Abstract
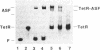
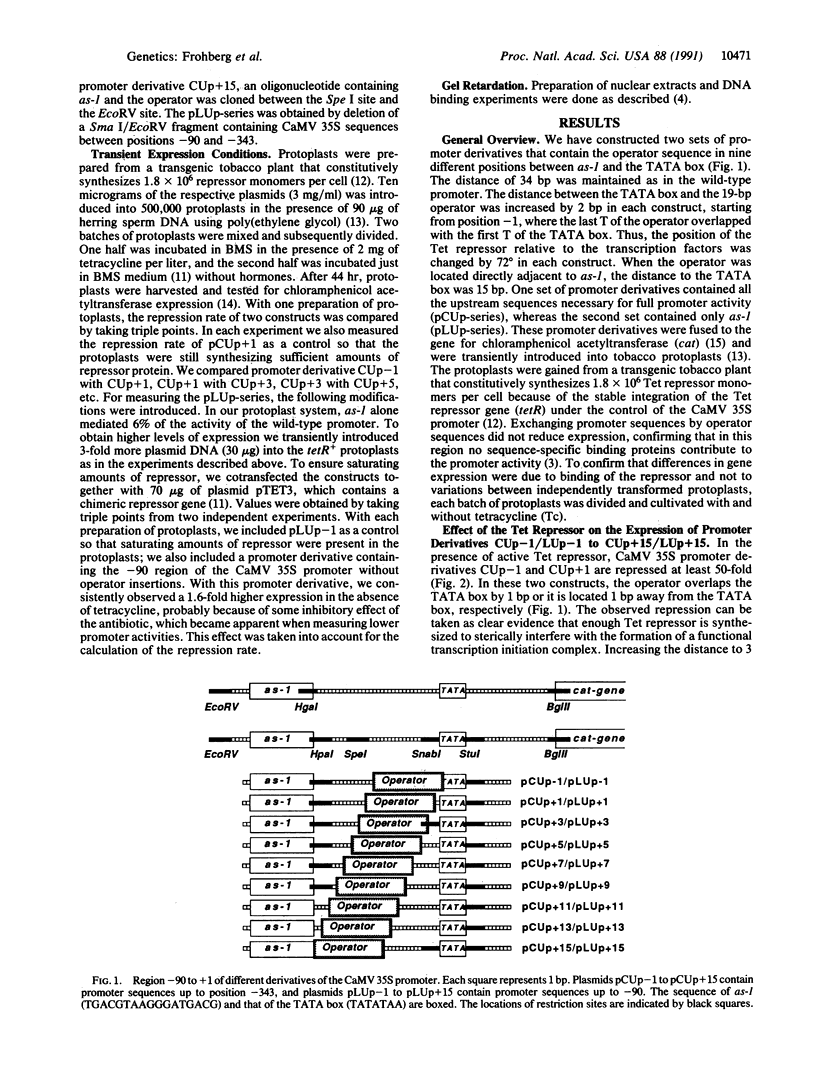
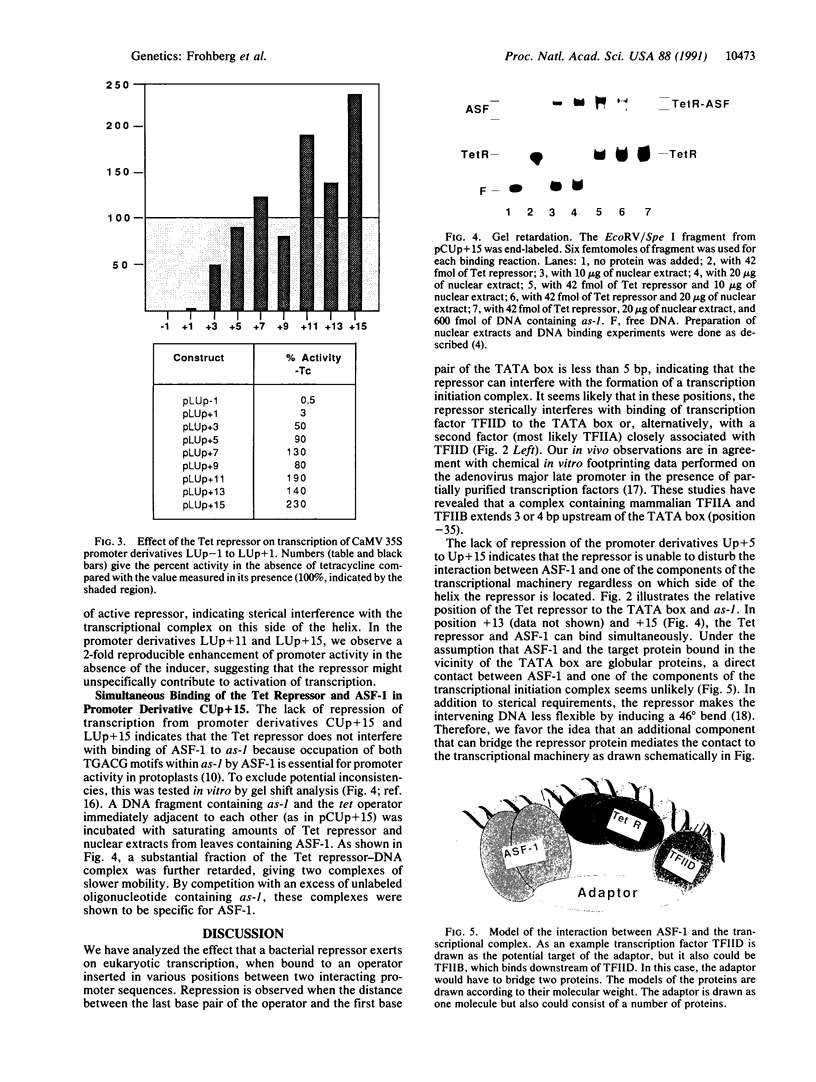
Transcription initiation from a eukaryotic polymerase II promoter requires a functional interaction of regulatory transcriptional activators with at least one of the basal transcription factors binding in the vicinity of the TATA box. To characterize this type of interaction in vivo, we have inserted the bacterial Tet repressor-operator complex in nine different positions between an enhancer element (as-1) and the TATA box of the cauliflower mosaic virus (CaMV) 35S RNA promoter. A direct contact between the transcriptional activator ASF-1, which binds to as-1, and the transcriptional machinery should be affected by a repressor protein bound between them, as the spacing of only 34 base pairs (bp) between as-1 and the TATA box is too short to allow looping of the DNA around the repressor. In each construct, the distance of 34 bp was kept constant, while the position of the 19-bp tet operator relative to the TATA box differed by 2 bp. Thus, the position of the Tet repressor relative to the plant transcription factors was consecutively changed by 72 degrees, which allowed us to investigate whether repression depended on the stereospecific alignment of the repressor with the transcription factors. Binding of the Tet repressor to the operator blocked transcription only when the operator was inserted less tha 5 bp from the TATA box. In all other promoter derivatives, no inhibitory effect of the repressor was observed, which suggests that ASF-1 does not directly interact with the general transcription machinery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Bouchez D., Tokuhisa J. G., Llewellyn D. J., Dennis E. S., Ellis J. G. The ocs-element is a component of the promoters of several T-DNA and plant viral genes. EMBO J. 1989 Dec 20;8(13):4197–4204. doi: 10.1002/j.1460-2075.1989.tb08605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A bacterial repressor protein or a yeast transcriptional terminator can block upstream activation of a yeast gene. Nature. 1984 Dec 13;312(5995):612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- Carey M., Lin Y. S., Green M. R., Ptashne M. A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature. 1990 May 24;345(6273):361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- Damm B., Schmidt R., Willmitzer L. Efficient transformation of Arabidopsis thaliana using direct gene transfer to protoplasts. Mol Gen Genet. 1989 May;217(1):6–12. doi: 10.1007/BF00330935. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Kaiser A., Wendenburg R. Regulation of a modified CaMV 35S promoter by the Tn10-encoded Tet repressor in transgenic tobacco. Mol Gen Genet. 1991 Jun;227(2):229–237. doi: 10.1007/BF00259675. [DOI] [PubMed] [Google Scholar]
