Abstract
Medicinal plants have been used by marginal communities to treat various ailments. However, the potential of endophytes within these bio-prospective medicinal plants remains unknown. The present study elucidates the endophytic diversity of medicinal plants (Caralluma acutangula, Rhazya stricta, and Moringa peregrina) and the endophyte role in seed growth and oxidative stress. Various organs of medicinal plants yielded ten endophytes, which were identified as Phoma sp. (6 isolates), Alternaria sp. (2), Bipolaris sp. (1), and Cladosporium sp. (1) based on 18S rDNA sequencing and phylogenetic analysis. The culture filtrates (CFs; 25%, 50%, and 100% concentrations) from these endophytes were tested against the growth of normal and dwarf mutant rice lines. Endophytic CF exhibited dose-dependent growth stimulation and suppression effects. CF (100%) of Phoma sp. significantly increased rice seed germination and growth compared to controls and other endophytes. This growth-promoting effect was due to the presence of indole acetic acid in endophytic CF. The gas chromatography/mass spectrometry (GC/MS) analysis showed the highest indole acetic acid content ((54.31±0.21) µmol/L) in Bipolaris sp. In addition, the isolate of Bipolaris sp. exhibited significantly higher radical scavenging and anti-lipid peroxidation activity than the other isolates. Bipolaris sp. and Phoma sp. also exhibited significantly higher flavonoid and phenolic contents. The medicinal plants exhibited the presence of bio-prospective endophytic strains, which could be used for the improvement of crop growth and the mitigation of oxidative stresses.
Keywords: Fungal endophytes, Diversity, Medicinal plants, Antioxidants, Indole acetic acid
1. Introduction
Endophytic microbes (bacteria and fungi) live in plant tissues without causing any symptoms of disease in the host. The initiation of fungal endophytic associations inside roots can alter the mineral nutrient composition, the phytohormonal balance, and the chemical constituents of root exudates, and protect the plant against abiotic and biotic stresses (Redman et al., 2011). Endophytic fungi provide the host with an arsenal of metabolites that improve plant defenses against environmental stimuli (Schulz and Boyle, 2005; Mandyam et al., 2013). A number of important endophytes (e.g. Piriformospora indica, Sebacina vermifera, and species of Penicillium and Colletotrichum) have been shown to improve plant growth in stressful environments (Waller et al., 2005; Redman et al., 2011; Khan et al., 2011; 2013).
Endophytic fungi produce bioactive secondary metabolites, including phytohormones. This potentially could result in improved host growth, and the diverse ecological niches of these organisms may be helpful in agriculture. Assisting crop growth and reducing cellular oxidative stress are important issues that need to be worked on. Endophytes play a crucial role in both of these processes, as reported recently by Murphy et al. (2014). Recent studies demonstrated that endophytic fungi can produce phytohormones, especially gibberellins (GAs), to improve crop growth and mitigate the negative impacts of abiotic stresses (Khan et al., 2013). Some fungal endophytes have also been reported to produce various classes of auxins, such as indole acetic acid (IAA). Waqas et al. (2014) and Ansari et al. (2013) demonstrated that some types of endophytic fungi can produce IAA. Like GA, IAA is essential for crop growth and development because it enhances root, axillary bud, and flower development, and influences other processes (Reinhardt et al., 2000).
Because of their abilities to secrete bioactive metabolites, endophytic fungi can also reduce oxidative stress. The production of radicals can hinder cellular functions (Gao et al., 2014). The anti- oxidative function of endophytic fungi might be due to the secretion of phenolic and flavonoid compounds into the growth medium. It was demonstrated previously that endophytes associated with medicinal plants have the potential to synthesize host-like bioactive chemical constituents (Schulz and Boyle, 2005). Endophytes can co-evolve with plant hosts and undergo species-specific interactions (Garcia et al., 2012). Medicinal plants and the diversity of the co-microbiota associated with these plants remain poorly understood (Nalini et al., 2014). In the present study, medicinal plants, viz. Caralluma acutangula, Rhazya stricta, and Moringa peregrina, growing in an arid region of Oman were collected and assessed for their endophytic microbial diversity. C. acutangula has been administered to children during chest congestion (Kipkore et al., 2014). R. stricta is an important ethnomedicinal plant that exhibits anticancer, antimicrobial, and enzyme inhibition activities (Mandyam et al., 2013). M. peregrina is used for abdominal pain and burns. The endophytic diversity of these plants was previously unknown.
This study was performed to investigate the endophytic diversity of these medicinally important plants. It also intended to explore the diversity of endophytic fungi and select fungi that can improve crop growth and ameliorate oxidative stress. In this regard, we isolated various endophytic fungi from the different organs i.e. leaves, stem, and roots of C. acutangula, R. stricta, and M. peregrina. Because these plants were growing in an extreme environment, we hypothesized that the endophytes inhabiting these plants might produce phytohormones that can help the host in the context of abiotic stress tolerance. Exploring the diversity of such endophytes is essential for improving the agricultural productivity of marginal lands.
2. Materials and methods
2.1. Fungal endophyte isolation
The three medicinal plants investigated in this study, viz. C. acutangula, R. stricta Decaisne, and M. peregrina, were collected from various localities in Jabal Al-Akhdar (23°04′22.00″ N; 57°40′07.00″ E) in the Sultanate of Oman. No specific permission was required to collect medicinal plants in these locations. It was confirmed that the collection process involved no endangered or protected species. The regional climatic condition is arid, and the plants grow in extremely water-deficient conditions (Ψ=−4.23 hPa). The collected plant organs (leaves, stems, and roots) were thoroughly washed in running tap water (Table 1). Finally for the isolation of endophytic microbes from the surface-sterilized small fragments (0.5 cm) of organs, the method described by Arnold and Lutzoni (2007) was adopted. In parallel, the sanitized fragments of different organs were also fixed onto separate Hagem plates to confirm the effectiveness of the surface disinfection (Arnold and Lutzoni, 2007). Newly appearing fungal spots from the tissues were removed and grown on new potato dextrose agar (PDA) medium under sterilized conditions.
Table 1.
Colonization and isolation rates of endophytic fungi from medicinal plants
| Organ | Plant | N T | N S | N I | R C (%) | R I |
| Stem* | RS | 200 | 120 | 2 | 60.00 | 0.017 |
| MP | 400 | 250 | 4 | 62.50 | 0.016 | |
| CA | 400 | 86 | 4 | 21.50 | 0.040 | |
| Leaves* | RS | 100 | 100 | 1 | 100.00 | 0.010 |
| MP | 100 | 0 | 0 | 0 | 0 | |
| CA | 100 | 0 | 0 | 0 | 0 | |
| Root* | RS | 200 | 75 | 2 | 37.50 | 0.020 |
| MP | 400 | 200 | 8 | 25.00 | 0.040 | |
| CA | 203 | 0 | 0 | 0 | 0 | |
| Total | 2103 | 1113 | 21 | 52.92 | 0.019 |
Based on seven tissue sections (0.5 cm) per plate. The number of isolation is based on the difference in morphological trait analysis of the endophytes isolated from individual plant organ. N T: total number of tissue sections; N S: number of samples yielding fungi; N I: number of isolation; R C: colonization rate; R I: isolation rate; RS: Rhazya stricta; MP: Moringa peregrina; CA: Caralluma acutangula
The colonization density, colonization rates, and isolation rates of fungal diversity were calculated as the percentage of segments infested by one or more isolates from the total number of segments of each plated tissue using the following method of Kumar and Hyde (2004):
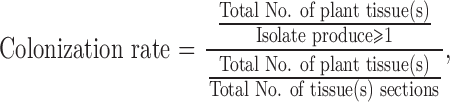 , ,
|
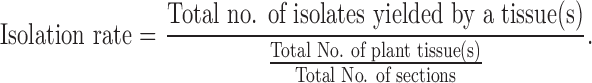 . .
