Abstract
Soluble amyloid precursor protein α (sAPPα), a secreted proteolytic fragment of non-amyloidogenic amyloid precursor protein (APP) processing, is known for numerous neuroprotective functions. These functions include but are not limited to proliferation, neuroprotection, synaptic plasticity, memory formation, neurogenesis and neuritogenesis in cell culture and animal models. In addition, sAPPα influences amyloid-β (Aβ) production by direct modulation of APP β-secretase proteolysis as well as Aβ-related or unrelated tau-pathology, hallmark pathologies of Alzheimer’s disease (AD). Thus, the restoration of sAPPα levels and functions in the brain by increasing non-amyloidogenic APP processing and/or manipulation of its signaling could reduce AD pathology and cognitive impairment. It is likely that identification and characterization of sAPPα receptors in the brain, downstream effectors, and signaling pathways will pave the way for an attractive therapeutic target for AD prevention or intervention.
Keywords: sAPPα, APP, Aβ, Alzheimer’s Disease, Receptor, Biomarker, Neuroprotection, Synaptic Plasticity, Memory, Neurogenesis, Aging, Cognitive Impairment, Therapeutics
Significance
Soluble amyloid precursor protein (sAPPα), a secreted proteolytic fragment of APP processing, elicits neuroprotection, synaptic plasticity, memory formation, neurogenesis and neuritogenesis, while reducing amyloid and tau pathology, in the brain. Since impairment of these processes underlies Alzheimer’s disease, restoration of sAPPα levels and function by increasing non-amyloidogenic APP processing and/or manipulation of its signaling could reduce AD-related amyloid pathology and cognitive impairment. The present review summarizes recent work on functional neural properties of sAPPα, as well as its potential signaling mechanisms, and discusses several potential sAPPα-based therapies for AD and other dementias.
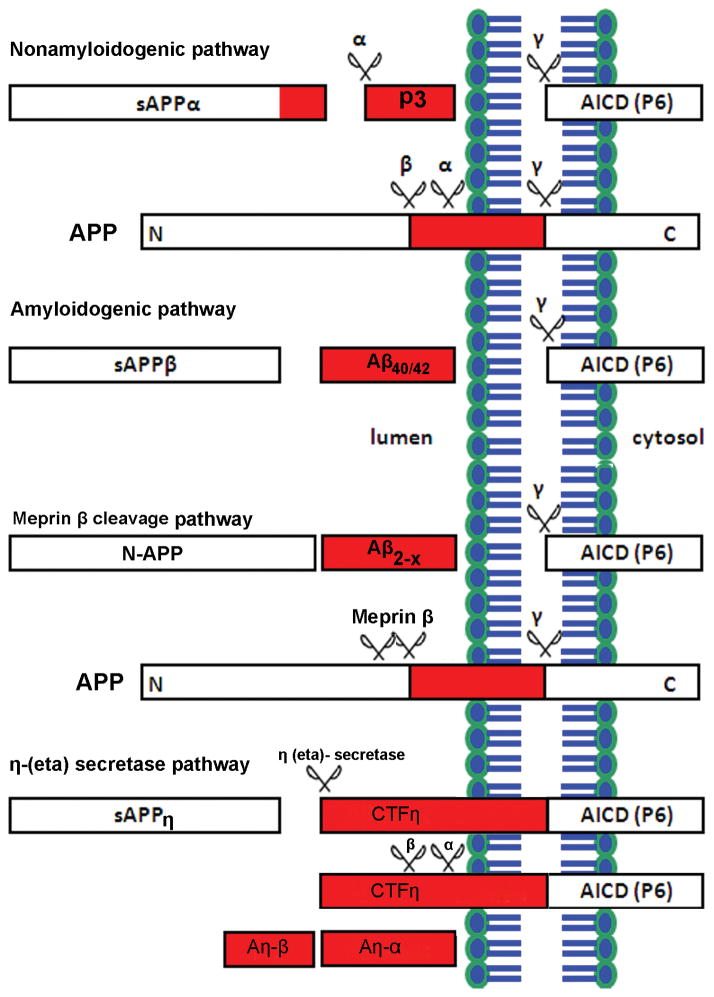
More than one hundred years have passed since Alois Alzheimer and Oskar Fisher’s discovery of the two neuropathological hallmarks of Alzheimer’s disease (AD), deposition of extracellular amyloid plaques and intracellular neurofibrillary tangles. Currently, AD is the most common type of age-associated dementia and there are no disease modifying treatments. The pathological features of AD are currently known to include: (a) extracellular amyloid plaques composed largely of amyloid-β (Aβ) peptides (Hardy and Allsop, 1991), (b) intracellular neurofibrillary tangles (NFTs) composed of the hyperphosphorylated microtubule associated protein tau (Goedert et al., 1991), (c) dysmorphic synapses and (d) neuronal loss (Palop and Mucke, 2010). The proteolytic cleavage of amyloid precursor protein (APP) by two different enzymes, β- (also called BACE1) and γ-secretases, is a critical step in AD development. In the non-amyloidogenic pathway, most of the APP is cleaved at the plasma membrane by α-secretase, which precludes Aβ formation but produces a large secreted N-terminal ectodomain of APP (sAPPα) of 105–125 kDa and small membrane-bound α-C-terminal fragment (CTF) (Haass and Selkoe, 1993). The membrane-bound α-CTF is cleaved by γ-secretase complex resulting in release of P3 peptide of 3 kDa and AICD (APP intracellular domain). In the amyloidogenic pathway, the remaining uncleaved APP is processed into the endosomal-lysosomal compartments by β-secretase results in soluble sAPPβ and membrane-bound β-CTF. The subsequent action of γ-secretase on β-CTF produces Aβ40/42 peptides and AICD (Kang et al., 1987). In addition to α-, β- and γ-secretases cleavage, a recent study identified that APP can be cleaved by the metalloprotease meprin β, generating soluble N-terminal truncated APP (N-APP) and N-terminally truncated Aβ2-X peptide variants, which show increased aggregation potential compared to non-truncated Aβ40 peptides (Jefferson et al., 2011); (Bien et al., 2012, Schonherr et al., 2016). Cleavage of APP by meprin β occurs prior to the endocytosis and different APP mutants affect the catalytic properties of the enzyme. More specifically, Swedish mutant APP does not undergo this cleavage and unable to produce this truncated Aβ variants. Another study showed that APP can also be cleaved by matrix metalloproteinases such as MT5-MMP, referred to as η-secretase, which releases a long-truncated ectodomain (sAPPη) and a membrane-bound CTF, termed CTFη (Willem et al. 2015). The membrane-bound CTFη is further cleaved by α- and β-secretases releasing both a long (Aη-α) and a short (Aη-β) peptide, respectively. The cleavage of η, cuts far from the N-terminus of the β-secretase cleavage site and produces fragments (92 or 108 amino acids), which end at either the β- or α-secretase site respectively (Willem et al., 2015) (Figure 1).
Figure 1. Schematic diagram of amyloid precursor protein (APP) processing pathways and cleavage products.
The non-amyloidogenic APP processing pathway (upper) involves cleavages by α- and γ-secretases. Sequential cleavage of APP by α-secretase generates sAPPα and c-terminal fragment C83 (not shown). The subsequent cleavage of C83 by γ-secretase complex generate APP intracellular domain (AICD) and a short fragment called P3. The amyloidogenic APP processing pathway (lower) involves cleavages by β- and γ-secretases. Cleavage of APP by β-secretase generate sAPPβ and c-terminal fragment C99 (not shown). Subsequent cleavage of C99 by γ-secretase complex generate toxic species Aβ (40 or 42, depends on the cutting site) and AICD. This is termed as amyloidogenic pathway due to generation and accumulation of Aβ species into plaque inside the brain. In addition to α-, β- and γ-secretases cleavage, APP is cleaved by metalloprotease meprin β, generating soluble N-terminal truncated APP (N-APP) or Aβ2-X variants. In addition to meprin β cleavage, the cleavage of APP by several matrix metalloproteinases such as MT5-MMP, referred to as η-secretase, releases a long-truncated ectodomain (sAPPη) and a membrane-bound carboxy-terminal fragment (CTF), termed CTFη. The membrane-bound CTFη is further cleaved by α- and β-secretases and release a long (Aη-α) and a short (Aη-β) peptide, respectively.
A number of in vitro and in vivo studies have demonstrated the toxic properties of Aβ peptides since the first identification of the APP gene in 1987 (Kang et al., 1987, Younkin, 1995). Administration of Aβ peptides (Maurice et al., 1996), their structural mimetics, and anti-Aβ antibodies (Cleary et al., 1995) have supported the deleterious functions of the peptide in terms of promoting cognitive deficits. Like sAPPα, sAPPβ has beneficial effects, is soluble in nature and secreted extracellularly but lacks 16 amino acids at the C-terminus. The potency of sAPPβ is found to be 100-times less than that of sAPPα, measured in its ability to protect hippocampal neurons against excitotoxicity, glucose deprivation, and Aβ toxicity (Furukawa et al., 1996b, Barger and Harmon, 1997). In accord with this finding, other studies also reported the reduced potency of sAPPβ as a neuroprotective fragment (Turner et al., 2003); (Li et al., 1997). Like sAPPα, sAPPβ also supports axonal outgrowth (Chasseigneaux et al., 2011) and neural differentiation of human embryonic stem cells (Freude et al., 2011). In contrast to above effects, sAPPβ also causes neuronal cell death by binding to the death receptor 6 (DR6) (Nikolaev et al., 2009) and does not protect cell death induced by proteasome inhibitors (Copanaki et al., 2010). Further study showed that sAPPβ fragments are not involved in long-term potentiation (LTP) (Taylor et al., 2008). Perneczky and colleagues have reported that plasma levels of sAPPβ were significantly decreased (Perneczky et al., 2013) in AD patients compared to control and frontotemporal dementia (FTD) patients. However, sAPPβ levels were increased in cerebrospinal fluid (CSF) (Perneczky et al., 2011) of mild cognitive impairment (MCI) patients who had progressed to probable AD compared to control and patients with frontotemporal dementia (FTD).
Unlike sAPPβ and Aβ, sAPPα has demonstrated neurotrophic and neuroprotective functions. In contrast to its neuroprotective effects, we and others have shown that sAPPα attenuates Aβ pathology by binding to the allosteric site of BACE1 (Obregon et al., 2012, Peters-Libeu et al., 2015). Additionally, sAPPα has been shown to reduce tau hyperphosphorylation by inhibiting BACE1 and glycogen synthase kinase (GSK) 3β activity in cell culture and transgenic PSAPP mouse model (Deng et al., 2015). Surprisingly, the physiological functions and therapeutic importance of this fragment have received little attention. In this review, we have compiled physiological functions of sAPPα, especially in the context of AD. It is possible that this is not a complete list of potential sAPPα actions. We believe comprehensive understanding of the sAPPα functions, signaling pathways and downstream effectors could provide new therapeutic opportunities for effective AD drug development.
Modulation of APP processing and Aβ clearance by sAPPα-receptor interaction
a. Interaction of LRP1 and sAPPα
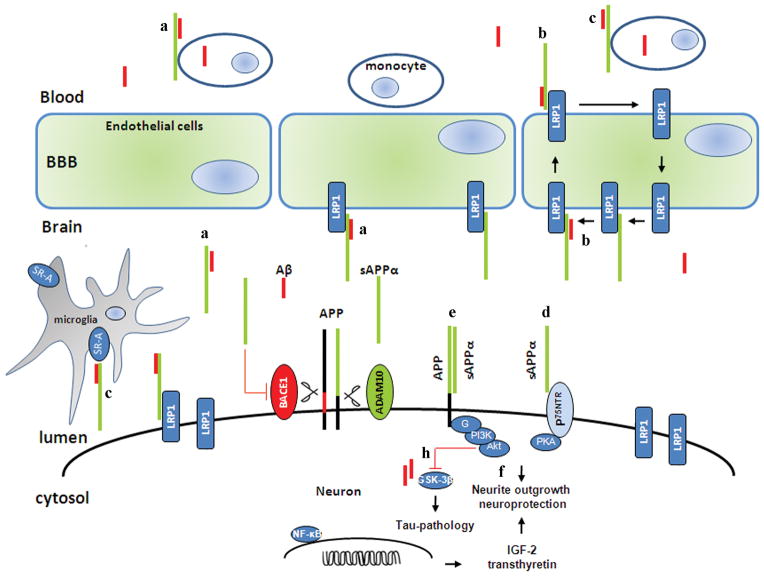
The physiological functions of sAPPα have been extensively studied in animal and in cell culture models. Earlier work pointed out the role of sAPPα as an extracellular ligand that modulates APP amyloidogenesis. LRP1 was initially identified as a receptor for APOE (Beisiegel et al., 1989), which regulates APP and Aβ metabolism (Kanekiyo and Bu, 2014). A study by Kounnas and colleagues (1995) first demonstrated that sAPP binds to the endocytic low-density lipoprotein (LDL) receptor-related protein (LRP1) via the Kunitz-type serine protease inhibitor (KPI) domain (Kounnas et al., 1995). Through the interaction with KPI, the LRP1 receptor enhances internalization of sAPPα into the endosomal and lysosomal compartments. As the KPI domain is essential for the sAPPα-LRP1 interaction, sAPPα695 isoform (lacking the KPI domain) acts as a weak LRP1 ligand. Both LRP1 antagonist (receptor-associated protein [RAP]) and heparin are able to inhibit the interaction. Interestingly, binding of RAP do not completely inhibit the effect and suggest the presence of receptors other than LRP1. Using LRP1-deficient cell lines, they showed that the binding is dependent of LRP1 receptor (Kounnas et al., 1995). These data provide evidence that secreted APP is internalized into the cell via the LRP1 receptors and heparin modulates the binding and internalization of sAPPα. As sAPPα does not influence Aβ production, it would be very interesting to see if the secreted APP751 could affect generation of Aβ through sAPPα-(KPI)-LRP1 interaction. Furthermore, Knauer and co-worker (1996) demonstrated that LRP1 is also critical for internalization and generation of Aβ by cell surface (unprocessed) APP751 (containing the KPI domain). The interaction between APP and LRP1 receptor leads to increased trafficking of APP into the amyloidogenic endocytic pathway (Knauer et al., 1996). This finding further supports the data of Kounnas and colleagues by showing that sAPPα695, which lacks the KPI domain, does not undergo this interaction. To identify the sAPPα receptor specifically, Hoffmann and colleagues used histidine-tag labeling techniques to identify cell surface-bound sAPPα. Using immunocytochemistry and surface plasmon resonance spectroscopy, they demonstrated that sAPPα (1 and 10 nM) binds specifically on the cell surface microdomain (Hoffmann et al., 1999). In 2000 and subsequent years, Goto and Tanzi further studied the sAPPα-LRP1 interaction and demonstrated that a mini domain within LRP1, known as LRP-cluster II region, can bind specifically with the sAPPα-KPI domain (Goto and Tanzi, 2002). They also showed that inhibition of interaction of KPI and the cluster II region of LRP1 reduces generation of Aβ in Chinese hamster ovary cells overexpressing wild-type APP (Goto and Tanzi, 2002). A recent study suggests that all three isoforms of APP are expressed in brain but APP695 is a predominant isoform in neurons (Guo et al., 2012a). This finding suggests that LRP1 may not be an exclusive receptor for sAPPα in the brain. It is plausible that inhibition of the sAPPα-(KPI)-LRP1 interaction may force sAPPα to interact with Aβ. A proposed tentative sAPPα-Aβ interaction and subsequent clearance by LRP1 receptor is illustrated in Figure 2a. More recently, Pietrzik group (2016) demonstrated that deletion of mouse LRP1 receptors from endothelium cells of the blood brain barrier (BBB) significantly elevated soluble Aβ42 in the brain and reduced it in blood plasma. This strongly supports LRP1-mediated clearance of Aβ across the BBB (Storck et al., 2016). It is plausible that sAPPα shuttles Aβ to the endothelial LRP1 at the abluminal side and shifts Aβ out the brain to the periphery as shown in Figure 2b. In accord with this hypothesis, our group (2015) found that sAPPα forms a complex with Aβ (corresponding to APP672–688 region) and thereby enhances phagocytosis by monocytes. In addition, sAPPα enhances scavenger receptor class A (SR-A) mediated phagocytosis of Aβ by microglia (brain) and monocytes (peripheral system) as shown in Figure. 2c (Darlington et al., 2015). There is a need of further study to prove this hypothesis. On the other hand, Moir and Tanzi have showed that LRP1-mediated removal of Aβ involved in the formation of a complex with Aβ and APOE or alpha (2)-macroglobulin. They demonstrated that the KPI domain inhibits LRP1-mediated clearance of Aβ (Moir and Tanzi, 2005). Further investigation on sAPPα-LRP1 interaction and subsequent endocytosis of APP will characterize sAPPα-mediated efflux of Aβ from the brain.
Figure 2. A schematic diagram presenting functions of APP processing metabolites Aβ (red) and sAPPα (green) inside the brain.
Membrane-associated APP is processed through non-amyloidogenic or amyloidogenic pathway, resulting in the production of sAPPα (green) and Aβ (red), respectively. Depending on the cellular conditions, these two fragments can exist as monomer, and/or homo/heterodimer. The interaction of sAPPα and LRP1 receptor may induce internalization of Aβ into neuronal cells as an sAPPα/Aβ heterodimer. Additionally, we also hypothesize that LRP1 can transfer this heterodimer across blood-brain-barrier into peripheral circulation, and which then could be phagocytosed by monocytes (Figure 2a). We hypothesize that sAPPα could shuttle Aβ to the endothelial LRP1 at the abluminal side and remove Aβ out the brain to the periphery (Figure 2b). Additionally. SR-A as another possible receptor expressed on microglia cell surface, may also involved in sAPPα-mediated Aβ clearance by microglia (brain) and monocyte (peripheral system) (Figure 2c). The secreted sAPPα (both N- and C- terminal) fragements bind to their neuron membrane receptor P75NTR and initiate neurite outgrowth (Figure 2d). In addition, sAPPα protects neuronal cells by disrupting the dimerization of APP (Figure 2e). Furthermore, sAPPα can triggers expression of neuroprotective genes (IGF-2 and transthyretin) through NF-κB and PI3K/Akt signaling which provides neuronal outgrowth and survival (Figure 2f). sAPPα also reduce tau-pathology by inhibiting GSK3β and BACE1 activity (Figure 2h).
b. Interaction of scavenger receptor SR-A and sAPPα
The microglial expression of SR-A has been shown to increase in brain injury (Bell et al., 1994) and in microglia surrounding the plaques (Honda et al., 1998). Santiago et al. (2001) found that activated platelets secrete sAPPα in the conditioned media, which competes with both LDL and SR-A receptors. They showed that both sAPP751 and sAPP695 equally bind to the SR-A receptor suggesting that the binding is independent of the KPI domain. Although, they found that sAPPα (residues of 191–264) is involved in SR-A binding (Santiago-Garcia et al., 2001), however, deletion of the SR-A receptors do not affect the plaque numbers and neurodegeneration in transgenic mice expressing human APP (Huang et al., 1999). Interestingly, our recent work indicates that sAPPα forms a complex with Aβ1–16 (corresponding to APP672–688) region, which augments the binding of this heterodimer complex with SR-A receptor. Our conclusion that scavenger receptor SR-A seems to be crucial for sAPPα-mediated clearance of Aβ (Darlington et al., 2015).
c. Interaction of sorting protein related receptor A (SORLA/LR11/SORL1) and sAPPα
A recent study demonstrated that variation within the two different clusters of intronic sequences of sorting protein related receptor A (SORLA/LR11/SORL1) is linked to sporadic AD (Rogaeva et al., 2007). Additional study data showed that overexpression of SORLA decreased production of Aβ and vice versa (Rogaeva et al., 2007). This finding is in good agreement with the reduced expression of SORLA in AD brain (Dodson et al., 2006). Using fluorescence resonance energy transfer (FRET) assay, Andersen et al. (2006) identified binding sites for APP-SORLA interactions (Andersen et al., 2006). They showed that two different sites of APP (APP28–123 and APP316–498) bind separately with SORLA (residues of 1044–1526). Utilizing plasmon resonance analysis and analytical ultracentrifugation techniques, the same study demonstrated that carbohydrate-linked (APP316–498) E2 domain of APP binds more favorably than the N-terminal (APP28–123) domain (Andersen et al., 2006). As the carbohydrate-linked domain (APP316–498) is an integral part of the sAPPα molecule, SORLA may be a plausible neuronal receptor. In agreement with this hypothesis, Hartl and colleagues have confirmed the sAPPα-SORLA interaction in cultured mouse cortical neurons showing that sAPPα downregulates cyclin dependent kinase (CDK) 5 activity by binding with the SORLA receptor (Hartl et al., 2013). As would be expected, the sAPPα-SORLA interaction increased expression of ORP150, which functions as a protective chaperone (Hartl et al., 2013). Like SORLA, Gustafsen et al. (2013) showed that sortilin acts as a neuronal receptors for sAPPα695. SORLA and sortilin both bind and mediate internalization of sAPPα into different intracellular compartments. Extracellular 6A domain of sAPPα interacts with sortilin in a pH dependent manner. As sortilin binds with both neuronal (sAPPα695) and non-neuronal (sAPPα751) isoforms, indicating the interaction could be independent of the KPI domain (Gustafsen et al., 2013).
d. Interaction of APP and of its different domains with sAPPα
Earlier works indicated APP as a cell-surface receptor that interacts with a variety of molecules in the extracellular environment. A recent investigation indicates that interaction of sAPPα ectodomain and full-length APP are crucial for neurite outgrowth. More specifically, they showed that neurotrophic activity of sAPPα is dependent on the membrane-bound full-length APP (Young-Pearse et al., 2008). To investigate if binding of sAPPα to the cell surface receptors is dependent on the membrane-bound (unprocessed) APP and of its homologues APLP1 and APLP2, Reinhard et al. (2013) demonstrated that sAPPα binds to the cell surface on a neuroblastoma cell line B103, which does not express APP, APLP1 and/or APLP2 (Reinhard et al., 2013). This suggests that binding of sAPPα is independent of full-length APP and of its family members. This finding is in disagreement with the Young-Pearse group (2007), where they demonstrated that activity of sAPPα is dependent on the full-length APP (Young-Pearse et al., 2008). Reinhard et al. (2013) also found that the growth factor like domain (GFLD) of sAPPα binds to the heparan sulfate proteoglycan (HSPG) at a concentration of 100 nM. They concluded that GFLD binds with heparin and the E2 domain mediates interaction with the HSPG (Reinhard et al., 2013).
e. Interaction and effect of dimerization of APP and its homologs (APLP1/APLP2) with sAPPα
APP and other homologs such as APLP1 and APLP2 form homo- and/or hetero- dimer, which modulate the trafficking of APP into the endocytic compartments. The homodimerization of APP at the plasmamembrane drives it into the endocytic compartments and generates Aβ upon cleavage by BACE1 (Scheuermann et al., 2001, Kaden et al., 2008). While studying the effect of sAPPα, Gralle and others have demonstrated that sAPPα protects neuronal cells by disrupting the dimerization of APP as shown in Figure 2e (Gralle et al., 2009); (Khalifa et al., 2010). While looking at the binding mechanism, Wang and colleagues demonstrated that heparin binds with the antiparallel dimer of APP (Wang and Ha, 2004). In agreement with the heparin-APP interaction, Gralle and colleagues demonstrated that heparin induces dimerization of sAPPα in solution at high concentrations (Gralle et al., 2006). Using single molecule FRET analysis, they showed that heparan sulfate (HS) induces dimerization of APP, which is crucial for intracellular signaling upon binding with an extracellular ligand (Gralle et al., 2009). Previous research indicated that both unprocessed and secreted APP can bind with the heparan sulfate (Williamson et al., 1996) and heparin (Mok et al., 1997), respectively. In recent years, Dahms and colleagues extensively investigated the interaction of heparin with the ectodomain of APP. They demonstrated that the heparin and E1 domain interaction is very specific (low dissociation constant, Kd, indicative of high affinity) (Dahms et al., 2010). In addition to heparin-E1 interaction, the E2 domain of APLP1 can bind with HS chain of HSPG in two different ways. The first mechanism involves the specific binding of E2 domain with the nonreducing end of the highly sulfated HS chain of HSPG. The second mechanism involves the general binding of the E2-HS chain. A different but similar study demonstrated that heparin-induced dimerization of APP is mediated by E1 (subdomains GFLD and CuBD) and regulated by acidic domain (Hoefgen et al., 2014). Dahms and colleagues have further demonstrated that sAPPα brings the E2 domain close to the nonreducing end of HS and the process is enhanced by heparinase modification (Dahms et al., 2015). Previous research suggested that the trans-dimerization of sAPPα or APP-E1 domain is crucial for synaptic functions, which also possess a copper binding domain (D2, CuBD, at amino acids 124–189). While investigating the role of copper on APP-dimerization, it has been shown that copper induced both cis-and trans-dimerization of APP in vitro (Kd = 18 nM) and in vivo (Kd = 100 μM) but the process is independent of the heparin interaction (Baumkotter et al., 2014).
The role of sAPPα in neuroprotection
Multiple lines of evidence demonstrate that sAPPα protect neurons against a variety of insults in cell cultures and animal models. Initial studies using rat hippocampal and human cortical neurons showed the protective role of sAPPα against hypoglycemic damage and glutamate mediated neurotoxicity (Mattson et al., 1993). In addition to the above findings, both sAPPα695 and sAPPα751 protect rat hippocampal neurons from iron mediated oxidative injury and Aβ-induced Ca2+ and free radical mediated neurotoxicity (Goodman and Mattson, 1994). These findings indicate that sAPPα regulates calcium homeostasis by inhibiting elevation of intracellular Ca2+ concentrations, the mechanism by which it enhances neuronal survival. Barger and Mattson further investigated the mechanism through which sAPPα shows the neuroprotective effect on hippocampal neurons. They showed that sAPPα increases the levels of cyclic nucleotides (cGMP) in neuronal cells, which inhibits elevation of cytosolic Ca2+ levels through inhibition of NMDA receptors (Barger et al., 1995). In a follow up study, the same group showed that elevation of cGMP by sAPPα is dependent on the activation of membrane-bound guanylate cyclase but independent of cytosolic (soluble) guanylate cyclase (Barger and Mattson, 1995). Using whole-cell patch-clamp and imaging techniques, Furukawa and colleagues extensively investigated sAPPα-mediated neuroprotective mechanisms in hippocampal neurons, showing that sAPPα (0.11 nM) suppresses neuronal excitability by activating K+ channels and modulates glutamate neurotoxicity by inhibiting NMDA-currents (Furukawa et al., 1996a, Furukawa and Mattson, 1998).
In addition to neuronal cells, both astrocytes and microglia express all three major forms of APP and process mostly via amyloidogenic pathway. The level and magnitude of APP expression by non-neuronal cells (astroglial) is much more subtle than the neuronal cells (neurons). Hence, very few studies have reported the exact role of sAPPα on regulating astroglial functions. Barger et al. (1997) showed that sAPPα activate microglial inflammation (Barger and Harmon, 1997) and activation via c-Jun N-terminal kinases (JNK) and p38-MAPK pathway (Bodles and Barger, 2005). In contrast, a different study showed that primary cytokine such as IL-1α stimulates α-secretase activity and expression of ADAM-10 and ADAM-17which enhanced APP processing and sAPPα secretion through non-amyloidogenic pathway (Bandyopadhyay et al., 2006). However, the secretion and production of sAPPα is independent of c-JNK pathway but dependent on p38-MAPK pathway. A very recent study showed that inflammatory cytokines such as TNFα and IL-1β treated astrocytes enhance sAPPα production through non-amyloidogenic processing of APP by increasing membrane fluidity in neuronal cells (Yang et al., 2015). Further study is needed to clarify the role of sAPPα in activating astroglial cells and subsequent effect on neurons in the brain.
In accord with the cell culture findings, sAPPα also exerts neuroprotective effects in animal models following CNS injury. Administration of recombinant sAPPα in a rat model reduced hippocampal neuronal deaths against ischemic (Smith-Swintosky et al., 1994), spinal cord (Bowes et al., 1994), and traumatic brain injury (TBI) (Thornton et al., 2006, Corrigan et al., 2011). In addition to enhanced neuronal survival, surviving neuronal cells synthesize new proteins, attenuate amyloid pathology, improve cognition, and motor functions in a moderately brain-injured APP knockout (KO) mouse model (Corrigan et al., 2012). A successive study by the same group showed that the heparin binding site of sAPPα (residues of 96–110) protects against TBI (Corrigan et al., 2014). The cellular receptors and the downstream effectors involving these effects are largely unknown. In brief, the neuroprotective effects of sAPPα could be through the modulation of ion channels and gene expressions (Mattson et al., 1997). While investigating the neuroprotective functions of sAPPα, several studies showed that sAPPα activates phosphatidylinositol-3-kinase (PI3K)/Protein Kinase B (PKB/Akt) (Cheng et al., 2002, Jimenez et al., 2011); (Milosch et al., 2014), nuclear factor kappa B (NF-kB) (Guo et al., 1998), extracellular signal regulated kinase (ERK) (Greenberg et al., 1995, Cheng et al., 2002), and inhibits stress-induced c-JNK signaling (Kogel et al., 2005). Lastly, sAPPα mediated neuroprotection also involves activation and transcription of different factors and enzymes such as insulin-like growth factor 2, manganese superoxide dismutase, catalase, and transthyretin (Stein et al., 2004, Kogel et al., 2005).
The role of sAPPα in learning and memory formation
Alterations or loss of synapses (Terry et al., 1991) and cognitive decline (DeKosky et al., 1996) have been reported in healthy aging and in neurodegenerative diseases including but not limited to AD. However, the processes of memory formation in the brain are still largely unknown. Nevertheless, to identify the cognitive impairment, researchers and physicians frequently measure long-term potentiation (LTP) in basic and clinical research. In addition to impaired LTP, hippocampal and cortical studies have showed significant correlation between cognitive impairment and synaptic protein loss; clearly indicating that synapses are critical for memory formation and storage (Winocur et al., 2010). Many researchers have studied the role of APP in synaptic plasticity and memory formation. Not surprisingly, APP, a key protein in AD development, is highly expressed in the presynaptic terminals and plays a critical role in synaptic functions (Turner et al., 2003), LTP, (Seabrook et al., 1999) and memory formation (Huber et al., 1997, Mileusnic et al., 2000). Muller and Zheng group have independently studied the role of APP and its fragments in synapse formation and correlated those abnormalities with cognitive impairment using several APP mutant mouse models [See reviews by (Aydin et al., 2012, Guo et al., 2012b, Muller and Zheng, 2012)]. In addition, Jung and Herms published a comprehensive review on the role of APP in dendritic spine formation [for review please see (Jung and Herms, 2012)]. The details of those studies are beyond the scope of this review.
Initial studies by blocking the extracellular domain of APP with anti-APP antibodies showed behavioral and memory impairment in rat models (Doyle et al., 1990, Huber et al., 1993). Subsequently, using an APP (KO) hypomorphic mouse models, Muller and Zheng have demonstrated impaired behavioral functions in rodent models (Muller et al., 1994, Zheng et al., 1995, Zheng et al., 1996). In contrast to the effects of Aβ, APP and its proteolytic fragments particularly sAPPα, promoted enhanced synaptic plasticity (Ishida et al., 1997, Hick et al., 2015) and memory formation (Bour et al., 2004). Additionally, the positive correlation between decreased CSF levels of sAPPα and impaired cognitive functions in animal (Anderson et al., 1999) and human studies (Van Nostrand et al., 1992, Lannfelt et al., 1995, Almkvist et al., 1997) further suggested a role of sAPPα in cognition. To identify the mechanism, intracerebroventricular (ICV) administration of total sAPP antibodies (combined sAPPα and sAPPβ) targeted against the N-APP demonstrated memory deficits in rat models (Doyle et al., 1990, Huber et al., 1993). To investigate the role of sAPPα in learning and memory formation, recombinant sAPPα (0.5 pg/4μl/mice) (Bour et al., 2004) and of its active domain (17 residues of sAPPα) (Roch et al., 1994) showed improved spatial memory in mice and memory retention in an aged rats, respectively. While investigating the role of sAPPα in LTP formation, induction of LTP has been shown to be associated with an increased secretion of APP and neural cell adhesion molecule in the dentate gyrus (DG) of a rat model (Fazeli et al., 1993). Furthermore, to investigate if sAPPα has a role in synaptic plasticity and spatial memory formation, administration of recombinant sAPPα increased and antibodies against endogenous sAPPα decreased LTP and NMDA transmission in an adult rat model (Taylor et al., 2008). NMDA receptors activation are shown to be involved in induction of nitric oxide from arginine, which subsequently increases cGMP, stimulates presynaptic (Arancio et al., 2001) soluble guanylyl cyclase (East and Garthwaite, 1991) and protein kinase G (PKG) signaling pathway (Zhuo et al., 1994). A different study in a drug induced-amnestic mouse model showed that both sAPPα695 and sAPPα751 are equally effective in enhancing memory at low doses (0.05–5000 pg) and the effect is independent of its KPI domain (Meziane et al., 1998). A recent study showed that sAPPα (10 nM) significantly increased protein synthesis at hippocampal synapses through cGMP signaling in an adult Sprague-Dawley rat model that might contribute synaptic plasticity (Claasen et al., 2009).
The APP KO mouse model exhibits anatomical, synaptic, and behavioral alterations. To investigate the role of APP and of its fragment sAPPα, Muller group have (2007) deleted the APP locus (APP-KO) and replaced it with an sAPPα knock-in (KI) gene at the same position, which constitutively expressed secreted sAPPα in the brain. They showed that sAPPα-KI mice had improved synaptic plasticity, cognition, and a rescue of all the deficits shown by APP-KO mice such as reductions in brain and body weight, grip strengths, exploratory impairments, alterations in circadian locomotor activity, as well as impairment of spatial learning and LTP (Ring et al., 2007). Interestingly, when sAPPα-KI mice were crossed with the APLP2 KO background model, most of the double mutants survived into adulthood. Despite the normal synaptic structure and transmission, these mice showed impaired LTP induction and maintenance coupled with working memory impairment. These findings suggest that sAPPα expression does not compensate the early developmental abnormalities in APLP2 KO mice and showed excessive nerve growth with widened nerve plates (Weyer et al., 2011). Contrary to the beneficial role of sAPPα, many studies have reported the increased level of sAPPα in autism studies (Bailey et al., 2008, Ray et al., 2011). The role of dysregulated secretion of sAPPα in autism is unknown.
Interestingly, to investigate the effect of sAPPβ in APP KO mouse, Li et al. (2010) constructed an sAPPβ KI mouse model. They found that secreted sAPPβ is highly stable in cell culture and in brain and CSF in this transgenic sAPPβ KI mouse model. Most of the offspring of the sAPPβ KI and APLP2 KO crossed mice died early due to the postnatal lethality. All surviving mice showed normal body weight and grip strength with abnormal nerve plate terminals (Li et al., 2010). The postnatal lethality of the APP/APLP2 double KO mouse was rescued by crossing sAPPα-KI with APLP2-deficient mice that showed impaired LTP function (Weyer et al., 2011). Due to the postnatal lethality of APP double KO mice, the effect of sAPPα was studied on conditional APP/APLP2 double KO mice model (Hick et al., 2015). These mice show reduced neurite length, dendritic branching, spine density, and spine head size in the hippocampus. They also demonstrated that exogenous administration of sAPPα (10 nM), but not sAPPβ (even at 50 nM, do not show this effect), rescued impairment of LTP and memory deficits in this APP/APLP2 double conditional KO mouse model (Hick et al., 2015). These findings suggest that sAPPα has a crucial role in improvement of synaptic plasticity and cognitive impairment in transgenic APP mice. Recently, Muller group (2016) have published data indicating a rescue of the structural, electrophysiological, and behavioral deficits in APP/PS1ΔE9 mice using adeno-associated virus (AAV)-mediated expression of sAPPα (Fol et al., 2016). They concluded that sAPPα activated microglial cells, which might reduce soluble Aβ species and plaques by up regulating insulin-degrading enzyme (IDE) and triggering receptor expressed on myeloid cells 2 (TREM2) receptors.
The proliferative role of sAPPα in neuritogenesis and neurogenesis
The early expression of APP mRNA (at embryonic day 9.5) in a mouse model underscored the significance of this molecule in nervous system development (Salbaum and Ruddle, 1994). Moreover, crystal structure and computer modeling studies indicate that sAPPα (residues of 18–350) has a cysteine-rich growth factor like domain (Rossjohn et al., 1999) and plays a key role in outgrowth and survival of neurons in cell culture studies (Araki et al., 1991, Milward et al., 1992, Qiu et al., 1995, Perez et al., 1997). In line with the above findings, both soluble and membrane-bound APP independently increased neurite outgrowth and branching (Whitson et al., 1990, Milward et al., 1992, Qiu et al., 1995). In contrast, Young-Pearse and co-workers (2008) demonstrated that sAPPα regulates neurite outgrowth through interaction with full-length APP and integrin beta1 signaling (Young-Pearse et al., 2008). They concluded that the activity of sAPPα is dependent on the membrane-bound APP. Earlier work indicated that neuritotropic and heparin-binding sites of APP are distinct and a heparinase-insensitive region is responsible for the effect (Ninomiya et al., 1993). In a different study, Jin and colleagues (1994) have showed that the neuritotropic activity of sAPPα is located on a stretch of 17 amino acids (residues of 319–335), which includes the RERMS (APP 328–332) sequence (Jin et al., 1994). Subsequently, Small and others have showed that interaction of APP and HSPG is critical for neurite outgrowth. To identify the heparin-binding domain in APP, deletion mutation and peptide mapping experiments have revealed four heparin-binding sites in APP. Among the four different binding sites, one site (residues of 96–110 of sAPPα) has more affinity than the other three sites (Clarris et al., 1994, Small et al., 1994, Small et al., 1999). Additionally, a delta NL mutation in the APP gene which produces less sAPPα but more sAPPβ further supports those findings by showing defective neurite extension (Li et al., 1997). Both early and recent works showed that sAPPα stimulates proliferation of neural stem cells (NSCs) (Hayashi et al., 1994, Ohsawa et al., 1999), embryonic stem cells (Porayette et al., 2009), and adult progenitor cells (Caille et al., 2004, Demars et al., 2011). To investigate the signaling pathways involved in the neurite extensions, one study showed that sAPPα activated MAPK/ERK signaling via activation of NMDA receptors (Gakhar-Koppole et al., 2008). A recent study demonstrated that both sAPPα and sAPPβ were able to enhance axonal growth in cell culture at low (nanomolar) concentrations through early growth response protein 1 signaling (Chasseigneaux et al., 2011).
Ohsawa et al. identified the extracellular matrix glycoprotein fibulin-1, mainly produced by neurons, as a potential sAPPα binding partner in the brain (Ohsawa et al., 2001). The binding of sAPPα and fibulin-1 is dependent on Ca2+ and blocked by an antibody against the N-terminal region of APP. In addition, they showed that the N-terminal region of APP binds to fibulin-1 and prevent sAPP-mediated proliferation of neural stem cells. Both sAPP and fibulin-1 are secreted in extracellular environment, the consequence of this interaction demands further study.
P75 neurotrophin receptor (p75NTR) belongs to a large family of transmembrane molecules of the tumor necrosis factor receptor superfamily. Ligand binding studies indicated the multiple functions of this receptor in regulating axonal growth, neuronal survival, synaptic transmission, and apoptosis (Dechant and Barde, 2002). A number of different studies showed that P75NTR interacts with full-length APP (Fombonne et al., 2009), Aβ (Knowles et al., 2009), N-terminal APP (APP1–286, EC50 = 300 nM) (Nikolaev et al., 2009), sAPPα and sAPPβ (Hasebe et al., 2013). More specifically, the carboxyl-terminal region of sAPPα (residues of 314–612) interacts with P75NTR (EC50 = 150 nM) and induced neurite outgrowth through activation of protein kinase A (PKA) signaling (Hasebe et al., 2013). These findings suggest that sAPPα (both N- and C- terminal) binds to P75NTR and initiates neurite outgrowth depending on the nature of binding as shown in Figure 2d.
The role of sAPPα in modulation of AD and aging
Multiple lines of evidence indicate that altered APP processing leads to an increased production of Aβ, which contributes to AD pathologies. Cleavage of APP by α- and γ-secretases not only prevents generation of toxic Aβ peptides but also produces neuroprotective sAPPα. Multiple lines of evidence indicate that sAPPα regulates the trafficking and processing of APP, which may decrease the risk of developing AD. The role of sAPPα as a modulator of γ-secretase complex came from an study which shows that sAPPα reduced the Aβ42/Aβ40 ratio by modulating the enzyme complex (Hou et al.). Additionally, modulation of BACE1 by sAPPα reduces generation of Aβ and plaques in cell culture and in a transgenic mouse model of AD (Obregon et al., 2012). In accord with this finding, Varghese group confirmed sAPPα as an endogenous inhibitor of BACE1 activity. They demonstrated that sAPPα decrease the enzymatic activity of BACE1 by binding to its allosteric site (Peters-Libeu et al., 2015). In addition, sAPPα, acting through unknown receptors, inhibited BACE1 and GSK3β activity, which reduced tau phosphorylation (Deng et al., 2015). This study also demonstrated that recombinant human sAPPα increased Ser-9 phosphorylation of GSK3β. Earlier work by Jimenez et al. (2011) demonstrated that GSK3β (Ser-9) phosphorylation decreased significantly in aged (18 months) APP/PS1 mice compared to young (6 months) mice (Jimenez et al., 2011). They also showed that soluble Aβ modulates the sAPPα-mediated neuroprotective PI3K/Akt/GSK3β signaling pathway in an aged mouse model as shown in Figure 2f. This indicated a key role of sAPPα in activation of survival pathway in an aged mouse model. The decreased level of hippocampal sAPPα coupled with reduced NMDA receptors and impaired LTP function further suggest the importance of this soluble fragment in an aged (24–27 months) rat model. In line with this finding, exogenous administration of sAPPα (100 nM) reduces age-associated deregulation of NMDA receptor function and LTP deficits (Moreno et al., 2015). Moreover, sAPPα-mediated inhibition of apoptosis and dendritic degeneration via c- JNK pathway further underscored the importance of the fragment in aging studies (Copanaki et al., 2010).
Diagnostic value of sAPPα as AD biomarker
Several studies have measured the metabolites of APP cleavage such as sAPPα, sAPPβ, and total sAPP (sAPPα and sAPPβ together) in AD and other neurodegenerative diseases. The results are inconsistent and contradictory in many cases. The inconsistencies are partly due to the heterogeneity of the disease, inconsistencies in mini-mental status exam (MMSE) scores, presence of co-morbid conditions, specificity and sensitivity of the assays, cross-reactivity of the antibodies, differences in sampling, as well as processing and storage of CSF samples. Initial studies (Ghiso et al., 1989); (Weidemann et al., 1989) as well as a recent one conducted on patients with MMSE score greater than 20 (Lewczuk et al., 2010) demonstrated high CSF levels of sAPPα and sAPPβ in patients with CSF findings characteristic of AD. The later study lacks the healthy controls and co-morbid conditions in the cohort. In contrast, other studies measured a slight or no significant change in total sAPP levels in the CSF of AD patients compared to non-demented controls (Palmert et al., 1990); (Hock et al., 1998). Notably, the antibodies used in early studies failed to demonstrate the difference between sAPPα and sAPPβ so instead measured total sAPP. On the other hand, many recent studies found no significant changes between the two soluble fragments in AD and non-demented controls (Zetterberg and Blennow, 2008, Rosen et al., 2012, Brinkmalm et al., 2013). In contrast to the above studies, while other studies show significantly decreased levels of total and sAPPα, however, sAPPβ was found to be unchanged in AD patients compared to controls (Prior et al., 1991, Van Nostrand et al., 1992, Sennvik et al., 2000). In accord with these findings, patients carrying the Swedish mutation (a double mutation in the APP gene) showed significantly decreased levels of sAPPα in the CSF (Lannfelt et al., 1995). Significant negative correlations between CSF levels of sAPPα and cognitive impairment have been reported in Swedish mutant AD patients (Almkvist et al., 1997). More recent work by Kim and colleagues corroborated this finding, demonstrating that mutations in A Disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) significantly reduced levels of α-secretase and sAPPα in familial late-onset AD (Kim et al., 2009). While the level of sAPPα significantly decreased in familial (Swedish mutation) and in moderate-to-severe AD, the levels did not change in the early stage of sporadic AD and mild cognitive impairment. In contrast to sAPPα, the higher level of p-tau181 and reduced level of Aβ42 serve as a diagnostic marker for AD (Blennow, 2004). Recently, in addition to sAPPα and sAPPβ, full-length soluble APP and sAPP complexes were detected in CSF samples (Cuchillo-Ibanez et al., 2015). Further investigation will clarify whether full-length sAPP complexes with itself and is falsely measured as sAPPα and/or sAPPβ.
A number of studies showed that apoliprotein 4 (APOE4), one of the variants of APOE, may also contribute to the CSF level of sAPPα in AD patients. APOE4, a risk factor for late-onset AD (Harold et al., 2009, Lambert et al., 2009), transports cholesterol in the brain (Liu et al., 2013). Most studies demonstrated that the APOE4 variant increased AD risk whereas the APOE2 variant decreased the risk of AD (Farrer et al., 1997). In line with these findings, addition of APOE4 to neuroblastoma SH-SY5Y cells (Cedazo-Minguez et al., 2001) and cortical neurons carrying the Swedish mutation co-cultured with APOE4 astrocytes showed decreased level of sAPPα generation. Accordingly, sAPPα levels are significantly decreased in AD patients having two APOE4 alleles compared to one APOE4 allele. However, the levels of Aβ42 and sAPPβ were found unchanged across other APOE genotypes (Vincent and Smith, 2001). This indicates that APOE4 might affect α-secretase cleavage of APP.
APP is involved in multiple physiological functions and variations in sAPPα generation occur in many different conditions in addition to AD. Reduced CSF concentrations of sAPPα has been reported in other conditions such as cerebrovascular and neurodegenerative diseases (Selnes et al., 2010, Steinacker et al., 2011), bipolar disorder (Jakobsson et al., 2013), amyotrophic lateral sclerosis (Steinacker et al., 2011) and idiopathic normal pressure hydrocephalus (Miyajima et al., 2013). The lower levels of sAPPα in other conditions indicate that critical clinical evaluation is necessary to rule out the other conditions. Although many association studies showed sAPPα as a predictive biomarker, more epidemiological data are needed to generate a robust and standard scale that is diagnostically precise and accurate.
The therapeutic potential of sAPPα
In this review, we summarized numerous physiological functions of sAPPα, which has been (Table 1) disrupted in the AD brain in several ways. These functions include, but are not limited to, neuroprotection (Goodman and Mattson, 1994, Gralle et al., 2009), neurite outgrowth (Araki et al., 1991, Gakhar-Koppole et al., 2008), elevation of LTP (Ishida et al., 1997, Hick et al., 2015), as well as stimulation and proliferation of neuronal (Ohsawa et al., 1999, Demars et al., 2011) and non-neuronal cells (Saitoh et al., 1989, Pietrzik et al., 1998). In addition, sAPPα directly inhibits β-secretase-mediated proteolysis of APP, thereby reducing generation of Aβ (Obregon et al., 2012). Furthermore, sAPPα also has the potential to reduce tau-pathology by inhibiting GSK3β and BACE1 activity as shown in Figure 2h (Deng et al., 2015). Moreover, both single and multiple low-dose infusion of human umbilical cord blood (Darlington et al., 2013) as well as derived monocytes significantly reduce Aβ and β-amyloid plaques, decrease APP processing, reactive microgliosis, associated astrocytosis and cognitive impairment in the PSAPP AD mouse model (Nikolic et al., 2008). While identifying the mechanism, further studies indicated that cord blood monocytes might have their own α-secretase or activate an endogenous α-secretase enzyme in the PSAPP mouse model. Interestingly, exogenous sAPPα reversed the deficiency of phagocytosis showed by aged blood monocytes (Darlington et al., 2015). Thus, restoration of sAPPα levels in the brain by shifting the amyloidogenic towards the non-amyloidogenic pathway could ameliorate AD-related amyloid and tau pathology, neuronal loss, and cognitive impairment. As such, increasing α-secretase activity is therefore an attractive strategy for treatment of AD.
Table 1.
Roles of sAPPα in neuroprotection, synaptic plasticity, neurogenesis and neurite outgrowth
| Citation | Test Model | Functional domain and concentration | Treatment | Effect |
|---|---|---|---|---|
| Araki et al. 1991 | Rat cerebral cortical neuron | sAPP695 and sAPP770 (40 nM) | Cortical neuron treated with sAPP695 and sAPP770 | Neurite extension |
| Goodman and Mattson et al. 1994 | Rat hippocampal cell culture | sAPP695 and sAPP751 | Hippocampal culture treated with sAPP695 and sAPP751 | Inhibit increase of intracellular Ca2+ level and free radical |
| Mattson et al. 1993 | Rat hippocampal, human cortical neuron | sAPP695 and sAPP751 | Hippocampal, human cortical neuron treated with sAPP695 and sAPP751 | Protect against hypoglycemic and glutamate neurotoxicity |
| Furukawa et al. 1996a and 1996b | Rat hippocampal neuron | N terminal- and C-terminal sAPPα(residues 591–612) | Hippocampal neuron treated with N terminal-sAPPα and C-terminal sAPPα (residues 591–612) | Suppress action potential; activation of K+ channel and cGMP; Heparinase reduces sAPPα activity |
| Furukawa and Mattson et al. 1998 | Rat hippocampal neuron | sAPPα (0.01–1 nM) | Rat hippocampal neuron treated with sAPPα | Neuroprotection by activation by cGMP and suppression of NMDA. |
| Smith-Swintosky et al. 1994 | Rat model | sAPP695 and sAPP751 | Intracerebroventricular (icv) infusion of sAPPα in post ischemic injured | Neuronal survival and synthesis of new proteins in CA1 |
| Bowes et al. 1994 | Rabbit spinal cord ischemia model | sAPPα 17-mer peptide at 500 nM | Intrathecal infusion of (once per 3 days) sAPPα 20 min prior to the ischemia. | Reduce necrotic tissue; Increased synaptophysin synthesis |
| Thornton et al. 2006 | Male Sprague-Dawley rat | sAPPα (0.2 mg/ml) | ICV infusion sAPPα (5 μl ) after 30 min of traumatic brain injury (TBI) rat model | Improved motor function; Reduced cortical and CA1 caspase-3; Axonal injury at corpus callosum |
| Copanaki et al. 2010 | Rat PC12 cells and mice hippocampal slices | sAPPα (0.1–50 nM) | sAPPα produced by HEK293 (APPWT) used to treat PC12 and mice hippocampal slices | Protect dendritic and neuronal damage in CA1; Inhibition of JNK and activation of PI3K/Akt signaling |
| Corrigan et al. 2011 | Sprague-Dawley rat | N-terminal D1 (APP28–123), and C-terminal D6a/E2 (APP316–498) of sAPPα (25 μM) | ICV infusions of sAPPα D1 and D6a domain after 30 min in TBI rat model | Improved motor and cognitive function; Reduced axonal injury; Signaling through HSPG |
| Corrigan et al. 2012 | APP KO mice | sAPPα (APP18–611) 25 μM | ICV infusions of sAPPα in APP KO after 30 min of moderate cortical injury | Improved motor and cognitive function; Reduced cortical and hippocampal damage |
| Corrigan et al. 2014 | APP KO mice | sAPPα (APP96–110) 25 μM of D1 domain | ICV infusions of sAPPα in APP KO after 30 min of cortical injury | Rescue motor and cognitive deficits in APP KO mice; Reduced axonal injury |
| Roch et al. 1994 | Rat (Adult) | sAPPα 17-mer peptide (residues 319–335) containing RERMS (APP328–332) (1 mM) | Intraventricular infusions of 17-mer peptide. After 14 days analyzed by behavioral and biochemical tests | Improved memory retention; Increased number of presynaptic terminals in the frontoparietal cortex |
| Ishida et al. 1997 | ND | sAPPα (1–612) purified HEK293 (APPWT) (100 nM) | sAPPα infusion for 30–120 min followed by LTP measurement in hippocampus | Induce cGMP and enhancedLTP in CA1 |
| Meziane et al. 1998 | Male Swiss mice | sAPPα695 and sAPPα751 (0.05 pg-5 ng) | ICV infusions of sAPPα immediately after drug induced amnesia | Inhibit drug induced amnesia; Improved short-and long-term memory. |
| Andersen et al. 1999 | Fischer 344-rat | Young (5–6 months) and Aged (24–25 months) | Total sAPP, sAPPα, Aβ measured in CSF of young and aged rat model | sAPPα reduce 50% in aged ; Improved spatial reference and working memory |
| Taylor et al. 2008 | Sprague Dawley rat (adult) | sAPPα (11 nM) purified from HEK293 (APPWT) cells | Intrahippocampal infusion of sAPPα and anti-sAPP antibody | Enhanced LTP and NMDA currents in CA1; Improved spatial memory |
| Classen et al. 2009 | Sprague Dawley rat (adult) | sAPPα and sAPPβ (10 nM) | Isolation of synaptoneurosome from hippocampus of adult (2–3 months) and aged (22–23 months) rat | Synaptic protein synthesis age and concentration dependent through PKG signaling; sAPPβ has no effect |
| Ring et al. 2007 | sAPPα-KI and APP-KO mice | APP gene is replaced with sAPPα gene | Deficits of APP KO mice was fully rescued by sAPPα-KI mice | Improved LTP and cognition; Rescue brain and body weight, grip strength, exploratory and locomotor activity. |
| Weyer et al. 2011 | sAPPα KI cross with APLP2 KO | sAPPα KI mice crossed with APLP2 KO background | Anatomical and behavioral assessment | Most of the mice survived; Cortical and hippocampal transmission normal; Impaired LTP and working memory; Excessive nerve growths |
| Li et al. 2010 | sAPPβ KI cross with APLP2 KO | sAPPβ KI cross with APLP2 KO background | Anatomical and behavioral assessment | Mice died early due to postnatal lethality; Normal body weight and grip strength but abnormal nerve terminal |
| Hick et al. 2015 | APP/APL2 double KO mice | sAPPα (10 nM), but sAPPβ (50 nM) | Conditional APP/APLP2 double KO in forebrain neurons using NexCre | sAPPα rescue impaired LTP; sAPPβ has no effect. |
| Fol et al. 2016 | APP/PS1delE9 mice | sAPPα-AAV, (1010vg/hippocampus) | sAPPα-AAV bilaterally injected into hippocampus and sacrificed after 5 months | Improved synaptic and cognitive deficits; Rescue spatial memory; Reduction of soluble Aβ and plaque loads. |
| Milward et al. 1992 | Rat PC12 cells | Membrane-bound APP (10 ng) sAPPα (100 ng) per ml at (10−10M) | Membrane-bound APP (10 ng) sAPPα (100 ng) treated for 18 hour. | Increased neurite length and branching; No change in neurite per cells. |
| Small et al. 1994;Clarris et al. 1994 and 1997 | Chick sympathetic and mice hippocampal neurons | sAPPα (residues 96–110) | sAPPα (10 μg/ml) | Binding of sAPPα (residues 96–110) to HSPG stimulates neurite outgrowth |
| Qiu et al. 1995 | Rat hippocampal neuronal culture | sAPPα (residues 361–648) (10 pM to 100 nM) | Rat hippocampal neurons treated with sAPPα for 26–28 hr | sAPPα751 and sAPPα770 promotes neurite outgrowth better than sAPPα695 in the presence of unprocessed APP |
| Ohsawa et al. 1995 and 1997 | Rat embryonic neocortical explants | sAPPα695 and sAPPα770 (30 ng/ml); 16-mer (APP66–81) and 17-mer peptide containing RERMS sequence | Neocortical explants incubated with sAPPα695 and sAPPα770 (30 ng/ml) | N-sAPPα770 (residues 16–290) promote neurite outgrowth but C-sAPPα770 (residues 380–663) do not show this effect. 16-mer enhances neurite outgrowth but 17-mer peptide show neuronal survival |
| Jin et al. 1994 and Ninomiya et al. 1994 | Rat neuronal line B103 | sAPPα (10–100 nM) containing RERMS sequence (APP319–335) | B103 cell lacks APP and treated with sAPPα (10–100 nM) containing RERMS sequence (APP319–335) | Induction of neurite outgrowth |
| Young-Pearse et al. 2008 | Primary E18 wild-type neurons;Sprague-Dawley rat | sAPPα (1–612-(His)6 | Primary E18 wild-type neurons treated with sAPPα for 3 days | sAPPα regulates the function of APP in neurite outgrowth |
| Gakhar-Koppole et al., 2008 | Mouse neural precursor cells | Human recombinant sAPPα695 and sAPLP2 | sAPPα treated with primary neuronal culture | Enhance neurite outgrowth through activation of cell surface APP, NMDAR and MAPK/ERK signaling |
| Chasseigneaux et al., 2011 | Primary neuronal culture; C57BL/6J mice | Recombinant sAPPα and sAPPβ (100 nM) | sAPPα (100 nM) added to differentiated neurons | Both sAPPα and sAPPβ increased axonal elongation through MAPK/ERK/Egr1 signaling; Decrease of dendrites |
| Hasebe et al. 2013 | Mice primary cortical neuron | sAPPα and sAPPβ (<100 nM) | sAPPα incubated with primary cortical culture for 24 h | Both sAPPα and sAPPβ bind to P75NTR. But, sAPPα promotes neurite outgrowth |
Although, sAPPα provides neuroprotection, the only way to increase sAPPα level in the brain is by increasing α-secretase and/or decreasing β-secretase activity. The currently known α-secretase enzymes ADAM10, ADAM17 (TNFα converting enzyme, TACE), and ADAM9 reduce some degree of AD pathology (De Strooper et al., 2010) but these enzymes have other substrates. Although, TACE, ADAM10, and ADAM9 are mainly involved in APP α-secretase cleavage, they also cleave various substrates involved in autoimmune and cardiovascular disease, neurodegeneration, neurodevelopmental disorders, infection, inflammation, and cancer (Arribas and Esselens, 2009; Crawford et al., 2009;(Peduto, 2009). Therefore, TACE and ADAM10 have been therapeutic targets for inflammation, cancer, and inflammation-associated cancer (Saftig and Reiss, 2011). Recently, dysregulation of ADAM10 activity has been shown to be associated with synaptic deficits in Fragile X Syndrome (Pasciuto et al., 2015). Despite the side effects, Farenholz and Postina have listed a variety of ways to enhance sAPPα production including but not limited to G-protein coupled muscarinic agonists, serotonin receptor 5HT4 agonists, neuropeptide pituitary adenylate cyclase-activating polypeptide, PKC activators, statins, retinoids, and caloric restriction (Fahrenholz and Postina, 2006); (Endres and Fahrenholz, 2010). In addition, a review by Vincent and Govitrapong summarized various natural and synthetic compounds such as acitretin, SirT1, statin, epigallocatechin-3 gallate, and, estrogen that are able to stimulate α-secretase activity selectively. They also emphasized the activation of protein kinase and G-protein-coupled receptors mediated upregulation of α-secretase activity (Vincent and Govitrapong, 2011).
It is imperative to identify the sAPPα-mediated signaling pathways and downstream effectors fully before using this fragment in therapeutic applications. Several studies suggest that sAPPα stimulates PI3K/Protein Kinase C (PKC)/Akt signaling in cell culture and animal models. Furthermore, Endres and Farenholz have summarized the modulation of the α-secretase ADAM10 gene expression by retinoic acid derivatives. They concluded that retinoids decrease generation of toxic Aβ and increase neuroprotective sAPPα (Endres and Fahrenholz, 2012). Recently, the Varghese group have summarized a review discussing the importance of sAPPα and enhancement of this fragments using many different approaches (Spilman P, 2015). They found that one of the α7- nicotinic acetylcholine receptor partial agonist, tropisetron, significantly increased sAPPα in cell culture and in a mouse model (Spilman et al., 2014). The size of sAPPα fragment is too large to cross the BBB. More research is necessary to identify the small functional unit of sAPPα that crosses the BBB but retain functional properties. It has proven to be quite difficult to obtain a small functional unit of sAPPα, since the function is dependent on the conformational structure of this molecule. Recently, the Varghese group have demonstrated that sAPPα and sAPPβ adopt a completely different structure, although they are differ by only 16 amino acids residues at the C-terminus (Peters-Libeu et al., 2015). Transgenic mice engineered to overexpress sAPPα could be another way to accomplish this function. Currently, only one transgenic mouse line overexpressing human sAPPα is available for AD and Autism studies (Bailey et al., 2012). AAV (Fol et al., 2016) and Lentivirus mediated sAPPα gene delivery into the specific region of the brain could be another strategy to increase expression of this fragment locally.
Conclusions
The presence of amyloid plaques and NFT’s is the pathognomonic feature delineating AD from other types of dementia. Currently, AD therapy focuses on the prevention and clearance of β-amyloid plaques and NFT from the brain. Unfortunately, none of the available strategies resulted in significant cognitive improvement in clinical trials. We believe that restoration of sAPPα function using this fragment, or a mimetic thereof, in the very early stage of the disease will reduce or prevent cognitive impairment in AD and in other neurodegenerative diseases.
Acknowledgments
The Silver Endowment and NIH/NIA (R01AG032432, J.T.) supported this work. We would like to thank Dr. Song Li for helping us in editing the article.
Footnotes
Competing interests
The authors declare that they have no conflict of interest.
Authors’ contribution
All authors drafted, contributed, read and approved our review.
References
- Almkvist O, Basun H, Wagner SL, Rowe BA, Wahlund LO, Lannfelt L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Archives of neurology. 1997;54:641–644. doi: 10.1001/archneur.1997.00550170111022. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Schmidt V, Spoelgen R, Gliemann J, Behlke J, Galatis D, McKinstry WJ, Parker MW, Masters CL, Hyman BT, Cappai R, Willnow TE. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. doi: 10.1021/bi052120v. [DOI] [PubMed] [Google Scholar]
- Anderson J, Holtz G, Baskin P, Wang R, Mazzarelli L, Wagner S, Menzaghi F. Reduced cerebrospinal fluid levels of α-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience. 1999;93:1409–1420. doi: 10.1016/s0306-4522(99)00244-4. [DOI] [PubMed] [Google Scholar]
- Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochemical and biophysical research communications. 1991;181:265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- Arancio O, Antonova I, Gambaryan S, Lohmann SM, Wood JS, Lawrence DS, Hawkins RD. Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:143–149. doi: 10.1523/JNEUROSCI.21-01-00143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin D, Weyer SW, Muller UC. Functions of the APP gene family in the nervous system: insights from mouse models. Experimental brain research. 2012;217:423–434. doi: 10.1007/s00221-011-2861-2. [DOI] [PubMed] [Google Scholar]
- Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, Sanberg CD, Sutton DT, Tan J. Peripheral biomarkers in Autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. International journal of clinical and experimental medicine. 2008;1:338–344. [PMC free article] [PubMed] [Google Scholar]
- Bailey AR, Hou H, Obregon DF, Tian J, Zhu Y, Zou Q, Nikolic WV, Bengtson M, Mori T, Murphy T, Tan J. Aberrant T-lymphocyte development and function in mice overexpressing human soluble amyloid precursor protein-alpha: implications for autism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1040–1051. doi: 10.1096/fj.11-195438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Hartley DM, Cahill CM, Lahiri DK, Chattopadhyay N, Rogers JT. Interleukin-1alpha stimulates non-amyloidogenic pathway by alpha-secretase (ADAM-10 and ADAM-17) cleavage of APP in human astrocytic cells involving p38 MAP kinase. Journal of neuroscience research. 2006;84:106–118. doi: 10.1002/jnr.20864. [DOI] [PubMed] [Google Scholar]
- Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of beta-amyloid precursor. Journal of neurochemistry. 1995;64:2087–2096. doi: 10.1046/j.1471-4159.1995.64052087.x. [DOI] [PubMed] [Google Scholar]
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Barger SW, Mattson MP. The secreted form of the Alzheimer’s beta-amyloid precursor protein stimulates a membrane-associated guanylate cyclase. The Biochemical journal. 1995;311(Pt 1):45–47. doi: 10.1042/bj3110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumkotter F, Schmidt N, Vargas C, Schilling S, Weber R, Wagner K, Fiedler S, Klug W, Radzimanowski J, Nickolaus S, Keller S, Eggert S, Wild K, Kins S. Amyloid precursor protein dimerization and synaptogenic function depend on copper binding to the growth factor-like domain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:11159–11172. doi: 10.1523/JNEUROSCI.0180-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- Bell MD, Lopez-Gonzalez R, Lawson L, Hughes D, Fraser I, Gordon S, Perry VH. Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. Journal of neurocytology. 1994;23:605–613. doi: 10.1007/BF01191555. [DOI] [PubMed] [Google Scholar]
- Bien J, Jefferson T, Causevic M, Jumpertz T, Munter L, Multhaup G, Weggen S, Becker-Pauly C, Pietrzik CU. The metalloprotease meprin beta generates amino terminal-truncated amyloid beta peptide species. The Journal of biological chemistry. 2012;287:33304–33313. doi: 10.1074/jbc.M112.395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodles AM, Barger SW. Secreted beta-amyloid precursor protein activates microglia via JNK and p38-MAPK. Neurobiology of aging. 2005;26:9–16. doi: 10.1016/j.neurobiolaging.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Bour A, Little S, Dodart JC, Kelche C, Mathis C. A secreted form of the beta-amyloid precursor protein (sAPP695) improves spatial recognition memory in OF1 mice. Neurobiology of learning and memory. 2004;81:27–38. doi: 10.1016/s1074-7427(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Bowes MP, Masliah E, Otero DA, Zivin JA, Saitoh T. Reduction of neurological damage by a peptide segment of the amyloid beta/A4 protein precursor in a rabbit spinal cord ischemia model. Experimental neurology. 1994;129:112–119. doi: 10.1006/exnr.1994.1152. [DOI] [PubMed] [Google Scholar]
- Brinkmalm G, Brinkmalm A, Bourgeois P, Persson R, Hansson O, Portelius E, Mercken M, Andreasson U, Parent S, Lipari F, Ohrfelt A, Bjerke M, Minthon L, Zetterberg H, Blennow K, Nutu M. Soluble amyloid precursor protein alpha and beta in CSF in Alzheimer’s disease. Brain research. 2013;1513:117–126. doi: 10.1016/j.brainres.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development (Cambridge, England) 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- Cedazo-Minguez A, Wiehager B, Winblad B, Huttinger M, Cowburn RF. Effects of apolipoprotein E (apoE) isoforms, beta-amyloid (Abeta) and apoE/Abeta complexes on protein kinase C-alpha (PKC-alpha) translocation and amyloid precursor protein (APP) processing in human SH-SY5Y neuroblastoma cells and fibroblasts. Neurochemistry international. 2001;38:615–625. doi: 10.1016/s0197-0186(00)00128-5. [DOI] [PubMed] [Google Scholar]
- Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B. Secreted amyloid precursor protein beta and secreted amyloid precursor protein alpha induce axon outgrowth in vitro through Egr1 signaling pathway. PloS one. 2011;6:e16301. doi: 10.1371/journal.pone.0016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu Z, Zhou D, Mattson MP. Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Experimental neurology. 2002;175:407–414. doi: 10.1006/exnr.2002.7920. [DOI] [PubMed] [Google Scholar]
- Claasen AM, Guevremont D, Mason-Parker SE, Bourne K, Tate WP, Abraham WC, Williams JM. Secreted amyloid precursor protein-alpha upregulates synaptic protein synthesis by a protein kinase G-dependent mechanism. Neuroscience letters. 2009;460:92–96. doi: 10.1016/j.neulet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Clarris HJ, Nurcombe V, Small DH, Beyreuther K, Masters CL. Secretion of nerve growth factor from septum stimulates neurite outgrowth and release of the amyloid protein precursor of Alzheimer’s disease from hippocampal explants. Journal of neuroscience research. 1994;38:248–258. doi: 10.1002/jnr.490380303. [DOI] [PubMed] [Google Scholar]
- Cleary J, Hittner JM, Semotuk M, Mantyh P, O’Hare E. Beta-amyloid(1–40) effects on behavior and memory. Brain research. 1995;682:69–74. doi: 10.1016/0006-8993(95)00323-i. [DOI] [PubMed] [Google Scholar]
- Copanaki E, Chang S, Vlachos A, Tschape JA, Muller UC, Kogel D, Deller T. sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Molecular and cellular neurosciences. 2010;44:386–393. doi: 10.1016/j.mcn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Pham CL, Vink R, Blumbergs PC, Masters CL, van den Heuvel C, Cappai R. The neuroprotective domains of the amyloid precursor protein, in traumatic brain injury, are located in the two growth factor domains. Brain research. 2011;1378:137–143. doi: 10.1016/j.brainres.2010.12.077. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Thornton E, Roisman LC, Leonard AV, Vink R, Blumbergs PC, van den Heuvel C, Cappai R. The neuroprotective activity of the amyloid precursor protein against traumatic brain injury is mediated via the heparin binding site in residues 96–110. Journal of neurochemistry. 2014;128:196–204. doi: 10.1111/jnc.12391. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Vink R, Blumbergs PC, Masters CL, Cappai R, van den Heuvel C. sAPPalpha rescues deficits in amyloid precursor protein knockout mice following focal traumatic brain injury. Journal of neurochemistry. 2012;122:208–220. doi: 10.1111/j.1471-4159.2012.07761.x. [DOI] [PubMed] [Google Scholar]
- Cuchillo-Ibanez I, Lopez-Font I, Boix-Amoros A, Brinkmalm G, Blennow K, Molinuevo JL, Saez-Valero J. Heteromers of amyloid precursor protein in cerebrospinal fluid. Molecular neurodegeneration. 2015;10:2. doi: 10.1186/1750-1326-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms SO, Hoefgen S, Roeser D, Schlott B, Guhrs KH, Than ME. Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5381–5386. doi: 10.1073/pnas.0911326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms SO, Mayer MC, Roeser D, Multhaup G, Than ME. Interaction of the amyloid precursor protein-like protein 1 (APLP1) E2 domain with heparan sulfate involves two distinct binding modes. Acta crystallographica Section D, Biological crystallography. 2015;71:494–504. doi: 10.1107/S1399004714027114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington D, Li S, Hou H, Habib A, Tian J, Gao Y, Ehrhart J, Sanberg PR, Sawmiller D, Giunta B, Mori T, Tan J. Human umbilical cord blood-derived monocytes improve cognitive deficits and reduce amyloid-beta pathology in PSAPP mice. Cell transplantation. 2015;24:2237–2250. doi: 10.3727/096368915X688894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nature reviews Neurology. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nature neuroscience. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- Demars MP, Bartholomew A, Strakova Z, Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem cell research & therapy. 2011;2:36. doi: 10.1186/scrt77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Habib A, Obregon DF, Barger SW, Giunta B, Wang YJ, Hou H, Sawmiller D, Tan J. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3beta signaling pathway. Journal of neurochemistry. 2015;135:630–637. doi: 10.1111/jnc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson SE, Gearing M, Lippa CF, Montine TJ, Levey AI, Lah JJ. LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. Journal of neuropathology and experimental neurology. 2006;65:866–872. doi: 10.1097/01.jnen.0000228205.19915.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle E, Bruce MT, Breen KC, Smith DC, Anderton B, Regan CM. Intraventricular infusions of antibodies to amyloid-beta-protein precursor impair the acquisition of a passive avoidance response in the rat. Neuroscience letters. 1990;115:97–102. doi: 10.1016/0304-3940(90)90524-d. [DOI] [PubMed] [Google Scholar]
- East SJ, Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the L-arginine-nitric oxide pathway. Neuroscience letters. 1991;123:17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- Endres K, Fahrenholz F. Upregulation of the alpha-secretase ADAM10--risk or reason for hope? The FEBS journal. 2010;277:1585–1596. doi: 10.1111/j.1742-4658.2010.07566.x. [DOI] [PubMed] [Google Scholar]
- Endres K, Fahrenholz F. Regulation of alpha-secretase ADAM10 expression and activity. Experimental brain research. 2012;217:343–352. doi: 10.1007/s00221-011-2885-7. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F, Postina R. Alpha-secretase activation--an approach to Alzheimer’s disease therapy. Neuro-degenerative diseases. 2006;3:255–261. doi: 10.1159/000095264. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fazeli MS, Corbet J, Dunn MJ, Dolphin AC, Bliss TV. Changes in protein synthesis accompanying long-term potentiation in the dentate gyrus in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:1346–1353. doi: 10.1523/JNEUROSCI.13-04-01346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fol R, Braudeau J, Ludewig S, Abel T, Weyer SW, Roederer JP, Brod F, Audrain M, Bemelmans AP, Buchholz CJ, Korte M, Cartier N, Muller UC. Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer’s disease mouse model. Acta neuropathologica. 2016;131:247–266. doi: 10.1007/s00401-015-1498-9. [DOI] [PubMed] [Google Scholar]
- Fombonne J, Rabizadeh S, Banwait S, Mehlen P, Bredesen DE. Selective vulnerability in Alzheimer’s disease: amyloid precursor protein and p75(NTR) interaction. Ann Neurol. 2009;65:294–303. doi: 10.1002/ana.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. The Journal of biological chemistry. 2011;286:24264–24274. doi: 10.1074/jbc.M111.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996a;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. Secreted amyloid precursor protein alpha selectively suppresses N-methyl-D-aspartate currents in hippocampal neurons: involvement of cyclic GMP. Neuroscience. 1998;83:429–438. doi: 10.1016/s0306-4522(97)00398-9. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. Journal of neurochemistry. 1996b;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. The European journal of neuroscience. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Tagliavini F, Timmers WF, Frangione B. Alzheimer’s disease amyloid precursor protein is present in senile plaques and cerebrospinal fluid: immunohistochemical and biochemical characterization. Biochemical and biophysical research communications. 1989;163:430–437. doi: 10.1016/0006-291x(89)92154-2. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Crowther RA. Tau proteins and neurofibrillary degeneration. Brain pathology (Zurich, Switzerland) 1991;1:279–286. doi: 10.1111/j.1750-3639.1991.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Experimental neurology. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Goto JJ, Tanzi RE. The role of the low-density lipoprotein receptor-related protein (LRP1) in Alzheimer’s A beta generation: development of a cell-based model system. Journal of molecular neuroscience : MN. 2002;19:37–41. doi: 10.1007/s12031-002-0008-4. [DOI] [PubMed] [Google Scholar]
- Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. The Journal of biological chemistry. 2009;284:15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralle M, Oliveira CL, Guerreiro LH, McKinstry WJ, Galatis D, Masters CL, Cappai R, Parker MW, Ramos CH, Torriani I, Ferreira ST. Solution conformation and heparin-induced dimerization of the full-length extracellular domain of the human amyloid precursor protein. Journal of molecular biology. 2006;357:493–508. doi: 10.1016/j.jmb.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Qiu WQ, Selkoe DJ, Ben-Itzhak A, Kosik KS. Amino-terminal region of the beta-amyloid precursor protein activates mitogen-activated protein kinase. Neuroscience letters. 1995;198:52–56. doi: 10.1016/0304-3940(95)11944-r. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li H, Gaddam SS, Justice NJ, Robertson CS, Zheng H. Amyloid precursor protein revisited: neuron-specific expression and highly stable nature of soluble derivatives. The Journal of biological chemistry. 2012a;287:2437–2445. doi: 10.1074/jbc.M111.315051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP. Secreted beta-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF-kappaB and stabilization of calcium homeostasis. The Journal of biological chemistry. 1998;273:12341–12351. doi: 10.1074/jbc.273.20.12341. [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang Z, Li H, Wiese M, Zheng H. APP physiological and pathophysiological functions: insights from animal models. Cell research. 2012b;22:78–89. doi: 10.1038/cr.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsen C, Glerup S, Pallesen LT, Olsen D, Andersen OM, Nykjaer A, Madsen P, Petersen CM. Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:64–71. doi: 10.1523/JNEUROSCI.2371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends in pharmacological sciences. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D, Klatt S, Roch M, Konthur Z, Klose J, Willnow TE, Rohe M. Soluble alpha-APP (sAPPalpha) regulates CDK5 expression and activity in neurons. PloS one. 2013;8:e65920. doi: 10.1371/journal.pone.0065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe N, Fujita Y, Ueno M, Yoshimura K, Fujino Y, Yamashita T. Soluble beta-amyloid Precursor Protein Alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PloS one. 2013;8:e82321. doi: 10.1371/journal.pone.0082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Kashiwagi K, Ohta J, Nakajima M, Kawashima T, Yoshikawa K. Alzheimer amyloid protein precursor enhances proliferation of neural stem cells from fetal rat brain. Biochemical and biophysical research communications. 1994;205:936–943. doi: 10.1006/bbrc.1994.2755. [DOI] [PubMed] [Google Scholar]
- Hick M, Herrmann U, Weyer SW, Mallm JP, Tschape JA, Borgers M, Mercken M, Roth FC, Draguhn A, Slomianka L, Wolfer DP, Korte M, Muller UC. Acute function of secreted amyloid precursor protein fragment APPsalpha in synaptic plasticity. Acta neuropathologica. 2015;129:21–37. doi: 10.1007/s00401-014-1368-x. [DOI] [PubMed] [Google Scholar]
- Hock C, Golombowski S, Muller-Spahn F, Naser W, Beyreuther K, Monning U, Schenk D, Vigo-Pelfrey C, Bush AM, Moir R, Tanzi RE, Growdon JH, Nitsch RM. Cerebrospinal fluid levels of amyloid precursor protein and amyloid beta-peptide in Alzheimer’s disease and major depression - inverse correlation with dementia severity. European neurology. 1998;39:111–118. doi: 10.1159/000007917. [DOI] [PubMed] [Google Scholar]
- Hoefgen S, Coburger I, Roeser D, Schaub Y, Dahms SO, Than ME. Heparin induced dimerization of APP is primarily mediated by E1 and regulated by its acidic domain. Journal of structural biology. 2014;187:30–37. doi: 10.1016/j.jsb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Pietrzik CU, Kummer MP, Twiesselmann C, Bauer C, Herzog V. Binding and selective detection of the secretory N-terminal domain of the alzheimer amyloid precursor protein on cell surfaces. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1999;47:373–382. doi: 10.1177/002215549904700311. [DOI] [PubMed] [Google Scholar]
- Honda M, Akiyama H, Yamada Y, Kondo H, Kawabe Y, Takeya M, Takahashi K, Suzuki H, Doi T, Sakamoto A, Ookawara S, Mato M, Gough PJ, Greaves DR, Gordon S, Kodama T, Matsushita M. Immunohistochemical evidence for a macrophage scavenger receptor in Mato cells and reactive microglia of ischemia and Alzheimer’s disease. Biochemical and biophysical research communications. 1998;245:734–740. doi: 10.1006/bbrc.1998.8120. [DOI] [PubMed] [Google Scholar]
- Hou H, Obregon D, Shahaduzzaman MD, Song M, Tian J, Giunta B, Mori T, Mattson M, Tan J. sAPP-alpha modulates gamma-secretase processing of APP. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 7:S402–S403. [Google Scholar]
- Huang F, Buttini M, Wyss-Coray T, McConlogue L, Kodama T, Pitas RE, Mucke L. Elimination of the class A scavenger receptor does not affect amyloid plaque formation or neurodegeneration in transgenic mice expressing human amyloid protein precursors. The American journal of pathology. 1999;155:1741–1747. doi: 10.1016/S0002-9440(10)65489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G, Bailly Y, Martin JR, Mariani J, Brugg B. Synaptic beta-amyloid precursor proteins increase with learning capacity in rats. Neuroscience. 1997;80:313–320. doi: 10.1016/s0306-4522(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Huber G, Martin JR, Loffler J, Moreau JL. Involvement of amyloid precursor protein in memory formation in the rat: an indirect antibody approach. Brain research. 1993;603:348–352. doi: 10.1016/0006-8993(93)91261-p. [DOI] [PubMed] [Google Scholar]
- Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- Jakobsson J, Zetterberg H, Blennow K, Johan Ekman C, Johansson AG, Landen M. Altered concentrations of amyloid precursor protein metabolites in the cerebrospinal fluid of patients with bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:664–672. doi: 10.1038/npp.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson T, Causevic M, auf dem Keller U, Schilling O, Isbert S, Geyer R, Maier W, Tschickardt S, Jumpertz T, Weggen S, Bond JS, Overall CM, Pietrzik CU, Becker-Pauly C. Metalloprotease meprin beta generates nontoxic N-terminal amyloid precursor protein fragments in vivo. The Journal of biological chemistry. 2011;286:27741–27750. doi: 10.1074/jbc.M111.252718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S, Torres M, Vizuete M, Sanchez-Varo R, Sanchez-Mejias E, Trujillo-Estrada L, Carmona-Cuenca I, Caballero C, Ruano D, Gutierrez A, Vitorica J. Age-dependent accumulation of soluble amyloid beta (Abeta) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPP(alpha)) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer mouse model. The Journal of biological chemistry. 2011;286:18414–18425. doi: 10.1074/jbc.M110.209718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LW, Ninomiya H, Roch JM, Schubert D, Masliah E, Otero DA, Saitoh T. Peptides containing the RERMS sequence of amyloid beta/A4 protein precursor bind cell surface and promote neurite extension. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:5461–5470. doi: 10.1523/JNEUROSCI.14-09-05461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CK, Herms J. Role of APP for dendritic spine formation and stability. Experimental brain research. 2012;217:463–470. doi: 10.1007/s00221-011-2939-x. [DOI] [PubMed] [Google Scholar]
- Kaden D, Munter LM, Joshi M, Treiber C, Weise C, Bethge T, Voigt P, Schaefer M, Beyermann M, Reif B, Multhaup G. Homophilic interactions of the amyloid precursor protein (APP) ectodomain are regulated by the loop region and affect beta-secretase cleavage of APP. The Journal of biological chemistry. 2008;283:7271–7279. doi: 10.1074/jbc.M708046200. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Frontiers in aging neuroscience. 2014;6:93. doi: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khalifa NB, Van Hees J, Tasiaux B, Huysseune S, Smith SO, Constantinescu SN, Octave JN, Kienlen-Campard P. What is the role of amyloid precursor protein dimerization? Cell adhesion & migration. 2010;4:268–272. doi: 10.4161/cam.4.2.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, Moir RD, Becker KD, Tanzi RE. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuate {alpha}-secretase activity. Human molecular genetics. 2009;18:3987–3996. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain research. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM. The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel D, Schomburg R, Copanaki E, Prehn JH. Regulation of gene expression by the amyloid precursor protein: inhibition of the JNK/c-Jun pathway. Cell death and differentiation. 2005;12:1–9. doi: 10.1038/sj.cdd.4401495. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lannfelt L, Basun H, Wahlund LO, Rowe BA, Wagner SL. Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nature medicine. 1995;1:829–832. doi: 10.1038/nm0895-829. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Kamrowski-Kruck H, Peters O, Heuser I, Jessen F, Popp J, Burger K, Hampel H, Frolich L, Wolf S, Prinz B, Jahn H, Luckhaus C, Perneczky R, Hull M, Schroder J, Kessler H, Pantel J, Gertz HJ, Klafki HW, Kolsch H, Reulbach U, Esselmann H, Maler JM, Bibl M, Kornhuber J, Wiltfang J. Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Molecular psychiatry. 2010;15:138–145. doi: 10.1038/mp.2008.84. [DOI] [PubMed] [Google Scholar]
- Li H, Wang B, Wang Z, Guo Q, Tabuchi K, Hammer RE, Sudhof TC, Zheng H. Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17362–17367. doi: 10.1073/pnas.1012568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Roch JM, Sundsmo M, Otero D, Sisodia S, Thomas R, Saitoh T. Defective neurite extension is caused by a mutation in amyloid beta/A4 (A beta) protein precursor found in familial Alzheimer’s disease. Journal of neurobiology. 1997;32:469–480. [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews Neurology. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. Journal of neuroscience research. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain research. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileusnic R, Lancashire CL, Johnston AN, Rose SP. APP is required during an early phase of memory formation. The European journal of neuroscience. 2000;12:4487–4495. [PubMed] [Google Scholar]
- Milosch N, Tanriover G, Kundu A, Rami A, Francois JC, Baumkotter F, Weyer SW, Samanta A, Jaschke A, Brod F, Buchholz CJ, Kins S, Behl C, Muller UC, Kogel D. Holo-APP and G-protein-mediated signaling are required for sAPP[alpha]-induced activation of the Akt survival pathway. Cell Death Dis. 2014;5:e1391. doi: 10.1038/cddis.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Nakajima M, Ogino I, Miyata H, Motoi Y, Arai H. Soluble amyloid precursor protein alpha in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. European journal of neurology. 2013;20:236–242. doi: 10.1111/j.1468-1331.2012.03781.x. [DOI] [PubMed] [Google Scholar]
- Moir RD, Tanzi RE. LRP-mediated clearance of Abeta is inhibited by KPI-containing isoforms of APP. Current Alzheimer research. 2005;2:269–273. doi: 10.2174/1567205053585918. [DOI] [PubMed] [Google Scholar]
- