Abstract
Objective(s)
Fibromyalgia (FM) is a chronic, common pain disorder characterized by hyperalgesia. A key mechanism by which Cognitive Behavioral Therapy (CBT) fosters improvement in pain outcomes is via reductions in hyperalgesia and pain-related catastrophizing, a dysfunctional set of cognitive-emotional processes. However, the neural underpinnings of these CBT effects are unclear. Our aim was to assess CBT’s effects on the brain circuitry underlying hyperalgesia in FM patients, and to explore the role of treatment-associated reduction in catastrophizing as a contributor to normalization of pain-relevant brain circuitry and clinical improvement.
Methods
Sixteen high-catastrophizing FM patients were enrolled in the study and randomized to 4 weeks of individual treatment with either CBT or a Fibromyalgia Education (control) condition. Resting state fMRI (rs-fMRI) scans evaluated functional connectivity between key pain-processing brain regions at baseline and post-treatment. Clinical outcomes were assessed at baseline, post-treatment and 6-month follow-up.
Results
Catastrophizing correlated with increased resting state functional connectivity between S1 and anterior insula. The CBT group showed larger reductions (compared to the Education group) in catastrophizing at post-treatment (p<0.05), and CBT produced significant reductions in both pain and catastrophizing at the 6-month follow-up (p<0.05). Patients in the CBT group also showed reduced resting state connectivity between S1 and anterior/medial insula at post-treatment; these reductions in resting state connectivity were associated with concurrent treatment-related reductions in catastrophizing.
Discussion
These results add to the growing support for the clinically important associations between S1-insula connectivity, clinical pain, and catastrophizing, and suggest that cognitive-behavioral therapy may, in part via reductions in catastrophizing, help to normalize pain-related brain responses in FM.
Keywords: Cognitive Behavioral Therapy (CBT), Insula, Fibromyalgia, fMRI, catastrophizing
Introduction
Fibromyalgia (FM) is a chronic musculoskeletal pain condition that affects 2–4% of the population and is characterized by anatomically widespread pain symptoms accompanied by fatigue, disturbed sleep and mood (1–5). Fibromyalgia pain is experienced predominantly in the muscles and soft tissue, though the diverse and widespread symptoms may also extend to nearly any anatomic region. This breadth of symptomatology is consistent with the view that FM is a pervasive nervous system disorder (6) involving a complex interaction of biopsychosocial mechanisms. Some of the hallmarks of FM include: 1) alterations in central pain-modulatory processes in the spinal cord and brain, 2) a prominent role of negative affective factors in maintaining pain and disability, 3) a relative lack of efficacy of many “peripheral” treatments such as local trigger point injections.
Though FM is often considered challenging to treat, some pharmacologic and non-pharmacologic interventions have shown promise in reducing its symptoms and impact. Recent meta-analyses and reviews suggest that Cognitive Behavioral Therapy (CBT), compared to other active treatments, reduces pain intensity, disability, and emotional distress among individuals with FM (7). CBT uses active, structured techniques to alter distorted thoughts and negative moods, and with as few as 2–4 sessions, can produce adaptive, lasting changes in pain-related outcomes (8–10).
Though the mechanisms supporting CBT’s benefits have not been fully elucidated (11), it is known that CBT acts to reduce negative affective responses to pain such as those characterized by pain-related catastrophizing (12). Catastrophizing, commonly measured by the Pain Catastrophizing Scale (PCS)(13), is a pain-specific psychosocial construct comprised of cognitive and emotional processes such as helplessness, pessimism, rumination about pain-related symptoms, and magnification of pain complaints (14, 15). While catastrophizing positively correlates with general measures of negative affect such as depressive symptoms and anxiety, it also has a unique and specific influence on pain-related outcomes (12). Overall, greater catastrophizing is associated with amplified attentional focus on pain (16–18), serves as a risk factor for long-term pain (19), and correlates with the presence of disproportionately-negative sequelae of pain (e.g., worsening physical and psychological disability and/or higher healthcare costs) (20–22). Process analyses of CBT treatment studies indicate that changes in catastrophizing and negative affect precede changes in clinical pain, and that CBT’s effects on catastrophizing last for months or years (23, 24). Despite a recent proliferation of research on CBT for chronic pain, however, there has been very limited investigation of its effects on the central nervous system’s processing of pain-related information in FM (25). Indeed, only one controlled, neuroimaging-based, study (26) of a CBT treatment has been conducted in FM patients; the CBT group showed changes in activation and connectivity within regions of the pre-frontal cortex at post-treatment, and reported reductions in anxiety and pain at the 3-month follow-up.