- Gatz C., Quail P. H. Tn10-encoded tet repressor can regulate an operator-containing plant promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1394–1397. doi: 10.1073/pnas.85.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer C., Hillen W. Tet repressor-tet operator contacts probed by operator DNA-modification interference studies. J Mol Biol. 1988 Aug 5;202(3):407–415. doi: 10.1016/0022-2836(88)90274-4. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Davidson N. The inducible lac operator-repressor system is functional in mammalian cells. Cell. 1987 Feb 27;48(4):555–566. doi: 10.1016/0092-8674(87)90234-0. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Lam E., Benfey P. N., Gilmartin P. M., Fang R. X., Chua N. H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. GT-1 binding site confers light responsive expression in transgenic tobacco. Science. 1990 Apr 27;248(4954):471–474. doi: 10.1126/science.2330508. [DOI] [PubMed] [Google Scholar]
- Lederer H., Tovar K., Baer G., May R. P., Hillen W., Heumann H. The quaternary structure of Tet repressors bound to the Tn10-encoded tet gene control region determined by neutron solution scattering. EMBO J. 1989 Apr;8(4):1257–1263. doi: 10.1002/j.1460-2075.1989.tb03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Carey M., Ptashne M., Green M. R. How different eukaryotic transcriptional activators can cooperate promiscuously. Nature. 1990 May 24;345(6273):359–361. doi: 10.1038/345359a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Maldonado E., Ha I., Cortes P., Weis L., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II: role of transcription factors IIA, IID, and IIB during formation of a transcription-competent complex. Mol Cell Biol. 1990 Dec;10(12):6335–6347. doi: 10.1128/mcb.10.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Jacobs J. D., Howell S. H. Functional regions of the cauliflower mosaic virus 35S RNA promoter determined by use of the firefly luciferase gene as a reporter of promoter activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4870–4874. doi: 10.1073/pnas.84.14.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat S., Willmitzer L., Sánchez-Serrano J. J. Nuclear proteins binding to a cauliflower mosaic virus 35S truncated promoter. Mol Gen Genet. 1989 Jun;217(2-3):209–214. doi: 10.1007/BF02464883. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Mileham K. A., Cooke S. E., Woolston S. J., George H. K., Charles A. D., Brammar W. J. The Escherichia coli LexA repressor-operator system works in mammalian cells. EMBO J. 1988 Dec 1;7(12):3975–3982. doi: 10.1002/j.1460-2075.1988.tb03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar K., Hillen W. Tet repressor binding induced curvature of tet operator DNA. Nucleic Acids Res. 1989 Aug 25;17(16):6515–6522. doi: 10.1093/nar/17.16.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]