Abstract
Astaxanthin (AST), a carotenoid molecule extensively found in marine organisms and increasingly used as a dietary supplement, has been reported to have beneficial effects against oxidative stress. In the current paper, the effects of AST on viability of prostate cells were investigated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay; cell apoptosis and intracellular reactive oxygen species (ROS) levels were determined by flow cytometry; the mitochondrial membrane potential (MMP) was measured by fluorospectrophotometer; and activities of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) were evaluated by a detection kit. The results show that copper ion (Cu2+) induced apoptosis, along with the accumulation of intracellular ROS and MDA, in both prostate cell lines (RWPE-1 and PC-3). AST treatments could decrease the MDA levels, increase MMP, and keep ROS stable in RWPE-1 cell line. An addition of AST decreased the SOD, GSH-Px, and CAT activities in PC-3 cell line treated with Cu2+, but had a contrary reaction in RWPE-1 cell lines. In conclusion, AST could contribute to protecting RWPE-1 cells against Cu2+-induced injuries but could cause damage to the antioxidant enzyme system in PC-3 cells.
Keywords: Oxidative stress, PC-3, RWPE-1, Astaxanthin, Copper ion
1. Introduction
Prostate cancer is the second most frequently diagnosed cancer and the second leading cause of cancer-related death in men; the incidence and mortality of this disease are high in both North America and Western Europe, and currently low, but increasing, in Asia. Considerable evidence indicates that both genetic and environmental factors are primarily involved in its evolution.
Copper ion (Cu2+) is an essential trace element for human health. An imbalance in the metabolism of Cu2+ could be an etiologic factor for prostate cancer development. It participates in a variety of important metabolic pathways in free radical forms, such as superoxide dismutase (SOD) scavenging intracellular free radicals and cytochrome oxidase transmitting respiratory chain electron. Low intracellular Cu2+ concentrations could influence the activities of these enzymes and the normal metabolisms of the cells. Interestingly, the redox properties of the metal also mediate its toxicity because uncontrolled production of reactive oxygen species (ROS) results in oxidative stress, which does not follow a correct antioxidant response and consequently damages the biological macromolecules such as nucleic acids, proteins, and lipids (Adler et al., 1999; Auten and Davis, 2009; Maltepe and Saugstad, 2009; Linder, 2012). Under normal conditions, all processes involved in copper intake, distribution, utilization, and excretion are precisely regulated (Rosenzweig and O'Halloran, 2000; Kim et al., 2008; de Feo et al., 2009; Banci et al., 2010; Festa and Thiele, 2011; Haas et al., 2011).
Both exogenous and endogenous sources contributed to the formation of intracellular ROS (Winterbourn, 2008). Exogenous sources include radiation and environmental agents. Major endogenous sources of cellular ROS are microsomes, peroxisomes, and mitochondria. Other endogenous sources of ROS include enzymes such as xanthine oxidase, amino-acid oxidases, lipoxygenase, and cyclo-oxygenase. Superoxide release, as a result of the activity of the latter two enzymes, could be especially important in prostate cancer because of prostaglandin biosynthesis (Schewe, 2002). In addition, deregulated androgen signaling increases ROS in prostate cancer (Ripple et al., 1997; Sun et al., 2001; Tam et al., 2003; Frohlich et al., 2008; Basu et al., 2009), which is consistent with the results of other studies that prostate cancer development is associated with oxidative stress (Paschos et al., 2013).
Antioxidants, especially carotenoids, play an important role in the regulation of the oxidative process. They have strong antioxidant effects due to their double-bonded structures, allowing for their delocalization of impaired electrons. In recent years, the interests in astaxanthin (AST; 3,3'-dihydroxy-β-β'-carotene-4,4'-dione) have been continuously growing. AST is a type of carotenoid, with antioxidant activity that is 100–1000 times greater than that of vitamin E. AST is commonly found in crustaceans such as shrimp and crab, as well as marine organisms such as salmon, krill, and algae (Barros et al., 2014). As reported, dietary supplementation with AST has beneficial effects in the treatments of inflammation, cardiovascular disease, and oxidative damages, suggesting that AST is a functional food ingredient (Ohgami et al., 2003; Pashkow et al., 2008; Fassett and Coombes, 2009; Preuss et al., 2009). However, there are no reports about the effect of AST on oxidative stress in prostate cell lines, especially in prostate epithelial (RWPE-1) and prostate cancer (PC-3) cell lines treated with Cu2+.
In this paper, the effects of AST on Cu2+-induced oxidative stress in prostate cells and prostate cancer cells are investigated.
2. Materials and methods
2.1. Materials
RWPE-1 and PC-3 cell lines were obtained from the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Purified preparations of AST and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St. Louis, MO, USA). RPMI 1640 was purchased from GIBCO (Grand Island, NY, USA), and Annexin V-fluorescein isothiocyanate (FITC), ROS, SOD, catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) detection kits were obtained from Beyotime (Nantong, China). Other reagents used in our research were of analytical grade.
