Abstract
Ancient oxygenic photosynthetic prokaryotes produced oxygen as a waste product, but existed for a long time under an oxygen-free (anoxic) atmosphere, before an oxic atmosphere emerged. The change in oxygen levels in the atmosphere influenced the chemistry and structure of many enzymes that contained prosthetic groups that were inactivated by oxygen. In the genome of Acaryochloris marina, multiple gene copies exist for proteins that are normally encoded by a single gene copy in other cyanobacteria. Using high throughput RNA sequencing to profile transcriptome responses from cells grown under microoxic and hyperoxic conditions, we detected 8446 transcripts out of the 8462 annotated genes in the Cyanobase database. Two-thirds of the 50 most abundant transcripts are key proteins in photosynthesis. Microoxic conditions negatively affected the levels of expression of genes encoding photosynthetic complexes, with the exception of some subunits. In addition to the known regulation of the multiple copies of psbA, we detected a similar transcriptional pattern for psbJ and psbU, which might play a key role in the altered components of photosystem II. Furthermore, regulation of genes encoding proteins important for reactive oxygen species-scavenging is discussed at genome level, including, for the first time, specific small RNAs having possible regulatory roles under varying oxygen levels.
Keywords: cyanobacteria, oxygen levels, transcriptome response, chlorophyll biosynthesis, reactive oxygen species
Photosynthesis uses solar energy, and transforms it into chemical energy, which is stored within the organic molecules of the organism. In essence, it provides the energy for all life on our planet. During oxygenic photosynthesis, sunlight is funneled toward a special pair of chlorophyll molecules, which produce a charge separation that results in the extraction of electrons from water. This initiates a chain of redox reactions that power the fixation of inorganic carbon into 3-phosphoglycerate, with oxygen (O2) generated as a side product of this reaction. In fact, before photosynthesis occurred in ancient cyanobacteria around 3.5–2.4 billion yr ago, the atmosphere was largely anaerobic (Blankenship and Hartman 1998). Over billions of years, oxygenic photosynthetic organisms changed the Earth’s atmosphere, steadily increasing its O2 levels to over 21% (v/v). This permitted the rise of multicellular organisms, dependent upon aerobic respiration (Dismukes et al. 2001; Blankenship 2008; Hedges et al. 2004).
Aerobic respiration is highly efficient in recovering the energy contained within the chemical bonds of organic molecules through oxidative phosphorylation. However, organisms living in aerobic environments also run the risk of being damaged by oxidants and reactive oxygen species (ROS). ROS include a number of reactive molecules derived from O2. Clearly, O2 in its ground state is harmless, as it has two unpaired electrons with parallel spin, making it paramagnetic. In this form, it is unlikely to participate in reactions with organic molecules, unless it is enzymatically or chemically activated by other reactions (Apel and Hirt 2004; Sharma et al. 2012). However, oxygen-derived ROS comprise superoxide, hydrogen peroxide, and hydroxyl radicals, which are a threat to the cell. Organisms mitigate ROS deleterious effects in various ways: by scavenging pathways (Ślesak et al. 2016; Pospíšil 2012), by changing the regulation of affected genes (Tamagnini et al. 2007), by separating the location of their product to oxygen-free compartments like heterocysts (Murry et al. 1984), or by evolving an alternative pathway resistant to oxidation (Busch and Montgomery 2015). Many metabolic pathways functioning today still contain enzymes sensitive to O2 levels, as illustrated by the coexisting oxygen-dependent, or oxygen-independent, reactions within the tetrapyrrole biosynthetic pathway (Busch and Montgomery 2015; Raymond and Blankenship 2004), and the activation of a counterpart D1 subunit of photosystem II (PSII) in response to changed O2 levels (Summerfield et al. 2008).
Acaryochloris marina (hereafter Acaryochloris) is a unicellular cyanobacterium, using chlorophyll d (Chl d), instead of chlorophyll a (Chl a), as the major pigment in its photosystems (Chen et al. 2002a, 2005b). Similar to all cyanobacteria, the thylakoids of Acaryochloris need to mitigate not only the oxidative stress generated by oxygenic photosynthetic activities, but also the oxidative stress produced because of aerobic respiration. Therefore, it is not surprising that under illumination, especially high-intensity light, singlet oxygen is mainly produced because of the interaction of unquenched Chl triplets with O2 generated within PSII, and the water-splitting complex. In photosystem I (PSI), the univalent reduction of O2 generates mainly superoxide anion radicals (reviewed in Latifi et al. 2009; Rutherford et al. 2012). To reduce the effects of ROS, a constant diffusion of O2 through the cell and photosynthetic membranes under illumination is crucial. This diffusion of O2, along with antioxidant enzymes that prevent accumulation of ROS, and the existence of remediation metabolites, such as ascorbic acid, glutathione, tocopherols, carotenoids, and flavonoids, prevent the interaction of O2 with electrons, other than those in the normal electron transfer pathways to O2, avoiding any possible oxidative stress (Latifi et al. 2009).
Because of the iconic character of Acaryochloris, in which the function of Chl a has been largely replaced by red-shifted Chl d, most of the research on this organism has been directed toward understanding Chl d biosynthesis (Schliep et al. 2010; Loughlin et al. 2014; Yoneda et al. 2016). In particular, this has focused on its role in photosynthesis (Chen et al. 2002b, 2005b; Hu et al. 1998; Tomo et al. 2007), and in far-red light acclimation (Duxbury et al. 2009). These studies have revealed direct oxidation of Chl a to Chl d, with participation of O2 (Schliep et al. 2010; Loughlin et al. 2013). Although the structural difference between Chl a and Chl d has a significant effect on its spectral characteristics, it does not affect the binding of Chl d with typical Chl a binding-peptides, as shown in in vitro reconstitution experiments (Chen et al. 2005a; Hoober et al. 2007; Chen and Cai 2007). In fact, so far, none of the studies carried out on the photosystems of Acaryochloris have revealed any significant difference to Chl a-containing photosystems, besides their distinct spectral characteristics, related to their pigment substitutions (Chen et al. 2005b).
In this study, we grew Acaryochloris under different O2 levels to test the effects of O2 on photo-pigment biosynthesis, photosynthetic reactions, and on their relationship with other essential metabolic reactions (including DNA and protein metabolism). Using high-throughput RNA sequencing (RNAseq), we obtained genome-level information on all expressed transcripts, under microoxic, normal air (control), and hyperoxic conditions. We detected genes coding for key proteins in photosynthesis and synthesis of chlorophyll, which were preferentially expressed under microoxic conditions. As expected, proteins involved in oxidative stress remediation also were induced under hyperoxic conditions. We also generated the first inventory of previously unknown small RNAs (sRNA), including untranslated regions (UTRs) and intergenic noncoding RNAs (ncRNAs), many of them differentially expressed upon O2 perturbation. The sRNAs that showed a strong induction were further investigated to predict their potential targets and their involvement in adaptation to these stress conditions. In this first systems-level study to include sRNAs performed on Acaryochloris, we uncover new insights on the particularities of “oxygenic” photosynthesis and its coevolutionary “anaerobic” metabolism.
Materials and Methods
Culture conditions
A. marina MBIC11017 was routinely kept in a culture room at 27° under 15–30 µmol photons m−2 s−1 of cool white light. Sterilized K+ES (artificial seawater), buffered with 25 mM TES at pH 8.0 was used as the culture medium for all three treatment groups. To make sure photosynthesis was not limited by CO2, NaHCO3 was dissolved in a small volume of autoclaved medium, and injected into the enclosed culture flasks every 2 d (yielding an initial concentration of 0.375 mM). The initial cell density of all culture groups was adjusted to an optical density at 750 nm of 0.2. The cultures were shaken on an orbital flat-bed shaker at ∼90 rpm.
Cultures under normal O2 levels were inoculated in 1-liter Erlenmeyer glass flasks containing 500 ml of medium, capped with a cotton stopper that permitted gas exchange. Thus, the O2 concentration of the gas phase inside the bottle was similar to atmospheric levels ∼21% (v/v).
Microoxic conditions were achieved by using a 500-ml two-necked round-bottom flask sealed tightly by a rubber stopper. Cultures were vacuumed, and refilled with pure nitrogen gas (99.95% purity) to ensure normal atmospheric pressure. We repeated this process several times, yielding a final O2 concentration of <0.2% inside the sealed culture flask. To maintain a microoxic condition, a positive pressure was created by bubbling nitrogen through the culture.
A similar set-up was used for hyperoxic conditions, with the exception that pure O2 gas was used to refill the flask after vacuuming. Because the cells generate O2 under illumination, ongoing input of O2 gas to maintain the high-oxygen concentration was not required. However, to avoid pressure build-up, the flask was revacuumed and refilled with O2 gas every 48 hr, as described above. The O2 concentration inside the flask remained within the range of 65–75% (v/v) during the experiment.
Total RNA extraction
Acaryochloris cultures were harvested after 7 d from all three different treatments. The harvested cell pellets were mixed with TRIzol (TRIzol Reagent, Life Technologies, Australia), and frozen immediately using liquid nitrogen. They were stored at −80° for at least 60 min. The frozen samples were thawed in a water bath at 37°, and spun down at 16,000 × g for 5 min to eliminate cellular debris. This supernatant was mixed at a volume ratio of 4:1, with chloroform, and spun down at 16,000 × g for 10 min at 4°. The upper layer, containing RNA and DNA, was carefully transferred to a new tube, without disturbing the white middle layer of solid components. The RNA was precipitated by addition of an equal volume of isopropanol, and incubated at −20° for at least 45 min. RNA pellets were washed with 70% (v/v) ethanol, and the DNA removed using the Baseline-ZERO DNase kit (Epicentre, WI), following the manufacturer’s instructions. Prior to RNA quality assessment, the absence of DNA was confirmed by polymerase chain reaction (data not shown).
The quality of RNA was assessed on a 2100 Bioanalyzer (Agilent Technologies, CA), using a RNA 6000 Nano RNA Kit (Agilent Technologies), to obtain an RNA integrity number of >8.0. Transcripts corresponding to rRNAs (5S, 16S, and 23S) were reduced from the samples with a Ribo-Zero Kit (Epicentre), following the manufacturer’s instructions. Further processing, including quality assessment, was undertaken prior to RNAseq analysis by Beijing Genomics Institution, China.
RNAseq data analysis
FASTQ files of the resulting sequences were processed using open source software. FASTQ files were aligned against the A. marina MBIC11017 reference genome on NCBI (http://www.ncbi.nlm.nih.gov/), using the “Tophat for Illumina” tool available in the Galaxy suite (Afgan et al. 2016). The BAM files obtained were superposed on the genome. and visualized in Artemis (Rutherford et al. 2000) to facilitate annotation of the predicted transcriptional units (TUs). The Java-based Rockhopper system (McClure et al. 2013) was used to process mapped sequence reads for differential analysis. The Rockhopper report, containing a summary of the number of reads aligned. is available in Supplemental Material, File S1.
sRNAs target prediction
All sRNA sequences (including ncRNAs and UTRs) were obtained from the transcriptional coordinates generated by the Rockhopper software, after mapping the obtained reads to unannotated regions of the Acaryochloris genome. The target protein-coding genes were predicted using the IntaRNA algorithm (Busch et al. 2008), with a window of 275 nucleotides around the respective start codons (200 upstream and 75 downstream).
Functional enrichment analysis
A standard functional enrichment analysis of differentially expressed genes (EADEG) was applied using hypergeometric tests, after Hernández-Prieto et al. (2012). Derived p-values were adjusted for multiple testing, while false discovery rates (FDR) were calculated using the Benjamini-Hochberg method. We used the gene annotation given in Cyanobase (Fujisawa et al. 2014) (http://genome.microbedb.jp/cyanobase/AM1), while gene associations with cellular functions are from the KEGG database (Kanehisa et al. 2016), and Gene Ontology (GO) terms in Uniprot (UniProt Consortium 2015) (Table S1). Our lists included genes associated with 116 KEGG pathways, and with 1196 GO terms. Only lists having a minimum of five genes annotated from the Acaryochloris genome (89 of 116 KEGG pathways, and 320 of 1196 GO terms) were investigated. To determine the functional composition of differentially expressed genes, enrichment was separately assessed for upregulated and downregulated genes.
Data availability
The Acaryochloris strain used in this study is available as an axenic culture through the NBRC culture collection (NBRC 102967). Raw gene expression data, and processed information, is available at GEO with the accession number GSE89387. File S1 contains detailed information of all supplemental files.
Results
Culture growth
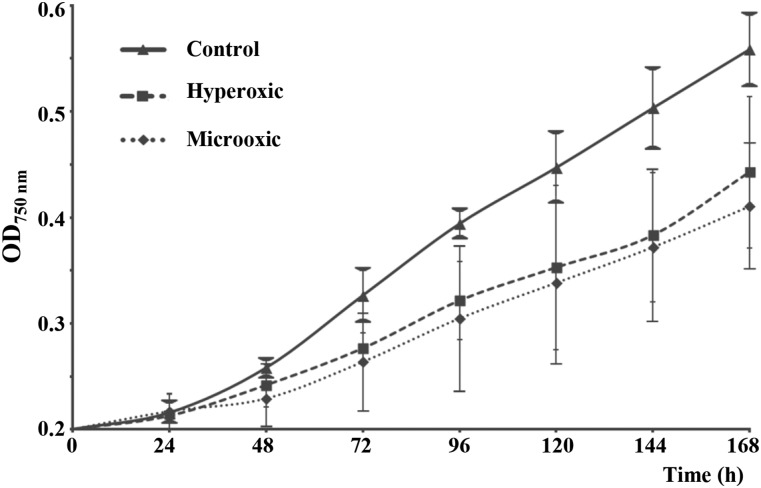
Monitoring of the O2 concentrations of the gas phase inside the culture bottles was performed daily with a Clark-type electrode. The concentration of O2 in the hyperoxic culture was maintained within the range of 65–75% (v/v), while in the microoxic culture, it was <0.2% (v/v). Thus, O2 concentration in the medium was equal to 350 and 1% that of air saturation at 25° for hyperoxic and microoxic cultures, respectively. These deviations from atmospheric conditions negatively affected Acaryochloris cells, as reflected in their low apparent growth rates (Figure 1). The control culture doubled its OD750nm every ∼57 hr during the exponential growth phase, while treated cultures had doubling times of > 72 hr (Figure 1). Thus, both treatment conditions had detrimental effects on apparent cell growth, given their ∼80% decrease in growth rate (Figure 1).
Figure 1.
Optical density curves of A. marina cultures under the three different oxygen concentrations (control, microoxic, and hyperoxic). Apparent growth was monitored daily by measuring the optical density of the cultures at 750 nm (OD750nm). Data were averaged from quadruplicate cultures; variability in these results is represented by error bars. Apparent growth was negatively affected under both treatment conditions.
Full transcriptome profiling of Acaryochloris
RNA was extracted from cells collected after 7 d under their respective treatments, at an OD750nm between 0.4 and 0.6 for all cultures. The gene expression profiling at genomic level was achieved by paired-end high-throughput sequencing of RNA, isolated from Acaryochloris exposed to different O2 concentrations. The experiment was duplicated for both test conditions, and triplicated for control conditions. To assess differential gene expression, we used the algorithms available in Rockhopper, because this software has been optimized for the analysis of RNAseq data obtained from prokaryotes (McClure et al. 2013). Transcript units (TUs) for 8446 of the 8462 TUs annotated for Acaryochloris (NCBI BioProject: PRJNA12997) were detected in both control and treated samples. Of the 16 undetected transcripts (Table S2), only one (AM1_A0163) has an annotated function in Cyanobase (as at June 2016), five were localized in the main chromosome, and the rest in the plasmids (two in pREB1, two in pREB2, three in pREB3, two in pREB4, and two in pREB5). RNA extracted from Acaryochloris cells grown under control culture conditions was used as a reference to evaluate transcript changes related to altered O2 levels. Under control conditions, the 50 most abundant transcripts correspond to 21 sRNAs (16 newly described in this study), and 29 to protein-coding genes, of which 12 encode subunits of PSI or PSII. Eight of these genes encoding photosystem subunits were among the 50 top transcripts in all three samples: five of them (psaA, psaB, psaC, psaJ, and psaM) encoding PSI subunits, two (psbA and psbK) encoding PSII subunits, and, intriguingly, a high-light induced protein (HLIP) involved in the incorporation of chlorophyll in newly assembled photosystems (Hernandez-Prieto et al. 2011) (Table 1).
Table 1. List of the top 50 most expressed open reading frames in Acaryochloris marina under control conditions.
| Gene | Product | Coordinates | Expression Control | Expression Microoxic | Expression Hyperoxic |
|---|---|---|---|---|---|
| AM1_6414a | 10Sa RNA (tmRNA), ssrA | 1113343, 1113638 | 8,862,827 | 1,108,900 | 6,593,223 |
| AM1_NC230a | Intergenic sRNA | 3815042, 3815332 | 1,522,785 | 95,333 | 2,440,135 |
| AM1_6418a | RNA subunit of RNase P, rnpB | 1701315, 1701673 | 235,615 | 105,408 | 245,204 |
| AM1_NC208a | Intergenic sRNA | 3587742, 3587961 | 118,457 | 96,521 | 21,716 |
| AM1_0390 | Hypothetical protein | 361900, 361736 | 96,439 | 7619 | 297,265 |
| AM1_4558 | Hypothetical protein | 4591968, 4591819 | 89,452 | 20,593 | 92,833 |
| AM1_NC288a | 3′UTR of AM1_4558 | 4591770, 4591805 | 65,792 | 8885 | 58,129 |
| AM1_NC36a | 5′UTR of AM1_0390 | 361932, 361942 | 64,575 | 6428 | 372,487 |
| AM1_5793 | Hypothetical protein | 5875753, 5875893 | 63,417 | 22,352 | 169,796 |
| AM1_1154 | DNA-binding protein HU | 1122737, 1123012 | 51,219 | 23,994 | 42,409 |
| AM1_1660 | PSI subunit, PsaC | 1638289, 1638044 | 47,437 | 16,284 | 29,939 |
| AM1_6426 | PSI subunit, PsaM | 3783989, 3783894 | 45,473 | 11,569 | 21,277 |
| AM1_6419a | 6Sa RNA, ssaA | 3661451, 3661282 | 44,034 | 17,418 | 19,246 |
| AM1_1530 | Hypothetical protein | 1513788, 1513489 | 43,502 | 25,195 | 53,880 |
| AM1_1942 | Hypothetical protein | 1936231, 1936359 | 40,184 | 17,863 | 30,491 |
| AM1_NC106a | 5′UTR of AM1_1530 | 1513809, 1513828 | 37,734 | 8185 | 61,960 |
| AM1_1140 | Hypothetical protein | 1111163, 1111005 | 35,060 | 16,626 | 27,461 |
| AM1_NC37a | Intergenic sRNA | 364322, 365501 | 29,258 | 8326 | 48,497 |
| AM1_3851 | PSII subunit, PsbK | 3905041, 3905178 | 29,102 | 10,430 | 19,366 |
| AM1_NC319a | Intergenic sRNA | 5411225, 5411416 | 25,090 | 4405 | 28,342 |
| AM1_1011 | PSII protein, PsbZ | 979177, 978989 | 24,093 | 14,982 | 14,471 |
| AM1_NC86a | 5′UTR of AM1_1114 | 1092698, 1092793 | 23,484 | 13,326 | 10,160 |
| AM1_3193 | High light inducible protein | 3230482, 3230634 | 23,357 | 10,335 | 195,987 |
| AM1_0039 | Hypothetical protein | 41120, 40992 | 23,217 | 9864 | 14,083 |
| AM1_1507 | Hypothetical protein | 1495267, 1495127 | 22,795 | 8451 | 18,798 |
| AM1_NC294a | Intergenic sRNA | 4826591, 4826827 | 22,636 | 27,816 | 31,549 |
| AM1_2457 | PSI core protein, PsaA | 2472897, 2475158 | 21,958 | 8776 | 27,807 |
| AM1_NC24a | 3′UTR of AM1_0345 | 317866, 318113 | 21,566 | 4680 | 23,373 |
| AM1_2458 | PSI core protein, PsaB | 2475181, 2477391 | 20,685 | 5754 | 15,938 |
| AM1_NC233a | 5′UTR of AM1_3627 | 3686575, 3686602 | 19,985 | 2682 | 36,489 |
| AM1_NC70a | Intergenic sRNA | 1198215, 1198479 | 19,545 | 4002 | 2138 |
| AM1_3627 | Hypothetical protein | 3686477, 3686334 | 19,092 | 7894 | 16,758 |
| AM1_2630 | Cyt b559 alpha subunit, PsbE | 2668907, 2668656 | 18,427 | 7885 | 11,051 |
| AM1_NC245a | 3′UTR of AM1_3885 | 3937055, 3937138 | 18,324 | 4911 | 8432 |
| AM1_NC154a | 5′UTR of AM1_2252 | 2259432, 2259766 | 17,851 | 34,718 | 5494 |
| AM1_2889 | PSII D1 protein, PsbA | 2929355, 2928273 | 16,679 | 7503 | 41,497 |
| AM1_NC120a | 5′UTR of AM1_1660 | 1638298, 1638415 | 16,076 | 7311 | 6156 |
| AM1_1114 | Conserved hypothetical protein | 1092691, 1092497 | 15,015 | 5172 | 13,010 |
| AM1_NC126a | Intergenic sRNA | 1742101, 1742419 | 14,826 | 27,364 | 447 |
| AM1_3119 | Conserved hypothetical protein | 3148026, 3147655 | 14,618 | 8294 | 15,236 |
| AM1_1439 | PSI protein, PsaJ | 1430979, 1430824 | 13,098 | 7467 | 13,772 |
| AM1_5515 | Ferredoxin, 2Fe-2S type, PetF1 | 5563740, 5564039 | 12,482 | 11,721 | 9641 |
| AM1_3950 | Hypothetical protein | 4000275, 4000505 | 12,345 | 10,183 | 16,966 |
| AM1_1440 | PSI protein, PsaF | 1431487, 1430984 | 12,016 | 5467 | 9543 |
| AM1_5512 | PSII protein, PsbH | 5561953, 5562168 | 10,385 | 3393 | 8835 |
| AM1_4405 | Hypothetical protein | 4432868, 4432984 | 10,120 | 1755 | 8134 |
| AM1_3885 | Cytochrome c550, PsbV | 3936566, 3937054 | 9500 | 6062 | 11,959 |
| AM1_1813 | Conserved hypothetical protein | 1801209, 1801415 | 9417 | 9037 | 5358 |
| AM1_6421a | 23S ribosomal RNA | 5638205, 5641084 | 6111 | 2859 | 20,215 |
| AM1_6416a | 23S ribosomal RNA | 1408620, 1405741 | 6110 | 2859 | 20,215 |
Coordinates of the transcripts are given to facilitate the location of the noncoding sRNAs. Expression values refer to RPKM normalized by the upper quartile of gene expression. UTR, untranslated region; PSI, Photosystem I; PSII, Photosystem II.
Rows corresponding to sRNAs.
Identification and classification of differentially expressed transcripts
A total of 8446 transcripts (99.8% of the genes annotated in the Cyanobase database) were detected as expressed in at least one of the test conditions. Imposing a stringent threshold for minimum expression in 50 reads, we identified 6635 protein-coding and 523 noncoding TUs as expressed, under at least one of the test conditions. We considered a transcript to be differentially expressed when it had an absolute log2FC (fold change) value ≥1.0 (i.e., a minimum twofold up or downregulation change). It is important to remark here that, since RNA sequencing data do not provide information on whether the differences in expression reflect induction or repression of transcription or changes in RNA stability under the new conditions, we use the term expression to indicate the number of detected transcripts. Using this FC criterion, 2896 TUs were identified as differentially expressed for at least one of the test conditions. These TUs consisted of 2536 mRNAs, 41 tRNAs, six rRNAs, three RNAs involved in RNA processing, and 310 unannotated sRNA, of which 248 were encoded in the chromosome (Table S3). Since the samples were treated to remove rRNAs (Materials and Methods), we eliminated data corresponding to 5S, 16S, and 23S rRNAs from our analysis, on the basis that differences in rRNA may reflect processing.
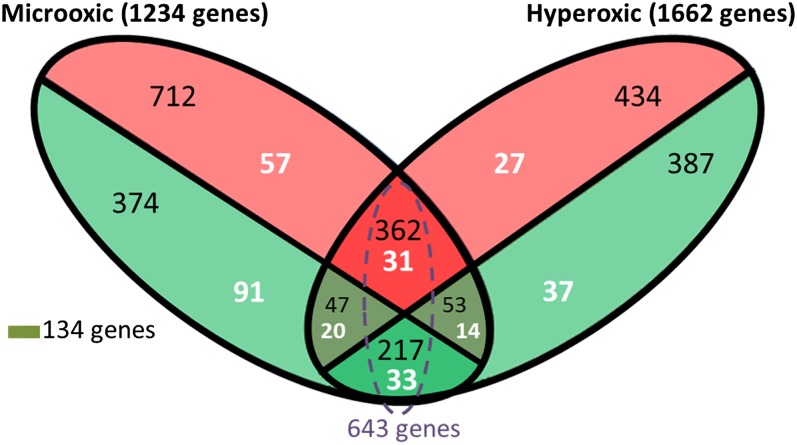
Of these 2896 TUs (1234 in microoxic, and 1662 in hyperoxic), 2119 had a significant expression change in only one of the test conditions, 643 showed a similar response under both, while 134 had opposing expression under microoxic vs. hyperoxic environments (Figure 2). Interestingly, among the genes with opposed expression profiles, five genes (two of them colocalized in the same genomic region) encode proteins involved in the metabolism of tetrapyrrole molecules (Table 2). Two out of five accumulated mainly under microoxic conditions (Log2FC >5), including AM1_0465 encoding the oxygen-dependent Mg-protoporphyrin IX monomethyl ester cyclase (AcsF), and AM1_0466 encoding a heme oxygenase (HO). In contrast, the transcripts of the genes encoding the three subunits of the light-independent protochlorophyllide reductase (ChlN, AM1_1444, ChlL, AM1_1445, and ChlB, AM1_1539) were significantly reduced under microoxic conditions. Similarly, the gene AM1_4366, encoding the uroporphyrin-III C-methyltransferase (cysG) at the branching point for the B12 (cobalamin) synthetic pathway, was significantly reduced under microoxic conditions. Another group of genes (AM1_1222, AM1_1223, and AM1_1224) in which expression was reduced under microoxic conditions, was the operon (SufBCD) coding for the proteins involved in the assembly of iron-sulfur clusters (Shen et al. 2007) (Table 2).
Figure 2.
Venn diagram showing transcriptional units differentially expressed under microoxic and hyperoxic conditions. The red and green ellipsoid areas represent genes up regulated and downregulated, respectively. The number of differentially expressed sRNAs is shown in white, while the number of mRNAs is shown in black. The sum of some areas is shown to facilitate understanding of our results.
Table 2. Expression levels of genes discussed in the text.
| Gene | Product | Expression Control | Expression Microoxic | Expression Hyperoxic | Log2 (Microoxic/Control) | Log2 (Hyperoxic/Control) |
|---|---|---|---|---|---|---|
| AM1_4394 | PSI assembly protein, Ycf37 | 534 | 433 | 525 | −0.30 | −0.02 |
| AM1_2827 | PSI assembly protein, Ycf3 | 971 | 636 | 649 | −0.61 | −0.58 |
| AM1_1082 | PSI assembly protein, Ycf4 | 319 | 346 | 297 | 0.12 | −0.10 |
| AM1_2457 | PSI core protein, PsaA | 27,521 | 11,603 | 45,090 | −1.25 | 0.71 |
| AM1_2458 | PSI core protein, PsaB | 25,295 | 8553 | 27,164 | −1.56 | 0.10 |
| AM1_1660 | PSI ferredoxin protein, PsaC | 79,831 | 18,946 | 44,342 | −2.07 | −0.85 |
| AM1_5144 | PSI protein, PsaD | 12,271 | 3740 | 6069 | −1.71 | −1.02 |
| AM1_2503 | PSI protein, PsaE | 13,114 | 3720 | 4762 | −1.82 | −1.46 |
| AM1_1440 | PSI protein, PsaF | 18,274 | 6802 | 16,128 | −1.43 | −0.18 |
| AM1_1439 | PSI protein, PsaJ | 26,686 | 10,852 | 24,983 | −1.30 | −0.10 |
| AM1_1120 | PSI protein, PsaK | 5411 | 2674 | 2936 | −1.02 | −0.88 |
| AM1_1637 | PSI protein, PsaK | 5345 | 2635 | 3063 | −1.02 | −0.80 |
| AM1_1437 | PSI protein, PsaL | 13,970 | 3888 | 6404 | −1.84 | −1.13 |
| AM1_6426 | PSI protein, PsaM | 85,093 | 14,553 | 27,494 | −2.55 | −1.63 |
| AM1_0448 | PSII D1 protein, PsbA | 40 | 251 | 25 | 2.62 | −0.66 |
| AM1_2166 | PSII D1 protein, PsbA | 14,425 | 6869 | 42,113 | −1.07 | 1.55 |
| AM1_2889 | PSII D1 protein, PsbA | 19,376 | 5675 | 65,901 | −1.77 | 1.77 |
| AM1_2026 | PSII CP47 protein, PsbB | 10,087 | 2155 | 6568 | −2.23 | −0.62 |
| AM1_1084 | PSII CP43 protein, PsbC | 4186 | 1012 | 4094 | −2.05 | −0.03 |
| AM1_4084 | PSII D2 protein, PsbD | 9385 | 4764 | 26,427 | −0.98 | 1.49 |
| AM1_1083 | PSII D2 protein, PsbD | 9452 | 4747 | 19,155 | −0.99 | 1.02 |
| AM1_6045 | PSII D2 protein, PsbD | 42 | 12 | 45 | −1.73 | 0.10 |
| AM1_1130 | Cytochrome b559 alpha subunit, PsbE | 47 | 21 | 33 | −1.13 | −0.50 |
| AM1_2630 | Cytochrome b559 alpha subunit, PsbE | 34,459 | 11,070 | 17,313 | −1.64 | −0.99 |
| AM1_1129 | Cytochrome b559 beta subunit, PsbF | 20 | 20 | 20 | 0.00 | 0.00 |
| AM1_5512 | PSII 10 kDa phosphoprotein, PsbH | 14,928 | 3783 | 12,054 | −1.98 | −0.31 |
| AM1_3799 | PSII protein, PsbI | 9360 | 3927 | 4252 | −1.25 | −1.14 |
| AM1_2629 | PSII protein, PsbJ | 7734 | 5426 | 17,622 | −0.51 | 1.19 |
| AM1_3851 | PSII protein, PsbK | 55,059 | 12,799 | 28,992 | −2.10 | −0.93 |
| AM1_6425 | PSII subunit, PsbL | 9796 | 4483 | 11,606 | −1.13 | 0.24 |
| AM1_2024 | PSII protein, PsbM | 1021 | 128 | 465 | −2.99 | −1.13 |
| AM1_5511 | PSII protein, PsbN | 125 | 25 | 116 | −2.28 | −0.11 |
| AM1_0526 | PSII manganese-stabilizing protein, PsbO | 6217 | 1533 | 5680 | −2.02 | −0.13 |
| AM1_0613 | PSII protein, PsbP | 767 | 460 | 908 | −0.74 | 0.24 |
| AM1_3795 | PSII protein, PsbQ | 8045 | 3177 | 4092 | −1.34 | −0.98 |
| AM1_5050 | PSII protein, PsbT | 247 | 61 | 97 | −2.00 | −1.34 |
| AM1_G0114 | PSII 12 kDa extrinsic protein, PsbU | 132 | 312 | 28 | 1.23 | −2.20 |
| AM1_D0138 | PSII 12 kDa extrinsic protein, PsbU | 481 | 438 | 1245 | −0.13 | 1.37 |
| AM1_3966 | PSII 12 kDa extrinsic protein, PsbU | 5964 | 2305 | 4019 | −1.37 | −0.57 |
| AM1_5046 | PSII 12 kDa extrinsic protein, PsbU | 687 | 250 | 52 | −1.45 | −3.70 |
| AM1_3885 | Cytochrome c550 subunit of PSII, PsbV | 14,802 | 7281 | 18,762 | −1.02 | 0.34 |
| AM1_3886 | Cytochrome c550 PsbV-like protein | 2641 | 272 | 1332 | −3.27 | −0.99 |
| AM1_2120 | PSII protein, PsbX | 1297 | 1064 | 644 | −0.29 | −1.01 |
| AM1_2631 | PSII stability/assembly factor, Ycf48 | 454 | 259 | 209 | −0.81 | −1.12 |
| AM1_1011 | PSII protein, PsbZ | 18,302 | 14,120 | 6270 | −0.37 | −1.55 |
| AM1_4426 | PSII protein, Psb27 | 459 | 331 | 120 | −0.47 | −1.93 |
| AM1_5552 | PSII protein, Psb28 | 178 | 284 | 254 | 0.67 | 0.51 |
| AM1_4891 | PSII biogenesis protein, Psb29 | 157 | 169 | 180 | 0.11 | 0.20 |
| AM1_C0117 | R-phycocyanin-2 subunit alpha | 392 | 67 | 626 | −2.53 | 0.67 |
| AM1_1558 | Allophycocyanin alpha subunit, ApcA | 17 | 43 | 83 | 1.29 | 2.22 |
| AM1_4469 | Allophycocyanin alpha subunit, ApcA | 30 | 31 | 11 | 0.05 | −1.37 |
| AM1_5810 | Allophycocyanin alpha subunit, ApcA | 3 | 3 | 14 | 0.00 | 1.91 |
| AM1_2376 | Allophycocyanin beta subunit, ApcB | 4936 | 5426 | 6393 | 0.14 | 0.37 |
| AM1_C0213 | Phycocyanin alpha subunit, CpcA | 17,975 | 4996 | 8646 | −1.85 | −1.06 |
| AM1_C0096 | Phycocyanin alpha subunit, CpcA | 18,218 | 4972 | 8449 | −1.87 | −1.11 |
| AM1_C0099 | Phycocyanin alpha subunit, CpcA | 16,251 | 3657 | 3128 | −2.15 | −2.38 |
| AM1_C0191 | Phycocyanin alpha subunit, CpcA | 16,262 | 3657 | 3158 | −2.15 | −2.36 |
| AM1_C0100 | Phycocyanin beta subunit, CpcB | 9490 | 3987 | 5119 | −1.25 | −0.89 |
| AM1_C0192 | Phycocyanin beta subunit, CpcB | 18,438 | 6232 | 11,198 | −1.56 | −0.72 |
| AM1_C0212 | Phycocyanin beta subunit, CpcB | 32,682 | 7338 | 60,428 | −2.15 | 0.89 |
| AM1_C0098 | Phycocyanin beta subunit, CpcB | 42,155 | 8403 | 23,434 | −2.33 | −0.85 |
| AM1_C0215 | PBS 32.1 kDa linker polypeptide, CpcC | 8683 | 2494 | 5084 | −1.80 | −0.77 |
| AM1_C0094 | PBS 32.1 kDa linker polypeptide, CpcC | 8631 | 2477 | 4950 | −1.80 | −0.80 |
| AM1_C0093 | PBS linker protein, CpcD | 19,292 | 6719 | 16,086 | −1.52 | −0.26 |
| AM1_C0216 | PBS linker protein, CpcD | 18,769 | 6471 | 16,609 | −1.54 | −0.18 |
| AM1_C0118 | Phycocyanobilin lyase subunit alpha, CpcE | 438 | 255 | 500 | −0.78 | 0.19 |
| AM1_C0272 | Phycocyanobilin lyase subunit beta, CpcF | 1156 | 731 | 971 | −0.66 | −0.25 |
| AM1_C0203 | PBS rod-core linker polypeptide, CpcG | 2103 | 933 | 1711 | −1.17 | −0.30 |
| AM1_C0092 | PBS rod-core linker polypeptide, CpcG | 4398 | 1669 | 1772 | −1.40 | −1.31 |
| AM1_C0102 | PBS rod-core linker polypeptide, CpcG | 4182 | 1090 | 2122 | −1.94 | −0.98 |
| AM1_0450 | Rieske iron-sulfur (cyt b6f) fusion protein | 16 | 276 | 10 | 4.03 | −0.63 |
| AM1_1552 | Transcriptional regulator, ChlR | 21 | 82 | 58 | 1.92 | 1.42 |
| AM1_0465 | Oxygen-dependent MPE-cyclase, AcsF | 16 | 1406 | 6 | 6.37 | −1.28 |
| AM1_0466 | Heme oxygenase | 33 | 1266 | 7 | 5.22 | −2.09 |
| AM1_1444 | D-POR, ChlN | 720 | 126 | 940 | −2.51 | 0.38 |
| AM1_1445 | D-POR, ChlL | 1657 | 421 | 3533 | −1.97 | 1.09 |
| AM1_1539 | D-POR, ChlB | 1659 | 417 | 2684 | −1.99 | 0.69 |
| AM1_4366 | Uroporphyrin-III C-methyltransferase, CysG | 195 | 92 | 430 | −1.08 | 1.14 |
| AM1_2801 | Protein with homology to HemJ | 182 | 178 | 162 | −0.03 | −0.17 |
| AM1_0467 | O2-independent coproporphyrinogen III oxidase, HemN | 6 | 822 | 5 | 6.88 | −0.22 |
| AM1_1283 | O2-independent coproporphyrinogen III oxidase, HemN | 53 | 46 | 44 | −0.20 | −0.26 |
| AM1_0615 | Coproporphyrinogen III oxidase, aerobic, HemF | 192 | 103 | 102 | −0.89 | −0.91 |
| AM1_2295 | Oxygen-dependent MPE-cyclase, AcsF | 3331 | 1722 | 3430 | −0.95 | 0.04 |
| AM1_1959 | Ferrochelatase, HemH | 74 | 57 | 50 | −0.37 | −0.56 |
| AM1_C0204 | Ferrochelatase, HemH | 946 | 788 | 762 | −0.26 | −0.31 |
| AM1_C0107 | Ferrochelatase, HemH | 907 | 729 | 745 | −0.31 | −0.28 |
| AM1_3193 | High light inducible protein, HLIP | 21,383 | 4241 | 38,036 | −2.33 | 0.83 |
| AM1_3366 | High light inducible protein, HLIP | 2 | 16 | 2 | 2.50 | 0.00 |
| AM1_1222 | FeS assembly protein, SufD | 123 | 17 | 273 | −2.78 | 1.14 |
| AM1_1223 | FeS assembly ATPase, SufC | 416 | 80 | 2067 | −2.36 | 2.31 |
| AM1_1224 | FeS assembly protein, SufB | 177 | 30 | 745 | −2.52 | 2.07 |
| AM1_5239 | Copper/Zinc superoxide dismutase, SodCC | 38 | 100 | 27 | 1.37 | −0.48 |
| AM1_2962 | Mn/Fe-containing superoxide dismutase, Sod | 98 | 168 | 126 | 0.77 | 0.36 |
| AM1_3669 | Mn/Fe-containing superoxide dismutase, Sod | 1061 | 1004 | 1779 | −0.08 | 0.75 |
| AM1_0511 | Ni-containing superoxide dismutase, SodN | 2744 | 1868 | 4456 | −0.55 | 0.70 |
| AM1_3715 | Catalase/peroxidase HPI, KatG | 122 | 55 | 1118 | −1.14 | 3.19 |
| AM1_3681 | Glutathione-disulfide reductase, Gor | 88 | 62 | 168 | −0.50 | 0.93 |
| AM1_A0300 | Peroxidase/ antioxidant protein | 40 | 21 | 44 | −0.90 | 0.13 |
| AM1_0449 | Rhodanese domain protein | 31 | 281 | 0 | 3.14 | −5.00 |
| AM1_0451 | Conserved hypothetical protein | 3 | 276 | 0 | 6.11 | −2.00 |
Expression values refer to RPKM normalized by the upper quartile of gene expression. MPE, Mg-protoporphyrin IX monomethyl ester; D-POR, protochlorophyllide reductase; PSI, Photosystem I; PSII, Photosystem II; PBS, Phycobilisome.
