins (Schirmer et al., 2003; Korfali et al., 2012), and some, such as those of the LEM (LAP2, emerin, and MAN1) family, specifically associate with the lamins. Heterochromatin at the nuclear periphery (Paddy et al., 1990; Solovei et al., 2013) and a wide range of transcription factors (Lloyd et al., 2002; Margalit et al., 2005; Rodríguez et al., 2010; Wilson and Foisner, 2010) also interact with the lamina. The nuclear envelope and its lamina are thus well positioned to serve as a multiplexing interface that can mechanotransduce in its regulation of the cell’s genome.
Recent approaches that range from methods for probing nuclear mechanics to mass spectrometry (MS)–based characterization of protein folding have expanded our understanding of nuclear mechanosensing. We start the review by discussing the insights these new technological advances have provided, in particular in the assessment of the direct physical effects that external force has on nuclear protein conformation and phosphorylation states. This is followed by summaries of stress-induced changes in localization of transcription factors, chromosome conformation and organization, nuclear envelope dilation, and finally, rupture. Links to embryonic development, disease, and aging are discussed, particularly in the context of the many “nuclear envelopathies” that result from mutations in structural components of the nucleus. Lastly, a “big picture” analysis of public transcriptome and proteome data for diverse tissues helps to establish stiffness-dependent scaling of key mechanosensory proteins as a broad, polymer physics foundation for nucleus mechanosensing.
Force-induced changes in protein conformation and phosphorylation states
Mechanical stress exerted on or by the cell can deform proteins, and in some cases, the stress-induced conformational changes regulate the activity of enzymes acting on the protein. In the ECM, tension stabilizes collagen I fibrils against enzymatic degradation by matrix metalloproteinase (Flynn et al., 2010). Given the primary role of collagen in maintaining the mechanical integrity of tissue, such resistance with stress seems reasonable. In the cytoskeleton, the Cas substrate domain protein p130Cas unfolds upon mechanical stretching, exposing cryptic tyrosine residues for subsequent phosphorylation by Src-family kinases (Sawada et al., 2006). In isolated nuclei, at least one domain of lamin A/C unfolds when nuclei are sheared, as indicated by increased reactivity of a cryptic cysteine residue (Cys522; Swift et al., 2013). MS analyses further revealed that in intact cells cultured on soft collagen-coated gels versus stiff gels, lamin A/C phosphorylation increases at all of four different sites in either the head domain (Ser22) or the tail domain (Ser390, Ser404, and Thr424; Swift et al., 2013). Conversely, culturing cells on soft gels results in rounded cells with wrinkled nuclei, as if there is excess membrane under little to no tension compared with cells grown on stiff gels that promote cell spreading and nuclear flattening. Total lamin A/C levels ultimately reach lower steady-state levels (by ∼50% or more) in cells cultured on soft gels (without affecting lamin B1/B2), suggesting that low tension in the cell and nucleus destabilizes the lamin A/C coiled-coil dimers, favoring phosphorylation by constitutive kinases and promoting subsequent degradation (Fig. 1 A). Increased turnover is evident in highly phosphorylated, low-molecular-mass bands in immunoblots (Buxboim et al., 2014). “Stress strengthening” thus seems to apply to lamin A/C as well as collagen I, which are both fibrous assemblies of helical multimers.
Figure 1.
Nucleus mechanosensation. Left and right sides indicate relaxed (soft) and mechanically stressed nuclei, respectively. (A and a) High nuclear tension can induce conformational changes in lamin coiled-coil dimers, which sterically inhibits access by kinases (Swift et al., 2013; Buxboim et al., 2014). In a relaxed nucleus, lamins are more phosphorylated and solubilized into the nucleoplasm (as during cell division). Phospho-solubilized lamins may ultimately become degraded (Bertacchini et al., 2013; Buxboim et al., 2014). Tension-inhibited turnover of lamins is similar to that of collagen I (Flynn et al., 2010) and is an example of structural proteins exhibiting stress-strengthening properties. (B and b) Pulling on nesprin-1 leads to phosphorylation of emerin by Src kinases (Guilluy et al., 2014) and results in stress stiffening of the nucleus. Emerin phosphorylation is high in cells cultured on stiff substrates and regulates many downstream mechanoresponses, including formation of stress fibers, migration, localization of YAP and TAZ, and SRF transcription. (C and c) Mechanosensitive transcription factors such as YAP and TAZ translocate into the nucleus under stress to modulate gene expression (Dupont et al., 2011). (D and d) Mechanical stress leads to nuclear localization of RARγ, which directly regulates LMNA transcription. Nuclear translocation of RARγ is facilitated by its interactions with SUN2 as well as lamin A/C, suggesting a feedback mechanism wherein the protein product lamin A/C regulates its own transcription (Swift et al., 2013). (E and e) Application of mechanical force may lead to changes in chromatin conformation (e.g., local stretching of genes), thereby altering transcriptional activity (Tajik et al., 2016). Mechanical perturbation can also affect the global arrangement of chromosome territories (Maharana et al., 2016). (F) High tension can induce membrane dilation and may lead to transient ruptures, allowing for the exchange and mislocalization of nucleoplasmic and cytoplasmic factors.
Interphase phosphorylation of nuclear lamins, as with cytoskeletal intermediate filament proteins (Chang and Goldman, 2004), is thought to be a major mechanism responsible for regulating filament assembly and localization. Another recent study identified 20 phosphosites within the lamin A/C protein, eight of which were high phosphate-turnover sites located within three “hot spot” regions (Kochin et al., 2014). Imaging of phosphomimetic mutants in interphase cells revealed that Ser22 and Ser392 (and to a lesser degree Ser390, Ser404, and Ser407) dominated the regulation of lamin assembly and dynamics. Phosphorylation favors dissociation from the lamina into the nucleoplasm and enhances nucleoplasmic mobility (Kochin et al., 2014), although degraded forms of these constructs should be considered (Buxboim et al., 2014). Even the precursor pre–lamin A is more degraded upon Akt-mediated phosphorylation at Ser404 (Fig. 1 a; Bertacchini et al., 2013). Mitotic phosphorylation of lamins is a key driver of nuclear envelope disassembly in cells rounded for division (Gerace and Blobel, 1980; Heald and McKeon, 1990) and is 10- to 20-fold higher than during interphase (Buxboim et al., 2014). The higher phosphorylation of lamin A/C (and lower total lamin A/C levels) observed in cells cultured on soft gels is consistent with such cells being more rounded under low stress, although key kinases that are up-regulated in mitosis (e.g., CDK1) are unlikely to have a role in interphase (Buxboim et al., 2014). These results collectively suggest that matrix stiffness–derived cell and nuclear tension induces conformational changes in lamin coiled-coil dimers (analogous to a rope being stretched from either side; Fig. 1, A and a), which sterically hinders access of kinases, including Cdks, PKC, and Akt (Buxboim et al., 2014). This mechanism of tension-inhibited phosphorylation provides the biophysical basis for a “lose it or use it” model (Dingal and Discher, 2014), whereby unstressed lamin A/C is degraded under low-stress conditions but stabilized under high-stress conditions.
Tensile forces can also alter phosphorylation states of emerin (Guilluy et al., 2014), another nuclear envelope protein that mediates the mechanical communication between the nucleus and the cytoplasm (Fig. 1, B and b). With isolated nuclei, application of sequential mechanical tension using magnetic tweezers on nesprin 1 antibody–coated beads led to stress stiffening and an increase in phosphorylation of emerin at Tyr74 and 95 by Src kinase. Mutation of these residues abolished the stiffening effect, indicating the importance of these phosphosites for emerin mechanosensitivity. Intact cells on stiff substrates showed high emerin phosphorylation that was reduced with myosin II inhibition by blebbistatin treatment. These results confirm nuclear regulation of signaling by intracellular tension. However, contrary to lamin A/C, lower expression of emerin stiffens the nucleus, and stress application increases emerin phosphorylation. Indeed, nonphosphorylatable mutants of Tyr74 and Tyr95 in intact cells led to fewer stress fibers, reduced migration, decreased nuclear localization of the transcription coactivators YAP and TAZ, and decreased transcription by the transcription factor serum response factor (SRF), which is a master regulator of numerous actin cytoskeletal proteins. Detailed mechanisms of how emerin phosphorylation initiates these downstream changes remain unclear, but these findings confirm the crucial role of nuclear envelope proteins not only in sensing mechanical stress but also in regulating cell behavior and phenotype.