|
Selected strains were inoculated into 50 ml of Czapek broth (1% (0.01 g/ml) glucose, 1% (0.01 g/ml) peptone, 0.05% (0.5 g/L) KCl, 0.05% (0.5 g/L) MgSO4·7H2O, and 0.001% (0.01 g/L) FeSO4·7H2O; pH 7.3±0.2) and grown for 7 d at 30 °C in a shaking incubator at 120 r/min to separate the liquid culture medium from the fungal mycelia; separation was accomplished via centrifugation at 2500g for 15 min at 4 °C. The culture medium (culture filtrate (CF), 50 ml) and mycelia were immediately moved to a −80 °C freezer and then freeze-dried for 4–7 d. The lyophilized CF was diluted with 1 ml of autoclaved double-distilled water (DDW). The CF was used to assess the presence of IAA and determine the antioxidant potential. The CF was also applied to differentiate growth-promoting strains for rice seeds. The mycelia of fungi were used for the extraction of genomic DNA and the identification of endophytic strains.
2.2. Rice seed germination assay
The production of phytohormones, particularly IAA, in pure cultures of endophytes was assessed using a screening bioassay on (1) Waito-C (GAs biosynthesis dy mutant with a dwarf phenotype) and (2) Oryza sativa L. cv. Dongjin-byeo (active GAs biosynthesis pathway and a normal growth pattern). The rice seeds were surface-disinfected with 2.5% (0.025 g/ml) sodium hypochlorite for 30 min, rinsed with distilled water (DW) and incubated for 24 h with 20 mg/L of uniconazole (except Dongjin-byeo) to obtain equally germinated seeds. The mutant seeds were treated with uniconazole to further inhibit the GAs pathway and validate the effects of IAA. The pre-germinated Waito-C and Dongjin-byeo seeds were shifted to autoclaved pots containing 0.8% (v/v) water:agar medium (Khan et al., 2011; Redman et al., 2011). After reaching the two-leaf stage, 20 µl of the CF collected from the endophytes was applied to the apex of the rice seedlings. After 7 d, rice growth was recorded and matched between CF- and fungus-free medium- treated rice plants.
2.3. Endophyte identification and phylogenetic analysis
The genomic DNA from endophytic fungal isolates was extracted according to the technique described by Arnold et al. (2007). Polymerase chain reaction (PCR) amplification and sequencing were performed using primers specific for 18S rDNA sequences. The obtained sequences were subjected to a BLASTn search to match the nucleotide sequence homology. The obtained closely related sequences were aligned with Clustal W using the MEGA software, Version 5.1 (Tamura et al., 2011), and the maximum likelihood and neighbor joining methods were used to construct a tree. Bootstrap replications (1000) were used as a statistical support for the nodes in the phylogenetic tree. Outgroups were considered to support differences among different species. The sequences were submitted in the National Center for Biotechnology Information (NCBI) GenBank for accession numbers. Detailed phylogenetic analyses of the strains were performed according to the method described by Tang et al. (2009) and Tamura et al. (2011) using MEGA 5.1 software.
2.4. IAA analysis
Estimations of IAA in the culture broth were made using a colorimetric assay and gas chromatography/ mass spectrometry (GC/MS) selected ion monitoring (SIM) according to Ullah et al. (2013). All the 21 endophytes were cultured in 20 ml of Czapek broth in the absence of tryptophan (0 g/L) and with a range of tryptophan concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 g/L). The cultures were incubated at (30±2) °C in a shaking incubator at 120 r/min for 10 d. The ethyl acetate fraction of the acidified CF (pH 2.8 using 1 mol/L HCl) was subjected for two analyses: initially using an enzyme-linked immunosorbent assay (ELISA) spectrophotometer (xMark ELISA, BioRad, USA) for all samples and finally a GC/MS SIM (Hewlett- Packard 6890, 5973N mass selective detector with an HA-1 capillary column) for 10 active fungal strains. The data were calculated in micro mole per 50 ml and each analysis was repeated twice. The experiment was repeated three times.
2.5. Antioxidant potential of endophytes
2.5.1 Determination of lipid peroxidation
The thiobarbituric acid reactive substance (TBARS) assay for the oxidative degradation of lipids was executed as described by Bajpai et al. (2014). Reading of the samples was taken at 532 nm and percent (%) anti-lipid peroxidation was determined in comparison to a dimethyl sulfoxide (DMSO) control. The percentage of anti-lipid peroxidation activity was obtained as percentage inhibition (%)=1− (sample/control)×100%. Butyl hydroxyanisole (BHA, 1.5 mmol/L) was used as a standard inhibitor.
2.5.2 Determination of total phenolic content
The total phenolic content was measured using an improved version of a previously described assay (Slinkard and Singleton, 1977) using the Folin- Ciocalteu reagent. The total phenolic values of all of the samples measured at 765 nm were stated in terms of gallic acid equivalents (μg/ml). For the blank (DMSO), the analysis procedure was kept the same as the samples. The dilution factor was taken into account for all diluted samples.
2.5.3 Analysis of total flavonoids
The total flavonoids were analyzed by modifying the assay reported by Huang et al. (2007). The results of the samples measured at 510 nm were evaluated as microgram of quercetin equivalents per milligram of dry mass of the culture samples using the standard curve obtained from 25 to 1000 mg/L of quercetin.
2.5.4 Radical scavenging activity using DPPH, ABTS, and NADH/PMS
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity was analyzed by modifying the assay reported by Gulati et al. (2012). The antioxidant activities of the samples recorded at 490 nm was expressed as percentage inhibition (%)=1−(absorbance of sample at 490 nm/absorbance of control at 490 nm)× 100%.
The 2,2-azinobis (3-ethylbenzthiazoline-6- sulphonic acid) (ABTS) cation scavenging activity was determined as reported by Saeed et al. (2012) and the percentage inhibition was calculated according to the formula: percentage inhibition (%)=1−(absorbance of sample at 475 nm/absorbance of control at 475 nm)×100%. The antioxidant capacity of the test samples was expressed as the EC50 (anti-radical activity), which is the concentration needed to achieve a 50% reduction of ABTS.
A superoxide anion radical scavenging assay was executed for the endophytic cultures as described by Ghosh et al. (2013) using non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS-NADH) system. The samples’ superoxide anion scavenging activities measured at 560 nm were calculated according to the equation: percentage scavenging (%)=1−(absorbance of sample at 560 nm/ absorbance of control at 560 nm)×100%. The experiment was repeated three times.