The Jensen et al. trial (26) represents an important step toward characterizing the neural mechanisms by which CBT shapes long-term improvements in pain-related outcomes. Unfortunately, there are no published studies of the role of catastrophizing in contributing to these outcomes. Given that previous non-neuroimaging CBT studies have identified catastrophizing as a crucial process variable, it seems likely that changes in catastrophizing may contribute critically to the putative “normalization” of brain function that CBT produces. Moreover, several recent fMRI studies have indicated that catastrophizing in patients with functional pain conditions such as fibromyalgia and irritable bowel syndrome is associated with the hyperalgesia that characterizes these conditions, and also with alterations in pain-related brain activation or functional connectivity (27–29). Specifically, Kim et al. (27) found that pain-evoked increase in primary somatosensory cortex (S1)-insula connectivity was correlated with catastrophizing – patients with greater PCS scores also demonstrated greater pain-evoked increases in S1-insula connectivity. Our aim in the present randomized, controlled trial was to assess CBT’s effects on brain circuitry underlying clinical pain and hyperalgesia, and to evaluate the association between treatment-related changes in catastrophizing and treatment-related changes in pain-related brain circuitry. Our primary hypothesis was that CBT, compared to a control condition matched for professional interaction, would reduce catastrophizing in FM patients, diminish functional connectivity between S1 and insula cortex and regions of the default mode network (which have previously been linked to elevated FM pain severity (28, 30–33)), and produce long-term reductions in clinical pain.
Methods
In total, we screened forty-four fibromyalgia patients (Figure 1). We required a diagnosis of fibromyalgia for at least 1 year (as confirmed by their rheumatologist and past medical records), and patients also had to meet the recently-promulgated American College of Rheumatology criteria (Wolfe et al., 2010), which require the presence of widespread pain as well as a number of somatic and cognitive symptoms (34). We enrolled a total of seventeen high catastrophizing patients that met the inclusion and exclusion criteria described below, and sixteen of them were randomized to treatment. The Partners Human Research Committee approved this study, and written informed consent was obtained from all participants. After the baseline visit, FM patients were randomly assigned into either a month-long 4-session individual CBT treatment program or a month-long 4-session FM education treatment program. The education group received CBT following completion of their post-treatment assessment and then both groups were followed up at 6 months post-treatment.
Figure 1.
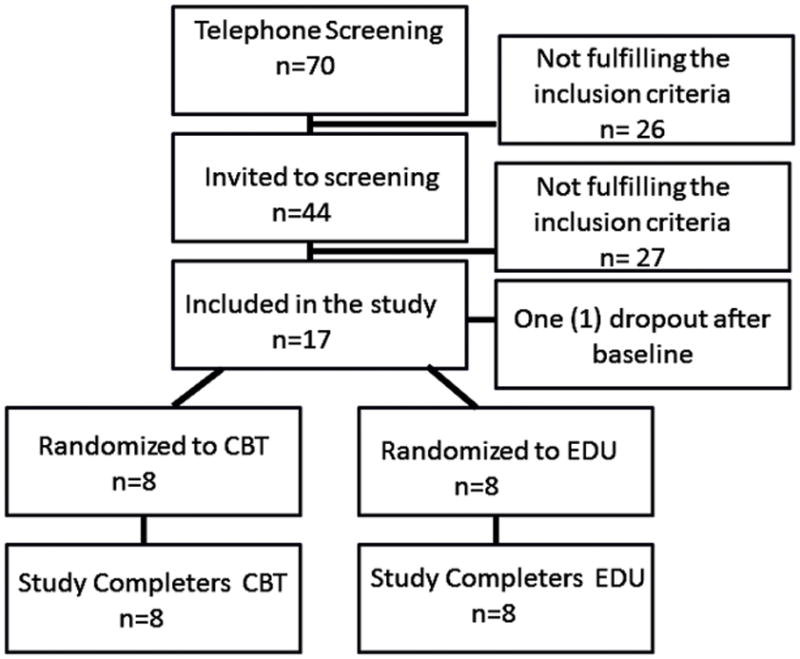
Study Flow
Specific trial eligibility criteria were as follows:
Inclusion criteria: 1. At least 18 years old, 2. Documented presence of rheumatologist-diagnosed FM for at least 1 year, 3. Meet the revised Wolfe et al. ACR criteria for FM (35), 4. Score on the Pain Catastrophizing Scale (PCS) of at least 21 (i.e., a range that represents the top 50% of FM patients in our earlier samples (28, 30).
Exclusion Criteria: 1. History of clinically significant anxiety symptoms interfering with fMRI procedures (e.g., claustrophobia, panic disorder), 2. Recent history of cardiac events such as myocardial infarction, 3. History of significant head injury, 4. Peripheral neuropathy, 5. Use of certain centrally-acting analgesic medications such as opioids, 6. History of substance abuse, 7. Concurrent autoimmune or inflammatory disease, 8. Implanted metallic objects, 9. Pregnancy, 10. Diseases affecting the central nervous system (e.g., multiple sclerosis, Parkinson’s disease), 11. Serious psychiatric conditions precluding participation (e.g., psychotic disorders).
