Abstract
Purpose of Review
Despite over a third of the world’s population being chronically infected with Toxoplasma gondii, little is known about this largely asymptomatic phase of infection. This stage is mediated in vivo by bradyzoites within tissue cysts. The absence of overt symptoms has been attributed to the dormancy of bradyzoites. In this review, we reexamine the conventional view of chronic toxoplasmosis in light of emerging evidence challenging both the nature of dormancy and the consequences of infection in the CNS.
Recent Findings
New and emerging data reveal a previously unrecognized level of physiological and replicative capacity of bradyzoites within tissue cysts. These findings have emerged in the context of a reexamination of the chronic infection in the brain that correlates with changes in neuronal architecture, neurochemistry, and behavior that suggest that the chronic infection is not without consequence.
Summary
The emerging data driven by the development of new approaches to study the progression of chronic toxoplasma infection reveals significant physiological and replicative capacity for what has been viewed as a dormant state. The emergence of bradyzoite and tissue cyst biology from what was viewed as a physiological “black box” offers exciting new areas for investigation with direct implications on the approaches to drug development targeting this drug-refractory state. In addition, new insights from studies on the neurobiology on chronic infection reveal a complex and dynamic interplay between the parasite, brain microenvironment, and the immune response that results in the detente that promotes the life-long persistence of the parasite in the host.
Keywords: Toxoplasma, Tissue cyst, Bradyzoite, Glycosylation, CNS infection
Introduction
The transmission of Toxoplasma gondii is mediated by two distinct cyst forms, the oocysts formed and shed into the environment by the definitive feline host and the tissue cyst, formed within and retained in all infected warm blooded animals [1]. Transmission of tissue cysts occurs during the act of carnivory or scavenging of infected tissues. T. gondii acquired by either form manifests as an acute infection associated with the rapid replication and spread within the body of the host of the tachyzoite form [1, 2]. This phase of infection within an immune competent host is typically asymptomatic as proliferating tachyzoites are cleared with the mounting of an aggressive innate and acquired immune response. However, unlike other infections, clearance of rapidly growing parasites fails to establish a sterile cure as a cohort of tachyzoites differentiate into the slow growing bradyzoite form establishing themselves within tissue cysts primarily in the CNS and muscle [1, 3]. These tissue cysts are maintained for the life of the host, progressing in their cycle during carnivory and completing the sexual cycle when the carnivore is a feline [1, 4].
The life-long persistence of tissue cysts and the bradyzoites they house is attributable to their relative invisibility to immune detection, a property lost when reactivation to tachyzoites occurs [5]. In the absence of immune function, most notably the loss of T cell immunity, as occurs in active HIV-AIDS, reactivation of bradyzoites to tachyzoites results in unrestricted growth [6]. Untreated active toxoplasmosis typically manifests as toxoplasmic encephalitis, on account of the CNS being a primary target for tissue cyst formation. This infection is lethal in the context of active HIV-AIDS [7]. Given the high seroprevalence of toxoplasma infections in the general human population that approaches 30 % worldwide [8], life-long persistence of the agent within tissue cysts represents a potential source of active infection following immune suppression [7]. In spite of its critical status in the pathogenesis of toxoplasma infections, surprisingly, little work has been done into dissecting bradyzoite and tissue cyst biology.
Much of what we know about bradyzoite biology comes from a combination of detailed morphological studies [3, 9–13] which reveal the organization of tissue cysts in vivo but offer little information on bradyzoite physiology. In contrast, cell culture-based systems have focused on the critical transition from tachyzoites to bradyzoites but fail to adequately address progression once in the chronic phase [14–16]. Studies focused on the transcriptomes of tissue cysts (both in culture and in vivo) by necessity [17, 18•] presented a weighted average that masks the behavior of individual tissue cysts and by extension that of individual bradyzoites within them. The recurring differences, in cyst burden and size, were largely attributed to variations with the host response [19, 20] with little consideration to the fact that bradyzoites themselves may be considerably more dynamic than has been previously imagined [21••].
Virtually, all of the functional studies to date, including those assessing the effectiveness of drugs, have used the tissue cyst (their numbers and size) as the metric. Inherent in defining the tissue cyst rather than the individual bradyzoite within it as the “unit” of the chronic phase is the assumption that cysts are largely equivalent and the bradyzoites within them uniform with regard to their physiology. The first systematic analysis of bradyzoite dynamics within cysts, recently published by our group [21••], directly challenges this notion necessitating a reassessment of how we approach understanding chronic toxoplasmosis and the development of drugs against this recalcitrant life cycle stage.
Organization of the In Vivo Tissue Cyst
The tissue cyst stage of T. gondii is defined by the elevated level of glycosylation and the transformation of the parasitophorous vacuole membrane (PVM) into the tissue cyst wall [22]. The cyst wall is 250–500 nm in thickness and is formed from the PVM with a dense outer layer sandwiched between the PVM and a more amorphous inner sponge-like layer [23]. The tachyzoite intravacuolar network is itself transformed into a meshwork of glycosylated proteins and glycolipids which form a filamentous meshwork that is connected to the tissue cyst wall [23]. The relative volume occupied by the bradyzoites and this matrix varies between cysts [11, 23, 24] and likely contributes to the different hydrodynamic densities in Percoll gradients that paradoxically shift from more dense to less dense with the progression of the chronic phase [21••].
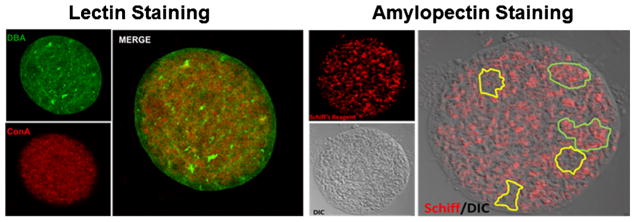
The cyst wall, clearly evident by electron microscopy, can also be visualized by fluorescence microscopy using labeled lectins. Glycosylation in both tachyzoites and tissue cysts is evident from spatial distribution of lectin reactivity [25–28]. Lectins binds their target glycans with high affinity and specificity [29]. The most prominent staining pattern is that of the tissue cyst wall using Dolichos lectin (DBA; recognizing terminal GalNAc) (Fig. 1) [27, 30, 31]. The primary target being glycosylated with GalNAc, designated TgCST1 [32••], was identified by the Weiss group as a high molecular weight mucin that is expressed both in tachyzoite vacuoles and tissue cysts, but only substantially glycosylated in the tissue cyst form [32••]. Importantly, TgCST1 plays an important structural role as knockout of this gene results in extremely fragile cysts that are susceptible to clearance [32••]. Of note, recent work from the Weiss laboratory has established that two distinct parasite-encoded ppGalNAc-Ts glycosyltransferases glycosylate TgCST1 sequentially and that this modification is critical for the structural rigidity of the cyst wall [33].