Nuclear localization of mechanosensitive transcription factors
Cell tension modulates nuclear translocation of mobile regulators (e.g., transcription factors), at least in cellular mechanotransduction, if not direct mechanosensing by the nucleus (Fig. 1, C and c; Halder et al., 2012; Ho et al., 2013). Perhaps the best-characterized mechanotransducing transcriptional regulators are YAP and TAZ, which influence growth in the canonical Hippo pathway and tend to localize to the nucleus in high-tension cells cultured on stiff substrates (Dupont et al., 2011). Although there have been many studies of exceptions and complexity in YAP and TAZ responses (Swift et al., 2013; Chopra et al., 2014), nuclear entry of YAP and TAZ can induce a wide range of downstream signaling cascades mediating complex cellular processes including differentiation (Dupont et al., 2011; Sun et al., 2014) and even contribute to storage of mechanical “memory” of past ECM interactions (Yang et al., 2014). Conversely, at least one transcription factor, NKX-2.5, enters the nucleus in response to low tension, and functions in the nucleus as a “mechanorepressor” to repress expression of genes contributing to high-tension states (e.g., α-smooth muscle actin [ACTA2]; Dingal et al., 2015). Translocation in and out of the nucleus can be regulated by a variety of mechanisms including phosphorylation (of YAP1; Murphy et al., 2014), but whether YAP/TAZ or NKX-2.5 interact directly or even indirectly with mechanosensitive factors in the nucleus or at the nuclear envelope remains unclear.
Translocation into the nucleus can indeed result from stresses affecting specific interactions with nuclear envelope proteins (Ho et al., 2013; Swift et al., 2013). Stiff substrates drive translocation of the transcription factor RARγ (retinoic acid receptor gamma) into the nucleus, a nuclear receptor modulated by retinoic acid agonists and antagonists that drives lamin A/C transcription (Fig. 1, D and d; Swift et al., 2013). Immunoprecipitation followed by MS identified several binding partners of RARγ, including SUN2, which shuttles between the ER and the INM (Fig. 1 A). Overexpression of SUN2 floods the ER with protein and results in rounded nuclei with decreased lamin A/C levels and increased cytoplasmic RARγ. Conversely, high lamin A/C effectively stabilizes nuclear retention of SUN2 and RARγ so that lamin A/C ultimately regulates its own transcription. This feedback mechanism between the level of a protein, as regulated by tension on the nucleus, and the level of its transcript is illustrative of a “mechanobiological gene circuit” (Swift et al., 2013). Additionally, lamin A/C and emerin modulate nuclear actin polymerization, which controls nuclear localization and transcriptional activity of MKL1 as a cofactor for the transcription factor SRF (Vartiainen et al., 2007; Ho et al., 2013). Perinuclear actin polymerization increases with stress (Shao et al., 2015), which could influence the state of nuclear actin and SRF regulation. High SRF drives expression of the actin–myosin cytoskeleton, which stresses ECM only up to a roughly constant strain in the matrix (Discher et al., 2005), with excess actin–myosin turning over and thereby limiting SRF as well as nuclear tension, lamin A/C, and nuclear RARγ. This current understanding suggests a tight coupling between a mechanobiological gene circuit for lamin A/C and another for the actin–myosin cytoskeleton, at least above a baseline level of cytoskeleton expression and tension that is independent of lamin A/C (Buxboim et al., 2014).
Stress-induced changes in chromatin organization and conformation
Although considerable force (e.g., in the nanonewton range) is typically required to significantly deform the nucleus in adherent mammalian cells (Neelam et al., 2015), recent studies show that even weak forces in the piconewton range can affect histone acetylation states (Li et al., 2011), chromatin dynamics (Hampoelz et al., 2011), and protein–protein interactions (e.g., coilin–SMN complexes) in the nucleus (Poh et al., 2012). Physical stress could also cause global or local rearrangement of chromosomes (Fig. 1, E and e), affecting the distinct “territories” that chromosomes occupy (Cremer and Cremer, 2001). Transcriptionally active euchromatin largely resides in the center (and near nuclear pores), and transcriptionally repressed heterochromatin typically anchors to the lamina at the nuclear periphery and also around nucleoli (Solovei et al., 2013). The organization of such domains is believed to influence differentiation; embryonic stem cells have no heterochromatin and exhibit more random and hyperdynamic arrangement of chromosome territories compared with differentiated cells (Maharana et al., 2016). Key chromatin proteins such as histones immobilize with differentiation (Meshorer et al., 2006), supporting the notion that chromosome arrangement becomes increasingly stabilized as cells commit to a lineage-specific fate. However, stresses that distort the nuclear envelope can directly reorganize chromosome domains, affecting transcriptional activity without any biochemical intermediates; in cell and nuclear flattening, for example, chromosome territories are seen to intermingle and overlap (Maharana et al., 2016). One possible explanation is that heterochromatin is tethered to nuclear envelope components that undergo structural remodeling in response to stress. Epitope masking in immunostaining of nuclear envelope components has long been a concern (Tunnah et al., 2005), and confocal imaging of cells in culture show that cell and nuclear compression induces basal-to-apical polarization of immunostained lamin A/C (but not B-type lamins; Ihalainen et al., 2015; Kim and Wirtz, 2015). This polarization could have its origins in the higher mobility of lamin A/C relative to B-type lamins (Dahl et al., 2006), combined perhaps with a stress-driven increase in lamin A/C multimerization at the basal nuclear envelope (Ihalainen et al., 2015). Extrinsic mechanical strain has also been shown to enrich emerin and nonmuscle myosin IIa at the outer nuclear membrane (Le et al., 2016). Corresponding loss of emerin at the INM associates with altered global histone modification states, coupled to defective heterochromatin anchorage to the lamina.
Single-cell studies in culture have elegantly probed strain propagation into engineered chromatin from the cell surface with magnetic beads using a large GFP-tagged transgene. The transgene has been seen to stretch when the bead is pulled, which up-regulates transcriptional activity (Fig. 1, E and e; Tajik et al., 2016). Stress-induced extension of chromatin depended on the direction of the applied stress as well as actomyosin contractility and the presence of nuclear envelope proteins (e.g., lamins and linker of nucleoskeleton and cytoskeleton components). For example, 2 min of 17.5-Pa stress at the cell surface increased transgene expression 20%, whereas knockdown of lamins, SUN1/2, or emerin only gave 5% more expression or less (lamin B receptor [LBR] knockdown had no effect in these cells), and basal expression of the transgene depended on actomyosin contractility. Physical forces propagating to the nuclear envelope can thus cause global and local chromosome rearrangements to affect transcriptional activity of genes. Similar results for some native loci within cells in native tissues (perhaps exploiting CRISPR/Cas9 methods) could be extremely interesting.
Membrane dilation and rupture
Large changes in nuclear shape or increases in nuclear volume are expected to increase tension in the nuclear envelope. In zebrafish, tissue damage induces osmotic nuclear swelling, which causes dilation of the nuclear membrane and accumulation of cytosolic phospholipase A2 (cPLA2) from the nucleoplasm to the INM (Enyedi et al., 2016). Activation of cPLA2 initiates lipid signaling, which results in the release of proinflammatory eicosanoids that play important roles in tissue damage repair. Perinuclear F-actin and the nuclear lamina help mediate this process, suggesting cPLA2 translocation and activation indeed depend on mechanical tension at the nuclear envelope.