Procedures
Subjects participated in two separate study baseline visits on different days: a behavioral visit and an imaging visit. The baseline behavioral visit included the process of informed consent, completion of self-report questionnaire measures, psychophysical testing, and confirmation of eligibility. The behavioral visit also served to determine the appropriate individually-tailored stimulus intensities to be used subsequently in the imaging session.
As described in prior studies (27, 28) we utilized cuff pain algometry (CPA) to produce tonic, deep-tissue pain. CPA stimuli can be applied tonically, and have a preferential effect on deep tissue nociceptors (36, 37). Mechanical stimuli were delivered on the calf using a 13.5cm-wide velcro-adjusted pressure cuff, connected to a rapid cuff inflator (Hokanson E20 AG101, Hokanson Inc, Bellevue, WA). At the behavioral visit, subjects were familiarized with the CPA procedures. Subjects sat comfortably on a chair with their feet resting on a support at a slightly elevated position. The cuff was then secured around the belly of the gastrocnemius muscle. Individual pressure calibration began by inflating the cuff to 60 mmHg of pressure and making adjustments in 10 mmHg increments until a pain intensity rating of ~50/100 (NRS ranged from 0, no pain, to 100, worst pain imaginable) was first obtained.
Standard demographic information and medical history information were collected by self-report. Questionnaires included the following:
Baseline Questionnaires
Widespread Pain Index and Symptom Severity Questionnaire (34)
We defined fibromyalgia using the modified 2010 American College of Rheumatology Diagnostic Criteria for Fibromyalgia (ACR 2010), which has been validated as a self-reported method for measuring fibromyalgia in a population with chronic pain.
Short form 36 health survey (SF-36) (38)
The SF-36 includes 36 questions assessing health-related quality of life. It has two global indices of quality of life, a Physical Health subscale and a Mental Health subscale Scores range from 0 to 100, with higher scores indicating better quality of life.
Visual Analog Scale for Fatigue (VAS-F (39))
A VAS was used for rating the severity of fatigue experienced by patients within the past two weeks. This scale consisted of a vertical line 10 cm in length, and the scale is anchored by “no fatigue” (score of 0) and “fatigue as bad as it could be” (score of 10).
Outcome measures (assessed at baseline, post-treatment, and 6-month follow-up)
The Brief Pain Inventory (BPI) (40) is a well-validated, widely used, and frequently recommended instrument that measures pain severity and pain-related interference for patients with fibromyalgia and other chronic pain conditions.
The Beck Depression Inventory (BDI) (41) is a well-validated, commonly-used, general measure of depressive symptomatology.
The Pain Catastrophizing Scale (PCS)(42) is a widely-used self-report measure of catastrophic thinking associated with pain. The Pain Catastrophizing Scale has good psychometric properties in pain patients and controls. The PCS includes three subscales: rumination, magnification, and helplessness.
FMRI Procedures
Prior to undergoing the scanning procedures, subjects completed a Safety Screening Checklist for MRI. Participants underwent fMRI scanning at a baseline timepoint, before being randomized to one of the interventions (i.e., the pre-intervention scan). Participants underwent the same fMRI procedures following completion of the intervention visits (i.e., the post-intervention scan).
FMRI data were acquired using a 3T Siemens TIM Trio MRI System (Siemens Medical, Erlangen, Germany) equipped for echo planar imaging with a 32-channel head coil. A whole brain T2*-weighted gradient echo BOLD EPI pulse sequence was used (TR/TE=2sec/30ms, f.a.=90°, 37 AC-PC aligned axial slices, voxel size=3.1×3.1×3.6mm). We also collected anatomical data, using a multi-echo MPRAGE pulse sequence (TR/TE1/TE2/TE3/T4= 2530/1.64/3.5/5.36/7.22 ms, flip angle=7°, voxel size=1mm isotropic). FMRI scans evaluated resting state connectivity over the course of a 6-minute resting-state fMRI acquisition period (180 scans), during which subjects were asked to remain awake with eyes open. During the imaging procedures, electrocardiography and pneumobelt respiratory volume data were collected concurrently, for the purpose of correction for cardiorespiratory artifacts in the fMRI data. Participants’ head motion was minimized using foam pads placed around the head along with a forehead strap.