Fig. 1.
Distribution of key glycans in tissue cysts. A distinguishing feature of Toxoplasma gondii tissue cysts is the high level of glycosylation. Left panel lectin staining with FITC-conjugated Dolichos biflorus (DBA-recognizing GalNAc) lectin labels the tissue cyst wall and the intra-cyst matrix (green). In contrast, Concanavalin A (ConA-recognizing mannose and glucose, and an indicator of N-linked glycosylation) selectively stains structures within the bradyzoites and in the matrix (red) but is excluded from the tissue cyst wall (merge). Right panel the distribution of amylopectin granules within bradyzoites detected using Schiff reagent (red) overlaid on a differential interference contrast of a purified tissue cyst reveals an uneven distribution of amylopectin within the tissue cyst with clusters of bradyzoites exhibiting high levels of amylopectin (outlined in green) adjacent to areas with low amylopectin levels (outlined in yellow)
Insights into machinery associated with glycosylation emerge from the targeted disruption of a nucleotide sugar transporter TgNST1 [34•]. Loss of this transporter, the substrate profile of which was confirmed experimentally, established that the glycosylation of the cyst wall is critical for the persistence of tissue cysts in vivo, despite the absence of any notable defect for tachyzoites [34•]. Another important recent development has been the use of unnatural sugars that can be chemically modified as tracers to identify novel glycoproteins in the parasite without the restrictions imposed by lectin reactivity [35•]. Such approaches can be applied to both tachyzoites and bradyzoites to establish glycoproteomes in both life cycle stages to better define how glycosylation may impact each of these life cycle stages [35•].
Examination of the major glycosylation pathways in toxoplasma reveals that the genes required to add complex N-linked sugars to proteins are incomplete [36]. In addition, our analysis of the genome fails to reveal any genes required for the synthesis or transfer of sialic acid (Datta and Sinai unpublished). Yet, lectins directed at complex N-linked sugars and sialic acid robustly label tissue cysts suggesting that toxoplasma may actively scavenge these sugars from the host cell as a potential mechanism of immune evasion as has been proposed [37, 38].
Carbohydrate metabolism plays another vital role in bradyzoite biology as evidenced by the earliest electron microscopic studies on tissue cysts in vivo [9, 11, 24], which revealed that bradyzoites accumulate amylopectin-like carbohydrate [39, 40]. Amylopectin is a plant glucose storage polymer (composed of α1,4-glucose residues with α1,6 linked branch points) [41] which has been believed to serve as a ready source of glucose (for energy and biosynthesis) upon transmission of cyst to a new host following carnivory [39, 40]. The distribution of amylopectin visualized using Schiff staining (Fig. 1) and electron microscopy [9] appears to be non-uniform within encysted bradyzoites. Tonkin and colleagues recently demonstrated that the Ca2+-dependent protein kinase TgCDPK2 is a key regulator of amylopectin metabolism [42••]. Notably, they identified enzymes involved in T. gondii amylopectin metabolism that are phosphorylated by TgCDPK2 [42••]. When they ablated TgCDPK2, they found massive accumulations of amylopectin in tachyzoites which are tolerated [42••]. In contrast, the exaggerated accumulation of amylopectin in bradyzoites leads to bradyzoite death [42••]. This strongly suggests that amylopectin levels are tightly regulated to ensure homeostasis within the bradyzoite making this pathway a potential target for drug development.
Dynamics of Tissue Cyst Burden and Size in Chronic Infection
Most of our knowledge of the chronic phase of toxoplasma infection comes from the murine infection and has focused on tissue cysts in the brain. In reality, tissue cysts form in other tissues as well, most notably in muscle and other organs [43]. Tissue cysts in muscle are an important source of infection of humans from the consumption of raw or undercooked meat making it among the most prevalent food borne infections in the USA [44]. Experimental studies on chronic toxoplasmosis in muscle are limited [45] although the recent development of specific skeletal muscle-based infection systems will undoubtedly accelerate this work [46–48].
The focus on chronic toxoplasmosis in the brain stems from the fact that in addition to being the primary site of tissue cyst formation, it is also the tissue site where the reactivation of cysts drives toxoplasmic encephalitis in the context of immune suppression [6, 7]. On a more practical note, the brain is easily recovered intact for histological studies and easily homogenized while maintaining the integrity of tissue cysts which can be quantified microscopically or purified using Percoll gradients as originally developed [49] and subsequently refined [21••]. Studies, to establish the tissue cyst burden in rodents, both mice and rats, have been reviewed in detail [50, 51••]. Of note, tissue cyst burdens can vary significantly, with these differences impacted by the vertebrate host, host strain, as well as the toxoplasma strain being used [24, 51••]. This variability appears to be intrinsic as seen with our experience using the Type II ME49 strain, serially passaged by i.p. injection of tissue cysts of infected brain homogenates in inbred female CBA/J mice [21••]. We found that, in 99 independent tissue cyst purifications from mouse brains, the average cyst burden per mouse could range from under 500 to close to 15,000 cysts [21••]. Cyst numbers, particularly at later time points, can be the result of reactivation mediated re-seeding in addition to the cysts established at the onset of the chronic infection. This does not appear to be the case in rats, where cyst rupture without reactivation occurred frequently [51••] or in CBA/J mice where the loss of a cyst was essentially balanced by its apparent replacement, implying that vast majority of bradyzoites within the cyst were cleared [21••]. This equilibrium appears to be responsible for the average cyst burden remaining relatively stable across the 5 time points tested (weeks 3, 4, 5, 6, and 8 post-infection) [21••].
The size of tissue cysts, measured in situ [11, 51••, 52], in homogenized brain tissues [51••, 53, 54] and following purification in Percoll gradients [21••] has been shown to cover a large range from 20 to over 100 microns in diameter. Of note, in rats, tissue cysts rarely reach a diameter of 70 microns, and despite variability between toxoplasma strains, no clear pattern is observed [51••]. These distributions varied somewhat based on the specimens being measured with measurements from histological and electron microscopy tending to be smaller than that seen for specimens measured in brain homogenates and following tissue cyst purification.