Functional composition of the set of differentially expressed genes
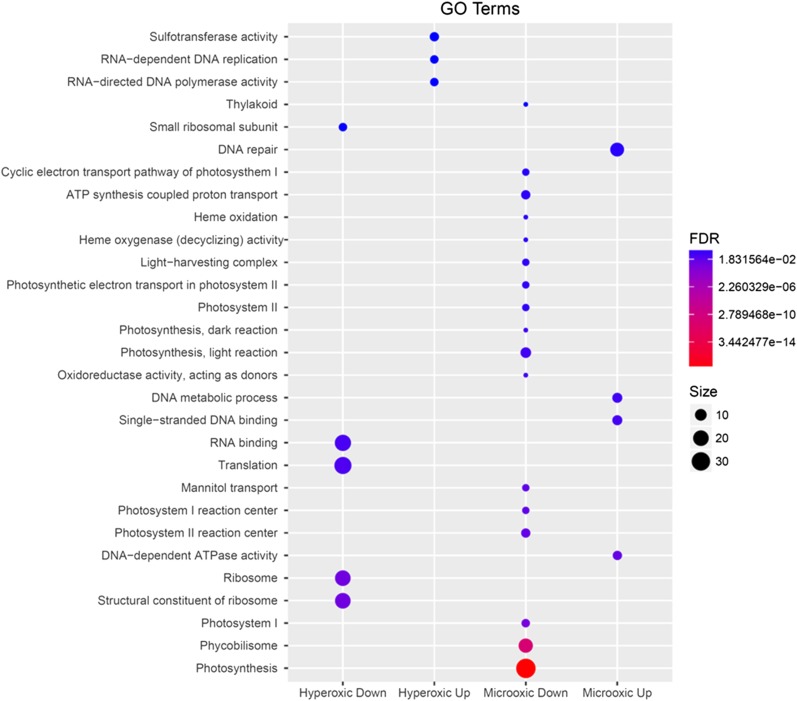
An EADEG was applied to identify functional categories significantly affected by our treatments. After a preliminary examination of the categorical classifications available in the Cyanobase, KEGG, and GO databases, we elected to use the KEGG and GO databases for categorization, because they cover a larger number of genes compared to Cyanobase (Table S1). The results for both databases indicated that, under microoxic conditions, a significant number of genes encoding subunits of both photosystems, as well as phycobilisomes (PBS) are downregulated, having a FDR <0.05 (Table S4). The results using the KEGG database categories did not show any significant upregulation for categories, while using the GO database classification, DNA processing reactions showed a significant upregulation under microoxic conditions (Figure 3). Although increased concentrations of O2 in the medium caused a significant downregulation of genes encoding subunits of the ribosome, and proteins involved in RNA translation, none of these categories were significantly upregulated (Figure 3). Nevertheless, these results should be viewed with caution, since >57% of the proteins-coding genes in Acaryochloris lack any annotated function, i.e., they are not categorized under any biological function.
Figure 3.
Representation of the results obtained after a standard functional enrichment analysis of differentially expressed genes using GO terms. Only categories with a FDR < 0.1 are shown (all other results are available in Table S4). The size of the circles is proportional to the number of genes in that category, reflecting differential expression, while color indicates their confidence level or FDR value. The graph was generated using the R package ggplot2.
Protein-coding genes differentially expressed within significantly affected categories
Based on results of our EADEG, genes encoding proteins involved in light harvesting, and subunits of both photosystems, were among the most affected by altered O2 levels in the cultures. None of the genes encoding PSI subunits showed a positive regulation in either microoxic or hyperoxic environments. In fact, only three genes (ycf3, ycf4, and ycf37) involved in PSI assembly/stability (Dühring et al. 2006a; Ozawa et al. 2009; Boudreau et al. 1997) showed stable expression under microoxic conditions (Table 2). A very similar downregulation was observed for genes localized in the plasmid pREB3, encoding the PBS subunits that form the phycocyanin units, and the linker proteins. Only one (AM1_1558, apcA) of the genes encoding the allophycocyanin subunits showed increased expression levels, while expression of others (AM1_4469, apcA, AM1_5810, apcA, and AM1_2376, apcB) did not change. It is important to note that expression of apcA under all test conditions is 100–1000 times lower than that of apcB, and >10,000 times lower than some of the phycocyanin-binding apo-proteins (Table 2).
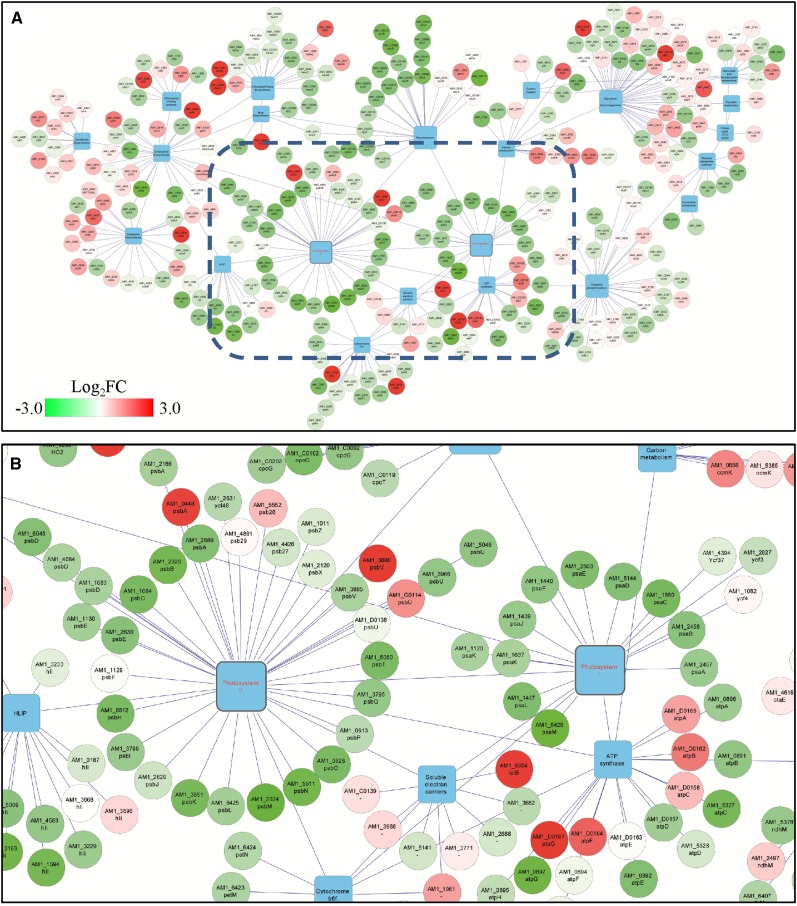
The expression patterns of genes coding PSII subunits were similar to those of PSI and phycobiliprotein complexes under microoxic conditions (Figure 4). Exceptions were noted for the induction of the normally cryptic psbA1 (AM1_0448) gene encoding the D1 protein (Summerfield et al. 2008), and the expression of one of the genes (AM1_G0114) encoding the PsbU subunit of the oxygen-evolving complex (Figure 4). Under hyperoxic conditions, the expression of psbA2 and psbA3 (encoding the D1 protein) increased, as did that of the gene encoding the PsbJ subunit, and AM1_D0138, encoding another homolog of the PsbU subunit (Table 2).
Figure 4.
Expression data mapped onto gene network generated using Cytoscape (Lopes et al. 2010). Protein-coding genes (circular nodes) were linked to their associated KEGG pathway (square nodes), and colored based on their Log2FC (fold change) under microoxic compared with control conditions, according to the gradational color bar shown in the top panel. (A) KEGG pathways relevant to our results. The blue rectangle in (A) marks the part of the network that is enlarged in (B), showing PSI, PSII, soluble electron carriers, and ATP synthase complexes.