Interventions
Following the baseline assessment, participants were randomized to one of the two 4-week individual therapy interventions: Cognitive Behavioral Therapy (CBT) or the Education (control) condition. The treatments were matched for degree of professional contact; both treatments involved four 60–70 minute visits conducted by the same licensed clinical psychologist. Study participants were informed that they would be randomized to receive “one of two behavioral interventions to improve quality of life in fibromyalgia patients.” After the end of the 4 treatment visits, subjects underwent a second scanning session which was identical to the baseline scan to assess intervention-related changes.
CBT
Treatment sessions used active, structured techniques to alter distorted thoughts, with a focus on acquiring and practicing cognitive and emotion-regulation skills. CBT was based on a pain self-management paradigm, and involved the identification and reduction of maladaptive pain-related cognitions (i.e., catastrophizing) using techniques such as relaxation, visual imagery, thought challenging, and distraction. CBT prominently emphasized in-vivo practice during each session, and featured home practice using written exercises. In particular, cognitive restructuring was used to help patients recognize the relationships between thoughts, feelings and behaviors. Patients learned to identify, evaluate, and challenge negative thoughts and to diminish the degree of catastrophizing about pain.
Education
This condition, matched for amount of professional contact, included information about fibromyalgia and about chronic pain. The sessions provided a variety of information about the nature and presumed causes of fibromyalgia, but they involved no active skills training or homework assignments. Education is often utilized as an active control condition that provides a comparator in CBT in controlled trials (43). This educational intervention was developed in order to control for important nonspecific factors related to therapist attention and outcome expectancy, as well as natural history and regression to the mean. Following completion of the education intervention, all patients in this arm of the study were offered the 4-session CBT treatment.
Data Analysis
The CBT and Education groups were compared from baseline to post-treatment using 2X2 factorial analyses of variance (ANOVAs) on the clinical outcome measures: BPI Pain severity, BPI Pain Interference, PCS, and BDI scores. A repeated measures ANOVA was used to evaluate whether, at the 6-month follow-up (when all patients had received CBT), changes from baseline were observed. We then evaluated relationships between changes in outcome variables and changes in brain connectivity (see below) using Pearson correlations.
Functional MRI data were processed using FMRI Expert Analysis Tool version 5.0, which is part of Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) (online at www.fmrib.ox.ac.uk/fsl). Data underwent the following preprocessing: physiologic (cardiorespiratory) artifact correction using RETROICOR, motion correction, slice timing correction, non-brain removal. Time-series statistical analysis was performed using FMRIB’s Improved Linear Model with local autocorrelation correction. Cortical surface reconstruction was performed using FreeSurfer software (online at http://surfer.nmr.mgh.harvard.edu/) for improved structural/functional co-registration purposes. A recently developed automated boundary-based registration algorithm (FreeSurfer’s bbregister tool) was used for co-registration. Scans were registered to the Montreal Neurological Institute (MNI) template MNI152 standard space using FMRIB’s Nonlinear Image Registration Tool. Data were then resampled to 2-mm isotopic voxels and spatially smoothed (full-width half-maximum of 6 mm), followed by high-pass temporal filtering (f = 0.006 Hz). Parameter estimates and relative variances for each explanatory variable were then included in mixed-effects group level analyses, performed using FMRIB’s Local Analysis of Mixed Effects, with enabled automatic outlier detection.
Functional connectivity was computed using seed-based correlation analysis. Based on our previous results (27, 28), we defined a seed representing lower leg area (S1leg: 8, −38, 68 mm in MNI coordinates). Our previous analysis found that this seed (S1leg) showed decreased resting connectivity to other S1 subregions in FM patients (27). For connectivity analyses, we extracted the averaged fMRI time series signal from a 4-mm radius sphere centered on the identified peak coordinates. Resultant connectivity maps, and their variance, from each individual were passed up to group level analyses to explore differences between the CBT and Education intervention groups from pre- to post-treatment using FMRIB’s Local Analysis of Mixed Effects (FLAME steps 1+2). We also performed whole brain voxel-wise linear regression analysis to investigate the link between changes in functional connectivity and changes in PCS scores. For this linear regression analysis, we combined subjects from both the CBT with EDU groups as both groups showed significantly decreased PCS scores after treatment. All brain maps were thresholded using cluster correction for multiple comparisons with a cluster-forming threshold of Z>2.3 and a cluster-size threshold of p<0.05 to control for family-wise error.
All statistical analyses for behavioral data were performed using SPSS 22.0 with an alpha level of 0.05.