Bradyzoite Replication and Growth Patterns Within Tissue Cysts During Chronic Infection
The fact that tissue cysts exhibit heterogeneity in their size suggests that they are dynamic growing entities despite being considered largely dormant and metabolically inert. If growth is indeed occurring, understanding the behavior of individual bradyzoites within the tissue cyst becomes crucial. In the course of imaging Percoll purified tissue cysts, we noted that bradyzoites nuclei stained with DAPI or Hoescht dye presented as discrete entities in optical sections. We therefore developed a unique imaging protocol and software, BradyCount 1.0 [21••] to directly enumerate nuclear cross-sections and by extension the number of bradyzoites within the widest optical section (diameter) of the tissue cyst. The development of BradyCount 1.0 and its implementation revealed that while in general larger tissue cysts contained more bradyzoites, this rule did not always hold [21••]. We therefore developed the concept of the “packing density” [21••] which quantified the number of bradyzoites within the imaged volume as a way to normalize for tissue cyst size. Interestingly, the relationship between tissue cyst size and the packing density was found to be an inverse one [21••]. As a result, larger tissue cysts contain, in general, proportionately fewer parasites and were typically less densely packed, i.e., they contained more matrix between the bradyzoites [21••]. The implication of this finding is significant as it conclusively establishes that tissue cyst size is not defined by bradyzoite replication. Rather, tissue cysts must expand in order for bradyzoites to have the space to replicate into [21••]. Thus, shifts in the packing density can be used as an indicator of the recency of bradyzoite replication within the tissue cyst.
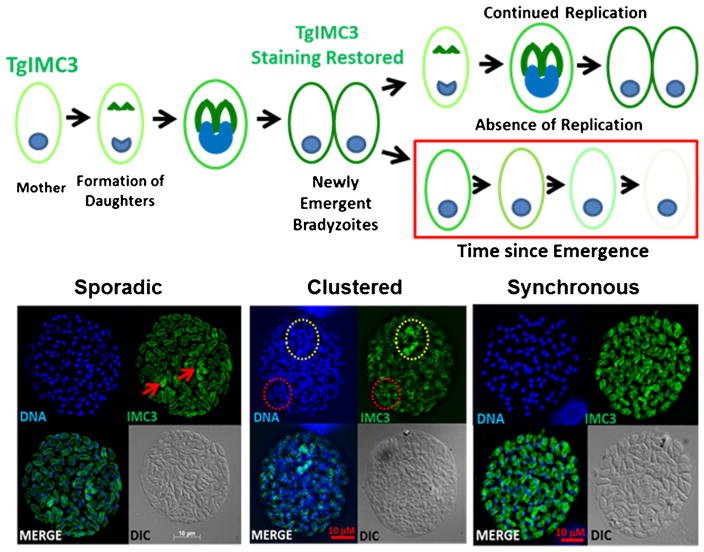
The very notion that bradyzoites can replicate has been the matter of some debate with a suggestion that should replication occur within a cyst, it must be due to a bradyzoite reverting to tachyzoite for the purpose of dividing [55]. In order to capture bradyzoite replication and establish if there were any patterns in replication, we labeled purified tissue cysts with an antibody against the inner membrane complex (IMC) component TgIMC3 [21••]. This protein has the added distinction of being more abundant in the developing daughter scaffolds and in recently emerged parasites relative to the gravid mother or other more mature parasites [56]. Furthermore, the Gubbels group established that TgIMC3 levels in the mother decrease over time unless a fresh round of replication is initiated [56] (Fig. 2). This provides for an internal marker of the recency of replication as “younger parasites” label more brightly for TgIMC3 than “aged” organisms. When examining tissue cysts for evidence of bradyzoite replication, we were able to capture evidence for replication at all time points tested [21••]. Somewhat surprisingly, distinct patterns of replication within tissue cysts emerged as well [21••]. As seen in Fig. 2, evidence of sporadic and clustered and even completely synchronized replication were all observed. The frequent detection of clustered replication that can extend to synchronous replication (a feature of tachyzoite replication within a vacuole) within a tissue cyst argues for a high level of coordination and signaling among bradyzoites within a cyst. Furthermore, this suggests that bradyzoites within a tissue cyst are heterogenous and not monolithic physiological entities [21••] as they have been assumed to be.
Fig. 2.
Evidence of replication and patterns of replication within tissue cysts. Tissue cysts and the bradyzoites within them have been viewed as dormant non-replicative entities. Replication by endodyogeny and cytokinesis in toxoplasma can be detected using antibodies against components of the inner membrane complex (IMC) including TgIMC3. Top panel the intensity of TgIMC3 is highest in developing daughter parasites and recently emerged parasites. Following emergence, the TgIMC3 signal loses intensity and can thus serve as a marker for the recency of replication. Bottom panel actively replicating bradyzoites within tissue cysts can be detected based on the level of TgIMC3 labeling. Evidence for sporadic replication (left panel, arrowheads), clustered replication (outlined in yellow) with a region where replication is not occurring (outlined in red) are seen in most cases. In several cases, highly synchronized replication whereby all the bradyzoites within tissue cyst were dividing at the same time is observed. Together, these findings suggest that the tissue cysts are dynamic entities containing replication competent bradyzoites
The likelihood of capturing replicating parasites was found to vary depending on the specific state of the infection [21••]. In contrast to tachyzoites within a vacuole, bradyzoites within tissue cysts are heterogenous with regard to their replicative potential and physiology [21••]. This greatly complicates the dissection of the chronic phase even though it progresses toward dormancy [21••]; its path to this state appears to be opportunistic and exhibits oscillatory behavior [21••] with a pattern suggestive of punctuated and potentially cyclical growth profiles evident both at the level of individual bradyzoites within tissue cysts at the population as a whole [21••]. The development of tools to address the behavior of individual bradyzoites within cysts such as BradyCount 1.0 together with ongoing development permitting quantitative measurement of other structural and physiological outputs (BradyCount 2.0, Patwardhan and Sinai, in progress) will greatly expand the range of measurable activities to gain new insights into bradyzoite biology. Given the nature of these studies where we are constrained by practical limitations regarding the amount of data that can be acquired, we are developing data-driven computational models to better understand the complex and understudied progression of chronic toxoplasmosis (Patwardhan and Sinai, in progress). The heterogeneity in bradyzoite replication and other physiological parameters [21••] suggests an opportunistic phenomenon that is not substantially affected by the events preceding it. Rather the behavior of a given bradyzoite within a tissue cyst or a population of tissue cysts within an animal is dominated by the specific physiological state (or distribution of physiological states—if examining the population) at the time of capture. This structure is less reliant on memory and lends itself to a Markov Chain model. Such models will be of great value to establish the mechanisms of both drug susceptibility and inherent resistance (see below).
How the dynamics of the chronic infection influences the host is as fascinating a question as to how the host status influences the progression of the chronic phase within the host. The first insights into how the presence of a chronic infection within the brain impacts gene expression in the brain has emerged from a deep sequencing RNA Seq study from the Knoll laboratory [18•]. Using the depth of RNA Seq and simultaneously profiling both the host and parasite transcriptomes, they establish that despite the absence of any symptoms or other evidence of the presence of the parasite dramatic changes in the expression of genes, including those involved in inflammation and immune responses, are observed [18•]. Notably, on the parasite side of the equation, expression of genes associated with an active cell cycle indicates that our notions of dormancy need to be reexamined [18•]. Thus, as regards the chronic infection in the brain, a dynamic picture emerges that is defined by bidirectional effects between the parasite and the host. Exciting new findings in the area are emerging against the backdrop of understanding the neurobiology of chronic infection.
Emerging Insights into Neurobiology of Chronic Toxoplasma Infection
With its long-term, potentially lifelong residence in the brains of healthy individuals and animals without any significant clinical consequence, chronic toxoplasma infection has been viewed as a benign condition. This absence of overt symptomology has contributed to the view that tissue cysts and the bradyzoites they house are dormant entities.
Recent developments in neurology, behavior, and neuroscience directly challenge the notion that chronic toxoplasma infections are without consequence. An emerging body of evidence suggests that the presence of an established chronic infection may contribute to the pathogenesis of diverse neurological conditions including schizophrenia [57, 58], epilepsy [59], and neurodegenerative conditions [60–62]. These studies and their impact on our understanding of chronic toxoplasmosis in human disease are reviewed elsewhere [57, 59, 60, 62]. In addition, evidence from rodent studies suggests that the chronic toxoplasmosis results in the modulation of the host’s behavior (reviewed in [63, 64]). These studies which have received considerable traction in the popular press are also discussed in several recent reviews and may have parallels in affecting human behavior as well [65].
The diverse spectrum of neurological and behavioral changes suggests that chronic toxoplasma infection in the brain does in fact manifest changes reinforcing the notion that these parasites are not truly dormant or latent. Recent studies have now begun to unveil the potential mechanistic insights into the neurobiology of chronic toxoplasmosis. These findings represent the first meaningful mechanistic steps into this complex interdisciplinary area that promises to be fertile area for investigation. As a cautionary note, care must be taken to try and untangle the effects that are a direct consequence of the parasite from those that are driven by persistent low level inflammation in the infected brain [18•, 66].
The spectrum of neurological and behavioral changes associated with chronic toxoplasmosis would suggest that the spatial distribution of cysts (which may number in the thousands, but still represent a miniscule number relative to the cellularity of the brain) may govern the phenotypic consequence [63]. While some mapping studies suggested the concentration of cysts in the amygdala [67] and hippocampus [68], others failed to find a strong association for this or any other site [51••, 69–71]. Some controversy also exists with regard to the specific cells within the brain being infected and able to house developing cysts. While there is agreement that neurons are likely to be the primary host cell of relevance [72–74], infection of microglia and astrocytes [73, 74] may also contribute as potential sites for tissue cyst formation.
The first studies on the neurochemistry of chronic toxoplasmosis are now emerging and reveal that the presence of the parasite drives changes in the levels of neurotransmitters, their precursors, and metabolites. Among the potential direct mechanisms for the modulation of neuronal action is the injection of primarily rhoptry derived host effector proteins (see reviewed in [75]) into both cells that the parasites invade as well as cells they interact with without invading [76, 77]. In this way, the parasite can directly influence not only the cell it infects but others in their vicinity.
The perturbation of dopamine, a neurotransmitter associated with several neurodegenerative and psychiatric conditions [78], is intriguing given that toxoplasma encodes and expresses a secreted tyrosine hydroxylase, a key enzyme in dopamine metabolism [79•, 80]. This presents the potential for direct manipulation of infected neurons as infected neurons release 3-fold higher levels of dopamine upon stimulation [80].
The effect of toxoplasma infection extends to gamma aminobutyric acid (GABA) synapses and signaling. GABA is a metabolite used by the parasite and is also an inhibitory neurotransmitter important in epilepsy. The Blader laboratory showed that, in the course of T. gondii infection, the redistribution (but not change in levels of) of synaptic glutamic acid decarboxylase 67 (GAD67) is associated with the development of seizures [81•]. Notably, the duration and severity of seizures triggered by GABA agonists were dependent on the infecting parasite strain arguing against a generalized effect caused by infection or inflammation [81•].
Toxoplasma effectively disrupts glutamate homeostasis in the infected brain [82••]. This is achieved by the selective downregulation of the primary astrocyte glutamate transporter GLT1 [82••]. Astrocytes serve as a critical buffer to clear extracellular glutamate which is neurotoxic and drives changes in neuronal architecture and morphology that interfere with efficient neuronal function [83]. The Wilson group, in a recent study, functionally demonstrated using microdialysis of the murine frontal cortex that levels of free glutamate were higher in the infected brain over the course of infection [82••]. While the specific molecular mechanism and parasite are not known, the finding that neuronal dysregulation in chronic toxoplasma infections can be driven by an effect on astrocyte functions reveals that this dysregulation is as sophisticated as it is complex.
Adding to the complexity of the pathogen-host relationship in the brain is the progression of the host immune response during the course of the infection (reviewed in [84]) which is beyond the scope of the current article.
Therapeutic Targeting of Chronic Toxoplasma Infections
Virtually, all studies to date to examine the effects of drugs against chronic toxoplasma infections have used the elimination of tissue cysts as the primary metric for efficacy. This is a relatively crude measure as it does not address the effect on the level of individual bradyzoites, their organization within cysts, and by extension their physiological state/replication potential. Given the heterogeneity of bradyzoites within tissue cysts and the diversity of tissue cysts within the infected brain [21••], the dissection of drug effects needs to be achieved at the level of bradyzoites. The imaging approached provided by BradyCount 1.0 allows for the direct enumeration of bradyzoites within cysts and insights into tissue cyst organization based on the computed packing density [21••]. The ongoing expansion of the capabilities in BradyCount 2.0 (Patwardhan and Sinai, in progress) will allow for more refined data regarding quantifiable physiological criteria that can serve as inputs for data-driven computational models.
The clinical management of toxoplasmosis relies on the targeting of actively growing tachyzoites [85, 86]. The primary drug combination targets folate metabolism on account of the synergizing activities of pyrimethamine and sulfadiazine targeting dihydrofolate reductase (DHFR) and dihydropterate synthase (DHPS), respectively [87]. Tissue cysts and bradyzoites within them appear refractory to these antifolate drugs likely due to the low level of DNA synthesis in the overall cyst population. The direct of demonstration of active bradyzoite replication within in vivo tissue cysts [21••] suggests that this subpopulation of bradyzoites should be susceptible. Just such an effect on bradyzoites is suggested by the reduction of genome equivalents in the presence of Pyr/Sulfa in vivo using quantitative PCR in infected brain samples [88].
The earliest indication of drug capable of reducing the tissue cyst burden was observed in the case of atovaquone [89–91]. This drug targets mitochondrial respiration at the level of the cytochrome bc1complex [92]. More recently, the endochin-like quinolones, which also target mitochondrial respiration were found to eliminate tissue cysts at between 76 and 88 % of the control, levels far greater than those achieved with atovaquone [93••]. That mitochondrial respiration targeting drugs appear to be effective suggests that mitochondrial respiration must play a role in the maintenance of the chronic state thereby providing a measurable physiological parameter for analysis. Other classes of drugs including guanabenz, an FDA-approved drug targeting translational control through eIF2α [94], both reduce the number of cysts formed when administered during the acute phase while also promoting a reduction in the cyst burden during the chronic phase [95•]. Finally, a recently developed inhibitor of the calcium-dependent protein kinase 1 (TgCDPK1) has also shown promise in reducing the cyst burden [96•] by levels similar to what was observed with the endochin-like quinolones [93••]. Importantly, unlike the encochin-like quinolones [93••], the TgCDPK1-directed compounds are effective with oral administration [96•]. Furthermore, the bradyzoite specific lethality associated with the TgCDPK2 knock-out [42••] presents this kinase and amylopectin metabolism as a legitimate drug target.
These recent developments, identifying multiple potential druggable targets coupled with an emerging appreciation for bradyzoite replication and physiology, point toward a new phase in the development of drugs targeting chronic toxoplasmosis.
Conclusions
A convergence of recent studies directly addressing the progression of chronic toxoplasmosis in vivo, advances in the neurobiology of infection, and the identification of new and effective drug targets presents new opportunities to understand this poorly studied though critical life cycle stage of the parasite. Thus, the study of tissue cyst and bradyzoite biology is emerging from the shadows where the area was treated largely as black box. The field is poised to exploit new technological developments in parasite molecular and cell biology to take on challenging questions at a level of sophistication that was unimaginable even a few years ago. Understanding the basis of bradyzoite physiology and metabolism in the context of both the immune competent and immune suppressed host will accelerate the development of much needed therapies.
Acknowledgments
Preparation of this article was supported in part by NIH/NIAID R21AI122894 awarded to APS and IDeA award from NIH/ NIGMS 5P30GM110787 (COBRE for the Center for Molecular Medicine. PI Louis B Hersh, University of Kentucky) project awarded jointly to APS and MSG.
Footnotes
Conflict of Interest Matthew Gentry, Anthony Sinai, Animesh Dhara, Elizabeth Watts, Abhijit Patwardhan, and Robert Murphy declare that they have no conflicts of interest.
Compliance of Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28(7):1019–24. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 2.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634–40. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11(2):267–99. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Chew WK, Wah MJ, Ambu S, Segarra I. Toxoplasma gondii: determination of the onset of chronic infection in mice and the in vitro reactivation of brain cysts. Exp Parasitol. 2012;130(1):22–5. doi: 10.1016/j.exppara.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Israelski DM, Chmiel JS, Poggensee L, Phair JP, Remington JS. Prevalence of Toxoplasma infection in a cohort of homosexual men at risk of AIDS and toxoplasmic encephalitis. J Acquir Immune Defic Syndr. 1993;6(4):414–8. [PubMed] [Google Scholar]
- 7.Nath A, Sinai AP. Cerebral Toxoplasmosis. Curr Treat Options Neurol. 2003;5(1):3–12. doi: 10.1007/s11940-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 8.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson DJ. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34(3):347–60. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson DJ, Graham DI, Hutchison WM. Pathological changes in the brains of mice infected with Toxoplasma gondii: a histological, immunocytochemical and ultrastructural study. Int J Exp Pathol. 1991;72(4):463–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73(6):483–91. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- 12.Fortier B, Coignard-Chatain C, Soete M, Dubremetz JF. Structure and biology of Toxoplasma gondii bradyzoites. C R Seances Soc Biol Fil. 1996;190(4):385–94. [PubMed] [Google Scholar]
- 13.Sims TA, Hay J, Talbot IC. Ultrastructural immunocytochemistry of the intact tissue cyst of Toxoplasma in the brains of mice with congenital toxoplasmosis. Ann Trop Med Parasitol. 1990;84(2):141–7. doi: 10.1080/00034983.1990.11812447. [DOI] [PubMed] [Google Scholar]
- 14.Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryotic Cell. 2004;3(4):992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44(3):721–33. doi: 10.1046/j.1365-2958.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- 16.White MW, Radke JR, Radke JB. Toxoplasma development-turn the switch on or off? Cellular microbiology. 2014 doi: 10.1111/cmi.12267. [DOI] [PubMed] [Google Scholar]
- 17.Fritz HM, Buchholz KR, Chen X, Durbin-Johnson B, Rocke DM, Conrad PA, et al. Transcriptomic analysis of toxoplasma development reveals many novel functions and structures specific to sporozoites and oocysts. PLoS One. 2012;7(2):e29998. doi: 10.1371/journal.pone.0029998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Pittman KJ, Aliota MT, Knoll LJ. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genomics. 2014;15:806. doi: 10.1186/1471-2164-15-806. The use of RNASeq to simultaneously establish the interplay between parasite and host at the level of gene expression hold the promise of dissecting the complex interplay during different stages of the chronic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoll LJ, Tomita T, Weiss LM. Bradyzoite Development. In: Weiss LM, Kim K, editors. Toxoplasma gondii The Model Apicomplexan: Perspectives and Methods. 2. London, United Kingdom: Academic Press (Elsevier); 2014. pp. 521–49. [Google Scholar]
- 20.Pittman KJ, Knoll LJ. Long-Term Relationships: the Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol Mole Biol Rev : MMBR. 2015;79(4):387–401. doi: 10.1128/MMBR.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP. Novel Approaches Reveal that Toxoplasma gondii Bradyzoites within Tissue Cysts Are Dynamic and Replicating Entities In Vivo. mBio. 2015;6(5):e01155–15. doi: 10.1128/mBio.01155-15. This work conclusively dispels the notion that parasite replication does not occur during the chronic phase of infection. The work reveals that bradyzoites within cysts are not uniform and that the properties of tissue cysts vary during the course of infection. Quantification of bradyzoites within tissue cysts vastly reveals an unappreciated level of complexity in the progression of chronic toxoplasmosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skariah S, McIntyre MK, Mordue DG. Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol Res. 2010;107(2):253–60. doi: 10.1007/s00436-010-1899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemgruber L, Lupetti P, Martins-Duarte ES, De Souza W, Vommaro RC. The organization of the wall filaments and characterization of the matrix structures of Toxoplasma gondii cyst form. Cell Microbiol. 2011;13(12):1920–32. doi: 10.1111/j.1462-5822.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson DJ, Huskinson-Mark J, Araujo FG, Remington JS. A morphological study of chronic cerebral toxoplasmosis in mice: comparison of four different strains of Toxoplasma gondii. Parasitol Res. 1994;80(6):493–501. doi: 10.1007/BF00932696. [DOI] [PubMed] [Google Scholar]
- 25.Scholtyseck E, Mehlhorn H, Muller BE. Fine structure of cyst and cyst wall of Sarcocystis tenella, Besnoitia jellisoni, Frenkelia sp. and Toxoplasma gondii. J Protozool. 1974;21(2):284–94. doi: 10.1111/j.1550-7408.1974.tb03655.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Zypen E, Piekarski G. On the ultrastructure of the Toxoplasma gondii cyst wall in the brain of the white mouse. Z Parasitenkd. 1966;28(1):45–59. [PubMed] [Google Scholar]
- 27.de Carvalho L, Souto-Padron T, de Souza W. Localization of lectin-binding sites and sugar-binding proteins in tachyzoites of Toxoplasma gondii. J Parasitol. 1991;77(1):156–61. [PubMed] [Google Scholar]
- 28.Fauquenoy S, Morelle W, Hovasse A, Bednarczyk A, Slomianny C, Schaeffer C, et al. Proteomics and glycomics analyses of N-glycosylated structures involved in Toxoplasma gondii–host cell interactions. Mol Cell Proteomics. 2008;7(5):891–910. doi: 10.1074/mcp.M700391-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Cummings RD, Etzler ME. Antibodies and Lectins in Glycan Analysis. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzo CR, et al., editors. Essentials of Glycobiology. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 633–49. [PubMed] [Google Scholar]
- 30.Derouin F, Beauvais B, Lariviere M, Guillot J. Binding of fluorescein-labelled lectins on trophozoites and cysts of 3 strains of Toxoplasma gondii. C R Seances Soc Biol Fil. 1981;175(6):761–8. [PubMed] [Google Scholar]
- 31.Sethi KK, Rahman A, Pelster B, Brandis H. Search for the presence of lectin-binding sites on Toxoplasma gondii. J Parasitol. 1977;63(6):1076–80. [PubMed] [Google Scholar]
- 32••.Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, et al. The Toxoplasma gondii Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence. PLoS Pathog. 2013;9(12):e1003823. doi: 10.1371/journal.ppat.1003823. Identification of the primary protein target responsible for lectin lableling of the cyst wall also establishes TgCST1 as a key strutural element in the mantaining cyst integrity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita T, Tatsuki T, Yakubu R, Tu V, Ma YF, Weiss LM. Making home sweet and sturdy: Toxoplasma gondii ppGalNAc-ts glycosylate in heirarchical order and confer cyst wall rigidity. mBio. 2016 doi: 10.1128/mBio.02048-16. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Caffaro CE, Koshy AA, Liu L, Zeiner GM, Hirschberg CB, Boothroyd JC. A nucleotide sugar transporter involved in glycosylation of the Toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites. PLoS Pathog. 2013;9(5):e1003331. doi: 10.1371/journal.ppat.1003331. Confirms the central role for glycoslylation in the maintenance of chronic toxoplasma infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Nazarova LA, Ochoa RJ, Jones KA, Morrissette NS, Prescher JA. Extracellular Toxoplasma gondii tachyzoites metabolize and incorporate unnatural sugars into cellular proteins. Microbes and infection/Institut Pasteur. 2016;18(3):199–210. doi: 10.1016/j.micinf.2015.11.004. This technical advance is the first use of chemical biology approaches to identify glycoproteins in Toxoplasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bushkin GG, Ratner DM, Cui J, Banerjee S, Duraisingh MT, Jennings CV, et al. Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryotic Cell. 2010;9(2):228–41. doi: 10.1128/EC.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuelson J, Robbins PW. Effects of N-glycan precursor length diversity on quality control of protein folding and on protein glycosylation. Semin Cell Dev Biol. 2015;41:121–8. doi: 10.1016/j.semcdb.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odenthal-Schnittler M, Tomavo S, Becker D, Dubremetz JF, Schwarz RT. Evidence for N-linked glycosylation in Toxoplasma gondii. Biochem J. 1993;291(Pt 3):713–21. doi: 10.1042/bj2910713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppin A, Dzierszinski F, Legrand S, Mortuaire M, Ferguson D, Tomavo S. Developmentally regulated biosynthesis of carbohydrate and storage polysaccharide during differentiation and tissue cyst formation in Toxoplasma gondii. Biochimie. 2003;85(3–4):353–61. doi: 10.1016/s0300-9084(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 40.Coppin A, Varre JS, Lienard L, Dauvillee D, Guerardel Y, Soyer-Gobillard MO, et al. Evolution of plant-like crystalline storage polysaccharide in the protozoan parasite Toxoplasma gondii argues for a red alga ancestry. J Mol Evol. 2005;60(2):257–67. doi: 10.1007/s00239-004-0185-6. [DOI] [PubMed] [Google Scholar]
- 41.Guerardel Y, Leleu D, Coppin A, Lienard L, Slomianny C, Strecker G, et al. Amylopectin biogenesis and characterization in the protozoan parasite Toxoplasma gondii, the intracellular development of which is restricted in the HepG2 cell line. Microbes Infect/Institut Pasteur. 2005;7(1):41–8. doi: 10.1016/j.micinf.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 42••.Uboldi AD, McCoy JM, Blume M, Gerlic M, Ferguson DJ, Dagley LF, et al. Regulation of Starch Stores by a Ca(2+)-Dependent Protein Kinase Is Essential for Viable Cyst Development in Toxoplasma gondii. Cell Host Microbe. 2015;18(6):670–81. doi: 10.1016/j.chom.2015.11.004. This work demonstrates that the dysregualtion of amylopectin metabolism is lethal to bradyzoites and promotes the clearance of tissue cysts. It demonstrates that amylopectin must play a role in bradyzoites rather than serve merely as an energy storage system for rapid growth following reactivation. This presents amylopecting metabolism as a potential drug target in chronic toxoplasmosis. [DOI] [PubMed] [Google Scholar]
- 43.Dubey JP. Distribution of tissue cysts in organs of rats fed Toxoplasma gondii oocysts. J Parasitol. 1997;83(4):755–7. [PubMed] [Google Scholar]
- 44.Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect. 2015;143(13):2795–804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonino P, Finol HJ, Marquez A. Skeletal muscle pathology in mice experimentally infected with Toxoplasma gondii. J Submicrosc Cytol Pathol. 1996;28(4):521–6. [PubMed] [Google Scholar]
- 46.da Ferreira-da-Silva MF, Takacs AC, Barbosa HS, Gross U, Luder CG. Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite-to-bradyzoite conversion at higher rates than fibroblasts. Int J Med Microbiol: IJMM. 2009;299(5):381–8. doi: 10.1016/j.ijmm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Guimaraes EV, de Carvalho L, Barbosa HS. Primary culture of skeletal muscle cells as a model for studies of Toxoplasma gondii cystogenesis. J Parasitol. 2008;94(1):72–83. doi: 10.1645/GE-1273.1. [DOI] [PubMed] [Google Scholar]
- 48.Swierzy IJ, Luder CG. Withdrawal of skeletal muscle cells from cell cycle progression triggers differentiation of Toxoplasma gondii towards the bradyzoite stage. Cell Microbiol. 2015;17(1):2–17. doi: 10.1111/cmi.12342. [DOI] [PubMed] [Google Scholar]
- 49.Cornelissen AW, Overdulve JP, Hoenderboom JM. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981;83(Pt 1):103–8. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- 50.Dubey JP. Comparative infectivity of Toxoplasma gondii bradyzoites in rats and mice. J Parasitol. 1998;84(6):1279–82. [PubMed] [Google Scholar]
- 51••.Dubey JP, Ferreira LR, Alsaad M, Verma SK, Alves DA, Holland GN, et al. Experimental Toxoplasmosis in Rats Induced Orally with Eleven Strains of Toxoplasma gondii of Seven Genotypes: Tissue Tropism, Tissue Cyst Size, Neural Lesions, Tissue Cyst Rupture without Reactivation, and Ocular Lesions. PloS One. 2016;11(5):e0156255. doi: 10.1371/journal.pone.0156255. This comprehensive study and review of the literature in rats and mice presents the simialrites and differences in these model rodent systems. Given the body of behavioral work in rats, the revisiting of the rat infection model will be useful for the integration of future behavioral studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Der Waaij D. Formation, growth and multiplication of Toxoplasma gondii cysts in mouse brain. Trop Georg Med. 1959;11:345–60. [Google Scholar]
- 53.Sullivan AM, Zhao X, Suzuki Y, Ochiai E, Crutcher S, Gilchrist MA. Evidence for finely-regulated asynchronous growth of Toxoplasma gondii cysts based on data-driven model selection. PLoS Comput Biol. 2013;9(11):e1003283. doi: 10.1371/journal.pcbi.1003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hooshyar H, Rostamkhani P, Arbabi M. Study on growth of Toxoplasma gondii tissue cyst in laboratory mouse. Jundishpur J Microbiol. 2009;2(4):140–3. [Google Scholar]
- 55.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, et al. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol. 2011;13(1):18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elsheikha HM, Busselberg D, Zhu XQ. The known and missing links between Toxoplasma gondii and schizophrenia. Metab Brain Dis. 2016;31(4):749–59. doi: 10.1007/s11011-016-9822-1. [DOI] [PubMed] [Google Scholar]
- 58.Sutterland AL, Fond G, Kuin A, Koeter MW, Lutter R, van Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta psychiatrica Scandinavica. 2015 doi: 10.1111/acps.12423. [DOI] [PubMed] [Google Scholar]
- 59.Ngoungou EB, Bhalla D, Nzoghe A, Darde ML, Preux PM. Toxoplasmosis and epilepsy–systematic review and meta analysis. PLoS Negl Trop Dis. 2015;9(2):e0003525. doi: 10.1371/journal.pntd.0003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahami-Oskouei M, Hamidi F, Talebi M, Farhoudi M, Taheraghdam AA, Kazemi T, et al. Toxoplasmosis and Alzheimer: can Toxoplasma gondii really be introduced as a risk factor in etiology of Alzheimer? Parasitol Res. 2016 doi: 10.1007/s00436-016-5075-5. [DOI] [PubMed] [Google Scholar]
- 61.Mohle L, Israel N, Paarmann K, Krohn M, Pietkiewicz S, Muller A, et al. Chronic Toxoplasma gondii infection enhances beta-amyloid phagocytosis and clearance by recruited monocytes. Acta Neuropathol Commun. 2016;4:25. doi: 10.1186/s40478-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prandota J. Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am J Alzheimer’s Dis Other Dementias. 2014;29(3):205–14. doi: 10.1177/1533317513517049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour - location, location, location? J Exp Biol. 2013;216(Pt 1):113–9. doi: 10.1242/jeb.074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster JP, Kaushik M, Bristow GC, McConkey GA. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J Exp Biol. 2013;216(Pt 1):99–112. doi: 10.1242/jeb.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33(3):757–60. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parlog A, Schluter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37(3):159–70. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- 67.Vyas A. Mechanisms of Host Behavioral Change in Toxoplasma gondii Rodent Association. PLoS Pathog. 