2.6. Statistical analysis
All samples were analyzed in triplicate and the data are showed as the mean±standard error of the mean (SEM). Differences were assessed using one- way analysis of variance (ANOVA) and considered significant at P<0.05 calculated by GraphPad Prism Version 5.0 for Windows (GraphPad Software, San Diego, CA, USA). The average values were compared using Duncan’s multiple range tests at P<0.05 (SAS 9.1, Cary, NC, USA).
3. Results
3.1. Endophyte isolation from medicinal plants
Endophytic fungi were isolated from different organs (leaves, stems, and roots) of C. acutangula (CA), R. stricta (RS) and M. peregrina (MP). These tissue pieces resulted in the isolation of 21 endophytic fungi (Table 1). From CA, MP, and RS, 4, 12, and 5 fungal endophytes were isolated, respectively (Table 1). Twelve fungal endophytes were isolated from the root and bark of MP, which yielded the highest fungal abundance level in comparison to RS and CA (Table 1). The colonization rate was also significantly higher for MP than for RS and CA (Table 1). The endophytes isolated from the bark of MP were primarily dark septate endophytes, which were grouped into five different endophytes based on morphological trait analysis, which included the color of aerial hyphae, margin characteristics, surface texture, colony shape, height from the medium, base color, growth rate and pattern, and growth depth into the medium (Arnold and Lutzoni, 2007). Similarly, the five fungal endophytes from RS were separated into three groups, and the four endophytes from CA were all unique.
3.2. Effects of endophytes on rice seed growth
The CFs of various endophytes from different plant samples were applied to Waito-C (dy mutant) and Dongjin-byeo (normal) rice seeds. Three different concentrations (100%, 50%, and 25%) of CF were used when testing the growth of the rice seeds. According to the results, with increasing concentrations of CFs, the germination and growth of Waito-C and Dongjin-byeo rice were reduced compared to controls. However, low concentrations of some of the CFs inhibited growth more than higher concentrations (Table 2). With respect to rice growth stimulatory strains, RSL1.2 and MPB6.1 significantly promoted the growth of both mutant and normal rice varieties. This effect was observed for all concentrations and for the growth of shoots and roots in comparison to the controls (Table 2). Two concentrations (25% and 50%) of CF from MPS10.1 significantly (P<0.02) increased root growth compared to the control, while the shoot length after the same treatment was similar to that observed after the control treatments. The maximum concentration (100%) of CF inhibited rice seed growth (Table 2). A similar response was also noted for the CA1 strain. A 25% application of CF from MPB6.2 significantly (P<0.001) increased the growth of roots and shoots compared to the control rice seeds. The opposite effect was observed for MPB5.2, for which a 100% concentration of CF significantly (P<0.0023) increased the growth of rice seeds. With respect to inhibitory CF from endophytic fungi, various concentrations of CA2, CA4, RSL8.1, RSOS1.3, and MPB8.1 significantly reduced (P<0.05) seed germination and growth compared to distilled water-treated control plants. The 100% concentration of CA4 inhibited rice seed germination, and the other concentrations significantly inhibited rice growth (Table 2).
Table 2.
Effects of different concentrations of endophytic culture filtrates on growth of Waito-C and Dongjin-byeo rice seeds
| Endophyte | 100% concentration |
50% concentration |
||||
|
Waito-C
|
Dongjin-byeo |
Waito-C
|
||||
| Root (cm) | Shoot (cm) | Root (cm) | Shoot (cm) | Root (cm) | Shoot (cm) | |
| Control | 3.60±0.30b | 2.60±0.47b | 3.20±0.17ab | 3.10±0.13c | 3.60±0.37c | 2.60±0.47c |
| MPB6.1 | 4.15±0.71a | 2.70±0.50b | 2.10±0.14c | 3.40±0.05c | 5.70±0.30a | 4.10±0.62a |
| MPB6.2 | 1.90±0.09d | 2.30±0.02bc | 2.72±0.19b | 3.77±0.06b | 2.57±0.89d | 3.30±0.39b |
| MPB5.2 | 4.12±0.04a | 3.30±0.40a | 3.35±0.12a | 4.00±0.13a | 2.25±0.82d | 2.30±0.54c |
| MPB8.1 | 2.30±0.20c | 1.70±0.30d | 2.42±0.16b | 3.25±0.10c | 2.15±0.49d | 1.20±0.69d |
| MPS10.1 | 1.70±1.50d | 1.20±0.00d | 0.67±0.03e | 3.20±0.33c | 4.12±0.40b | 3.20±0.84b |
| RSOS1.3 | 0.27±0.15e | 1.60±0.70d | 1.90±0.15c | 1.85±0.30e | 1.95±0.67e | 2.20±0.36c |
| RSL1.2 | 3.85±0.70ab | 3.10±0.61a | 3.70±0.49a | 4.07±0.70a | 2.00±0.84e | 1.20±0.28d |
| RSL8.1 | 0.75±0.80e | 0.80±0.02e | 0.00±0.00e | 2.50±0.50d | 1.25±0.29f | 1.20±0.29d |
| CA2 | 2.00±0.10d | 3.00±0.03a | 1.62±0.84d | 3.05±0.50c | 4.75±0.08b | 2.50±0.61c |
| CA4 | 0.00±0.00f | 0.00±0.00f | 0.00±0.00e | 0.00±0.00e | 2.32±0.98d | 2.40±0.83c |
|
| ||||||
|
| ||||||
| Endophyte | 50% concentration | 25% concentration | ||||
|
| ||||||
| Dongjin-byeo |
Waito-C
|
Dongjin-byeo |
||||
| Root (cm) | Shoot (cm) | Root (cm) | Shoot (cm) | Root (cm) | Shoot (cm) | |
|
| ||||||
| Control | 4.20±0.70b | 3.12±0.03d | 3.65±0.30d | 2.62±0.40d | 4.20±0.70c | 3.10±1.00d |
| MPB6.1 | 6.10±0.83a | 5.20±0.08a | 4.25±0.68c | 2.87±0.60d | 4.20±0.70c | 5.40±0.60b |
| MPB6.2 | 3.87±0.80c | 3.55±0.30c | 6.07±0.19a | 5.90±0.60a | 5.50±1.90b | 5.80±1.60a |
| MPB5.2 | 3.37±0.90c | 4.30±0.70b | 1.62±0.63f | 1.75±0.80e | 2.37±0.50e | 2.80±3.00d |
| MPB8.1 | 6.90±0.76a | 5.10±0.80a | 2.02±0.01e | 0.75±0.01f | 2.10±0.10e | 4.70±0.80c |
| MPS10.1 | 4.70±0.50b | 3.20±0.90c | 5.50±0.28b | 2.65±0.44d | 5.20±1.00b | 4.20±0.90c |
| RSOS1.3 | 4.10±0.75b | 3.60±0.90c | 1.00±1.06f | 1.40±0.04e | 3.70±0.50d | 3.10±0.30d |
| RSL1.2 | 3.70±0.40c | 4.15±0.90b | 4.52±0.11c | 2.30±0.98d | 6.90±1.10a | 4.90±0.10b |
| RSL8.1 | 0.37±0.64e | 1.25±0.20f | 1.75±0.04f | 0.87±0.80f | 5.90±0.60b | 4.70±0.10c |
| CA2 | 4.62±0.90b | 5.00±0.60a | 6.38±0.00a | 3.62±0.50c | 7.00±2.10a | 5.20±0.10b |
| CA4 | 2.50±0.20d | 2.05±0.10e | 1.60±0.01f | 4.60±0.50b | 1.13±0.00f | 1.50±0.00e |
Values in each cell are the mean of three replications and are shown with standard error of the mean. The values having different letters in the same column are significantly different as evaluated by DMRT test (P<0.05)
3.3. IAA production capacity of endophytic fungi
IAA elicits seed growth by cell elongation. We observed a significantly higher (P<0.0021; (54.32±0.21) μmol) quantity of IAA in the 50 ml of CF obtained from RSL1.2. MPB6.1 produced (47.78±0.21) μmol of IAA/50 ml of CF (Fig. 1) using GC/MS SIM analysis. The MPB6.2 and CA2 endophytic strains produced similar amounts ((41.32±0.63) and (40.01±0.81) μmol, respectively) of IAA. RSL8.1 produced (39.01±1.21) μmol of IAA. The IAA levels of MPB5.2, MPS10.1, CA4, and RSOS1.3 were not significantly different from each other. The endophytes MPB5.2, MPS10.1, CA4, and RSOS1.3 produced (36.87±1.21), (34.91±1.01), (35.71±0.98), and (37.02±0.92) μmol of IAA, respectively. MPB8.1 produced significantly lower levels of IAA than the other strains (Fig. 1). These results suggested that these endophytes have the potential to secrete IAA into the growth medium.
Fig. 1.
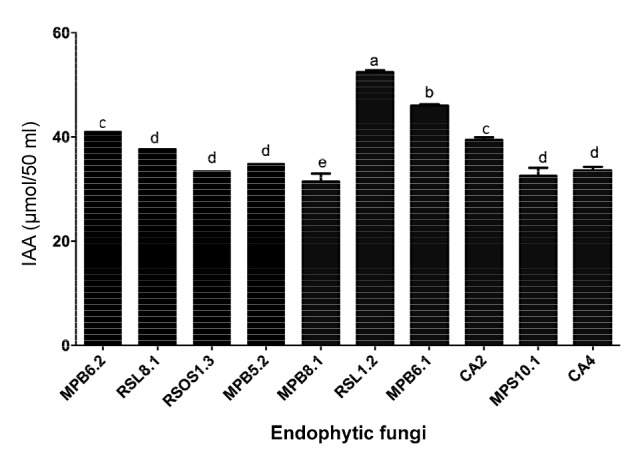
IAA production by the isolated endophytes in their growing culture medium
The bars represent the standard error of the mean. The different letter(s) depicts a significant (P<0.05) difference with respect to each other as evaluated by DMRT analysis. The IAA results are based on the 50 ml growth medium of the endophytes
3.4. Anti-oxidative potential of endophytes
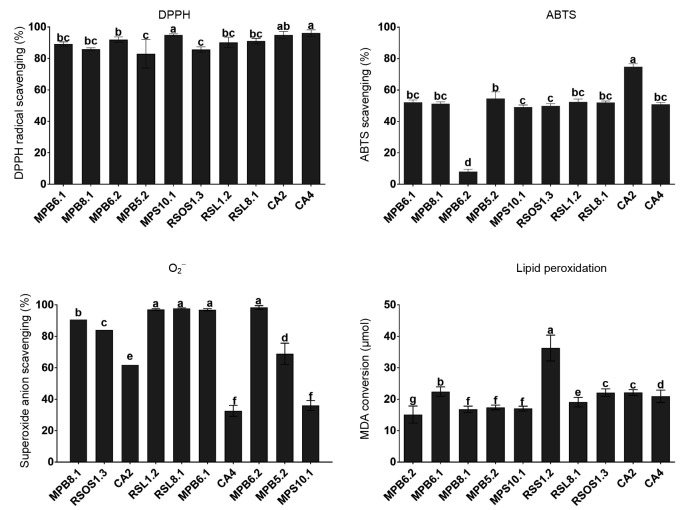
To explore the antioxidant potential of the ten isolated endophytes, the CFs were analyzed for their capacity to scavenge oxidative radicals. The endophytic DPPH radical scavenging potential was assessed in contrast to the positive control (ascorbic acid). The results demonstrated that MPS10.1 and CA4 had significantly higher (P<0.0452) radical scavenging abilities, followed by CA2 and MPB6.2; RSOS1.3 and MPB5.2 had significantly lower radical scavenging activities (Fig. 2). Similarly, with respect to ABTS radical scavenging activity, CA2 again recorded significantly higher (P<0.0041) radical scavenging activity than the other endophytic strains. MPB6.1, MPB5.1, RSL1.2, RSL8.1, and CA4 had nearly identical ABTS scavenging effects. The radical scavenging activities did not differ significantly among these strains. MPB6.2 had the lowest ABTS radical scavenging activity compared to the other strains (Fig. 2).
Fig. 2.
Potential of fungal endophytes in scavenging radicals and avoiding oxidative stress
The bars represents the standard error of the mean. The different letter(s) depicts a significant (P<0.05) difference with respect to each other as evaluated by DMRT analysis
Superoxide anion radical conversion was assessed using the NADH/PMS reaction with the endophytic CFs. The results demonstrated that MPB6.2, RSL1.2, RSL8.1, and MPB6.1 have significantly higher (P<0.0103) levels of superoxide anion scavenging potential than the other strains. Endophytic strains such as MPB8.1 and RSOS1.3 exhibited moderate activities (Fig. 2), while CA4 exhibited the lowest activity among the tested endophytic strains. The endophytes were also assessed for their anti-lipid peroxidation abilities. The level of lipid peroxidation is often analyzed using the secondary breakdown of lipid bilayers, which forms malondialdehyde (MDA). The endophyte RSL1.2 exhibited significantly higher (P<0.0074) levels of anti-lipid peroxidation capacity than the other nine endophytic fungal strains. This level was followed by MPB6.1. Other fungal endophytes (CA2, CA4, RSOS1.3, RSL8.1, MPS10.1, MPB5.2, and MPB6.2) exhibited moderate to lower anti-lipid peroxidation activities (Fig. 2).
3.5. Bioactive metabolite content of endophytes
The CF analysis of the 10 endophytes revealed that the total flavonoid content was significantly higher in MPB6.1 (P<0.00185) than in the other fungal strains. MPS10.1 was the second highest with respect to flavonoid content. The flavonoid contents of CA4, RSL1.2, RSOS1.3, MPB6.2, and RSL8.1 did not differ significantly (Fig. 3). The flavonoid contents of MPB5.2 and CA2 were significantly lower than those of the other endophytic fungal strains. With respect to phenolics, RSL1.2 and MPB6.1 had significantly higher (P<0.0039) contents than the other strains. Endophytes CA2 and MPB6.2 also produced higher amounts of phenolic compounds. However, these levels were significantly lower than those produced by RSL1.2 and MPB6.1. The phenolic contents of CA4, MPS10.1, RSOS1.3, and MPB5.2 were not significantly different from each other, but MPB8.1 had significantly lower levels of phenolics than the other endophytic fungal strains (Fig. 3).
Fig. 3.
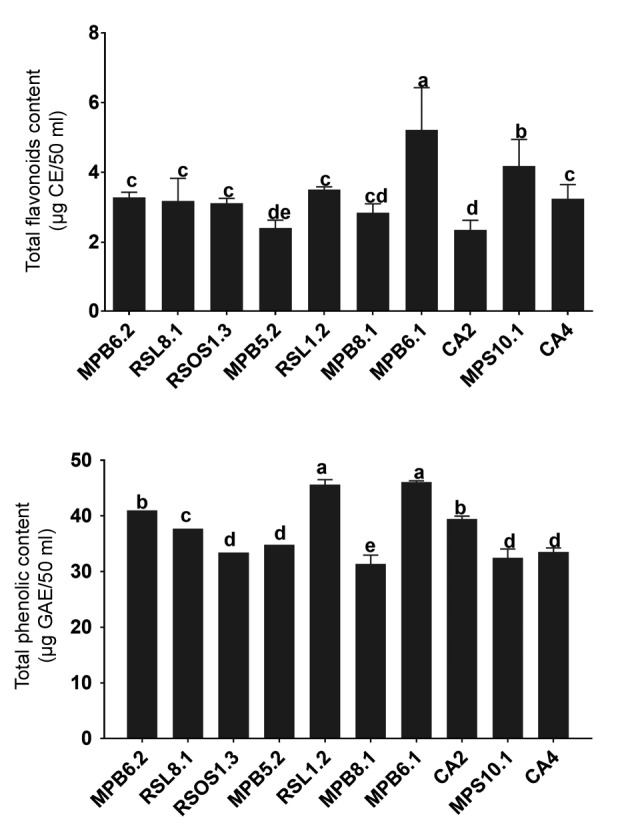
Flavonoid and phenolic contents of endophytic fungal strains
The bars represent the standard error of the mean. The different letter(s) shows a significant (P<0.05) difference with respect to each other as evaluated by DMRT analysis. The results are based on the 50 ml growth medium of the endophytes and correlative to cathachol (CE) and gallic acid equivalent (GAE) for flavonoid and phenolic, respectively
3.6. Endophyte identification and phylogenetic analysis
3.6.1 Sequence analysis and identification of fungal strains
PCR amplification of the 18S rDNA sequences from the ten fungal strains revealed fragment lengths of 522–564 bp. The sequence of each of the PCR-amplified products from the ten fungal strains was subjected to an NCBI BLAST search, and the sequence homology to the fungal species available in the NCBI database was between 90% and 99%. Based on 95%–99% sequence similarity, we identified the fungal strains as Phoma sp. (6 strains), Alternaria sp. (2 strains), Bipolaris sp. (1 strain), and Cladosporium sp. (1 strain) (Fig. S1). The 95%–99% sequence homologies and an individual phylogenetic analysis of each strain performed using the sequences available in the NCBI database using Aspergillus niger as an outgroup revealed that MPB5.2, MPB6.1, MPB6.2, CA2, RSOS1.3, and MPS10.1 were Phoma sp., CA4 was Alternaria alternata, RSL8.1 was Alternaria sp., RSL1.2 was Bipolaris sorokiniana, and MPB8.1 was Cladosporium sphaerospermum (Fig. S1). The sequence analysis demonstrated that all ten fungal strains contained 18S (20–37 bp), ITS1 (138–175 bp), 5.8S (157–159 bp), ITS2 (151–172 bp), and 28S (56–61 bp) sequences (Table S1). When the ten fungal strains were compared to identify sequence homologies among the strains, the homology levels were 84%–99%.
3.6.2 Phylogenetic analysis
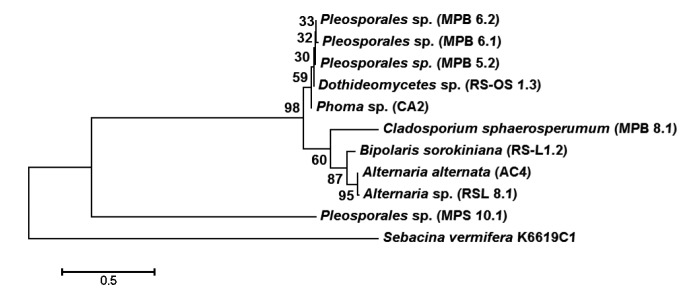
Phylogenetic analysis revealed two major clusters, separating C. sphaerospermum (MPB8.1) from the other nine fungal strains, which were clustered together in another major cluster (Fig. 4). The second major cluster was sub-divided into sub-clusters; the first sub-cluster grouped all the six Phoma sp. (MPB5.2, MPB6.1, MPB6.2, MPS10.1, RSOS1.3, and CA2), and the second sub-cluster grouped the Alternaria sp. (AC4 and RSL8.1) and B. sorokiniana (RSL1.2) together (Fig. 4). An interesting pattern was also observed for the ITS2 sequence, for which the first sub-cluster had a size of 138–139 bp, and the second sub-cluster (AC4, RSL8.1, and RSL1.2) had sequence lengths of 162–175 bp (Table S1). The most distinct C. sphaerospermum also exhibited higher evolutionary divergence than the other nine strains (sequences) (Tables S2 and S3).
Fig. 4.
Molecular phylogenetic analysis of fungal strains by maximum likelihood method
Sebacina vermifera K6619C1 was taken as an outgroup
3.6.3 Single nucleotide polymorphisms
When the sequences of the ten fungal strains were aligned, the total number of single nucleotide polymorphisms (SNPs) observed was 215 nucleotide sites. When the SNP variation was evaluated among four Phoma sp. (MPB5.2, MPB6.1, MPB6.2, and MPS10.1), only 8 SNPs were found. The sub-cluster containing all six Phoma sp. (MPB5.2, MPB6.1, MPB6.2, MPS10.1, RSOS1.3, and CA2) had 12 SNP variations. The second sub-cluster of B. sorokiniana (RSL1.2), A. alternata (AC4), and Alternaria sp. exhibited 63 SNP variations. When the nine fungal strains in the major sub-cluster were analyzed, 121 SNP variations were observed. The results confirmed that of the ten strains with 215 SNP variations, 94 SNPs occurred in C. sphaerospermum (MPB8.1), which was separated into a second major sub-cluster (Tables S2 and S3).
4. Discussion
After the discovery of taxol from a host-associated endophytic fungus, the exploration of novel endophytes from medicinal plants has attracted much attention because these organisms produce host-like metabolites (Kusari et al., 2014). Numerous studies reported the isolation of large numbers of endophytes from medicinal plants (Chen et al., 2011; Khan et al., 2013). To date, no reports on the fungal diversity or biological activity of C. acutangula, R. stricta, or M. peregrina collected from the arid regions of the Sultanate of Oman are available. In the present study, after the initial morphological trait analysis of 21 fungal endophytes, we identified the following fungal endophytes from various organs of medicinal plants: Phoma sp. (6 strains), Alternaria sp. (2 strains), Bipolaris sp. (1 strain), and Cladosporium sp. (1 strain). The abundance level and frequency of Phoma sp. were significantly higher than those of the other endophytes. However, in comparison to medicinal plants from temperate regions, the endophytes in this study exhibited less diversity. This difference could be attributed to the environmental conditions in which the host plants live (Nalini et al., 2014). Various strains of Phoma sp. were previously isolated from a number of medicinal plants, such as Arisaema erubescens (Wang et al., 2012), Araxacum mongolicum (Zhang et al., 2013), Solanum cernuum, Sapindus saponaria (Garcia et al., 2012), Camptotheca acuminata (Ding et al., 2013), Piper hispidum (Orlandelli et al., 2012), and Tylophora asthmatica (Nalini et al., 2014). Similarly, species and strains of Alternaria are also frequently isolated and identified from medicinal plants, as reported by Garcia et al. (2012) and Orlandelli et al. (2012). With respect to Bipolaris sp., this organism has been only isolated from P. hispidum (Orlandelli et al., 2012) in Brazil. Similarly, Cladosporium sp. is not well known for associations with medicinal plants. This species was isolated from Tinospora cordifolia (Thakur et al., 2013). The present results demonstrated that the composition and abundance of endophytes differed among the three medicinal plants and among the plant organs.