If lamin A/C is compromised through knockdown or mutation, cells on stiff 2D substrates can apply sufficient tension to strain and even rupture the nuclear envelope transiently during interphase (Fig. 1 F; De Vos et al., 2011; Vargas et al., 2012; Tamiello et al., 2013). Rupture has been seen to regulate localization of transcription factors (e.g., RELA and OCT1) as well as constructs of GFP–nuclear localization sequence. Importantly, rupture is suppressed by culturing cells on soft gels (Tamiello et al., 2013), which minimize nuclear and cell tension (Discher et al., 2005).
Cell migration in 3D through narrow, rigid pores (∼3 µm in diameter) can likewise stress the nucleus sufficiently to disrupt the lamina (Harada et al., 2014) and to increase DNA damage throughout the nucleus based on quantitation of repair foci of gH2AX and phosphorylated ATM kinase as well as single-cell electrophoreses (comet assays; Irianto et al., 2016b). Transient rupture of GFP–nuclear localization sequence into the cytoplasm and local accumulations of the DNA repair factor GFP-53BP1 in the nucleus (Denais et al., 2016; Raab et al., 2016) has led to speculation that constitutive nucleases leak into the nucleus to cleave DNA during envelope rupture events. Alternatively, repair factors have been seen to leak into the cytoplasm after constricted migration (Irianto et al., 2016a,b), which is consistent with rupture-induced loss of nuclear factors from lamin A–defective cells cultured on rigid substrates. For the latter cells, at least some DNA repair factors exhibit low steady-state levels attributable to their degradation, and the slow repair of DNA damage caused by ionizing radiation can be rescued by overexpression of 53BP1 (Gonzalo, 2014).
Migration-induced DNA damage could be mechanistically similar but more transient and could also involve additional mechanisms. For example, mobile DNA repair factors always segregate away from DNA, which is squeezed and aligned in a pore (Irianto et al., 2016a). In addition, live imaging of a chromatin locus in constricted nuclei demonstrated stretching by more than 10-fold (Irianto et al., 2016c), which could modulate repair of preexisting breaks. Regardless of mechanism, constricted migration of cancer cell clones has been shown by genotype and phenotype analyses to cause heritable changes that affect cell shape (Irianto et al., 2016b).
Transient ruptures are not selective for entry or exit of specific proteins, but mechanosensitive factors that are already “primed” to favor entry into the nucleus under high-stress conditions (e.g., YAP1, RARγ, and SRF) might bind accessible loci and accumulate more readily upon rupture than other factors. Subsequent up-regulation of major structural and cytoskeletal genes might thus better equip a cell for resisting large mechanical strains, as seen with RARγ nuclear entry, which drives up LMNA expression to produce a stiffer nucleus (Swift et al., 2013). Further kinetics-focused studies are required to assess whether such protective responses can indeed be observed in different contexts, especially with cells such as those of the immune system that undergo repetitive constrictive events throughout their lifetime.
Nuclear mechanosensing in development, disease, and aging
Early embryos are uniformly soft and compliant, with correspondingly low levels of collagenous ECM (Majkut et al., 2013). Nuclei of embryonic stem cells are likewise very soft, with low levels of lamin A/C (Pajerowski et al., 2007; Eckersley-Maslin et al., 2013). However, from an initial embryonic disk stiffness of ∼0.3 kPa (which is 100-fold softer than a gummy bear), the embryonic chick heart stiffens every day by ∼0.3 kPa/day, largely because of accumulation of collagenous ECM made by cardiac fibroblasts (Majkut et al., 2013). The brain, on the other hand, remains throughout life soft and as low in collagen as the embryo. Surprisingly, Lmna-knockout mice survive the tissue stiffening of embryogenesis and generate all tissues but fail to grow after birth (small skeleton) and die within weeks because of chronic injury and dystrophy in cardiac and skeletal muscle, among other stiff tissues (Sullivan et al., 1999; Kubben et al., 2011; Jahn et al., 2012). The lack of a strict need for a robust nucleus during the earliest stages of development is understandable for ultrasoft embryonic tissue that does not generate or bear large mechanical stresses while protected inside the womb. In normal development, however, lamin A/C is expressed after tissue differentiation, and the timing of initial expression varies depending on the tissue considered in both chick embryos (which only express lamin A isoform; Lehner et al., 1987) and mouse embryos (Rober et al., 1989). Interestingly, LBR tends to show an opposite expression pattern as lamin A/C, with either one able to control chromatin tethering at the envelope (Solovei et al., 2013). One plausible model is that LBR is progressively displaced by lamin A/C as tissue-specific stiffening in the embryo drives the expression of lamin A/C. This mechanoregulation of lamin A/C in the embryo is likely maintained throughout tissue maturation until steady-state levels are reached in adulthood (Swift et al., 2013).
Defects in nuclear mechanosensory proteins are linked to a large number of postnatal progressive diseases. Nearly all of these diseases affect stiff tissues including heart and skeletal muscle, as well as cartilage and bone, which generate and/or sustain considerable mechanical stress. Fat can also be affected, as it has intermediate levels of collagens that suggests it bears some stress (Swift et al., 2013). Cardiomyopathies are common (Narula et al., 2012), with more than 120 different LMNA mutations linked to dilated cardiomyopathy (DCM), characterized by progressive thinning of the ventricular wall and weakened cardiac contractility. Mutant forms of other nuclear envelope proteins, including emerin, nesprins 1/2, Lap2α, and LUMA (Bione et al., 1994; Taylor et al., 2005; Bengtsson and Otto, 2008), also cause DCM and various forms of Emery–Dreifuss muscular dystrophy (Bione et al., 1994; Bonne et al., 1999; Zhang et al., 2007; Bengtsson and Otto, 2008). Impaired mechanotransduction (Lammerding et al., 2005) and nuclear envelope fragility (with low lamin A/C levels; Narula et al., 2012) are often considered to be part of disease pathogenesis. The large number of different genetic diseases caused by mutations in nuclear envelope proteins (Worman, 2012) reflects the importance of nuclear mechanosensing in normal cell function, but “epigenetic” changes in lamin A/C levels during diseases such as cancer remain an active topic of research (Harada et al., 2014).
An inability of the nucleus to respond dynamically to mechanical stress might also contribute to normal and accelerated aging. In Hutchinson–Gilford progeria syndrome (HGPS), a rare premature aging disorder, a farnesylated mutant product of the LMNA gene called progerin causes the nucleus to be more brittle and “solid-like” (as opposed to a viscous or “fluid-like” lamina; Dahl et al., 2006). FRAP experiments confirm that progerin is immobile compared with lamin A/C (Dahl et al., 2006), consistent with an inability to flow and remodel dynamically in response to mechanical stress. Phosphorylation of progerin is also lower than that of normal lamin A/C (Moiseeva et al., 2016), supporting the notion that farnesylated lamins (i.e., pre–lamin A, progerin, and B-type lamins) are all more tightly anchored to the membrane and less soluble. HGPS cells also exhibit elevated levels of DNA damage (Liu et al., 2005, 2006; Gonzalez-Suarez et al., 2009; Burtner and Kennedy, 2010), which again suggests a mechanistic link between nuclear mechanics and the accumulation of DNA breaks (Irianto et al., 2016b). Indeed, all other premature aging disorders that are also pan-tissue (such progeroid syndromes affect more than just on tissue such as brain in Alzheimer’s disease) result from mutations in DNA repair factors. Consistent with a shift from lamin A/C to a more lamin B–like progerin, the accelerated aging phenotype in HGPS patients and progeria mouse models is like that of lamin A/C–deficient mice in that they exhibit more pronounced effects on stiff tissues such as heart and skeletal muscle with increasingly fibrotic, collagen-rich ECM and, for progeria mice, death in 3–8 mo (Osorio et al., 2011).