2015;11(7):e1004935. doi: 10.1371/journal.ppat.1004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasitol Res. 2012;111(1):53–8. doi: 10.1007/s00436-011-2800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berenreiterova M, Flegr J, Kubena AA, Nemec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6(12):e28925. doi: 10.1371/journal.pone.0028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Afonso C, Paixao VB, Costa RM. Chronic Toxoplasma infection modifies the structure and the risk of host behavior. PLoS One. 2012;7(3):e32489. doi: 10.1371/journal.pone.0032489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. Temporal and spatial distribution of Toxoplasma gondii differentiation into Bradyzoites and tissue cyst formation in vivo. Infect Immun. 2008;76(8):3491–501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, et al. Neurons are the Primary Target Cell for the Brain-Tropic Intracellular Parasite Toxoplasma gondii. PLoS Pathog. 2016;12(2):e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melzer TC, Cranston HJ, Weiss LM, Halonen SK. Host Cell Preference of Toxoplasma gondii Cysts in Murine Brain: A Confocal Study. J Neuroparasitology. 2010:1. doi: 10.4303/jnp/N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sibley LD, Qiu W, Fentress S, Taylor SJ, Khan A, Hui R. Forward genetics in Toxoplasma gondii reveals a family of rhoptry kinases that mediates pathogenesis. Eukaryotic Cell. 2009;8(8):1085–93. doi: 10.1128/EC.00107-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, et al. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 2012;8(7):e1002825. doi: 10.1371/journal.ppat.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6(1):79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 78.Rangel-Barajas C, Coronel I, Floran B. Dopamine Receptors and Neurodegeneration. Aging Dis. 2015;6(5):349–68. doi: 10.14336/AD.2015.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PloS One. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801. The potential for direct inteference with dopamine metabolism establishes a potential mechanism for parasite mediated modulation of CNS function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.Brooks JM, Carrillo GL, Su J, Lindsay DS, Fox MA, Blader IJ. Toxoplasma gondii Infections Alter GABAergic Synapses and Signaling in the Central Nervous System. mBio. 2015;6(6):e01428–15. doi: 10.1128/mBio.01428-15. Exposition of infection mediated neurological changes and their association with structural changes in GABAergic responses begins to examine the basis for potential paasrite driven changes during chronic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82••.David CN, Frias ES, Szu JI, Vieira PA, Hubbard JA, Lovelace J, et al. GLT-1-Dependent Disruption of CNS Glutamate Homeostasis and Neuronal Function by the Protozoan Parasite Toxoplasma gondii. PLoS Pathogens. 2016;12(6):e1005643. doi: 10.1371/journal.ppat.1005643. This study is important in that it not only describes the changes instituted in the infected mouse brain but also provides a mechansitic framework supported by biochemical data in addition to quantitative moprhometry to document infection-related changes in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch - Eur J Physiol. 2010;460(2):525–42. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 84.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10(11):766–78. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaye A. Toxoplasmosis: diagnosis, treatment, and prevention in congenitally exposed infants. J Pediatr Health Care. 2011;25(6):355–64. doi: 10.1016/j.pedhc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Rajapakse S, Chrishan Shivanthan M, Samaranayake N, Rodrigo C, Deepika FS. Antibiotics for human toxoplasmosis: a systematic review of randomized trials. Pathog Glob Health. 2013;107(4):162–9. doi: 10.1179/2047773213Y.0000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyde JE. Targeting purine and pyrimidine metabolism in human apicomplexan parasites. Curr Drug Targets. 2007;8(1):31–47. doi: 10.2174/138945007779315524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Notarangelo FM, Wilson EH, Horning KJ, Thomas MA, Harris TH, Fang Q, et al. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr Res. 2014;152(1):261–7. doi: 10.1016/j.schres.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Araujo FG, Huskinson-Mark J, Gutteridge WE, Remington JS. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36(2):326–30. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferguson DJ, Huskinson-Mark J, Araujo FG, Remington JS. An ultrastructural study of the effect of treatment with atovaquone in brains of mice chronically infected with the ME49 strain of Toxoplasma gondii. Int J Exp Pathol. 1994;75(2):111–6. [PMC free article] [PubMed] [Google Scholar]
- 91.Huskinson-Mark J, Araujo FG, Remington JS. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 1991;164(1):170–1. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- 92.McFadden DC, Boothroyd JC. Cytochrome b mutation identified in a decoquinate-resistant mutant of Toxoplasma gondii. J Eukaryot Microbiol. 1999;46(5):81S–2S. [PubMed] [Google Scholar]
- 93••.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Nat Acad Sci USA. 2012;109(39):15936–41. doi: 10.1073/pnas.1208069109. The first family of drugs that exhibit signficant efficacy in the clearance of tissue cysts in the mouse model. The study also provides valuable insights into the metabolic and physiological state of bradyzoites supporting a view for their being more active than previously considered. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang M, Joyce BR, Sullivan WJ, Jr, Nussenzweig V. Translational control in Plasmodium and toxoplasma parasites. Eukaryotic Cell. 2013;12(2):161–7. doi: 10.1128/EC.00296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Benmerzouga I, Checkley LA, Ferdig MT, Arrizabalaga G, Wek RC, Sullivan WJ., Jr Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob Agents Chemother. 2015;59(11):6939–45. doi: 10.1128/AAC.01683-15. The establishment of translational control as a potential target for drug dvelopment. The study also highlights the utility of drug repurposing as a strategy to streamline drug dvelopment for what may be considered orphan infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Vidadala RS, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, et al. Development of an Orally Available and Central Nervous System (CNS) Penetrant Toxoplasma gondii Calcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitor with Minimal Human Ether-a-go-go-Related Gene (hERG) Activity for the Treatment of Toxoplasmosis. J Med Chem. 2016;59(13):6531–46. doi: 10.1021/acs.jmedchem.6b00760. Identification of TgCDPK’s as targets for drug development against chronic toxoplasma infections presents an additional and legitimate target. The development of an orally available compound holds promise for treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]