Expression of ROS-scavenging genes
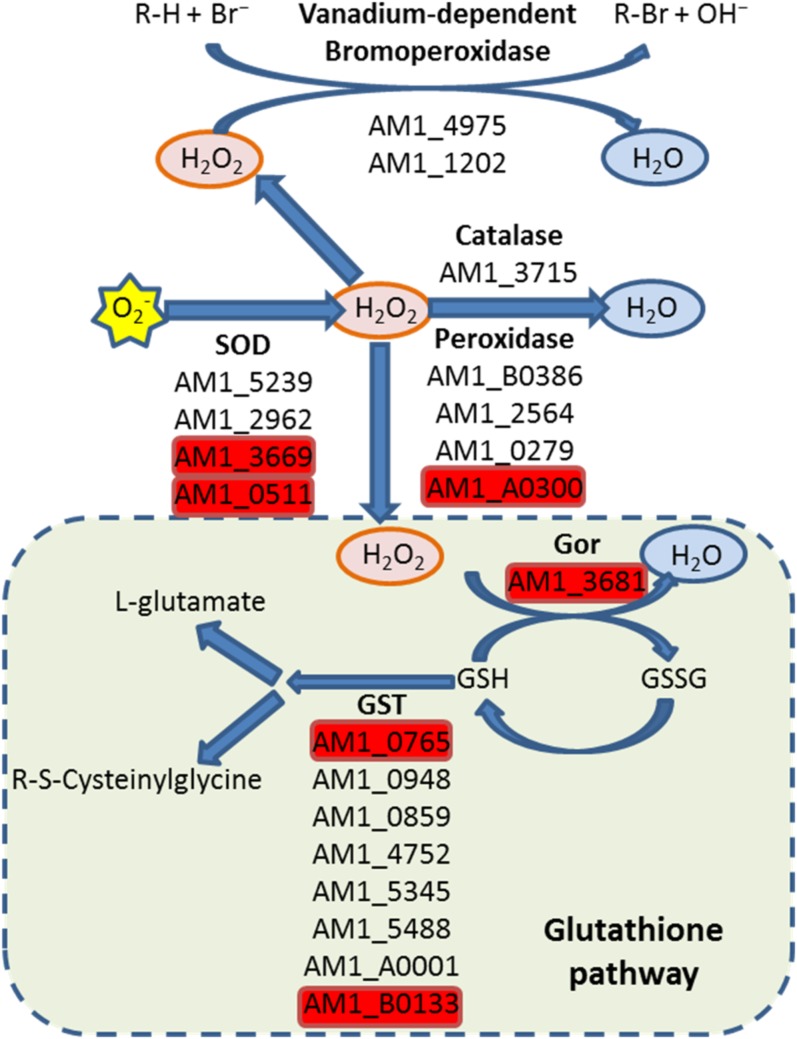
ROS are by-products of both respiration and photosynthesis. Thus, efficient scavenging is crucial to prevent photo-oxidative damage, especially in cyanobacteria, in which both processes occur simultaneously. Enzymes, like superoxide dismutase (SOD), efficiently scavenge the superoxide (O2−). In Acaryochloris, four open reading frames, AM1_5239 (Cu2+/Zn2+-SOD), AM1_2962 (Mn2+/Fe2+-SOD), AM1_3669 (Mn2+/Fe2+-SOD), and AM1_0511 (Ni-SOD), encode proteins resembling SOD. Of these, only AM1_3669 and AM1_0511 accumulated under hyperoxic conditions, but decreased under microoxic conditions (Figure 5). The highest induction of genes encoding scavenging proteins under hyperoxic conditions was for AM1_3715 (catalase, katG) (log2FC > 3), which participates in the elimination of H2O2. Other genes, related to H2O2 scavenging, such as AM1_A0300 (thiol-specific peroxidase), increased their expression under hyperoxic conditions, but had decreased expression under microoxic conditions. Interestingly, the genes encoding enzymes involved in glutathione metabolism either did not change markedly, or else their transcripts increased more under microoxic conditions than under hyperoxic conditions. The only gene for which expression under hyperoxic conditions was significantly higher than under control conditions was AM1_3681, encoding glutathione-disulfide reductase; its expression decreased with respect to the control under microoxic conditions (Figure 5).
Figure 5.
Putative ROS-scavenging pathways. Genes encoding antioxidant enzymes or involved in ROS-scavenging are shown under the reaction that they catalyze. Genes highlighted in red were identified as upregulated in the hyperoxic environment. SOD, Superoxide dismutase; Gor, Glutathione-disulfide reductase; GST, Glutathione S-transferase (GST); GSH, Glutathione; GSSG, Glutathione disulfide.
Terminal oxidases also play an important role in ROS prevention. The presence of terminal oxidases in the thylakoid membrane is key to balancing metabolic flow between the respiratory and photosynthetic electron transport chains, as well as reducing the amount of O2 in the vicinity of the thylakoid membrane thus, preventing ROS (Schmetterer 2016). In Acaryochloris, genes encoding four terminal oxidases have been previously annotated: (i) AM1_4621 (coxB), AM1_4620 (coxA), and AM1_4619 (coxC), encoding subunits of a mitochondrial-type cytochrome c oxidase (cox) complex; (ii) AM1_A0138; (iii) AM1_0843; and (iv) AM1_1551, encoding plastidic-type terminal oxidases (ptox) (Schmetterer 2016). In Anabaena variabilis, coxB, the first gene within the cox locus (coxBAC), was apparently transcribed more often than the other two genes in the operon (Schmetterer et al. 2001). A similar expression pattern is apparent in our results, with coxB (AM1_4621) transcript levels at least three times higher than the other two subunits (Figure S1A). In fact, coxB expression level increased up to eight times under hyperoxic conditions. The expression of coxA and coxC showed no significant changes under altered O2 conditions. Intriguingly, the expression of two (AM1_A0138 and AM1_0483) of the three ptox genes was very low under all conditions, while the expression level of AM1_1551 was >45 times higher under microoxic than under control conditions (Figure S1B).
Potential transacting sRNAs involved in the adaptation to aerobic variations
Alignment of the reads resulted in a large number of sRNAs corresponding to either UTRs or intergenic ncRNAs. We differentiated sRNAs within these two main groups, based on whether the distance between the detected sRNA and the closest annotated mRNA was ≤20 nucleotides (in the case of UTRs), or >20 nucleotides (in the case of ncRNAs). The transcription start site and orientation of the transcript were determined from predictions obtained using algorithms embedded in PePPER (de Jong et al. 2012). Using this criterion for the 248 differentially expressed chromosome-detected transcripts, we identified 190 sRNAs as UTRs and 58 as ncRNAs. The UTR expression level was mostly correlated (Spearman correlation coefficient, rS > 0.6) with the closest gene, except for four of the sRNAs (AM1_NC24, AM1_NC96, AM1_NC169, and AM1_NC181) (Figure S2).
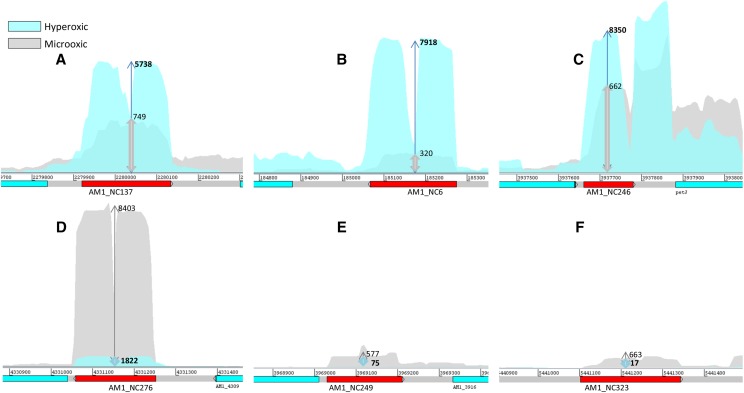
Some of the intergenic ncRNAs were among the most highly expressed transcripts under all three conditions profiled (Table 1). Of the 58 differentially expressed ncRNAs localized in the chromosome, only six showed significant opposing expression profiles for microoxic and hyperoxic conditions. Three were less abundant under microoxic conditions (AM1_NC12, AM1_NC161, and AM1_NC254), while transcripts for the other three (AM1_NC270, AM1_NC256, and AM1_NC315) were enhanced in the hyperoxic culture (Figure 6). Given that most bacterial ncRNAs act through sequence-specific binding to regions close to the ribosome-binding site of mRNAs, we sought to predict potential RNA targets using a window of 275 nucleotides around their respective start codons. Setting a p-value threshold of 0.01 for results obtained from the IntaRNA server, the number of predicted targets was 95 for AM1_NC6, 96 for AM1_NC246, 50 for AM1_NC137, 89 for AM1_NC276, 45 for AM1_NC249, and 84 for AM1_NC323. The number of targets was much larger than expected based on available literature. To reduce these targets, as well as the number of false positives, we calculated the correlation between the numbers of reads obtained for each of the three conditions. We reasoned that any potential target should show an inverse correlation with the particular ncRNA, given that most bacterial ncRNAs act as negative regulators of gene expression, even when other mechanisms cannot be disregarded (Storz et al. 2011). Defining a threshold of rS ≤ −0.5 for inverse correlation, the list of candidates was reduced to 38 potential targets for AM1_NC12, 43 for AM1_NC254, 22 for AM1_NC161, 21 for AM1_NC270, 15 for AM1_NC256, and 24 for AM1_NC315 (Table S5). Functional enrichment analyses of these targets returned significant results (FDR < 0.05) for only two ncRNAs: AM1_NC6 and AM1_NC276. Specifically, AM1_NC6 targets were significantly enriched in genes involved on “DNA integration,” while AM1_NC276 targets showed enrichment in genes encoding proteins involved in “aerobic respiration” (Table 3).
Figure 6.
Noncoding RNAs regulated in opposing directions, under the two oxygen treatment conditions. (A–C) were induced under hyperoxic conditions; while (D–F) were induced under microoxic conditions. The arrows and the numbers adjacent to them represent relative expression. Chromosome coordinates are given above the gene representation. RPKM, reads per kilobase per million mapped reads.
Table 3. Functional enrichment analysis of the predicted targets for intergenic small noncoding RNAs targets.
| GO ID | Function | Targets of AM1_NC276 | P-Value | FDR |
|---|---|---|---|---|
| GO:0009060 | Aerobic respiration | 2 | 5.29 × 10−6 | 0.006 |
| GO:0020037 | Heme binding | 2 | 0.0014 | 0.839 |
| GO:0005506 | Iron ion binding | 2 | 0.0026 | 1 |
| GO:0009055 | Electron carrier activity | 2 | 0.0059 | 1 |
| GO:0019898 | Extrinsic to membrane | 1 | 0.0083 | 1 |
| GO:0042549 | Photosystem II stabilization | 1 | 0.0083 | 1 |
| GO:0009654 | Oxygen evolving complex | 1 | 0.0097 | 1 |
| GO ID | Function | Targets of AM1_NC6 | P-Value | FDR |
| GO:0015074 | DNA integration | 5 | 5.71 × 10−5 | 0.048 |
| GO:0003676 | Nucleic acid binding | 5 | 0.0036 | 0.985 |
| GO:0003952 | NAD+ synthase (glutamine-hydrolyzing) activity | 1 | 0.0074 | 0.985 |
| GO:0004127 | Cytidylate kinase activity | 1 | 0.0074 | 0.985 |
| GO:0004553 | Hydrolase activity, hydrolyzing O-glycosyl compounds | 2 | 0.0023 | 0.985 |
| GO:0004592 | Pantoate-beta-alanine ligase activity | 1 | 0.0074 | 0.985 |
Our analysis was performed similarly to the enrichment analysis of differentially expressed genes described in the Materials and Methods. The top six categories with the lowest p-values are shown. Only GO categories having a FDR ≤ 0.05 (in bold) were considered significant.
Discussion
In the laboratory, Acaryochloris can grow as a free-living form, under conditions very different from those in which it was initially isolated. In nature, it forms part of an algal mat, associated with colonial ascidians (Miyashita et al. 1996). It has been speculated that the multiplicity of homologous genes, and the relatively large genome size, of Acaryochloris reflect its evolutionary adaptation to its niche (Swingley et al. 2008), in contrast to the reduced genome size of the picoplanktonic Prochlorococcus genus (Delaye and Moya 2010; Dufresne et al. 2005). Here, we have shown that, under different O2 conditions, expression levels among homologous genes varies. Given such adaptive capability, it is tempting to speculate on the flexibility of Acaryochloris to adapt to changing environmental conditions. This would be one of the benefits obtained from having coexisting multiple copies of genes, in spite of the cost of such a large genome size.