Extrinsic feedback with ECM stiffness is likely to be defective in aging-related disease. Indeed, a mosaic mouse model in which 50% of cells in all tissues express farnesylated pre–lamin A is normal and long-lived, which is surprising given that the homozygous mouse dies in weeks like other progeria and lamin A–null mice (de la Rosa et al., 2013). Because culture studies further showed that the ECM can rescue the proliferative defects of pre–lamin A–expressing fibroblasts (de la Rosa et al., 2013), soft ECM could be suppressing nuclear stress, DNA damage, and the resulting senescence. Consistent with such outside-in signals, the same authors separately reported a mouse knockout for a collagenolytic protease (MMP14) that exhibits a progeria-like course of disease, including anomalous lamin A/C, in which premature death was delayed by administration of retinoic acid (Gutierrez-Fernandez et al., 2015). Understanding the interplay of collagenous matrix stiffness and mechanosensitive lamin A/C expression as modulated by natural soluble factors such as retinoic acid is thus beginning to impact therapeutic approaches to aging-related diseases.
“Universal” stiffness-dependent scaling of lamin A/C and other nuclear envelope proteins
Tissue microelasticity or “stiffness,” Et, is measured in units of stress (kilopascal) and is largely determined by the concentration of collagens and other ECM components (Fig. 1; Brower et al., 2006). At the scale of a cell, the magnitude of Et spans at least two logs from soft brain or marrow to the very stiff osteoid that osteoblasts calcify to bone (Discher et al., 2009). Identifying log-scale variations is crucial to recognizing any potential polymer physics–based trends (Gennes, 1979), and recent MS-based studies of adult mouse tissue proteomes (Swift et al., 2013) indeed reveal a power-law scaling relationship over several orders of magnitude between tissue stiffness (Et; units of kilopascals) and the molar concentration of collagen I:
| (1) |
where n is the scaling exponent (i.e., the slope that results from a log–log plot of the two quantities). Of course, such scaling expressions leave out proportionality factors (in units of kilopascals per molarn) and ignore small offsets (e.g., critical concentrations to percolate a network), but they make clear that high levels of fibrillar collagen are found in stiffer tissues (e.g., cardiac or skeletal muscle or osteoid). Indeed, direct perturbation of collagens in intact tissue, either by enzymatic degradation or cross-linking, generally changes tissue stiffness even for a soft embryonic heart (Majkut et al., 2013). As the most abundant proteins in our bodies, comprising more than 30% of all proteins and 90% of all ECM (van der Rest and Garrone, 1991; Shoulders and Raines, 2009), it should not be surprising that tissue stiffness exhibits power-law scaling with the concentration of this prominent structural biopolymer. Scaling is indeed seen for the stiffness of gels made from purified collagen I (Yang and Kaufman, 2009) and is generally found for the physical properties of polymer networks (Gennes, 1979).
In addition to collagenous ECM, MS-based proteomic profiling of hundreds of the most abundant structural proteins in adult mouse tissues (Swift et al., 2013) revealed that the concentration of A-type lamins scales over several orders of magnitude with tissue stiffness Et:
| (2) |
where m denotes the scaling exponent. This scaling expression quantifies up-regulation of A-type lamins (by 30-fold from soft brain to rigid bone) in response to tissue stiffness; the result does not imply that nuclei contribute to tissue stiffness. B-type lamin levels remain relatively constant (for lamin B1, m ∼0.2, and for lamin B2, m ∼0.0). Thus, whereas collagens and other ECM proteins set the stiffness of the tissue, lamin A/C at the nuclear envelope responds (as shown in Fig. 1) to resist cell tension that is largely matrix-driven. Importantly, rearrangement of the equations above gives a simple and useful correlation between the concentrations of collagen and lamin A: [lamin A] ∼ [collagen I]α with αLmna = m × n ≈ 0.45, where αLmna denotes the scaling exponent obtained by combining Eqs. 1 and 2. Causality for any such relationship must be established of course by in-depth cell biological studies such as those reviewed in the context of Fig. 1, but emerging trends might at least be sought in publicly available, standardized omics datasets (as outlined below).
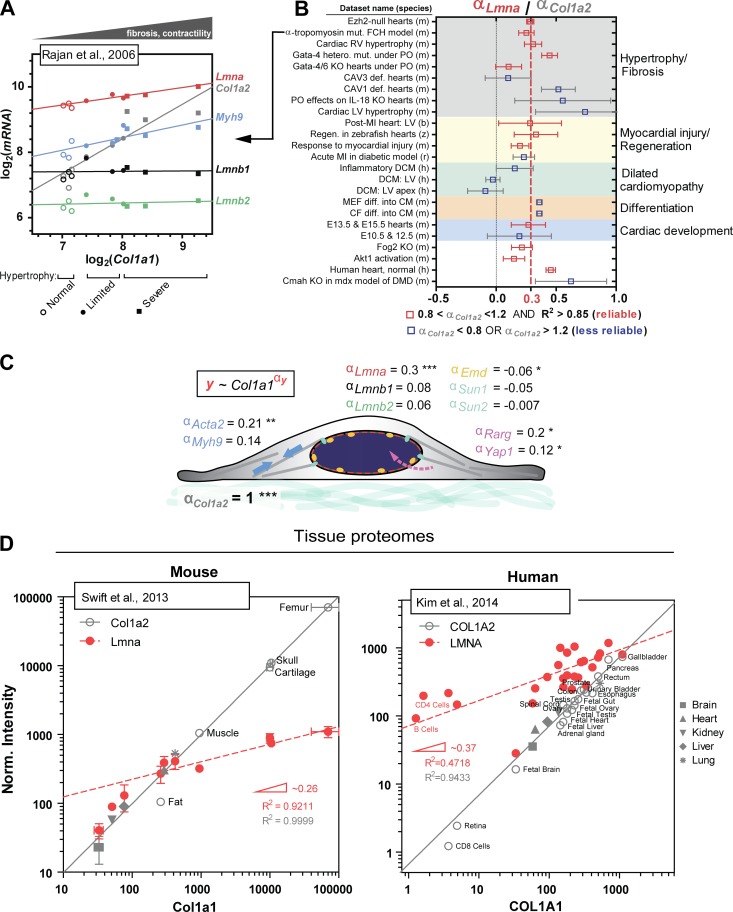
As an example of a broad meta-analysis in today’s big-data era, we focus on heart tissue. The heart offers the largest number of normal and diseased transcriptomic and/or proteomic datasets relevant to mechanosensation. Open-access datasets are available for normal development and aging, as well as fibrosis, myocardial injury, and hypertrophy. Second, datasets span a wide range of species, including mouse, human, rat, boar, dog, and zebrafish (Barrett et al., 2013; Vizcaino et al., 2016). Once a dataset is selected, a first check on quality is provided by collagen I’s two stoichiometric subunits: if collagen I-α1 increases or decreases in level, then collagen I-α2 should do the same in proportion. Changes in collagen I-α1 between samples in a dataset could be caused by normal variation, experimental perturbation, or even perhaps experimental noise in other components of analysis. However, provided one finds for a given dataset an exponent (αCol1a2) close to 1 and a reasonable fit (R2 > 0.85) of the form: [collagen I-α2] ∼ [collagen I-α1]α with αCol1a2 = 1.0 ± 0.2, then the dataset passes a first validation. Of course, although collagen I seems a reasonable surrogate for tissue stiffness, most tissues in the body exhibit some heterogeneity in their mechanical properties (Koser et al., 2016), and other ECM proteins add complexity to rheology measurements. Therefore, to take into account different sources of variation, a large number of datasets should be carefully analyzed for a diversity of tissue samples and disease models before developing broad hypotheses.
For illustration, transcript data for genes of interest from a mouse model of familial cardiac hypertrophy and fibrosis (Rajan et al., 2006) are plotted in log–log form versus Col1a1 (Fig. 2 A). A statistically robust positive correlation between Col1a2 and Col1a1 (Pearson coefficient [r] = 0.93), with a suitable slope (αCol1a2 ≈ 1.0) and goodness of fit (R2 = 0.87), provides some validation for further analysis. In comparison, Lmna increases more weakly (αLmna ≈ 0.22; R2 = 0.81), but the very good fit is factor-of-two consistent with the proteomics-based scaling (αLmna ≈ 0.45) and thus supports the model wherein increased deposition of collagenous ECM results in correspondingly higher lamin A/C levels. One might expect an increase in contractility (per Fig. 1), and indeed nonmuscle myosin IIa (Myh9) and smooth muscle actin (Acta2) exhibit similar positive correlations. Not everything changes: Lmnb1 and Lmnb2 showed little to no correlation with collagen I (r = 0.012 and 0.21, respectively), which is consistent with constant B-type lamin levels quantified for adult mouse tissue proteomes (Swift et al., 2013).
Figure 2.
Meta-analysis of universal stiffness-dependent scaling of lamin A/C and other nuclear envelope proteins. Published omics datasets of relevance were collected from various open-access databases (Barrett et al., 2013; Vizcaino et al., 2016), and as a first simple check for quantitative reliability, log–log plots of Col1a1 versus Col1a2 were generated for each dataset, because the two should in principle correlate well with each other as components of collagen’s stoichiometric structure. Only those datasets that gave Col1a2 scaling exponents (= slopes on a log–log plot) of αCol1a2 = 1 ± 0.2, with high R2 > 0.85, were selected for analysis, with the assumption that a robust correlation between Col1a1 and Col1a2 indicates minimal error arising from sample preparation and/or normalization. Such data provides an added advantage in that type I collagen content becomes a proxy for tissue stiffness (Swift et al., 2013). Once reliable datasets were identified, other proteins of interest (e.g., nuclear lamins) were plotted against Col1a1 to determine scaling exponents relative to that of Col1a2. (A) Representative transcriptomics dataset for mouse model of familial cardiac hypertrophy (FCH; Rajan et al., 2006) illustrating robust scaling between Col1a1 and Col1a2 (αCol1a2 = 0.95). Lmna and Myh9, among many other key mechanosensory proteins and genes, also correlate with Col1a1, whereas Lmnb1 and Lmnb2 remain constant. Samples were parsed into three groups: “normal,” “limited,” and “severe” hypertrophy. (B) The mean scaling exponent for Lmna (αLmna) normalized to that for Col1a2 (αCol1a2) obtained from ∼25 transcriptomics datasets is equal to <αLmna> = 0.3. Datasets span embryonic, fetal, and adult cardiac tissue samples from six different species (h, human; m, mouse; r, rat; z, zebrafish; b, boar; and d, dog) and at least five different disease models, including DCM, hypertrophy, fibrosis, and myocardial injury. Datasets that are deemed most quantitatively reliable with 0.8 < αCol1a2 < 1.2 and R2 > 0.85 are in red. CF, cardiac fibroblast; CM, cardiomyocyte; E, embryonic day; KO, knockout; LV, left ventricle; MEF, mouse embryonic fibroblast; MI, myocardial infarction; PO, pressure overload; RV, right ventricle. (C) Mean scaling exponents (αy) of several key proteins involved in nucleus mechanosensing. Col1a2, Lmna, Emd, Acta2, Myh9, Rarg, and Yap1 have statistically nonzero exponents. ***, P < 0.0001; **, P < 0.01; *, P < 0.05. (D) MS-based profiling of mouse (left) and human (right) tissue proteomes shows comparable scaling of LMNA with collagen I over several orders of magnitude (αLMNA ≈ 0.3), consistent with αLmna determined for heart transcriptomes.
Based on more than 20 datasets for heart, the scaling exponent for Lmna versus Col1a1 converges to αLmna = 0.3 ± 0.04 (Fig. 2 B). The majority of the highly diverse datasets (different species and perturbations) showed the expected collagen I scaling of αCol1a2 ≈ 1.0 and were therefore included in the best estimate of αLmna. The implied stiffness-dependent scaling of lamin A/C thus appears to be a highly conserved phenomenon, perhaps generalizable to a broader range of cell types and tissue or organ systems. Phylogenetic analyses have indeed indicated that lamins are the most ancient of the intermediate filament proteins (Dittmer and Misteli, 2011), and so it is sensible that, in animals, lamins have evolved their ability to mechanosense—some isoforms more so than others.
Other nuclear envelope proteins that interact closely with lamins do not exhibit the same scaling relationships with collagen I (Fig. 2 C). For example, Sun1 and Sun2 remain constant in most datasets, with mean scaling exponents of αSun1 = −0.05 and αSun2 = −0.007. The results for Sun2 message are nonetheless consistent with past analyses that showed the nuclear fraction of Sun2 protein does scale with tissue stiffness (Swift et al., 2013); the mRNA understandably reflects the total level of a factor in a cell, whereas proteins that partition between ER and nucleus (e.g., SUN1/2 and LBR) or between cytoplasm and nucleus (perhaps transcription factors) can exhibit nuclear fractions that are more revealing of mechanosensitivity. Emerin (Emd) correlates inversely with collagen I (P < 0.05). This seems consistent with lower emerin expression in a stiffer nucleus (Guilluy et al., 2014). However, transcripts of Acta2 and Myh9 (readouts for basal cytoskeletal contractility) as well as Rarg (transcriptional regulator of Lmna) and (most weakly) Yap1 all scaled with collagenous ECM. Such positive scaling of transcripts does not prove causality but nonetheless supports the general model of mechanotransduction from ECM to nucleus, involving contractile strain as well as transcriptional activation (Fig. 2 C).
The larger exponents in these datasets are likely to be the easiest to demonstrate as significant by cell biology methods. Conversely, if one discovers a relationship between transcripts in vitro that is not evident in such meta-analyses of real, 3D tissues, then many questions should be asked about the relevance of the culture systems as well as the source of the datasets.
Proteomics datasets are currently less standardized than transcriptomics datasets, but two proteomics datasets for diverse adult tissues were examined. Both exhibit the expected linear scaling of collagen subunits over many logs and are therefore reasonable for further meta-analysis. For mouse (Swift et al., 2013), Lmna protein scales linearly with Col1a1 protein for softer tissue with low collagen, whereas for a larger range of higher collagen I, αLmna ≈ 0.3 (Fig. 2 D). The unexpectedly low amount of lamin A/C is most evident in brain, which is notable for having abundant miR-9 that represses lamin A isoform expression (Jung et al., 2012). However, the transition to weaker scaling suggests the miR-9 mechanism does not apply to stiffer tissues, although this requires deeper investigation. Additionally, because the weaker scaling in stiffer tissues applies to a larger range of data, an overall exponent of αLmna = m × n ≈ 0.45 is close to the weighted average. For the one human dataset (Kim et al., 2014b), the lamin A/C data are much noisier but yield a similar result: αLMNA ≈ 0.3. These results are thus reasonably consistent with transcriptomics analyses of heart and therefore suggest some universality and robustness to the stiffness-dependent scaling of lamin A/C protein and message levels. Of course, all of this analysis of protein and mRNA levels in tissues merely motivates molecularly detailed cell biological studies of nuclear mechanosensing by the lamins among other nuclear components.
Conclusion
Many recent studies now demonstrate that the nuclear envelope, as well as chromatin itself, can sense and respond to mechanical forces exerted on or by the cell’s cytoskeleton. Nuclear mechanosensing is achieved via several pathways, including stress-induced changes in protein conformation (interaction with binding partners, such as enzymes), translocation of transcriptional regulators, chromosome conformation and organization, and membrane dilation and/or rupture. An omics-based meta-analysis suggests that at least some of these mechanosensitive processes, particularly those pertaining to the nuclear lamina, are applicable to a broad range of species, tissues, and diseases. Deeper insight into downstream effects will likely improve our basic understanding of how our cells and tissue are shaped by mechanical cues and might also potentiate novel approaches to therapy for the very large number of diseases linked to changes in components of the nuclear envelope.