Results
In this trial, all 16 randomized participants completed the study successfully; one participant dropped out following the baseline assessment (the subject did not attend any treatment visits and was unreachable following the baseline assessment). Among the 16 completers, all subjects completed all four treatment visits. Demographic and clinical data are presented in Table 1. The Education and CBT groups did not differ at baseline in BPI, PCS, or BDI scores. ANOVAs on the pre- to post-treatment data revealed a significant effect of Time and a Group X Time interaction for PCS scores: [F(1,15) = 4.5, p<0.05, for the interaction] (Figure 2). Both groups showed reductions in PCS scores, but the decrease was larger in the CBT relative to the Education group. The follow-up assessment was conducted six months after treatment, and was completed by fifteen patients. Repeated measures ANOVAs revealed that PCS (p<0.01) and BDI Pain Interference (p<0.05) scores were significantly reduced from baseline at the 6-month follow-up (see Table 2, and Figures 2 and 3). Overall, treatment-related changes in catastrophizing tended to be larger than changes in other outcome variables (e.g., a 64% reduction in PCS scores at the completion of 6-month follow-up). Collectively, changes in catastrophizing were strongly related to long-term changes in pain over the course of the study: The correlation of 6-month changes in PCS with 6-month changes in BPI Pain Severity is r=0.74, p<0.01, and with 6-month changes in BPI Pain Interference is r=0.79, p<0.01.
Table 1.
Sociodemographics and clinical variables at baseline
| Age (Mean ±SD) | 45.7 ± 12.2 |
| % Male | 17.1% |
| % Married | 37.1% |
| %White | 81.4% |
| %Employed | 27.1% |
| %Post-secondary degree | 14.3% |
| Fibromyalgia symptom duration (years) | 12.5 ± 12.2 |
| Weight (pounds) | 177.4 ± 41.7 |
| Questionnaire Data | |
| BPI (Severity) | 5.6 ± 1.7 |
| BPI (Interference) | 6.2 ± 1.7 |
| Pain Catastrophizing Scale | 33.9 ± 6.7 |
| Beck Depression Inventory | 18.2 ± 8.8 |
| Fatigue Severity (VAS), 0–10 scale | 2.9 ± 2.0 |
| SF-36 Physical Health | 37.4 ± 22.2 |
| SF-36 Mental Health | 50.0 ± 19.2 |
Figure 2.
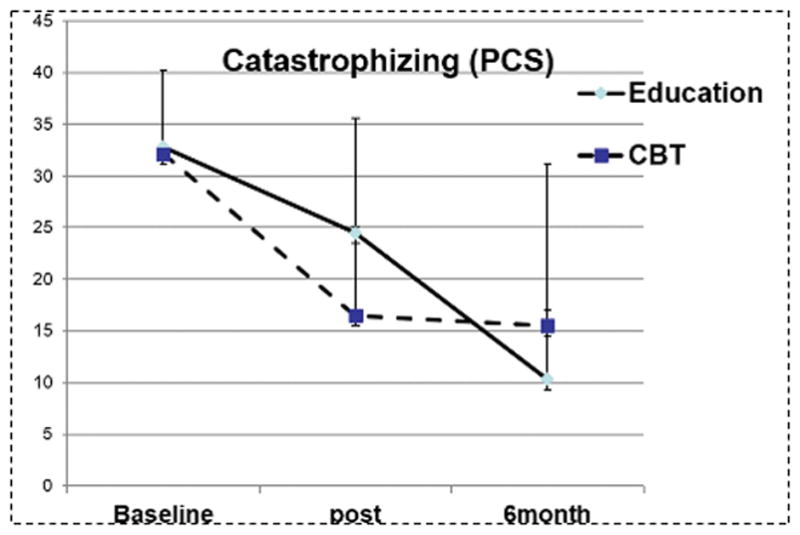
Pain Catastrophizing Scale scores at baseline, post and 6 month follow-up
Table 2.
Changes in BPI, PCS and BDI between baseline, post-treatment and 6-month follow-up
| Outcome Variables | Group | post- baseline | 6months- baseline* | ||
|---|---|---|---|---|---|
|
|
|||||
| Mean diff (SD) | P value | Mean diff (SD) | P value | ||
| Pain Severity (BPI) | |||||
| (0–10 scale) | CBT | −.35 (2.0) | .47 | −1.23 (2.19) | .18 |
| EDU | −.28 (1.8) | .17 | −1.85 (3.34) | .19 | |
| Pain Interference (BPI) | |||||
| (0–10 scale) | CBT | −1.5 (2.9) | .21 | −2.5 (2.7) | .05 |
| EDU | −.39 (1.6) | .78 | −1.5 (3.1) | .21 | |
| Pain Catastrophizing Scale (PCS) | |||||
| (0–52) | CBT | −14.1 (6.6) | .001 | −16.2 (12.7) | .01 |
| EDU | −8.5 (9.2) | .06 | −20.5 (13.1) | .006 | |
| Beck Depression Inventory (BDI) | |||||
| (0–63) | CBT | −3.5 (7.9) | .23 | −5.6 (11.1) | .22 |
| EDU | −2.0 (4.4) | .25 | −0.07 (4.8) | .97 | |
The Education group received CBT between the “post” evaluation and 6-month follow-up
Figure 3.