The endophytic fungi were identified based on morphological trait analysis and 18S rDNA sequencing, which is a famous and well-known method for fungal identification. Previously, various reports suggested the successful identification of fungal endophytes using 18S internal transcribed spacer (ITS) regions. Ghimire et al. (2011) identified 18 taxa of fungal endophytes from switch grass (Panicum virgatum L.). Similarly, various reports identified and phylogenetically analyzed endophytes from medicinal plants using the same sequencing regions (Ghimire et al., 2011; Khan et al., 2013). Our results are consistent with these reports. Sequence diversity, which was assessed using LSU, ITS1, ITS2, and 5.8S rDNA, was studied in endophytes from the seagrass Enhalus acoroides (Sakayaroj et al., 2010). rDNA ITS sequence analysis of the ITS1, 5.8S, and ITS2 regions in Cannabis sativa identified thirty fungal endophytes; among these endophytes, the dominant species was Penicillium copticola (Kusari et al., 2014). In our study, phylogenetic analysis demonstrated that A. alternata and Alternaria sp. could be isolated using a phylogenetic tree, which is consistent with the studies published by de Hoog and Horre (2002), who reported a clear demarcation between A. alternata and A. infectoria, with a 26-bp insert in the latter species. In the dendrogram, all six strains of Phoma sp. from Pleosporales, which is the largest order of the class Dothideomycetes, as mentioned by Zhang et al. (2011), clustered together based on sequence variations in 18S. The clustering of these six fungal strains confirms their assignment to the same genus. However, the small number of observed nucleotide variations (8–12 SNPs) may suggest that these strains may be sub-species or species of Phoma, particularly those isolated from medicinal plants.
Endophytes that inhabit medicinal plants exhibit potent biological activity, such as antioxidant, antimalarial, antimicrobial, and anticancer activities (Strobel et al., 2004; Xiao et al., 2014). In the current study, endophytes isolated from medicinal plants were assessed for their antioxidant activity. DPPH radical scavenging activity is frequently investigated in endophytes (e.g. Botryosphaeria dothidea and Aspergillus awamori) isolated from medicinal plants (Melia azedarach L. and Rauwolfia serpentina Benth, respectively) (Xiao et al., 2014; Nath et al., 2015). Liu et al. (2007) isolated Xylaria sp. from the stem of Ginkgo biloba and studied the potential of this species to produce antioxidants; the organism exhibited a strong radical scavenging activity. Similarly, Huang et al. (2007) investigated the total antioxidant and total phenolic contents of a repository of 292 endophytes from 29 traditional Chinese medicinal plants using improved ABTS methods. However, Toxocarpus wightianus, Plumeria rubra, and Artemisia capillaris exhibited the highest levels of total antioxidant capacity, which had a highly positive linear correlation with the corresponding total phenolic content. Endophytes also possess the ability to detoxify reactive oxygen species (ROS). The superoxide anion scavenging potential of our endophytes is consistent with the investigation reported by Gubiani et al. (2014) and Zhao et al. (2014). Gubiani et al. (2014) found four biologically active metabolites in the CF of the endophytic fungus Camarops sp., which was isolated from Alibertia macrophylla. These metabolites scavenge the superoxide anion indirectly via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibition in neutrophils and exhibit anti-inflammatory and antioxidant potentials. Aspergillus fumigatus isolated from the well-known medicinal pigeon pea plant was analyzed for antioxidant potential, and luteolin, which is a product of the same fungus, was found to inhibit lipid peroxidation (Huang et al., 2007).
This higher antioxidant potential may occur due to endophyte metabolomic profiles, particularly flavonoids and phenolic compounds, which are well-known antioxidants (Gao et al., 2014). Flavonoids and phenolic compounds scavenge hydroxyl groups, inactivate lipid free radicals, and inhibit free radical formation by reducing the disintegration of hydroperoxides. The anti-oxidative potential of endophytes is measured in terms of phenolic and flavonoid contents (Nath et al., 2015). For this reason, various organic solvents are used to optimize the extraction of total phenolic and flavonoid contents (e.g. the methanol extract from Xylaria sp. was of a higher quantity than hexane, chloroform, ethyl acetate, and acetone) (Zhao et al., 2014). Flavonoid and phenolic compounds are also qualitatively evaluated for specific compounds, and the host plant and endophytes frequently share an origin for a compound (Hilbert et al., 2012). Chlorogenic acid is one of the main bioactive phenolic compounds and was found in both foliar endophytic fungi and their host plants (Hilbert et al., 2012). Here, endophytes of medicinal plants could represent an additional source of endogenous flavonoid and phenolic compounds, further increasing the antioxidant potential of the hosts.
Phytohormone-like compounds are among the other important metabolites produced by endophytes. These compounds include IAA, which is an important phytohormone that is beneficial for plant growth and development. IAA is fabricated by microbes via tryptophan-dependent and -independent pathways (Chutima and Lumyong, 2012). In the current study, we found that the synthesis of IAA occurs via a tryptophan-dependent pathway, which is consistent with the reports by Rai et al. (2014) and Waqas et al. (2014). Very few endophytes associated with medicinal plants have been shown to produce IAA (Vadassery et al., 2008). However, this study is the first report related to C. acutangula, R. stricta, and M. peregrina. Among endophytes, only Phoma sp. was previously reported to secrete IAA into the growth medium (Waqas et al., 2014); the other strains are reported for the first time in the current study. The IAA-producing ability of these endophytes shows a growth-promoting effect and the induction of stress resistance against drought in the host plant under harsh environmental conditions. One of the possible contributions of these IAA-producing endophytes may be the extension of root growth (Kende, 2001; White and Torres, 2010). In this experiment, the use of mutant dwarf and normal rice for the sensitive detection of plant growth regulators in the CF of endophytes correlates with previous findings (Nishijima et al., 1994; Schulz and Boyle, 2005). This result suggests that CF containing IAA can improve the germination and growth of rice seeds (Keerthi and Jumpponen, 2014). The current study demonstrates that the application of CF to both normal and mutant rices stimulated seed growth, even in a mutant line with impaired hormonal biosynthesis (Nishijima et al., 1994; Torres et al., 2012). In conclusion, fungal endophytes isolated from medicinal plants growing in harsh environmental conditions comprise a community of diverse and biologically active endophytes. Such endophytes can increase the tolerance of host plants, which can be replicated in agricultural crops exposed to abiotic stresses.
5. Conclusions
Endophytic fungi have been known to possess a dynamic potential in improving growth of host plants during favorable and non-favorable conditions. In addition to the resilience of medicinal plants in the traditional health care system, they offer a unique opportunity to offer bio-prospective endophytes, which could be used for a diverse array of beneficial applications. In the present study, we subjected for the first time three arid land medicinal plants, viz. C. acutangula, R. stricta, and M. peregrina, to isolate such endophytic fungal strains. The results showed a possible role against oxidative stress developing because of environmental stimuli. Additionally, the cultures of fungal isolates increase the seed germination of mutant and wild-type seeds, which suggests the presence of phytohormone-like metabolites. Our preliminary screening and then chromatographic analysis revealed various quantities of IAA, which is a known stimulator of seed germination and seedling growth. Such bio-prospective endophytic application can extend greater benefits to the crops growing in marginal lands.
List of electronic supplementary materials
Individual phylogenetic analysis of the 10 fungal endophytes isolated from medicinal plants
Location and length of 18S, 28S, 5.8S and ITS1, ITS2 sequences of various fungal strains
Estimates of evolutionary divergence between sequences
Sequence homology matrix of fungal strains
Footnotes
Project supported by the Oman Research Council (FURAP Program), and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Research Center Support Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (716001-7)
Electronic supplementary materials: The online version of this article ((http://dx.doi.org/10.1631/jzus.B1500271) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Abdul Latif KHAN, Syed Abdullah GILANI, Muhammad WAQAS, Khadija AL-HOSNI, Salima AL-KHIZIRI, Yoon-Ha KIM, Liaqat ALI, Sang-Mo KANG, Sajjad ASAF, Raheem SHAHZAD, Javid HUSSAIN, In-Jung LEE, and Ahmed AL-HARRASI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ansari MW, Trivedi DK, Sahoo RK, et al. A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol Biochem. 2013;70:403–410. doi: 10.1016/j.plaphy.2013.06.005. (Available from: http://dx.doi.org/10.1016/j.plaphy.2013.06.005) [DOI] [PubMed] [Google Scholar]
- 2.Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots. Ecology. 2007;88(3):541–549. doi: 10.1890/05-1459. (Available from: http://dx.doi.org/10.1890/05-1459) [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Hu KX, Hou XQ, et al. Endophytic fungi assemblages from 10 Dendrobium medicinal plants (Orchidaceae) World J Microbiol Biotechnol. 2011;27(5):1009–1016. (Available from: http://dx.doi.org/10.1007/s11274-010-0544-y) [Google Scholar]
- 4.Chutima R, Lumyong S. Production of indole-3-acetic acid by Thai native orchid-associated fungi. Symbiosis. 2012;56(1):35–44. (Available from: http://dx.doi.org/10.1007/s13199-012-0158-2) [Google Scholar]
- 5.de Hoog GS, Horre R. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses. 2002;45(7-8):259–276. doi: 10.1046/j.1439-0507.2002.00747.x. (Available from: http://dx.doi.org/10.1046/j.1439-0507.2002.00747.x) [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Liu K, Deng B, et al. Isolation and characterization of endophytic fungi from Camptotheca acuminata . World J Microbiol Biotechnol. 2013;29(10):1831–1838. doi: 10.1007/s11274-013-1345-x. (Available from: http://dx.doi.org/10.1007/s11274-013-1345-x) [DOI] [PubMed] [Google Scholar]
- 7.Gao JM, Xiao J, Zhang Q, et al. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J Agric Food Chem. 2014;62(16):3584–3590. doi: 10.1021/jf500054f. (Available from: http://dx.doi.org/10.1021/jf500054f) [DOI] [PubMed] [Google Scholar]
- 8.Garcia A, Rhoden SA, Rubin-Filho CJ, et al. Diversity of foliar endophytic fungi from the medicinal plant Sapindus saponaria L. and their localization by scanning electron microscopy. Biol Res. 2012;45(2):139–148. doi: 10.4067/S0716-97602012000200006. (Available from: http://dx.doi.org/10.4067/S0716-97602012000200006) [DOI] [PubMed] [Google Scholar]
- 9.Ghimire SR, Charlton ND, Bell JD, et al. Biodiversity of fungal endophyte communities inhabiting switchgrass (Panicum virgatum L.) growing in the native tall grass prairie of northern Oklahoma. Fungal Div. 2011;47(1):19–27. (Available from: http://dx.doi.org/10.1007/s13225-010-0085-6) [Google Scholar]
- 10.Ghosh S, Derle A, Ahire M, et al. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera . PLoS ONE. 2013;8(12):e82529. doi: 10.1371/journal.pone.0082529. (Available from: http://dx.doi.org/10.1371/journal.pone.0082529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubiani JR, Zeraik ML, Oliveira CM, et al. Biologically active eremophilane-type sesquiterpenes from Camarops sp., an endophytic fungus isolated from Alibertia macrophylla . J Nat Prod. 2014;77(3):668–672. doi: 10.1021/np400825s. (Available from: http://dx.doi.org/10.1021/np400825s) [DOI] [PubMed] [Google Scholar]
- 12.Gulati V, Harding IH, Palombo EA. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemia. BMC Comp Alter Med. 2012;12:77. doi: 10.1186/1472-6882-12-77. (Available from: http://dx.doi.org/10.1186/1472-6882-12-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilbert M, Voll LM, Ding Y, et al. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012;196(2):520–534. doi: 10.1111/j.1469-8137.2012.04275.x. (Available from: http://dx.doi.org/10.1111/j.1469-8137.2012.04275.x) [DOI] [PubMed] [Google Scholar]
- 14.Huang WY, Cai YZ, Xing J, et al. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot. 2007;61(1):14–30. (Available from: http://dx.doi.org/10.1663/0013-0001(2007)61[14:APAREF]2.0.CO;2) [Google Scholar]
- 15.Keerthi M, Jumpponen A. Unraveling the Dark Septate Endophyte Functions: Insights from the Arabidopsis Model. Advances in Endophytic Research. Springer India; 2014. pp. 115–141. [Google Scholar]
- 16.Kende H. Hormone response mutants: a plethora of surprises. Plant Physiol. 2001;125(1):81–84. doi: 10.1104/pp.125.1.81. (Available from: http://dx.doi.org/10.1104/pp.125.1.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AL, Hamayun M, Kim YH, et al. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol . Biochem. 2011;49(8):852–862. doi: 10.1016/j.plaphy.2011.03.005. (Available from: http://dx.doi.org/10.1016/j.plaphy.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 18.Khan AL, Hussain J, Al-Harrasi A, et al. Endophytic fungi: a source of gibberellins and crop resistance to abiotic stress. Crit Rev Biotech. 2013;35(1):1–13. doi: 10.3109/07388551.2013.800018. (Available from: http://dx.doi.org/10.3109/07388551.2013.800018) [DOI] [PubMed] [Google Scholar]
- 19.Kipkore W, Wanjohi B, Rono H, et al. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed. 2014;10(1):24. doi: 10.1186/1746-4269-10-24. (Available from: http://dx.doi.org/10.1186/1746-4269-10-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar DSS, Hyde KD. Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii . Fungal Divers. 2004;17:69–90. [Google Scholar]