Acknowledgments
Seminal contributions from many groups to the topic of mechanosensing were not able to be included here because of length restrictions, but additional papers can be found within the references.
This work was supported by the National Institutes of Health, National Cancer Institute (U54-CA193417); National Heart, Lung, and Blood Institute (R21-HL128187); the Charles E. Kaufman Foundation Grant (KA2015-79179); the American Heart Association (14GRNT20490285); the US/Israel Binational Science Foundation; and the National Science Foundation (Materials Research Science and Engineering Center).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- cPLA
- cytosolic phospholipase
- DCM
- dilated cardiomyopathy
- HGPS
- Hutchinson–Gilford progeria syndrome
- INM
- inner nuclear membrane
- LBR
- lamin B receptor
- MS
- mass spectrometry
- SRF
- serum response factor
References
- Alam S.G., Lovett D., Kim D.I., Roux K.J., Dickinson R.B., and Lele T.P.. 2015. The nucleus is an intracellular propagator of tensile forces in NIH 3T3 fibroblasts. J. Cell Sci. 128:1901–1911. 10.1242/jcs.161703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., et al. . 2013. NCBI GEO: Archive for functional genomics data sets–update. Nucleic Acids Res. 41(D1):D991–D995. 10.1093/nar/gks1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson L., and Otto H.. 2008. LUMA interacts with emerin and influences its distribution at the inner nuclear membrane. J. Cell Sci. 121:536–548. 10.1242/jcs.019281 [DOI] [PubMed] [Google Scholar]
- Bertacchini J., Beretti F., Cenni V., Guida M., Gibellini F., Mediani L., Marin O., Maraldi N.M., de Pol A., Lattanzi G., et al. . 2013. The protein kinase Akt/PKB regulates both prelamin A degradation and Lmna gene expression. FASEB J. 27:2145–2155. 10.1096/fj.12-218214 [DOI] [PubMed] [Google Scholar]
- Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G., and Toniolo D.. 1994. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 8:323–327. 10.1038/ng1294-323 [DOI] [PubMed] [Google Scholar]
- Bonne G., Di Barletta M.R., Varnous S., Becane H.M., Hammouda E.H., Merlini L., Muntoni F., Greenberg C.R., Gary F., Urtizberea J.A., et al. . 1999. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21:285–288. 10.1038/6799 [DOI] [PubMed] [Google Scholar]
- Brower G.L., Gardner J.D., Forman M.F., Murray D.B., Voloshenyuk T., Levick S.P., and Janicki J.S.. 2006. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur. J. Cardiothorac. Surg. 30:604–610. 10.1016/j.ejcts.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Burtner C.R., and Kennedy B.K.. 2010. Progeria syndromes and ageing: What is the connection? Nat. Rev. Mol. Cell Biol. 11:567–578. 10.1038/nrm2944 [DOI] [PubMed] [Google Scholar]
- Buxboim A., Swift J., Irianto J., Spinler K.R., Dingal P.C., Athirasala A., Kao Y.R., Cho S., Harada T., Shin J.W., and Discher D.E.. 2014. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 24:1909–1917. 10.1016/j.cub.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor T.J., Lee J., Thodeti C.K., and Lele T.. 2010. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 99:115–123. 10.1016/j.bpj.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., and Goldman R.D.. 2004. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5:601–613. 10.1038/nrm1438 [DOI] [PubMed] [Google Scholar]
- Chopra A., Murray M.E., Byfield F.J., Mendez M.G., Halleluyan R., Restle D.J., Raz-Ben Aroush D., Galie P.A., Pogoda K., Bucki R., et al. . 2014. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 35:71–82. 10.1016/j.biomaterials.2013.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T., and Cremer C.. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2:292–301. 10.1038/35066075 [DOI] [PubMed] [Google Scholar]
- Crisp M., Liu Q., Roux K., Rattner J.B., Shanahan C., Burke B., Stahl P.D., and Hodzic D.. 2006. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J. Cell Biol. 172:41–53. 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K.N., Scaffidi P., Islam M.F., Yodh A.G., Wilson K.L., and Misteli T.. 2006. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 103:10271–10276. 10.1073/pnas.0601058103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K.N., Ribeiro A.J., and Lammerding J.. 2008. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 102:1307–1318. 10.1161/CIRCRESAHA.108.173989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa J., Freije J.M., Cabanillas R., Osorio F.G., Fraga M.F., Fernandez-Garcia M.S., Rad R., Fanjul V., Ugalde A.P., Liang Q., et al. . 2013. Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion. Nat. Commun. 4:2268 10.1038/ncomms3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais C.M., Gilbert R.M., Isermann P., McGregor A.L., te Lindert M., Weigelin B., Davidson P.M., Friedl P., Wolf K., and Lammerding J.. 2016. Nuclear envelope rupture and repair during cancer cell migration. Science. 352:353–358. 10.1126/science.aad7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos W.H., Houben F., Kamps M., Malhas A., Verheyen F., Cox J., Manders E.M., Verstraeten V.L., van Steensel M.A., Marcelis C.L., et al. . 2011. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum. Mol. Genet. 20:4175–4186. 10.1093/hmg/ddr344 [DOI] [PubMed] [Google Scholar]
- Dingal P.C., and Discher D.E.. 2014. Systems mechanobiology: Tension-inhibited protein turnover is sufficient to physically control gene circuits. Biophys. J. 107:2734–2743. 10.1016/j.bpj.2014.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingal P.C., Bradshaw A.M., Cho S., Raab M., Buxboim A., Swift J., and Discher D.E.. 2015. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater. 14:951–960. 10.1038/nmat4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D.E., Janmey P., and Wang Y.L.. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science. 310:1139–1143. 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- Discher D.E., Mooney D.J., and Zandstra P.W.. 2009. Growth factors, matrices, and forces combine and control stem cells. Science. 324:1673–1677. 10.1126/science.1171643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer T.A., and Misteli T.. 2011. The lamin protein family. Genome Biol. 12:222 10.1186/gb-2011-12-5-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. . 2011. Role of YAP/TAZ in mechanotransduction. Nature. 474:179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Eckersley-Maslin M.A., Bergmann J.H., Lazar Z., and Spector D.L.. 2013. Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus. 4:53–60. 10.4161/nucl.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi B., Jelcic M., and Niethammer P.. 2016. The cell nucleus serves as a mechanotransducer of tissue damage-induced inflammation. Cell. 165:1160–1170. 10.1016/j.cell.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn B.P., Bhole A.P., Saeidi N., Liles M., Dimarzio C.A., and Ruberti J.W.. 2010. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS One. 5:e12337 10.1371/journal.pone.0012337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennes P.-G. 1979. Scaling Concepts in Polymer Physics. Cornell University Press, Ithaca, NY. 324 pp. [Google Scholar]
- Gerace L., and Blobel G.. 1980. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 19:277–287. 10.1016/0092-8674(80)90409-2 [DOI] [PubMed] [Google Scholar]
- Goldman R.D., Gruenbaum Y., Moir R.D., Shumaker D.K., and Spann T.P.. 2002. Nuclear lamins: Building blocks of nuclear architecture. Genes Dev. 16:533–547. 10.1101/gad.960502 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I., Redwood A.B., and Gonzalo S.. 2009. Loss of A-type lamins and genomic instability. Cell Cycle. 8:3860–3865. 10.4161/cc.8.23.10092 [DOI] [PubMed] [Google Scholar]
- Gonzalo S. 2014. DNA damage and lamins. Adv. Exp. Med. Biol. 773:377–399. 10.1007/978-1-4899-8032-8_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Margalit A., Goldman R.D., Shumaker D.K., and Wilson K.L.. 2005. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 6:21–31. 10.1038/nrm1550 [DOI] [PubMed] [Google Scholar]