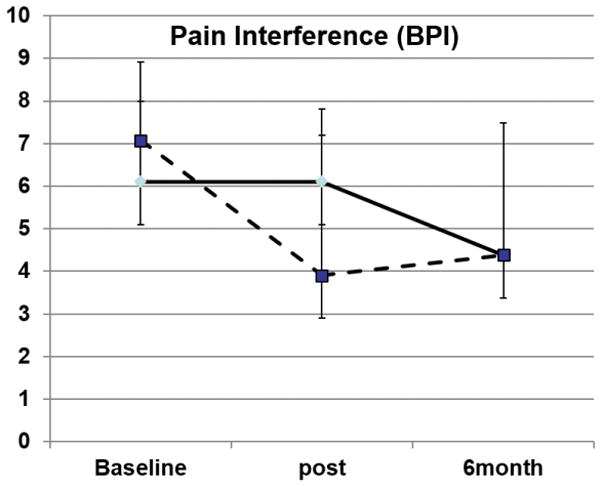
BPI Pain Interference scores at baseline, post and 6 month follow-up
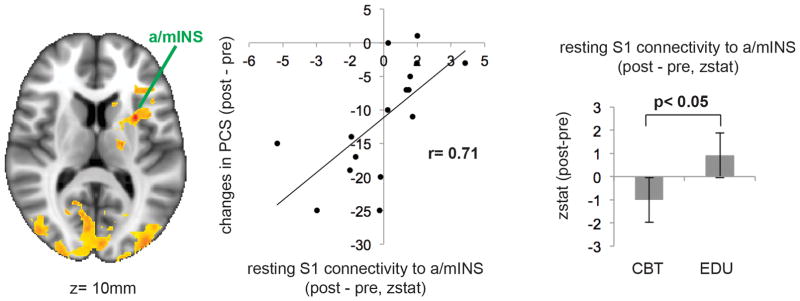
Our brain connectivity analysis then explored whether changes in S1 connectivity following therapy were associated with the reductions in PCS reported by subjects. Whole brain voxel-wise linear regression analysis demonstrated that changes in connectivity between S1 and anterior/medial insula was correlated with post-treatment (combined CBT and EDU) changes in PCS scores (Figure 4). Other regions demonstrating associations between changes in PCS and changes in S1 connectivity were cuneus, precuneus, occipital cortex, inferior frontal gyrus, thalamus, and cerebellum (Table 3).
Figure 4.
Functional connectivity effects, with group differences in pre- to post-treatment changes in S1-a/mINS connectivity and a scatterplot of the association between pre- to post-treatment changes in PCS and pre- to post-treatment changes in S1-a/mINS connectivity
Table 3.
Brain regions showing significant correlation between changes in S1leg connectivity (Post-Pre) and changes in PCS (Post-Pre) scores.
| side | size (mm3) | MNI coordinates
|
peak z-stat | |||
|---|---|---|---|---|---|---|
| X (mm) | Y (mm) | Z (mm) | ||||
| cuneus | R | 1,600 | 6 | −88 | 18 | 5.31 |
| cuneus | L | 6,584 | −6 | −90 | 34 | 6.79 |
| precuneus | L | 3,984 | −18 | −72 | −24 | 4.58 |
| occipital cortex | R | 2,312 | 36 | −86 | −4 | 4.66 |
| occipital cortex | L | 944 | −42 | −70 | −10 | 4.07 |
| anterior/mid insula | L | 1,272 | −30 | 6 | 10 | 5.21 |
| thalamus | L | 1,272 | −16 | −12 | 10 | 3.26 |
| inferior frontal gyrus | L | 560 | −32 | 26 | 8 | 4.11 |
| cerebellum | L | 440 | −26 | −42 | −44 | 3.60 |
Furthermore, z statistics extracted from the S1 - anterior/medial insula connectivity cluster demonstrated that patients randomized to CBT showed more reduced resting state connectivity between S1-anterior/medial insula at post-treatment compared to the Education group (see bar graph in Figure 4). Such differences between groups were not found for the other regions whose S1 connectivity changes were associated with PCS changes – i.e. cuneus, precuneus, occipital cortex, inferior frontal gyrus, thalamus, and cerebellum.