2.2. Cell culture
Both cell lines were cultured in RPMI 1640 supplemented with 10% (0.1 g/ml) fetal bovine serum and 100 U/ml penicillin-streptomycin. The cells were maintained at 37 °C and 5% CO2 in a humid environment, and stock solutions of AST and CuSO4 were prepared.
2.3. Determination of cell viability
Cells were harvested and counted, then seeded on a 96-well plate (1×105 PC-3 cells/well, 5×104 RWPE-1 cells/well) for 12 h. Different concentrations of CuSO4 and AST were prepared with serial dilutions ranging from 100 to 180 μmol/L and 0.001 to 10 μmol/L, respectively (Shen, 2014). AST was dissolved in dimethyl sulphoxide (DMSO) as a stock solution. For cell treatment, the AST was diluted in RPMI 1640 to obtain the final concentration, which was then added in sextuplicate to the cells (DMSO concentration in a dilution was <0.01%). After a 24-h or 48-h incubation, 20 μl of 5 mg/ml MTT reagent was added to each well. The cells were further incubated for 4 h in the dark, before 150 μl of DMSO was added and fully vibrated. Absorbance was measured at 490 nm (A 490) using a microplate reader (Thermal Lab system, Finland). The relative cell viability (%) was calculated as (A 490 of treated samples/A 490 of untreated samples)×100%.
2.4. Annexin-V/PI double-staining and flow cytometry
Apoptosis was double-stained with Annexin V-FITC and propidium iodide (PI) according to the manufacturer’s instructions (Annexin V-FITC apoptosis detection kit) (Saha et al., 2003). Live cells were shown as Annexin V-negative and PI-negative (A−/PI−, B3). Since cells with sustained plasma membrane integrity did not take up the PI, cells stained with Annexin V but not with PI were considered to be in the early stages of apoptosis (A+/PI−, B4). In the late apoptosis stage or the necrosis secondary stage to apoptosis, the cell membrane lost its integrity, allowing staining of the cells with both Annexin V and PI (A+/PI+, B2). Cells stained only with PI (A−/PI+, B1) were considered necrotic. Briefly, RWPE-1 (5×104 cells/well) and PC-3 (1×105 cells/well) were seeded into six-well plates and treated with Cu2+ and AST for 48 h, washed with an ice-cold phosphate buffer solution (PBS), and subsequently stained with a 100-μl incubation buffer containing 50 μg/ml Annexin V-FITC and 50 μg/ml PI at 37 °C in the dark for 15 min. Treated cells were immediately analyzed in a flow cytometer (FC500, Beckman Coulter, USA).
Intracellular ROS levels were measured using the cell permeable substrate 2',7'-dichlorofluorescein diacetate (DCFH-DA), a converted detectable fluorescent product (Rajeshkumar et al., 2015). After 48 h incubation, the cells were washed with a cold PBS solution prior to adding 1 ml PBS containing 10 μmol/L DCFH-DA (excitation: 488 nm; emission: 525 nm), and then continually incubated for 20 min in the dark at 37 °C. The treated cells were immediately washed and resuspended in PBS before ROS production was measured by using flow cytometry (FC500, Beckman Coulter, USA). The data were analyzed with CellQuest software.
2.5. Measurement of mitochondrial membrane potential
In order to measure the mitochondrial membrane potential (MMP), the cell suspension was incubated with 10 μmol/L Rh123 (excitation: 488 nm; emission: 534 nm) for 10 min in the dark at 37 °C, then immediately washed and resuspended in PBS for three separate times. The fluorescence was analyzed via a fluorospectrophotometer (Cary Eclipse, Varian, USA) (Brawek et al., 2010).
2.6. Measurements of MDA, SOD, CAT, and GSH-Px
RWPE-1 and PC-3 cells were seeded in 6-well plates at a density of 5×104 and 1×105 cells/well, respectively. Subsequently, the cells were treated with Cu2+ and AST. After a 48-h incubation, cells were collected for measurements of MDA, SOD, CAT and GSH-Px using a detection kit according to the manufacturer’s instructions. The results were corrected for their protein levels based on the reference manual for a BCA protein assay kit (Changsha Auragene Bioscience, Hunan, China).
2.7. Statistical analysis
All data were reported as the mean±standard deviation (SD). Comparison between groups was made by one-way analysis of variance (ANOVA), using SPSS software (Version 21.0). Each experiment was carried out in triplicate. A P-value of <0.05 is considered statistically significant.
3. Results
3.1. Effects of AST and Cu2+ on viabilities of RWPE-1 and PC-3 cells
To investigate the cytotoxicities of AST and Cu2+ on the viabilities of RWPE-1 and PC-3 cells, concentration-and time-dependent experiments were designed and carried out (Fig. 1). Cells were incubated with AST and Cu2+ of increasing initial concentrations (0.01 to 10 μmol/L and 100 to 180 μmol/L, respectively) for either 24 or 48 h and the cell viability was determined by performing MTT assay. AST induced a significant increase of PC-3 cells. Treatment with higher concentrations of AST (more than 0.01 μmol/L) for 48 h resulted in significant increases of RWPE-1 cells. Supplementation of Cu2+ to PC-3 cells resulted in an increase of cell viability after 24 h, while the growth was significantly suppressed after 48 h. For RWPE-1 cells, Cu2+ treatment did not lead to significant decreases after 24 h. Moreover, it was shown that Cu2+ treatment at low concentrations could promote cell growth (up to 129.8% for the viability) after 48 h, while 180 μmol/L Cu2+ treatment inhibited the growth of cells to 70.6%. Eventually, the effects of different concentrations of AST (0.001, 0.1, and 10 μmol/L as low dose (LD), middle dose (MD), and high dose (HD), respectively) on the oxidative damage of 120 μmol/L Cu2+-treated prostate cells were studied.
Fig. 1.
Effects of AST and Cu2+ on cell viabilities of RWPE-1 and PC-3 cells
AST promoted PC-3 cell proliferation in 24 and 48 h. AST promoted RWPE-1 cell proliferation in 48 h while inhibited cell proliferation in high concentrations in 24 h. Cu2+ increased cell viability of PC-3 cells in 24 h, while suppressed cell viability in 48 h. Cu2+ treatment did not decreased viability of RWPE-1 cells in 24 h. Cu2+ promoted RWPE-1 cell growth in low concentrations, but inhibited RWPE-1 cell growth in high concentrations in 48 h. Data are expressed as mean±SD (n=3)
3.2. Effects of AST on apoptosis of RWPE-1 and PC-3 cells in the presence of Cu2+
To further confirm effects of AST on apoptosis in both cells induced by Cu2+, Annexin V-FITC/PI double staining was performed. Representative dot plot diagrams and specific percentages, obtained through flow cytometry of the RWPE-1 and PC-3 cells treated with different concentrations of AST for 48 h, were shown in Figs. 2 and 3 and Table 1. In the non-apoptotic, viable control cells, Annexin V-FITC-negative and PI-negative staining was found in B3. The Cu2+ had a greater effect on the apoptosis of PC-3 cells because the percentage of cells in the late apoptosis stage or the necrosis secondary stage to apoptosis (A+/PI+, B2) increased significantly in the presence of Cu2+, which increased the dot numbers of RWPE-1 and PC-3 in B2 from 0.01% to 15.47% and 21.50%, respectively. However, similar results were obtained in AST treatment groups and there were no significant differences when compared with the Cu2+ treatment group. The population of cells progressed to advanced apoptosis because Cu2+ could induce cell apoptosis progression and the AST treatment seemed to have no clear indication of protective effects on apoptosis for the RWPE-1 or PC-3 cells.
Fig. 2.
Effects of AST on cell apoptosis in Cu2+-treated RWPE-1 cells
RWPE-1-CK: control group; RWPE-1-Cu2+: RWPE-1 cells treated with 120 μmol/L Cu2+; RWPE-1-10 μmol/L: RWPE-1 cells treated with 10 μmol/L AST and 120 μmol/L Cu2+; RWPE-1-0.1 μmol/L: RWPE-1 cells treated with 0.1 μmol/L AST and 120 μmol/L Cu2+; RWPE-1-0.001 μmol/L: RWPE-1 cells treated with 0.001 μmol/L AST and 120 μmol/L Cu2+
Fig. 3.
Effects of AST on cell apoptosis in Cu2+-treated PC-3 cells
PC-3-CK: control group; PC-3-Cu2+: PC-3 cells treated with 120 μmol/L Cu2+; PC-3-10 μmol/L: PC-3 cells treated with 10 μmol/L AST and 120 μmol/L Cu2+; PC-3-0.1 μmol/L: PC-3 cells treated with 0.1 μmol/L AST and 120 μmol/L Cu2+; PC-3-0.001 μmol/L: PC-3 cells treated with 0.001 μmol/L AST and 120 μmol/L Cu2+
Table 1.
Effects of AST on the apoptosis of the RWPE-1 and PC-3 cells treated with Cu2+
| Treatment | B1 (%) |
B2 (%) |
B3 (%) |
B4 (%) |
||||
| RWPE-1 | PC-3 | RWPE-1 | PC-3 | RWPE-1 | PC-3 | RWPE-1 | PC-3 | |
| CK | 0.02±0.01c | 0.03±0.01c | 0.01±0.01b | 0.01±0.00b | 99.97±0.01a | 99.96±0.01a | 0.00±0.00c | 0.00±0.00b |
| Cu2+ | 0.72±0.04a | 0.23±0.01a | 15.47±0.33a | 21.50±0.42a | 80.98±0.60b | 75.64±0.55b | 2.82±0.31b | 2.64±0.15a |
| HD | 0.51±0.10b | 0.15±0.01b | 16.28±1.12a | 21.63±2.14a | 79.35±1.43b | 75.57±2.11b | 3.85±0.35a | 2.65±0.16a |
| MD | 0.43±0.08b | 0.23±0.05a | 14.32±0.63a | 21.02±0.59a | 80.74±0.83b | 76.64±0.69b | 4.50±0.22a | 2.11±0.30a |
| LD | 0.51±0.04b | 0.23±0.03a | 16.28±0.22a | 23.38±1.75a | 79.15±0.35b | 74.24±1.84b | 4.06±0.19a | 2.15±0.11a |
Results were presented as the mean±SD (n=3). Different lowercase letters in the same column indicated significant differences, P<0.05. CK: control group; Cu2+: 120 μmol/L Cu2+ group; HD: 10 μmol/L AST+120 μmol/L Cu2+ group; MD: 0.1 μmol/L AST+120 μmol/L Cu2+ group; LD: 0.001 μmol/L AST+120 μmol/L Cu2+ group. B1: necrotic cells; B2: late apoptotic cells; B3: live cells; B4: early apoptotic cells
3.3. Effects of AST on ROS production in RWPE-1 and PC-3 cells treated with Cu2+
The cells were stained with DCFH-DA to examine a level of ROS before a fluorospectrophotometer was applied. The data in Fig. 4 show that the ROS level in PC-3 cells treated with 120 μmol/L Cu2+ was higher than that in the untreated groups. The addition of AST significantly impaired Cu2+-induced ROS production. The HD-treated groups showed better scavenge ability resulting in a decrease of ROS production as compared with the control groups. In contrast, Cu2+ induced a significant decrease of ROS accumulation in RWPE-1 cells, implying that Cu2+ treatment can induce greater damage to PC-3 cells.
Fig. 4.

Effects of AST on ROS production in Cu2+-treated RWPE-1 and PC-3 cells
Values are expressed as mean±SD (n=3). ** P<0.01 compared with control; ## P<0.01 compared with Cu2+ group
3.4. Effects of AST on MMP levels in RWPE-1 and PC-3 cells treated with Cu2+
In order to characterize the changes in the mitochondrial events induced by Cu2+ treatment, we observed the collapse of MMP in both cells with Rh123. When the MMP level decreased, the mitochondrial cell membrane was partially destroyed, so that Rh123 could be released into a matrix, causing the enhancement of the fluorescence intensity. As shown in Fig. 5, the addition of different concentrations of AST (LD, MD, and HD) on RWPE-1 cells reversely reduced MMP; therefore, the fluorescence intensity decreased from 143.0% to 79.6%, 67.0%, and 26.0% of the level in the control group (100%), respectively. Moreover, the addition of AST resulted in MMP reduction in a concentration-dependent manner in PC-3 cells induced by Cu2+ treatment. These results suggest that AST protected RWPE-1 cells against apoptosis induced by Cu2+ by reversing the down-regulation of MMP.
Fig. 5.
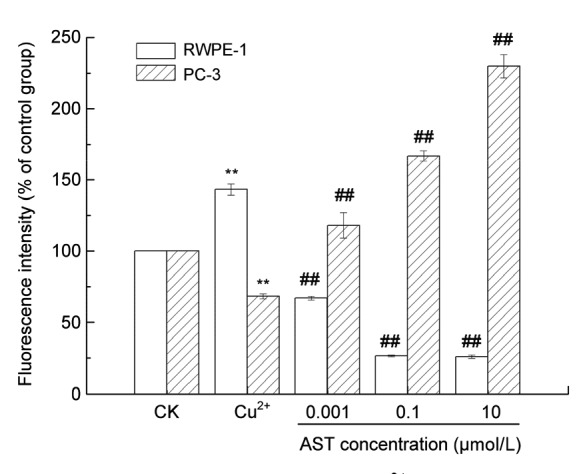
Effects of AST on MMP in Cu2+-treated RWPE-1 and PC-3 cells
Values are expressed as mean±SD (n=3). ** P<0.01 compared with control; ## P<0.01 compared with Cu2+ group
3.5. Effects of AST on MDA level and SOD activity in RWPE-1 and PC-3 cells treated with Cu2+
ROS overproduction could interfere with the intracellular redox homeostasis leading to oxidative stress. To determine whether AST was able to release cell oxidative stress induced by Cu2+, the intracellular MDA levels were determined after treatment as described above. As shown in Fig. 6, exposure of both kinds of cells to Cu2+ resulted in significant increases in the intracellular MDA levels, but the MDA levels in PC-3 cells were lower. However, an addition of LD AST effectively reduced the intracellular MDA generation in RWPE-1 cells, whereas MD and HD AST treatments resulted in a significant increase of MDA in PC-3 cells, indicating that AST accelerated oxidative stress induced by Cu2+ in PC-3 cells.
Fig. 6.
Effects of AST on the oxidative enzyme system in Cu2+-treated RWPE-1 and PC-3 cells
Values are expressed as mean±SD (n=3). ** P<0.01 compared with control; # P<0.05, ## P<0.01 compared with Cu2+ group
SOD is an important enzyme that is crucial for the prevention of diseases correlated to oxidative stress. As shown in Fig. 6, the treatment with Cu2+ resulted in a significant decrease of SOD activity in RWPE-1 and PC-3 cells to 57.1% and 53.8%, respectively. In contrast, HD treatment of AST significantly increased SOD activity, as compared with the control group, while LD treatment resulted in a significant decrease of SOD activity in PC-3 cells, indicating that a low concentration of AST may decrease its ability to scavenge ROS in PC-3 cells.
3.6. Effects of AST on GSH-Px and CAT in RWPE-1 and PC-3 cells treated with Cu2+
GSH-Px enzyme, one of the most important antioxidant enzymes in the human body, functions as a detoxification of hydrogen peroxide. As shown in Fig. 6, the treatment with Cu2+ resulted in a significant decrease of GSH-Px activity in RWPE-1 and PC-3 cells, only reaching to 54.2% and 71.8%, respectively, of the level of the control group; the addition of AST significantly increased GSH-Px activity in RWPE-1 cells and displayed concentration-dependent decreases in both cells, as compared with the cells only treated with Cu2+. However, after AST treatment, the GSH-Px activity in PC-3 cells was lower than that after only Cu2+ treatment in all groups. In summary, AST could increase the activity of GSH-Px in RWPE-1 cells treated with Cu2+, resulting in oxidative injury.
CAT enzyme could remove hydrogen peroxide in vivo to protect the cells; thus, it is one of the key enzymes in the antioxidant enzyme system. As shown in Fig. 6, treatment with Cu2+ still resulted in a significant decrease of CAT in both types of cells, and the CAT level in RWPE-1 cells was significantly higher than that in PC-3 cells in all the treated groups. The addition of AST did not reverse CAT reduction in PC-3 induced by Cu2+, while the LD concentration of AST increased CAT level significantly in RWPE-1 cells.
4. Discussion
The intrinsic balance could be interfered by several environmental stresses. ROS are among the most potent and omnipresent threats faced by any living organism. Intracellular accumulation of ROS such as superoxide anion, hydrogen peroxide, singlet oxygen, hydroxyl radical, and peroxy radical, usually results from toxic reactions or normal metabolic processes. These oxidative modified products might interfere with several functions in cancer cells, such as cell proliferations and genetic mutations. Inherent oxidative stress is a potent factor of angiogenesis and is considered to be involved in pathophysiology of cancers (Kuroki et al., 1996). ROS, also termed oxidants, were common by-products of the standard aerobic cellular metabolism, and were continuously generated in cells and synchronously scavenged by an array of antioxidant mechanisms (Dröge, 2002). Oxidative stress results from an imbalance between ROS production and the cell antioxidant defense capability, and causes reactions among those oxidative molecules and lipids, proteins and DNA (Montezano and Touyz, 2012; Ambati et al., 2014). Oxidative stress had been clarified as an interference factor in the prooxidant-antioxidant balance, resulting in potential cell damage (Sies, 1985). In our results, Cu2+ accelerated ROS production in prostate cancer cells. We determined that prostate cancer cells could be more sensitive to oxidative stress induced by Cu2+.
AST is an orange-pinkish carotenoid extensively found in marine organisms, which have various biological activities such as antioxidant, anti-cancer, and anti-inflammatory activities (Preuss et al., 2009; Yang et al., 2013; Kimura et al., 2014). Some results showed that AST protected neuronal cells against oxidative damage, and thus it has been a potent candidate for brain food (Liu and Osawa, 2009), and additionally could protect PC12 cells against glucose toxicity (Zhang et al., 2015). On the other hand, Cu2+ could lead to various cell injuries by inducing oxidative stress and mitochondrial dysfunction (Murphy and Taiz, 1997; Mira et al., 2002; Ma et al., 2014). Cu2+-induced production of hydrogen peroxide and hydroxyl radicals has been directly correlated with the damage to proteins and lipids (Wang et al., 2015). However, there have been no reports about the effects of AST on oxidative stress induced by Cu2+ in prostate cells. As an excellent anti-oxidant, AST suppressed ROS production in prostate cancer cells. Also, AST increased the ROS of normal prostate cells treated with Cu2+ to the same level as the control. Our results confirmed that AST could regulate oxidant status in prostate cells.
ROS initiated autocatalytic lipid peroxidation, and generated various potential genotoxic breakdown products, including alkoxyl radicals, peroxyl radicals, and aldehydes such as MDA. MDA has been used for many years as a convenient biomarker for lipid peroxidation because of its facile reaction with thiobarbituric acid to form an intensely colored chromogen (Rodríguez-Sureda et al., 2015). In this study, Cu2+ induced apoptosis along with the accumulation of intracellular ROS and increased MDA levels in both cells. In turn, AST treatment could reduce ROS and MMP accumulation and increase their MDA levels. According to our results, AST could have protective effects against Cu2+-induced oxidative damage.
There were several enzyme systems (SOD, CAT, and GSH-Px) that catalyzed reactions clearing free radicals and ROS. These form the body’s endogenous defense mechanisms to protect against cell damages induced by free radicals. First, SOD, a major cytoplasmic antioxidant enzyme, catalyzed the dismutation of superoxide radicals to molecular oxygen and hydrogen peroxides which, in turn, were converted by either GSH-Px or CAT to water and oxygen, thus providing a combined enzymatic action against oxygen toxicity. The imbalance of SOD to GSH-Px and CAT resulted in the accumulation of H2O2 which might participate in the Fenton’s reaction and consequently lead to the formation of noxious hydroxyl radicals (de Haan et al., 1996). The present study investigated the antioxidant enzyme expression in long-term cultures of prostate cells. Considering the results, Cu2+ could significantly reduce SOD, GSH-Px, and CAT in both cells as shown in Fig. 6. The addition of AST decreased the SOD, GSH-Px, and CAT activities in PC-3 cells induced by Cu2+, while they were up-regulated in RWPE-1 cells. It is speculated that AST could be able to protect RWPE-1 cells against Cu2+-induced damage on the cell antioxidant enzyme system, and to a certain extent, promote enzyme activity.
5. Conclusions
Our data clearly demonstrated that AST has a potential ability to inhibit Cu2+-induced oxidative stress in RWPE-1 cells. The underlying pathway was associated with the properties of AST to scavenge ROS, to restore MMP, and to increase antioxidant enzyme activity. However, AST induced the damage of the antioxidant system induced by Cu2+ in PC-3 cells, specifically through its regulation on the levels of CAT, GSH-Px, and MMP. This study therefore provided insight into the protective effects of AST on Cu2+-induced oxidative stress. The molecular mechanism of AST against Cu2+-induced oxidative stress still needs further exploration. Importantly, in vivo investigation is also required to assess whether AST could be a potential therapeutic agent for releasing Cu2+-induced toxicity in prostate cancer.
Footnotes
Project supported by the Plan of Medicine and Health Science and Technology of the Zhejiang Province of China (No. 2012RCA022)
Compliance with ethics guidelines: Hong-zhou MENG, Xiao-feng NI, Hai-ning YU, Shan-shan WANG, and Sheng-rong SHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Adler V, Yin Z, Tew KD, et al. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18(45):6104–6111. doi: 10.1038/sj.onc.1203128. (Available from: http://dx.doi.org/10.1038/sj.onc.1203128) [DOI] [PubMed] [Google Scholar]
- 2.Ambati RR, Phang SM, Ravi S, et al. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs. 2014;12(1):128–152. doi: 10.3390/md12010128. (Available from: http://dx.doi.org/10.3390/md12010128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66(2):121–127. doi: 10.1203/PDR.0b013e3181a9eafb. (Available from: http://dx.doi.org/10.1203/PDR.0b013e3181a9eafb) [DOI] [PubMed] [Google Scholar]
- 4.Banci L, Bertini I, Cantini F, et al. Cellular copper distribution: a mechanistic systems biology approach. Cell Mol Life Sci. 2010;67(15):2563–2589. doi: 10.1007/s00018-010-0330-x. (Available from: http://dx.doi.org/10.1007/s00018-010-0330-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barros MP, Poppe SC, Bondan EF. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients. 2014;6(3):1293–1317. doi: 10.3390/nu6031293. (Available from: http://dx.doi.org/10.3390/nu6031293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu HS, Thompson TA, Church DR, et al. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69(19):7689–7695. doi: 10.1158/0008-5472.CAN-08-2472. (Available from: http://dx.doi.org/10.1158/0008-5472.CAN-08-2472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brawek B, Lӧffler M, Wagner K, et al. Reactive oxygen species (ROS) in the human neocortex: role of aging and cognition. Brain Res Bull. 2010;81(4-5):484–490. doi: 10.1016/j.brainresbull.2009.10.011. (Available from: http://dx.doi.org/10.1016/j.brainresbull.2009.10.011) [DOI] [PubMed] [Google Scholar]
- 8.de Feo CJ, Aller SG, Siluvai GS, et al. Three-dimensional structure of the human copper transporter hCTR1. PNAS. 2009;106(11):4237–4242. doi: 10.1073/pnas.0810286106. (Available from: http://dx.doi.org/10.1073/pnas.0810286106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Haan JB, Cristiano F, Iannello R, et al. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5(2):283–292. doi: 10.1093/hmg/5.2.283. (Available from: http://dx.doi.org/10.1093/hmg/5.2.283) [DOI] [PubMed] [Google Scholar]
- 10.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. (Available from: http://dx.doi.org/10.1152/physrev.00018.2001) [DOI] [PubMed] [Google Scholar]
- 11.Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5(4):333–342. doi: 10.2217/fca.09.19. (Available from: http://dx.doi.org/10.2217/fca.09.19) [DOI] [PubMed] [Google Scholar]
- 12.Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 2011;21(21):R877–R883. doi: 10.1016/j.cub.2011.09.040. (Available from: http://dx.doi.org/10.1016/j.cub.2011.09.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frohlich DA, McCabe MT, Arnold RS, et al. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27(31):4353–4362. doi: 10.1038/onc.2008.79. (Available from: http://dx.doi.org/10.1038/onc.2008.79) [DOI] [PubMed] [Google Scholar]
- 14.Haas KL, Putterman AB, White DR, et al. Model peptides provide new insights into the role of histidine residues as potential ligands in human cellular copper acquisition via Ctr1. J Am Chem Soc. 2011;133(12):4427–4437. doi: 10.1021/ja108890c. (Available from: http://dx.doi.org/10.1021/ja108890c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–185. doi: 10.1038/nchembio.72. (Available from: http://dx.doi.org/10.1038/nchembio.72) [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Iida M, Yamauchi H, et al. Astaxanthin supplementation effects on adipocyte size and lipid profile in OLETF rats with hyperphagia and visceral fat accumulation. J Funct Foods. 2014;11:114–120. (Available from: http://dx.doi.org/10.1016/j.jff.2014.08.001) [Google Scholar]
- 17.Kuroki M, Voest EE, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98(7):1667. doi: 10.1172/JCI118962. (Available from: http://dx.doi.org/10.1172/JCI118962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder MC. The relationship of copper to DNA damage and damage prevention in humans. Mutat Res. 2012;733(1-2):83–91. doi: 10.1016/j.mrfmmm.2012.03.010. (Available from: http://dx.doi.org/10.1016/j.mrfmmm.2012.03.010) [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Osawa T. Yoshikawa, T. (Ed.), Food Factors for Health Promotion. Karger, Basel: Forum of Nutrition; 2009. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food; pp. 129–135. (Available from: http://dx.doi.org/10.1159/000212745) [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Li X, Wang Y, et al. Cu(II) inhibits hIAPP fibrillation and promotes hIAPP-induced beta cell apoptosis through induction of ROS-mediated mitochondrial dysfunction. J Inorg Biochem. 2014;140:143–152. doi: 10.1016/j.jinorgbio.2014.07.002. (Available from: http://dx.doi.org/10.1016/j.jinorgbio.2014.07.002) [DOI] [PubMed] [Google Scholar]
- 21.Maltepe E, Saugstad OD. Oxygen in health and disease: regulation of oxygen homeostasis-clinical implications. Pediatr Res. 2009;65(3):261–268. doi: 10.1203/PDR.0b013e31818fc83f. (Available from: http://dx.doi.org/10.1203/PDR.0b013e31818fc83f) [DOI] [PubMed] [Google Scholar]
- 22.Mira L, Tereza Fernandez M, Santos M, et al. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36(11):1199–1208. doi: 10.1080/1071576021000016463. (Available from: http://dx.doi.org/10.1080/1071576021000016463) [DOI] [PubMed] [Google Scholar]
- 23.Montezano AC, Touyz RM. Molecular mechanisms of hypertension–reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28(3):288–295. doi: 10.1016/j.cjca.2012.01.017. (Available from: http://dx.doi.org/10.1016/j.cjca.2012.01.017) [DOI] [PubMed] [Google Scholar]
- 24.Murphy A, Taiz L. Correlation between potassium efflux and copper sensitivity in 10 Arahidopsis ecotypes . New Phytol. 1997;136(2):211–222. (Available from: http://dx.doi.org/10.1046/j.1469-8137.1997.00738.x) [Google Scholar]
- 25.Ohgami K, Shiratori K, Kotake S, et al. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44(6):2694–2701. doi: 10.1167/iovs.02-0822. (Available from: http://dx.doi.org/10.1167/iovs.02-0822) [DOI] [PubMed] [Google Scholar]
- 26.Paschos A, Pandya R, Duivenvoorden WCM, et al. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013;16(3):217–225. doi: 10.1038/pcan.2013.13. (Available from: http://dx.doi.org/10.1038/pcan.2013.13) [DOI] [PubMed] [Google Scholar]
- 27.Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101(10):S58–S68. doi: 10.1016/j.amjcard.2008.02.010. (Available from: http://dx.doi.org/10.1016/j.amjcard.2008.02.010) [DOI] [PubMed] [Google Scholar]
- 28.Preuss HG, Echard B, Bagchi D, et al. Astaxanthin lowers blood pressure and lessens the activity of the renin-angiotensin system in Zucker Fatty Rats. J Funct Foods. 2009;1(1):13–22. (Available from: http://dx.doi.org/10.1016/j.jff.2008.09.001) [Google Scholar]
- 29.Rajeshkumar RK, Vennila R, Karthikeyan S, et al. Antiproliferative activity of marine stingray Dasyatis sephen venom on human cervical carcinoma cell line. J Venomous Anim Toxins Incl Trop Dis. 2015;21:41. doi: 10.1186/s40409-015-0036-5. (Available from: http://dx.doi.org/10.1186/s40409-015-0036-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ripple MO, Wilding G, Henry WF, et al. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst. 1997;89(1):40–48. doi: 10.1093/jnci/89.1.40. (Available from: http://dx.doi.org/10.1093/jnci/89.1.40) [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Sureda V, Vilches Á, Sánchez O, et al. Intracellular oxidant activity, antioxidant enzyme defense system, and cell senescence in fibroblasts with trisomy 21. Oxid Med Cell Longev. 2015;2015:509241. doi: 10.1155/2015/509241. (Available from: http://dx.doi.org/10.1155/2015/509241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenzweig AC, O'Halloran TV. Structure and chemistry of the copper chaperone proteins. Curr Opin Chem Biol. 2000;4(2):140–147. doi: 10.1016/s1367-5931(99)00066-6. (Available from: http://dx.doi.org/10.1016/S1367-5931(99)00066-6) [DOI] [PubMed] [Google Scholar]
- 33.Saha NR, Usami T, Suzuki Y. A double staining flow cytometric assay for the detection of steroid induced apoptotic leucocytes in common carp (Cyprinus carpio) Dev Comp Immunol. 2003;27(5):351–363. doi: 10.1016/s0145-305x(02)00116-7. (Available from: http://dx.doi.org/10.1016/S0145-305X(02)00116-7) [DOI] [PubMed] [Google Scholar]
- 34.Schewe T. 15-Lipoxygenase-1: a prooxidant enzyme. Biol Chem. 2002;383(3-4):365–374. doi: 10.1515/BC.2002.041. (Available from: http://dx.doi.org/10.1515/BC.2002.041) [DOI] [PubMed] [Google Scholar]
- 35.Shen YZ. Biological behaviors of prostate cells with PUFAs supplementation. Hangzhou, China: Zhejiang University; 2014. MS Thesis. (in Chinese) [Google Scholar]
- 36.Sies H. Sies, H. (Ed.), Oxidative Stress. London: Academic Press; 1985. Oxidative stress: introductory remarks; pp. 1–8. (Available from: http://dx.doi.org/10.1016/B978-0-12-642760-8.50005-3) [Google Scholar]
- 37.Sun XY, Donald SP, Phang JM. Testosterone and prostate specific antigen stimulate generation of reactive oxygen species in prostate cancer cells. Carcinogenesis. 2001;22(11):1775–1780. doi: 10.1093/carcin/22.11.1775. (Available from: http://dx.doi.org/10.1093/carcin/22.11.1775) [DOI] [PubMed] [Google Scholar]
- 38.Tam NNC, Gao Y, Leung YK, et al. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163(6):2513–2522. doi: 10.1016/S0002-9440(10)63606-1. (Available from: http://dx.doi.org/10.1016/S0002-9440(10)63606-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JY, Lee YJ, Chou MC, et al. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse Leydig cells. Mar Drugs. 2015;13(3):1375–1388. doi: 10.3390/md13031375. (Available from: http://dx.doi.org/10.3390/md13031375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4(5):278–286. doi: 10.1038/nchembio.85. (Available from: http://dx.doi.org/10.1038/nchembio.85) [DOI] [PubMed] [Google Scholar]
- 41.Yang DJ, Lin JT, Chen YC, et al. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264. 7 cells via NF-κB and JNK inactivation. J Funct Foods. 2013;5(2):607–615. (Available from: http://dx.doi.org/10.1016/j.jff.2013.01.001) [Google Scholar]
- 42.Zhang Y, Wang W, Hao C, et al. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J Funct Foods. 2015;16:137–151. (Available from: http://dx.doi.org/10.1016/j.jff.2015.04.008) [Google Scholar]