- Guilluy C., Osborne L.D., Van Landeghem L., Sharek L., Superfine R., Garcia-Mata R., and Burridge K.. 2014. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16:376–381. 10.1038/ncb2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A., Soria-Valles C., Osorio F.G., Gutierrez-Abril J., Garabaya C., Aguirre A., Fueyo A., Fernandez-Garcia M.S., Puente X.S., and Lopez-Otin C.. 2015. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 34:1875–1888. 10.15252/embj.201490594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Dupont S., and Piccolo S.. 2012. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13:591–600. 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- Hampoelz B., Azou-Gros Y., Fabre R., Markova O., Puech P.H., and Lecuit T.. 2011. Microtubule-induced nuclear envelope fluctuations control chromatin dynamics in Drosophila embryos. Development. 138:3377–3386. 10.1242/dev.065706 [DOI] [PubMed] [Google Scholar]
- Harada T., Swift J., Irianto J., Shin J.W., Spinler K.R., Athirasala A., Diegmiller R., Dingal P.C., Ivanovska I.L., and Discher D.E.. 2014. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204:669–682. 10.1083/jcb.201308029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., and McKeon F.. 1990. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 61:579–589. 10.1016/0092-8674(90)90470-Y [DOI] [PubMed] [Google Scholar]
- Ho C.Y., Jaalouk D.E., Vartiainen M.K., and Lammerding J.. 2013. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 497:507–511. 10.1038/nature12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen T.O., Aires L., Herzog F.A., Schwartlander R., Moeller J., and Vogel V.. 2015. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater. 14:1252–1261. 10.1038/nmat4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D.E. 2006. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 20:811–827. 10.1096/fj.05-5424rev [DOI] [PubMed] [Google Scholar]
- Irianto J., Pfeifer C.R., Bennett R.R., Xia Y., Ivanovska I.L., Liu A.J., Greenberg R.A., and Discher D.E.. 2016a Nuclear constriction segregates mobile nuclear proteins away from chromatin. Mol. Biol. Cell. 27:4011–4020. 10.1091/mbc.E16-06-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irianto J., Xia Y., Pfeifer C.R., Athirasala A., Ji J., C. Alvey M. Tewari R. Bennett S. Harding A. Liu R.A. Greenberg, and Discher D.E.. 2016b DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 10.1016/j.cub.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irianto J., Xia Y., Pfeifer C.R., Greenberg R.A., and Discher D.E.. 2016c As a nucleus enters a small pore, chromatin stretches and maintains integrity, even with DNA breaks. Biophys. J. 10.1016/j.bpj.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D., Schramm S., Schnolzer M., Heilmann C.J., de Koster C.G., Schutz W., Benavente R., and Alsheimer M.. 2012. A truncated lamin A in the Lmna −/− mouse line: Implications for the understanding of laminopathies. Nucleus. 3:463–474. 10.4161/nucl.21676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.J., Coffinier C., Choe Y., Beigneux A.P., Davies B.S., Yang S.H., Barnes R.H. II, Hong J., Sun T., Pleasure S.J., et al. . 2012. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. USA. 109:E423–E431. 10.1073/pnas.1111780109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatau S.B., Hale C.M., Stewart-Hutchinson P.J., Patel M.S., Stewart C.L., Searson P.C., Hodzic D., and Wirtz D.. 2009. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 106:19017–19022. 10.1073/pnas.0908686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., and Wirtz D.. 2015. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials. 48:161–172. 10.1016/j.biomaterials.2015.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Cho S., and Wirtz D.. 2014a Tight coupling between nucleus and cell migration through the perinuclear actin cap. J. Cell Sci. 127:2528–2541. 10.1242/jcs.144345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Li B., Si F., Phillip J.M., Wirtz D., and Sun S.X.. 2015. Volume regulation and shape bifurcation in the cell nucleus. J. Cell Sci. 128:3375–3385. 10.1242/jcs.166330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Pinto S.M., Getnet D., Nirujogi R.S., Manda S.S., Chaerkady R., Madugundu A.K., Kelkar D.S., Isserlin R., Jain S., et al. . 2014b A draft map of the human proteome. Nature. 509:575–581. 10.1038/nature13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochin V., Shimi T., Torvaldson E., Adam S.A., Goldman A., Pack C.G., Melo-Cardenas J., Imanishi S.Y., Goldman R.D., and Eriksson J.E.. 2014. Interphase phosphorylation of lamin A. J. Cell Sci. 127:2683–2696. 10.1242/jcs.141820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfali N., Wilkie G.S., Swanson S.K., Srsen V., de Las Heras J., Batrakou D.G., Malik P., Zuleger N., Kerr A.R., Florens L., and Schirmer E.C.. 2012. The nuclear envelope proteome differs notably between tissues. Nucleus. 3:552–564. 10.4161/nucl.22257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koser D.E., Thompson A.J., Foster S.K., Dwivedy A., Pillai E.K., Sheridan G.K., Svoboda H., Viana M., Costa L.D., Guck J., et al. . 2016. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 19:1592–1598. 10.1038/nn.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N., Voncken J.W., Konings G., van Weeghel M., van den Hoogenhof M.M., Gijbels M., van Erk A., Schoonderwoerd K., van den Bosch B., Dahlmans V., et al. . 2011. Post-natal myogenic and adipogenic developmental: Defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2:195–207. 10.4161/nucl.2.3.15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J., Hsiao J., Schulze P.C., Kozlov S., Stewart C.L., and Lee R.T.. 2005. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 170:781–791. 10.1083/jcb.200502148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H.Q., Ghatak S., Yeung C.Y., Tellkamp F., Gunschmann C., Dieterich C., Yeroslaviz A., Habermann B., Pombo A., Niessen C.M., and Wickstrom S.A.. 2016. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18:864–875. 10.1038/ncb3387 [DOI] [PubMed] [Google Scholar]
- Lehner C.F., Stick R., Eppenberger H.M., and Nigg E.A.. 1987. Differential expression of nuclear lamin proteins during chicken development. J. Cell Biol. 105:577–587. 10.1083/jcb.105.1.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chu J.S., Kurpinski K., Li X., Bautista D.M., Yang L., Sung K.L., and Li S.. 2011. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys. J. 100:1902–1909. 10.1016/j.bpj.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang J., Chan K.M., Tjia W.M., Deng W., Guan X., Huang J.D., Li K.M., Chau P.Y., Chen D.J., et al. . 2005. Genomic instability in laminopathy-based premature aging. Nat. Med. 11:780–785. 10.1038/nm1266 [DOI] [PubMed] [Google Scholar]
- Liu Y., Rusinol A., Sinensky M., Wang Y., and Zou Y.. 2006. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J. Cell Sci. 119:4644–4649. 10.1242/jcs.03263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D.J., Trembath R.C., and Shackleton S.. 2002. A novel interaction between lamin A and SREBP1: Implications for partial lipodystrophy and other laminopathies. Hum. Mol. Genet. 11:769–777. 10.1093/hmg/11.7.769 [DOI] [PubMed] [Google Scholar]
- Lovett D.B., Shekhar N., Nickerson J.A., Roux K.J., and Lele T.P.. 2013. Modulation of nuclear shape by substrate rigidity. Cell. Mol. Bioeng. 6:230–238. 10.1007/s12195-013-0270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana S., Iyer K.V., Jain N., Nagarajan M., Wang Y., and Shivashankar G.V.. 2016. Chromosome intermingling-the physical basis of chromosome organization in differentiated cells. Nucleic Acids Res. 44:5148–5160. 10.1093/nar/gkw131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S., Idema T., Swift J., Krieger C., Liu A., and Discher D.E.. 2013. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol. 23:2434–2439. 10.1016/j.cub.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit A., Segura-Totten M., Gruenbaum Y., and Wilson K.L.. 2005. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc. Natl. Acad. Sci. USA. 102:3290–3295. 10.1073/pnas.0408364102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marganski W.A., Dembo M., and Wang Y.L.. 2003. Measurements of cell-generated deformations on flexible substrata using correlation-based optical flow. Methods Enzymol. 361:197–211. 10.1016/S0076-6879(03)61012-8 [DOI] [PubMed] [Google Scholar]