Discussion
The present results support prior findings that individual CBT in patients with FM produces long-term improvements in pain, and that reductions in catastrophizing may serve as an important process factor in shaping these outcomes. It is noteworthy that treatment-related changes in catastrophizing in this study were much larger than changes in a more general affective factor such as depressive symptomatology (i.e., BDI scores). A substantial portion of CBT’s effectiveness for pain management is likely attributable to its emphasis on cognitive skills (e.g., cognitive restructuring, distraction, etc.) that can be honed through practice and deployed to manage daily pain symptoms (44, 45). It is important to note that the present trial enrolled only high-catastrophizing patients, and there is some recent evidence suggesting that the highest-catastrophizing patients may benefit most from these cognitive coping skills (46). It is unknown how effective this CBT treatment program would be in low-catastrophizing patients, though a larger trial that will eventually be able to answer this question is underway.
Our neuroimaging results add to a growing body of literature suggesting important links between clinical outcomes in FM and the degree of connectivity between the anterior/middle insula cortex and brain regions not typically connected to the insula in a resting state (i.e. primary somatosensory and default mode network areas) (30, 32, 33). These results extend our previous work on FM patients showing increased connectivity (relative to a matched group of healthy controls) between S1 and anterior insula in response to pain (27) at baseline. The present pilot findings suggest that CBT reduces potentially dysfunctional brain states and improve clinical outcomes such as pain-related disability in part by reducing catastrophizing. Such a conclusion is supported by the substantial reduction in connectivity between insula and primary somatosensory cortex that was observed in the CBT group, and by the large proportion of shared variance between changes in PCS scores and changes in this connectivity metric. Our findings hint that CBT’s effectiveness may result directly from its ability to reduce catastrophizing and “normalize” connectivity between salience processing areas such as the insula cortex and primary somatosensory regions that are known to both localize pain and ascribe magnitude to this perception. However, we did not compare CBT to other active, empirically-supported behavioral treatments (e.g., exercise, meditation), and are thus unable to definitively determine the specificity of these effects to CBT.
While the present study has some crucial strengths, such as the carefully matched (for duration and type of professional contact) education condition, a number of limitations will need to be addressed in future studies. First, we did not formally assess the amount of time patients continued to practice specific skills during and following completion of the treatment programs. Therefore, we cannot draw clear conclusions on which particular CBT skills were most beneficial to patients in reducing pain interference and catastrophizing. Second, because the Education group underwent CBT after completing their post-treatment assessment, we did not have a control condition for CBT at the 6 month post-treatment time point. While we did observe improvements in BPI pain interference across this time frame, and we suspect that these improvements are CBT-related, we are not able to definitively quantify that effect in a controlled manner. A final limitation was the relatively small sample size for this type of functional connectivity MRI analysis, which may have restricted our overall power, though even with this small sample we were able to observe significant associations between changes in brain connectivity and long-term changes in clinical outcomes.
Collectively, these preliminary results support the potential effectiveness of CBT in reducing catastrophizing, improving pain interference, and resolving amplified insula-S1 connectivity in patients with FM who are high in catastrophizing. Catastrophizing may serve both as an important phenotyping variable (which could guide the selection of optimal treatments for individual patients) and as a key process variable (changes in which are partly responsible for changes in pain-related outcomes). Moreover, it is important to highlight the potential sequential associations that were apparent in this treatment study. Catastrophizing and insula-S1 resting state connectivity changed over the short-term course of treatment (i.e., over approximately one month, after four sessions of individual CBT) while significant changes in pain interference were only observed at 6-month follow-up. Such temporal sequences highlight the possibility that fMRI might be used to identify early-treatment biomarkers that predict long-term treatment benefits. Future longitudinal studies using fMRI-derived variables as biomarkers, and including substantially larger sample sizes will be needed to identify the clinical potential of these preliminary findings.
Footnotes
The authors of the article declare that they have no competing financial interests.
Disclosures: This research was supported by NIH grant R01-AR064367, by grants to RRE from the Arthritis Foundation and the American College of Rheumatology and grant P01-AT006663, R01-AT007550 to VN by the National Center for complementary and Integrative Health (NCCIH). The project was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health andthe KIOM grant K16051.