- 21.Kusari S, Singh S, Jayabaskaran C. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol. 2014;32(6):297–303. doi: 10.1016/j.tibtech.2014.03.009. (Available from: http://dx.doi.org/10.1016/j.tibtech.2014.03.009) [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Dong M, Chen X, et al. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba . Food Chem. 2007;105(2):548–554. (Available from: http://dx.doi.org/10.1016/j.foodchem.2007.04.008) [Google Scholar]
- 23.Mandyam KG, Roe J, Jumpponen A. Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol. 2013;117(4):250–260. doi: 10.1016/j.funbio.2013.02.001. (Available from: http://dx.doi.org/10.1016/j.funbio.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 24.Murphy BR, Doohan FM, Hodkinson TR. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 2014;62(1):29–39. (Available from: http://dx.doi.org/10.1007/s13199-014-0268-0) [Google Scholar]
- 25.Nalini MS, Sunayana N, Prakash HS. Endophytic fungal diversity in medicinal plants of Western Ghats, India. Int J Biodiv. 2014;2014:1–9. (Available from: http://dx.doi.org/10.1155/2014/494213) [Google Scholar]
- 26.Nath A, Chattopadhyay A, Joshi SR. Biological activity of endophytic fungi of Rauwolfia serpentine Benth: an ethnomedicinal plant used in folk medicines in northeast India. PNAS. 2015;85(1):233–240. (Available from: http://dx.doi.org/10.1007/s40011-013-0184-8) [Google Scholar]
- 27.Nishijima T, Koshioka M, Yamazaki H. Use of several gibberellin biosynthesis inhibitors in sensitized rice seedling bioassays. Biosci Biotechnol Biochem. 1994;58(3):572–573. (Available from: http://dx.doi.org/10.1271/bbb.58.572) [Google Scholar]
- 28.Orlandelli RC, Alberto RN, Rubin Filho CJ, et al. Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genet Mol Res. 2012;11(2):1575–1585. doi: 10.4238/2012.May.22.7. (Available from: http://dx.doi.org/10.4238/2012.May.22.7) [DOI] [PubMed] [Google Scholar]
- 29.Rai MR, Rathod D, Agarkar G, et al. Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis. 2014;62(2):63–79. (Available from: http://dx.doi.org/10.1007/s13199-014-0273-3) [Google Scholar]
- 30.Redman RS, Kim YO, Woodward CJDA, et al. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS ONE. 2011;6(7):e14823. doi: 10.1371/journal.pone.0014823. (Available from: http://dx.doi.org/10.1371/journal.pone.0014823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12(4):507–518. doi: 10.1105/tpc.12.4.507. (Available from: http://dx.doi.org/10.2307/3871065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Comp . Alter Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. (Available from: http://dx.doi.org/10.1186/1472-6882-12-221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakayaroj Y, Preedanon S, Supaphon O, et al. Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Div. 2010;42(1):27–45. (Available from: http://dx.doi.org/10.1007/s13225-009-0013-9) [Google Scholar]
- 34.Schulz B, Boyle C. The endophytic continuum. Mycol Res. 2005;109(6):661–686. doi: 10.1017/s095375620500273x. (Available from: http://dx.doi.org/10.1017/S095375620500273X) [DOI] [PubMed] [Google Scholar]
- 35.Slinkard K, Singleton L. Total phenol analyses: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 36.Strobel G, Daisy B, Castillo U, et al. Natural products from endophytic microorganisms. J Nat Prod. 2004;67(2):257–268. doi: 10.1021/np030397v. (Available from: http://dx.doi.org/10.1021/np030397v) [DOI] [PubMed] [Google Scholar]
- 37.Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. (Available from: http://dx.doi.org/10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang AMC, Jeewon R, Hyde KD. A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Div. 2009;34:127–155. [Google Scholar]
- 39.Thakur A, Kaur S, Kaur A, et al. Enhanced resistance to Spodoptera litura in endophyte infected cauliflower plants. Environ Entomol. 2013;42(2):240–246. doi: 10.1603/EN12001. (Available from: http://dx.doi.org/10.1603/EN12001) [DOI] [PubMed] [Google Scholar]
- 40.Torres MS, White JFJr, Zhang X, et al. Endophyte-mediated adjustments in host morphology and physiology and effects on host fitness traits in grasses. Fungal Ecol. 2012;5(3):322–330. (Available from: http://dx.doi.org/10.1016/j.funeco.2011.05.006) [Google Scholar]
- 41.Ullah I, Khan AR, Park GS, et al. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci Biotechnol. 2013;22(S1):25–31. (Available from: http://dx.doi.org/10.1007/s10068-013-0044-6) [Google Scholar]
- 42.Vadassery V, Ritter C, Venus Y, et al. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica . MPMI. 2008;21(10):1371–1383. doi: 10.1094/MPMI-21-10-1371. (Available from: http://dx.doi.org/10.1094/MPMI-21-10-1371) [DOI] [PubMed] [Google Scholar]
- 43.Waller F, Achatz B, Baltruschat H, et al. The endophytic fungus Piriformis indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. PNAS. 2005;102(38):13386–13391. doi: 10.1073/pnas.0504423102. (Available from: http://dx.doi.org/10.1073/pnas.0504423102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LW, Xu BG, Wang JY, et al. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens . Appl Microbiol Biotechnol. 2012;93(3):1231–1239. doi: 10.1007/s00253-011-3472-3. (Available from: http://dx.doi.org/10.1007/s00253-011-3472-3) [DOI] [PubMed] [Google Scholar]
- 45.Waqas M, Khan AL, Lee IJ. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J Plant Interact. 2014;9(1):478–487. (Available from: http://dx.doi.org/10.1080/17429145.2013.860562) [Google Scholar]
- 46.White JFJr, Torres MS. Is plant endophyte mediated defensive mutualism the result of oxidative stress protection? Physiol Plant. 2010;138(4):440–446. doi: 10.1111/j.1399-3054.2009.01332.x. (Available from: http://dx.doi.org/10.1111/j.1399-3054.2009.01332.x) [DOI] [PubMed] [Google Scholar]
- 47.Xiao J, Zhang Q, Gao YQ, et al. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J Agric Food Chem. 2014;62(16):3584–3590. doi: 10.1021/jf500054f. (Available from: http://dx.doi.org/10.1021/jf500054f) [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Xiong H, Zhao H, et al. An antimicrobial compound from the endophytic fungus Phoma sp. isolated from the medicinal plant Taraxacum mongolicum . J Taiwan Inst Chem Eng. 2013;44(2):177–181. (Available from: http://dx.doi.org/10.1016/j.jtice.2012.11.013) [Google Scholar]
- 49.Zhang Y, Crous PW, Schoch CL, et al. Pleosporales. Fungal Div. 2011;53(1):1–221. doi: 10.1007/s13225-011-0117-x. (Available from: http://dx.doi.org/10.1007/s13225-011-0117-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao JT, Ma DH, Luo M, et al. In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.] Food Res Int. 2014;56:243–251. (Available from: http://dx.doi.org/10.1016/j.foodres.2013.12.028) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual phylogenetic analysis of the 10 fungal endophytes isolated from medicinal plants
Location and length of 18S, 28S, 5.8S and ITS1, ITS2 sequences of various fungal strains
Estimates of evolutionary divergence between sequences
Sequence homology matrix of fungal strains