- Meshorer E., Yellajoshula D., George E., Scambler P.J., Brown D.T., and Misteli T.. 2006. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 10:105–116. 10.1016/j.devcel.2005.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O., Lopes-Paciencia S., Huot G., Lessard F., and Ferbeyre G.. 2016. Permanent farnesylation of lamin A mutants linked to progeria impairs its phosphorylation at serine 22 during interphase. Aging. 8:366–381. 10.18632/aging.100903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A.J., Pierce J., de Caestecker C., Libes J., Neblett D., de Caestecker M., Perantoni A.O., Tanigawa S., Anderson J.R., Dome J.S., et al. . 2014. Aberrant activation, nuclear localization, and phosphorylation of Yes-associated protein-1 in the embryonic kidney and Wilms tumor. Pediatr. Blood Cancer. 61:198–205. 10.1002/pbc.24788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula N., Favalli V., Tarantino P., Grasso M., Pilotto A., Bellazzi R., Serio A., Gambarin F.I., Charron P., Meder B., et al. . 2012. Quantitative expression of the mutated lamin A/C gene in patients with cardiolaminopathy. J. Am. Coll. Cardiol. 60:1916–1920. 10.1016/j.jacc.2012.05.059 [DOI] [PubMed] [Google Scholar]
- Neelam S., Chancellor T.J., Li Y., Nickerson J.A., Roux K.J., Dickinson R.B., and Lele T.P.. 2015. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc. Natl. Acad. Sci. USA. 112:5720–5725. 10.1073/pnas.1502111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F.G., Navarro C.L., Cadinanos J., Lopez-Mejia I.C., Quiros P.M., Bartoli C., Rivera J., Tazi J., Guzman G., Varela I., et al. . 2011. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 3:106ra107 10.1126/scitranslmed.3002847 [DOI] [PubMed] [Google Scholar]
- Paddy M.R., Belmont A.S., Saumweber H., Agard D.A., and Sedat J.W.. 1990. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell. 62:89–106. 10.1016/0092-8674(90)90243-8 [DOI] [PubMed] [Google Scholar]
- Pajerowski J.D., Dahl K.N., Zhong F.L., Sammak P.J., and Discher D.E.. 2007. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA. 104:15619–15624. 10.1073/pnas.0702576104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh Y.C., Shevtsov S.P., Chowdhury F., Wu D.C., Na S., Dundr M., and Wang N.. 2012. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat. Commun. 3:866 10.1038/ncomms1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M., Gentili M., de Belly H., Thiam H.R., Vargas P., Jimenez A.J., Lautenschlaeger F., Voituriez R., Lennon-Dumenil A.M., Manel N., and Piel M.. 2016. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 352:359–362. 10.1126/science.aad7611 [DOI] [PubMed] [Google Scholar]
- Rajan S., Williams S.S., Jagatheesan G., Ahmed R.P., Fuller-Bicer G., Schwartz A., Aronow B.J., and Wieczorek D.F.. 2006. Microarray analysis of gene expression during early stages of mild and severe cardiac hypertrophy. Physiol. Genomics. 27:309–317. 10.1152/physiolgenomics.00072.2006 [DOI] [PubMed] [Google Scholar]
- Rober R.A., Weber K., and Osborn M.. 1989. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: A developmental study. Development. 105:365–378. [DOI] [PubMed] [Google Scholar]
- Rodríguez J., Calvo F., Gonzalez J.M., Casar B., Andres V., and Crespo P.. 2010. ERK1/2 MAP kinases promote cell cycle entry by rapid, kinase-independent disruption of retinoblastoma-lamin A complexes. J. Cell Biol. 191:967–979. (published erratum appears in J. Cell Biol. 2011. 192:201) 10.1083/jcb.201004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin-Thaler B.J., Cherniavskaya O., Sakai R., Tanaka S., and Sheetz M.P.. 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 127:1015–1026. 10.1016/j.cell.2006.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer E.C., Florens L., Guan T., Yates J.R. III, and Gerace L.. 2003. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 301:1380–1382. 10.1126/science.1088176 [DOI] [PubMed] [Google Scholar]
- Shao X., Li Q., Mogilner A., Bershadsky A.D., and Shivashankar G.V.. 2015. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc. Natl. Acad. Sci. USA. 112:E2595–E2601. 10.1073/pnas.1504837112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders M.D., and Raines R.T.. 2009. Collagen structure and stability. Annu. Rev. Biochem. 78:929–958. 10.1146/annurev.biochem.77.032207.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I., Wang A.S., Thanisch K., Schmidt C.S., Krebs S., Zwerger M., Cohen T.V., Devys D., Foisner R., Peichl L., et al. . 2013. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 152:584–598. 10.1016/j.cell.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Sullivan T., Escalante-Alcalde D., Bhatt H., Anver M., Bhat N., Nagashima K., Stewart C.L., and Burke B.. 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147:913–920. 10.1083/jcb.147.5.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yong K.M., Villa-Diaz L.G., Zhang X., Chen W., Philson R., Weng S., Xu H., Krebsbach P.H., and Fu J.. 2014. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 13:599–604. 10.1038/nmat3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., et al. . 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 341:1240104 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajik A., Zhang Y., Wei F., Sun J., Jia Q., Zhou W., Singh R., Khanna N., Belmont A.S., and Wang N.. 2016. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 15:1287–1296. 10.1038/nmat4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiello C., Kamps M.A., van den Wijngaard A., Verstraeten V.L., Baaijens F.P., Broers J.L., and Bouten C.C.. 2013. Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus. 4:61–73. 10.4161/nucl.23388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.R., Slavov D., Gajewski A., Vlcek S., Ku L., Fain P.R., Carniel E., Di Lenarda A., Sinagra G., Boucek M.M., et al. . 2005. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum. Mutat. 26:566–574. 10.1002/humu.20250 [DOI] [PubMed] [Google Scholar]
- Tunnah D., Sewry C.A., Vaux D., Schirmer E.C., and Morris G.E.. 2005. The apparent absence of lamin B1 and emerin in many tissue nuclei is due to epitope masking. J. Mol. Histol. 36:337–344. 10.1007/s10735-005-9004-7 [DOI] [PubMed] [Google Scholar]
- van der Rest M., and Garrone R.. 1991. Collagen family of proteins. FASEB J. 5:2814–2823. [PubMed] [Google Scholar]
- Vargas J.D., Hatch E.M., Anderson D.J., and Hetzer M.W.. 2012. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 3:88–100. 10.4161/nucl.18954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen M.K., Guettler S., Larijani B., and Treisman R.. 2007. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 316:1749–1752. 10.1126/science.1141084 [DOI] [PubMed] [Google Scholar]
- Versaevel M., Grevesse T., and Gabriele S.. 2012. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 3:671 10.1038/ncomms1668 [DOI] [PubMed] [Google Scholar]
- Vizcaino J.A., Csordas A., del-Toro N., Dianes J.A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., et al. . 2016. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44(D1):D447–D456. 10.1093/nar/gkv1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K.L., and Foisner R.. 2010. Lamin-binding proteins. Cold Spring Harb. Perspect. Biol. 2:a000554 10.1101/cshperspect.a000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H.J. 2012. Nuclear lamins and laminopathies. J. Pathol. 226:316–325. 10.1002/path.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., and Kaufman L.J.. 2009. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys. J. 96:1566–1585. 10.1016/j.bpj.2008.10.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tibbitt M.W., Basta L., and Anseth K.S.. 2014. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13:645–652. 10.1038/nmat3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bethmann C., Worth N.F., Davies J.D., Wasner C., Feuer A., Ragnauth C.D., Yi Q., Mellad J.A., Warren D.T., et al. . 2007. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16:2816–2833. 10.1093/hmg/ddm238 [DOI] [PubMed] [Google Scholar]