References
- 1.Clauw DJ. Fibromyalgia: more than just a musculoskeletal disease. Am Fam Physician. 1995;52(3):843–51. 53–4. [PubMed] [Google Scholar]
- 2.Clauw DJ. The pathogenesis of chronic pain and fatigue syndromes, with special reference to fibromyalgia. Med Hypotheses. 1995;44(5):369–78. doi: 10.1016/0306-9877(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 3.Clauw DJ, Katz P. The Overlap Between Fibromyalgia and Inflammatory Rheumatic Disease: When and Why Does it Occur? J Clin Rheumatol. 1995;1(6):335–42. doi: 10.1097/00124743-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Clauw DJ. Elusive syndromes: treating the biologic basis of fibromyalgia and related syndromes. Cleve Clin J Med. 2001;68(10):830, 2–4. doi: 10.3949/ccjm.68.10.830. [DOI] [PubMed] [Google Scholar]
- 5.Clauw DJ, Russel IJ. Toward optimal health: the experts discuss fibromyalgia. J Womens Health Gend Based Med. 2000;9(10):1055–60. doi: 10.1089/152460900445965. [DOI] [PubMed] [Google Scholar]
- 6.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 7.Bernardy K, Klose P, Busch AJ, Choy EH, Hauser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;9:CD009796. doi: 10.1002/14651858.CD009796.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JA, Clancy S. Comparison of operant behavioral and cognitive-behavioral group treatment for chronic low back pain. J Consult Clin Psychol. 1988;56(2):261–6. doi: 10.1037//0022-006x.56.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Bennett R, Nelson D. Cognitive behavioral therapy for fibromyalgia. Nat Clin Pract Rheumatol. 2006;2(8):416–24. doi: 10.1038/ncprheum0245. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Palacios A, Herrero R, Belmonte MA, Castilla D, Guixeres J, Molinari G, et al. Ecological momentary assessment for chronic pain in fibromyalgia using a smartphone: a randomized crossover study. Eur J Pain. 2014;18(6):862–72. doi: 10.1002/j.1532-2149.2013.00425.x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–24. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MJL, Bishop SR, Pivik J. Psychological Assessment. 1995;7:524–32. [Google Scholar]
- 14.Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91(1–2):147–54. doi: 10.1016/s0304-3959(00)00430-9. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998;75(2–3):187–98. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]
- 17.Crombez G, Eccleston C, Van den Broeck A, Van Houdenhove B, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain Res Manag. 2002;7(1):31–9. doi: 10.1155/2002/576792. [DOI] [PubMed] [Google Scholar]
- 18.Peters ML, Vlaeyen JW, Weber WE. The joint contribution of physical pathology, pain-related fear and catastrophizing to chronic back pain disability. Pain. 2005;113(1–2):45–50. doi: 10.1016/j.pain.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Burton AW, Fine PG, Passik SD. Transformation of acute cancer pain to chronic cancer pain syndromes. J Support Oncol. 2012;10(3):89–95. doi: 10.1016/j.suponc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51–6. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 21.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil. 2005;86(6):1147–54. doi: 10.1016/j.apmr.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77(3):253–60. doi: 10.1016/S0304-3959(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 23.Burns JW, Day MA, Thorn BE. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med. 2012;2(1):22–9. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, et al. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain. 2011;152(12):2710–20. doi: 10.1016/j.pain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14(12):1573–84. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, et al. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153(7):1495–503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Kim J, Loggia ML, Cahalan C, Garcia RG, Vangel MG, et al. Fibromyalgia is characterized by altered frontal and cerebellar structural covariance brain networks. Neuroimage Clin. 2015;7:667–77. doi: 10.1016/j.nicl.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66(1):203–12. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients. eNeuro. 2014;1(1):e20, 14. doi: 10.1523/ENEURO.0006-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner F, Garcia RG, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395–405. doi: 10.1002/art.39043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napadow V, Harris RE. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of 'centralized' pain? Arthritis Res Ther. 2014;16(5):425. doi: 10.1186/s13075-014-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe F. New American College of Rheumatology criteria for fibromyalgia: a twenty-year journey. Arthritis Care Res (Hoboken) 2010;62(5):583–4. doi: 10.1002/acr.20156. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 36.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain. 2002;100(1–2):19–26. doi: 10.1016/s0304-3959(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 37.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff algometry. J Pain. 2002;3(1):28–37. doi: 10.1054/jpai.2002.27140. [DOI] [PubMed] [Google Scholar]
- 38.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Crawford BK, Piault EC, Lai C, Bennett RM. Assessing fibromyalgia-related fatigue: content validity and psychometric performance of the Fatigue Visual Analog Scale in adult patients with fibromyalgia. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S34–43. [PubMed] [Google Scholar]
- 40.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 41.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40(6):1365–7. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 42.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognit Ther Res. 2012;36(5):427–40. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumari N, Blackburn IM. How specific are negative automatic thoughts to a depressed population? An exploratory study. Br J Med Psychol. 1992;65(Pt 2):167–76. doi: 10.1111/j.2044-8341.1992.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 45.Palos R, Viscu L. Anxiety, automatic negative thoughts, and unconditional self-acceptance in rheumatoid arthritis: a preliminary study. ISRN Rheumatol. 2014;2014:317259. doi: 10.1155/2014/317259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber KL, Campbell C, Martel MO, Greenbaum S, Wasan AD, Borsook D, et al. Distraction analgesia in chronic pain patients: the impact of catastrophizing. Anesthesiology. 2014;121(6):1292–301. doi: 10.1097/ALN.0000000000000465. [DOI] [PubMed] [Google Scholar]