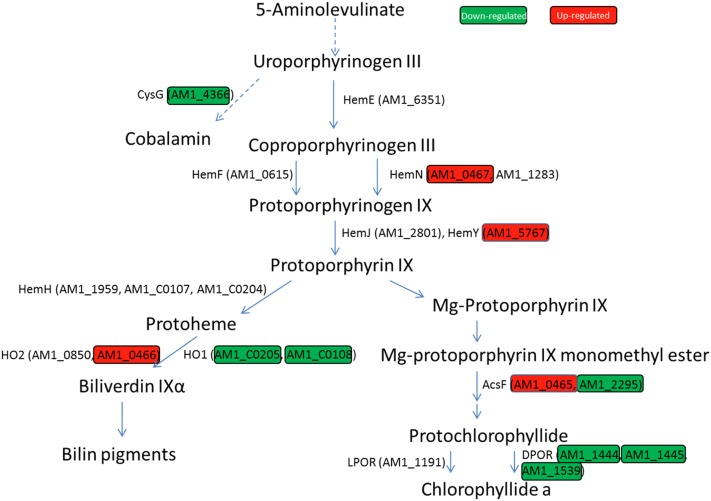
The combination of porphyrin-containing molecules, O2, and light often results in photo-oxidative damage to cellular structures. Hence, it has been speculated that divergence of the biosynthesis of bacteriochlorophyll and chlorophyll occurred to reduce photo-oxidative damage under an increasingly oxic atmosphere (Reinbothe et al. 1996). The syntheses of various tetrapyrrole molecules (heme, bilins, cobalamin, and chlorophyll) share a common pathway from ALA to uroporphyrinogen III. At this point, the cobalamin biosynthesis pathway branches from the uroporphyrinogen III pathway via a methylation reaction, catalyzed by the multifunctional chelatase CysG. The downregulation of cysG expression under microoxic conditions indicates that O2 levels are an important regulatory element for the synthesis of cobalamin. In fact, CysG directs the uroporphyrinogen III pathway toward the synthesis of cobalamin either via an oxygen-independent or dependent pathway (Figure 7). Alternatively, it can be redirected toward the heme or chlorophyll biosynthetic pathways. In these pathways, O2 levels influence the expression of genes encoding enzymes involved in oxidation. The part common to the heme and chlorophyll biosynthetic pathways, from uroporphyrinogen III to protoporphyrin IX, contains several oxidation reactions. The first oxidation step converts coproporphyrinogen III to protoporphyrinogen IX. This oxidation is catalyzed by either HemF or HemN, using O2 or a 5′deoxyadenosil radical generated from S-adenosylmethionine, respectively. HemN contains a [4Fe-4S] cluster, as a prosthetic group, sensitive to the presence of O2. In Synechocystis, a mutant lacking hemF was able to grow under microoxic conditions, but did not grow under aerobic conditions. In contrast, an hemN knockout mutant showed impaired growth only under microoxic conditions (Goto et al. 2010). Thus, in the presence of O2, it could be expected that hemN expression would be minimal compared with hemF. In Acaryochloris, the product of two genes (AM1_0467 and AM1_1283) resembles HemN, but only AM1_0467 had a strong induction (Log2FC ∼6.8) under microoxic conditions (Figure 7). Expression of HemF (AM1_0615) did not show any significant change. This suggests that AM1_0467 is the homolog to hemN in Acaryochloris, based on its expression profile. The product of the HemN/HemF reaction (protoporphyrinogen IX) is converted to protoporphyrin IX, becoming the final precursor common to Chl and heme, as well as heme-derived bilins. The protoporphyrinogen IX oxidation to protoporphyrin IX can be catalyzed by three enzymes, namely HemG, HemY, and HemJ (Kato et al. 2010). Only HemG, which is absent in most cyanobacteria, seems to be oxygen-independent (Boynton et al. 2009). Most cyanobacteria use the oxygen-dependent HemJ, although a few use HemY (Kobayashi et al. 2014). Interestingly, Acaryochloris contains both a HemJ- and a HemY-like enzyme, and, like most cyanobacteria, it lacks a gene homologous to HemG. AM1_5767, having enhanced expression in the microoxic environment, encodes a protein containing a HemY-like domain. A BLAST search, using the sequence for the Synechocystis HemJ (encoded by slr1790) (Kato et al. 2010), returned AM1_2801 as being highly homologous to HemJ. Unlike AM1_5767, the expression of AM1_2801 was not influenced by altered O2 levels, suggesting that the main pathway for the oxidation of protoporphyrinogen IX in Acaryochloris under microoxic conditions is through HemY (AM1_5767). After this step, iron or magnesium is incorporated into protoporphyrin IX by a ferro- or magnesium chelatase, leading to the synthesis of heme and bilins (in the case of iron insertion), or Chl (in the case of magnesium insertion) (Chen 2014). In the Chl branch, the next substrate that is oxidized is Mg-protoporphyrin IX monomethyl ester (MPE). This reaction is catalyzed by MPE cyclase, which converts MPE into protochlorophyllide (Beale 1999). MPE can follow two pathways: one through an oxygen-dependent MPE-cyclase (AcsF, encoded by AM1_0465, or AM1_2295); and the other through an oxygen-independent MPE-cyclase (BchE) (Raymond and Blankenship 2004). BLAST results did not return any gene homologous to BchE in Acaryochloris. Nevertheless, our results show that the expression of both ascF (AM1_0465, AM1_2295) homologs differ. AM1_0465 was strongly induced under microoxic conditions (log2FC > 6), while the expression of AM1_2295 was reduced by almost half (Figure 7). In Synechocystis, a similar expression profile was described for two homologous genes encoding AcsF; deletion of these genes impaired growth under aerobic conditions (Minamizaki et al. 2008). Based on the conclusions drawn for Synechocystis, it is likely that AM1_0465 is the main MPE-cyclase in Acaryochloris under microoxic conditions, while, under aerobic conditions, our results show its expression is null, compared to that of AM1_2295 (Figure 7).
Figure 7.
Diagram representing the tetrapyrrole biosynthetic pathway. Only genes encoding proteins discussed in the text are shown. Genes differentially expressed are highlighted in green and red, indicating downregulation and upregulation under microoxic conditions, respectively. Specific values are given in Table 2.
Based on published work, the FeS cluster within the ChlL subunit of the light-independent protochlorophyllide reductase (D-POR) shows a high vulnerability to O2 (Nomata et al. 2006; Yamazaki et al. 2006). Such susceptibility might explain why this multimeric enzyme functions in the dark, when O2 levels are low. Intriguingly, downregulation of the genes encoding the subunits of D-POR under microoxic conditions was observed, confirming that expression levels of this gene are controlled by the reduction state of the photosynthetic electron transport chain, and not by O2 levels (Horiuchi et al. 2010). In contrast, the expression level of the light-dependent protochlorophyllide reductase (L-POR) did not change under microoxic conditions, but decreased under hyperoxic conditions (Table 2).
Because of the potential deleterious effects of a misregulation of the tetrapyrrole biosynthetic pathway, it is expected that several regulatory factors (including sRNAs) control its activity. However, how a cell senses O2 levels, and controls the expression of multiple genes is not fully understood. In Synechocystis, O2 levels are sensed by the transcriptional factor ChlR (sll1512), which positively regulates acsF, ho2, and hemN expression (Aoki et al. 2012). The homolog to ChlR in Acaryochloris is encoded by AM1_1552. Expression of AM1_1552, as expected for a positive regulator of genes sensitive to O2, increased under microoxic conditions (log2FC ∼1.9). A search using the FIMO tool within the MEME suite using the ChlR recognition motif (TTMCC-N4/3-GGWAA) provided by Aoki et al. (2012) returned a putative site (p-value <0.0005), located 22 bp upstream of AM1_0466 (ho2).
Furthermore, the expression control performed by regulatory factors, the synthesis of the final products, and their assembly into the apoprotein moiety, have to be tightly regulated to avoid their accumulation as free pigments in the membrane. For example, members of the HLIP family appear to mediate between both pathways in the assembly of photosynthetic complexes (Hernandez-Prieto et al. 2011; Yao et al. 2012; Sobotka et al. 2008; Adamska et al. 2001). In Synechocystis, HLIPs accumulate under multiple-stress conditions, while, under laboratory growth conditions, they are expressed at a low level (He et al. 2001). In Acaryochloris, there are 13 hlip genes; our data showed that AM1_3193 is among the most expressed transcripts in the control sample, contrary to what was observed in Synechocystis (He et al. 2001). In general, hlip expression levels decreased both under microoxic and hyperoxic conditions, following the trend observed for both photosystems. The regulatory role of HLIPs is most evident when examining the C-terminal extension of the ferrochelatase gene in cyanobacteria and chloroplasts, which shares a high homology with HLIPs (Funk and Vermaas 1999). This HLIP-like extension appears to induce or repress ferrochelatase activity, depending on the amount of free chlorophyll in the thylakoid membrane (Sobotka et al. 2008). In most cyanobacteria, only one gene encodes for ferrochelatase, while in Acaryochloris there are three ferrochelatase gene copies (AM1_1959, AM1_C0107, and AM1_C0204), and all of them have HLIP-like C-terminal extensions. AM1_C0107 and AM1_C0204, localized in the plasmid pRBE3, encode identical ferrochelatases; and their transcripts are >100 times more abundant than those generated from the copy (AM1_1959) localized in the chromosome, under all test conditions. The identification of ferrochelatase, as a pivotal enzyme at the intersection between Chl and heme syntheses (Figure 7), makes the regulation of its function by the HLIP-like extension essential to the flux of metabolites toward one or other of its final products.
In cyanobacteria, a large portion of the heme generated by ferrochelatase is funneled toward heme oxygenase. This is the first step in the synthesis of bilins, the pigments bound in the phycobiliproteins of the PBS. The PBS antenna found in Acaryochloris consists of a single rod structure (Chen et al. 2009; Hu et al. 1999), in which phycocyanin (pc)-containing subunits are the main component, and allophycocyanin (Apc)-containing subunits are only a minor component of the bottom disc of the rod-structured phycobiliprotein complex (Marquardt et al. 1997). This structure explains the larger number of reads for the genes encoding the pc-binding apoproteins (Table 2), compared with the Apc-containing subunits (ApcA and ApcB). Furthermore, their gene loci are physically separated from each other, with the genes encoding the Apc proteins, ApcA (AM1_1558, AM1_4469, and AM1_5810) and ApcB (AM1_2376), localized in the main chromosome, and the ones encoding the pc-binding apoproteins localized in the pREB3 plasmid. Our results and previously published work (Lin et al. 2013) show that, under microoxic conditions, the expression of genes encoding PBS subunits and their assembly in the antenna complex, are significantly decreased. Similarly, experimental conditions that limit access of cultures to essential nutrients also induce a downregulation of PBS encoding genes (Foster et al. 2007; Wang et al. 2004; Zhang et al. 2008). Downregulation of PBS encoding genes under microoxic conditions also has been observed in Synechocystis sp. PCC6803 (Summerfield et al. 2011). This downregulation may reflect decreased formation of bilins by the oxygen-dependent heme oxygenase (HO). In most cyanobacteria, two genes encode for heme oxygenases (ho1, and ho2), with HO2 having a higher affinity for O2, and being most active under microoxic conditions (Aoki et al. 2011). In Acaryochloris, two genes encode proteins with high similarity to HO1 (AM1_C0205 and AM1_C0108), while two others encode HO2 (AM1_0850 and AM1_0466). The expression of both genes encoding for HO1 decreased under microoxic conditions, while the expression of AM1_0466 increased under microoxic conditions (log2FC > 5), revealing this gene as most probably HO2.
In Synechocystis, it was noted that the expression of genes encoding PBS subunits is highly correlated with genes encoding subunits of the ATP synthase (ATPase) complex (Hernández-Prieto et al. 2016; Summerfield et al. 2011). Hence, a similar downregulation of ATPase subunits would also be expected for Acaryochloris under microoxic conditions. In Acaryochloris, two sets of genes encoding for ATPase subunits exist (one set is localized in the chromosome, and another in the pREB4 plasmid) (Swingley et al. 2008). The expression of the genes encoding ATPase located in the plasmid increased slightly under microoxic conditions, while the ATPase from the main chromosome showed decreased expression under microoxic conditions, consistent with the results for Synechocystis (Hernández-Prieto et al. 2016). In fact, the ATPase encoding genes localized in the chromosome are phylogenetically closer to ATPase from other cyanobacteria (Swingley et al. 2008) than those localized in the plasmid. In addition to their downregulation under microoxic conditions, the ATPase genes localized in the chromosome are transcribed more frequently under all test conditions than the copies localized in the plasmid. These results indicate that the ATPase gene copies localized in the plasmid might be cryptic, or function in conditions different from the ones tested here.
A well-documented effect observed under microoxic conditions in cyanobacteria is the induction of the psbA1 gene encoding a homolog of the D1 protein of PSII (Summerfield et al. 2008), with repression of psbA2 and psbA3. D1 differential expression also was confirmed in this study, consistent with previously published results (Kiss et al. 2012). Noticeably, a similar expression profile was observed here for one (AM1_G0114) of the four genes (AM1_G0114, AM1_D0138, AM1_3966, and AM1_5046) encoding the PsbU subunit of PSII (Figure 4). The PsbU subunit is associated with the oxygen-evolving complex, and functions to stabilize the PSII complex under high-intensity light conditions, protecting it from ROS (Abasova et al. 2011). The differential induction of this gene is interesting, since it has been assumed that, because of differences in the sequence of the psbA1 encoded D1 protein, the PSII complexes assembled with this protein lack the capacity to evolve O2 (Kiss et al. 2012; Murray 2012). The expression of the rieske-containing subunit (PetC) (Wenk et al. 2005) of the cytochrome b6f subunits is affected in a similar manner to psbA1. Similar to D1, PetC can be transcribed from three genes (AM1_4450, AM1_0450, and AM1_1961), with AM1_0450 being the only one induced under microoxic conditions (Table 2). It is important to note that AM1_0450 is upstream of psbA1. Thus, it is likely that they are cotranscribed in Acaryochloris, together with another two genes encoding proteins of unknown function: one (AM1_0449) containing a bacteria-conserved domain (DUF2892), and the other (AM1_0451) containing a rhodanese-like domain linked with assimilation of thiosulfate under anaerobic conditions in other bacteria (Schedel and Trüper 1980). A similar gene cluster is observed in Synechocystis genome, where psbA1 (slr1181) is the first gene of a set of 10 genes orientated in the same direction, including slr1184 encoding a rhodanese-like protein, and slr1185 encoding PetC; expression levels in these genes were also higher under microoxic conditions (Summerfield et al. 2008). Based on the microarray meta-analysis presented in CyanoEXpress, only the expression of slr1182, slr1183, and slr1184 seem to follow the same trend under different environmental conditions, as expected for genes in an operon (Hernández-Prieto et al. 2016). Nevertheless, results obtained from genes for which expression levels are low under most conditions (as is the case for psbA1) should be viewed with caution, especially when interpreting correlations. Further investigation to confirm whether expression of these proteins results in a restructuring of the photosynthetic complexes, and rerouting of the electron transport chain under microoxic conditions, is needed, but is beyond the scope this paper.
Transcriptome data (Mitschke et al. 2011; Kopf et al. 2014; Voigt et al. 2014; Hernández-Prieto et al. 2012), as well as computational predictions (Voß et al. 2009; Voigt et al. 2014), of sRNAs in cyanobacteria have revealed a large number of previously unidentified noncoding protein transcripts, exceeding all previous predictions. Although the roles of some of these sRNAs have been partially characterized in cyanobacteria (Nakamura et al. 2007; Voß et al. 2007; Dühring et al. 2006b), the function of most of them is still unknown. A well understood process in Escherichia coli is the degradation of complementarily paired ncRNAs and mRNAs, mediated by the protein Hfq (Massé et al. 2003). Such processes affect protein synthesis at the transcriptional level, saving valuable resources that can be used to synthesize a different protein complement that is more suitable to the new environmental conditions. In cyanobacteria, a gene encoding a homolog to Hfq has been identified, but its role in ncRNA/mRNA degradation has not yet been demonstrated (Bøggild et al. 2009; Dienst et al. 2008). Of the 58 ncRNAs localized in the chromosome (Table 1), only six (AM1_NC12, AM1_NC161, AM1_NC254, AM1_NC270, AM1_NC256, and AM1_NC315) showed significant opposing expression profiles for microoxic and hyperoxic conditions, as would be expected for ncRNAs involved in adaptation to different O2 levels (Figure 6). Of these, functional enrichment analyses of their potential targets returned significant results (FDR < 0.05) for only two ncRNAs: AM1_NC6 and AM1_NC276. The targets predicted for AM1_NC276 comprised genes within the category “aerobic respiration” (Table 3), indicating that expression of this ncRNA might be relevant for adaptation to altered O2 levels. In E. coli, several ncRNAs have been shown to play a role in adaptation to oxidative stress (Berghoff and Klug 2012), but further experimental work is necessary to determine whether they have the same functions in Acaryochloris.
In conclusion, the large number of protein-coding genes and sRNAs detected as differentially expressed under our test conditions revealed that there is a high level of regulation related to O2 in cyanobacteria. The multiplicity of genes encoding homologous proteins in Acaryochloris exceeds that of many cyanobacteria, indicating a complex regulatory network in this organism. Multiple ROS-scavenging pathways, and their different transcriptomic responses, may represent the history of alternative ROS-scavenging mechanisms, which has evolved and developed in parallel to new metabolic pathways that produce ROS.
Ultimately, the lack of efficient methods to generate mutants in Acaryochloris makes environmental studies, such as this one, key to understanding its regulation and annotating its yet unknown gene functions.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.036855/-/DC1.
Acknowledgments
The authors thank Patrick Loughlin and Robert Willows for their valuable discussions and suggestions that contributed to experimental design and data processing. This manuscript presents part of a Ph.D. study conducted by Y. L. M.C. holds an Australian Research Council (ARC) Future Fellow (FT120100464). The project is financially supported by the ARC (DP0878174 and CE140100015).
Footnotes
Communicating editor: B. J. Andrews
Literature Cited
- Abasova L., Deák Z., Schwarz R., Vass I., 2011. The role of the PsbU subunit in the light sensitivity of PSII in the cyanobacterium Synechococcus 7942. J. Photochem. Photobiol. B 105(2): 149–156. [DOI] [PubMed] [Google Scholar]
- Adamska I., Kruse E., Kloppstech K., 2001. Stable insertion of the early light-induced proteins into etioplast membranes requires chlorophyll a. J. Biol. Chem. 276(11): 8582–8587. [DOI] [PubMed] [Google Scholar]
- Afgan E., Baker D., van den Beek M., Blankenberg D., Bouvier D., et al. , 2016. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 44(W1): W3–W10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R., Goto T., Fujita Y., 2011. A heme oxygenase isoform is essential for aerobic growth in the cyanobacterium Synechocystis sp. PCC 6803: modes of differential operation of two isoforms/enzymes to adapt to low oxygen environments in cyanobacteria. Plant Cell Physiol. 52(10): 1744–1756. [DOI] [PubMed] [Google Scholar]
- Aoki R., Takeda T., Omata T., Ihara K., Fujita Y., 2012. R-type transcriptional regulator ChlR activates expression of tetrapyrrole biosynthesis genes in response to low-oxygen conditions in cyanobacteria. J. Biol. Chem. 287(16): 13500–13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H., 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Beale S. I., 1999. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 60(1): 43–73. [Google Scholar]
- Berghoff B., Klug G., 2012. Small RNAs with a role in the oxidative stress response of bacteria, pp. 1–14 in Regulatory RNAs in Prokaryotes. Springer, Vienna. [Google Scholar]
- Blankenship R. E., 2008. Molecular Mechanisms of Photosynthesis. John Wiley & Sons, New York. [Google Scholar]
- Blankenship R. E., Hartman H., 1998. The origin and evolution of oxygenic photosynthesis. Trends Biochem. Sci. 23(3): 94–97. [DOI] [PubMed] [Google Scholar]
- Bøggild A., Overgaard M., Valentin-Hansen P., Brodersen D. E., 2009. Cyanobacteria contain a structural homologue of the Hfq protein with altered RNA-binding properties. FEBS J. 276(14): 3904–3915. [DOI] [PubMed] [Google Scholar]
- Boudreau E., Takahashi Y., Lemieux C., Turmel M., Rochaix J. D., 1997. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 16(20): 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton T. O., Daugherty L. E., Dailey T. A., Dailey H. A., 2009. Identification of Escherichia coli HemG as a novel, menadione-dependent flavodoxin with protoporphyrinogen oxidase activity. Biochemistry 48(29): 6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A., Richter A. S., Backofen R., 2008. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics 24(24): 2849–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. W., Montgomery B. L., 2015. Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol. 4: 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., 2014. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 83(1): 317–340. [DOI] [PubMed] [Google Scholar]
- Chen M., Cai Z.-L., 2007. Theoretical study on the thermodynamic properties of chlorophyll d-peptides coordinating ligand. Biochim. Biophys. Acta 1767(6): 603–609. [DOI] [PubMed] [Google Scholar]
- Chen M., Quinnell R. G., Larkum A. W. D., 2002a Chlorophyll d as the major photopigment in Acaryochloris marina. J. Porphyr. Phthalocyanines 6(12): 763–773. [Google Scholar]
- Chen M., Quinnell R. G., Larkum A. W. D., 2002b The major light-harvesting pigment protein of Acaryochloris marina. FEBS Lett. 514(2–3): 149–152. [DOI] [PubMed] [Google Scholar]
- Chen M., Eggink L. L., Hoober J. K., Larkum A. W. D., 2005a Influence of structure on binding of chlorophylls to peptide ligands. J. Am. Chem. Soc. 127(7): 2052–2053. [DOI] [PubMed] [Google Scholar]
- Chen M., Telfer A., Lin S., Pascal A., Larkum A. W. D., et al. , 2005b The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina. Photochem. Photobiol. Sci. 4(12): 1060–1064. [DOI] [PubMed] [Google Scholar]
- Chen M., Floetenmeyer M., Bibby T. S., 2009. Supramolecular organization of phycobiliproteins in the chlorophyll d-containing cyanobacterium Acaryochloris marina. FEBS Lett. 583(15): 2535–2539. [DOI] [PubMed] [Google Scholar]
- de Jong A., Pietersma H., Cordes M., Kuipers O. P., Kok J., 2012. PePPER: a webserver for prediction of prokaryote promoter elements and regulons. BMC Genomics 13(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye L., Moya A., 2010. Evolution of reduced prokaryotic genomes and the minimal cell concept: variations on a theme. BioEssays 32(4): 281–287. [DOI] [PubMed] [Google Scholar]
- Dienst D., Dühring U., Mollenkopf H.-J., Vogel J., Golecki J., et al. , 2008. The cyanobacterial homologue of the RNA chaperone Hfq is essential for motility of Synechocystis sp. PCC 6803. Microbiology 154(10): 3134–3143. [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Klimov V. V., Baranov S. V., Kozlov Y. N., DasGupta J., et al. , 2001. The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 98(5): 2170–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A., Garczarek L., Partensky F., 2005. Accelerated evolution associated with genome reduction in a free-living prokaryote. Genome Biol. 6(2): R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühring U., Irrgang K.-D., Lünser K., Kehr J., Wilde A., 2006a Analysis of photosynthetic complexes from a cyanobacterial ycf37 mutant. Biochim. Biophys. Acta 1757(1): 3–11. [DOI] [PubMed] [Google Scholar]
- Dühring U., Axmann I. M., Hess W. R., Wilde A., 2006b An internal antisense RNA regulates expression of the photosynthesis gene isiA. Proc. Natl. Acad. Sci. USA 103(18): 7054–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury Z., Schliep M., Ritchie R. J., Larkum A. W. D., Chen M., 2009. Chromatic photoacclimation extends utilisable photosynthetically active radiation in the chlorophyll d-containing cyanobacterium, Acaryochloris marina. Photosynth. Res. 101(1): 69–75. [DOI] [PubMed] [Google Scholar]
- Foster J. S., Singh A. K., Rothschild L. J., Sherman L. A., 2007. Growth-phase dependent differential gene expression in Synechocystis sp. strain PCC 6803 and regulation by a group 2 sigma factor. Arch. Microbiol. 187(4): 265–279. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Okamoto S., Katayama T., Nakao M., Yoshimura H., et al. , 2014. CyanoBase and RhizoBase: databases of manually curated annotations for cyanobacterial and rhizobial genomes. Nucleic Acids Res. 42(D1): D666–D670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C., Vermaas W., 1999. A cyanobacterial gene family coding for single-helix proteins resembling part of the light-harvesting proteins from higher plants. Biochemistry 38(29): 9397–9404. [DOI] [PubMed] [Google Scholar]
- Goto T., Aoki R., Minamizaki K., Fujita Y., 2010. Functional differentiation of two analogous coproporphyrinogen III oxidases for heme and chlorophyll biosynthesis pathways in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 51(4): 650–663. [DOI] [PubMed] [Google Scholar]
- He Q., Dolganov N., Bjorkman O., Grossman A. R., 2001. The high light-inducible polypeptides in Synechocystis PCC6803. Expression and function in high light. J. Biol. Chem. 276(1): 306–314. [DOI] [PubMed] [Google Scholar]
- Hedges S. B., Blair J. E., Venturi M. L., Shoe J. L., 2004. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Prieto M. A., Tibiletti T., Abasova L., Kirilovsky D., Vass I., et al. , 2011. The small CAB-like proteins of the cyanobacterium Synechocystis sp. PCC 6803: their involvement in chlorophyll biogenesis for Photosystem II. Biochim. Biophys. Acta 1807(9): 1143–1151. [DOI] [PubMed] [Google Scholar]
- Hernández-Prieto M. A., Schon V., Georg J., Barreira L., Varela J., et al. , 2012. Iron deprivation in Synechocystis: inference of pathways, non-coding RNAs, and regulatory elements from comprehensive expression profiling. G3 2(12): 1475–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Prieto M. A., Semeniuk T. A., Giner-Lamia J., Futschik M. E., 2016. The transcriptional landscape of the photosynthetic model cyanobacterium Synechocystis sp. PCC6803. Sci. Rep. 6: 22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Eggink L. L., Chen M., 2007. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 94(2–3): 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M., Nakamura K., Kojima K., Nishiyama Y., Hatakeyama W., et al. , 2010. The PedR transcriptional regulator interacts with thioredoxin to connect photosynthesis with gene expression in cyanobacteria. Biochem. J. 431(1): 135–140. [DOI] [PubMed] [Google Scholar]
- Hu Q., Miyashita H., Iwasaki I., Kurano N., Miyachi S., et al. , 1998. A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 95(22): 13319–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Marquardt J., Iwasaki I., Miyashita H., Kurano N., et al. , 1999. Molecular structure, localization and function of biliproteins in the chlorophyll a/d containing oxygenic photosynthetic prokaryote Acaryochloris marina. Biochim. Biophys. Acta 1412(3): 250–261. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M., 2016. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44(D1): D457–D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Tanaka R., Sano S., Tanaka A., Hosaka H., 2010. Identification of a gene essential for protoporphyrinogen IX oxidase activity in the cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 107(38): 16649–16654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss É., Kós P. B., Chen M., Vass I., 2012. A unique regulation of the expression of the psbA, psbD, and psbE genes, encoding the D1, D2 and cytochrome b559 subunits of the Photosystem II complex in the chlorophyll d containing cyanobacterium Acaryochloris marina. Biochim. Biophys. Acta 1817(7): 1083–1094. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Masuda T., Tajima N., Wada H., Sato N., 2014. Molecular phylogeny and intricate evolutionary history of the three isofunctional enzymes involved in the oxidation of protoporphyrinogen IX. Genome Biol. Evol. 6(8): 2141–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Klahn S., Scholz I., Matthiessen J. K., Hess W. R., et al. , 2014. Comparative analysis of the primary transcriptome of Synechocystis sp. PCC 6803. DNA Res. 21(5): 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A., Ruiz M., Zhang C.-C., 2009. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 33(2): 258–278. [DOI] [PubMed] [Google Scholar]
- Lin, Y., B. Crossett, and M. Chen, 2013 Effects of anaerobic conditions on photosynthetic units of Acaryochloris marina, pp. 121–124 in Photosynthesis Research for Food, Fuel and the Future: 15th International Conference on Photosynthesis. Springer, Berlin . [Google Scholar]
- Lopes C. T., Franz M., Kazi F., Donaldson S. L., Morris Q., et al. , 2010. Cytoscape web: an interactive web-based network browser. Bioinformatics 26(18): 2347–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin P., Lin Y., Chen M., 2013. Chlorophyll d and Acaryochloris marina: current status. Photosynth. Res. 116(2): 277–293. [DOI] [PubMed] [Google Scholar]
- Loughlin P. C., Willows R. D., Chen M., 2014. In vitro conversion of vinyl to formyl groups in naturally occurring chlorophylls. Sci. Rep. 4: 6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt J., Senger H., Miyashita H., Miyachi S., Mörschel E., 1997. Isolation and characterization of biliprotein aggregates from Acaryochloris marina, a Prochloron-like prokaryote containing mainly chlorophyll d. FEBS Lett. 410(2–3): 428–432. [DOI] [PubMed] [Google Scholar]
- Massé E., Escorcia F. E., Gottesman S., 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 17(19): 2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R., Balasubramanian D., Sun Y., Bobrovskyy M., Sumby P., et al. , 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 41: e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamizaki K., Mizoguchi T., Goto T., Tamiaki H., Fujita Y., 2008. Identification of two homologous genes, chlaI and chlaII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 283(5): 2684–2692. [DOI] [PubMed] [Google Scholar]
- Mitschke J., Georg J., Scholz I., Sharma C. M., Dienst D., et al. , 2011. An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 108(5): 2124–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita H., Ikemoto H., Kurano N., Adachi K., Chihara M., et al. , 1996. Chlorophyll d as a major pigment. Nature 383(6599): 402–402. [Google Scholar]
- Murray J. W., 2012. Sequence variation at the oxygen-evolving centre of photosystem II: a new class of ‘rogue’ cyanobacterial D1 proteins. Photosynth. Res. 110(3): 177–184. [DOI] [PubMed] [Google Scholar]
- Murry M. A., Horne A. J., Benemann J. R., 1984. Physiological studies of oxygen protection mechanisms in the heterocysts of Anabaena cylindrica. Appl. Environ. Microbiol. 47(3): 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Naito K., Yokota N., Sugita C., Sugita M., 2007. A cyanobacterial non-coding RNA, Yfr1, is required for growth under multiple stress conditions. Plant Cell Physiol. 48(9): 1309–1318. [DOI] [PubMed] [Google Scholar]
- Nomata J., Kitashima M., Inoue K., Fujita Y., 2006. Nitrogenase Fe protein-like Fe–S cluster is conserved in L-protein (BchL) of dark-operative protochlorophyllide reductase from Rhodobacter capsulatus. FEBS Lett. 580(26): 6151–6154. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Nield J., Terao A., Stauber E. J., Hippler M., et al. , 2009. Biochemical and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21(8): 2424–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospíšil P., 2012. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817(1): 218–231. [DOI] [PubMed] [Google Scholar]
- Raymond J., Blankenship R. E., 2004. Biosynthetic pathways, gene replacement and the antiquity of life. Geobiology 2(4): 199–203. [Google Scholar]
- Reinbothe S., Reinbothe C., Apel K., Lebedev N., 1996. Evolution of chlorophyll biosynthesis—the challenge to survive photooxidation. Cell 86(5): 703–705. [DOI] [PubMed] [Google Scholar]
- Rutherford A. W., Osyczka A., Rappaport F., 2012. Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett. 586(5): 603–616. [DOI] [PubMed] [Google Scholar]
- Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., et al. , 2000. Artemis: sequence visualization and annotation. Bioinformatics 16(10): 944–945. [DOI] [PubMed] [Google Scholar]
- Schedel M., Trüper H. G., 1980. Anaerobic oxidation of thiosulfate and elemental sulfur in Thiobacillus denitrificans. Arch. Microbiol. 124(2): 205–210. [Google Scholar]
- Schliep M., Crossett B., Willows R. D., Chen M., 2010. 18O labeling of chlorophyll d in Acaryochloris marina reveals that chlorophyll a and molecular oxygen are precursors. J. Biol. Chem. 285(37): 28450–28456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer G., 2016. The respiratory terminal oxidases (RTOs) of cyanobacteria, pp. 331–355 in Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling, edited by Cramer A. W., Kallas T. Springer, Dordrecht, Netherlands. [Google Scholar]
- Schmetterer G., Valladares A., Pils D., Steinbach S., Pacher M., et al. , 2001. The coxBAC operon encodes a cytochromec oxidase required for heterotrophic growth in the cyanobacterium Anabaena variabilis strain ATCC 29413. J. Bacteriol. 183(21): 6429–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M., 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012: 26. [Google Scholar]
- Shen G., Balasubramanian R., Wang T., Wu Y., Hoffart L. M., et al. , 2007. SufR coordinates two [4Fe-4S]2+, 1+ clusters and functions as a transcriptional repressor of the sufBCDS operon and an autoregulator of SufR in cyanobacteria. J. Biol. Chem. 282(44): 31909–31919. [DOI] [PubMed] [Google Scholar]
- Ślesak I., Ślesak H., Zimak-Piekarczyk P., Rozpądek P., 2016. Enzymatic antioxidant systems in early anaerobes: theoretical considerations. Astrobiology 16(5): 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka R., McLean S., Zuberova M., Hunter C. N., Tichy M., 2008. The C-terminal extension of ferrochelatase is critical for enzyme activity and for functioning of the tetrapyrrole pathway in Synechocystis strain PCC 6803. J. Bacteriol. 190(6): 2086–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Vogel J., Wassarman K. M., 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43(6): 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield T. C., Toepel J., Sherman L. A., 2008. Low-oxygen induction of normally cryptic psbA genes in cyanobacteria. Biochemistry 47(49): 12939–12941. [DOI] [PubMed] [Google Scholar]
- Summerfield T. C., Nagarajan S., Sherman L. A., 2011. Gene expression under low-oxygen conditions in the cyanobacterium Synechocystis sp. PCC 6803 demonstrates Hik31-dependent and -independent responses. Microbiology 157(2): 301–312. [DOI] [PubMed] [Google Scholar]
- Swingley W. D., Chen M., Cheung P. C., Conrad A. L., Dejesa L. C., et al. , 2008. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc. Natl. Acad. Sci. USA 105(6): 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnini P., Leitão E., Oliveira P., Ferreira D., Pinto F., et al. , 2007. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol. Rev. 31(6): 692–720. [DOI] [PubMed] [Google Scholar]
- Tomo T., Okubo T., Akimoto S., Yokono M., Miyashita H., et al. , 2007. Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium. Proc. Natl. Acad. Sci. USA 104(17): 7283–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium , 2015. UniProt: a hub for protein information. Nucleic Acids Res. 43(D1): D204–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt K., Sharma C. M., Mitschke J., Lambrecht S. J., Voss B., et al. , 2014. Comparative transcriptomics of two environmentally relevant cyanobacteria reveals unexpected transcriptome diversity. ISME J. 8(10): 2056–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voß B., Gierga G., Axmann I. M., Hess W. R., 2007. A motif-based search in bacterial genomes identifies the ortholog of the small RNA Yfr1 in all lineages of cyanobacteria. BMC Genomics 8(1): 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voß B., Georg J., Schön V., Ude S., Hess W. R., 2009. Biocomputational prediction of non-coding RNAs in model cyanobacteria. BMC Genomics 10(1): 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-L., Postier B. L., Burnap R. L., 2004. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 279(7): 5739–5751. [DOI] [PubMed] [Google Scholar]
- Wenk S.-O., Schneider D., Boronowsky U., Jäger C., Klughammer C., et al. , 2005. Functional implications of pigments bound to a cyanobacterial cytochrome b6f complex. FEBS J. 272(2): 582–592. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Nomata J., Fujita Y., 2006. Differential operation of dual protochlorophyllide reductases for chlorophyll biosynthesis in response to environmental oxygen levels in the cyanobacterium Leptolyngbya boryana. Plant Physiol. 142(3): 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D. C., Brune D. C., Vavilin D., Vermaas W. F., 2012. Photosystem II component lifetimes in the cyanobacterium Synechocystis sp. strain PCC 6803: small Cab-like proteins stabilize biosynthesis intermediates and affect early steps in chlorophyll synthesis. J. Biol. Chem. 287(1): 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A., Wittmann B. J., King J. D., Blankenship R. E., Dantas G., 2016. Transcriptomic analysis illuminates genes involved in chlorophyll synthesis after nitrogen starvation in Acaryochloris sp. CCMEE 5410. Photosynth. Res. 129(2): 171–182. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Pendse N. D., Phillips K. N., Cotner J. B., Khodursky A., 2008. Gene expression patterns of sulfur starvation in Synechocystis sp. PCC 6803. BMC Genomics 9: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Acaryochloris strain used in this study is available as an axenic culture through the NBRC culture collection (NBRC 102967). Raw gene expression data, and processed information, is available at GEO with the accession number GSE89387. File S1 contains detailed information of all supplemental files.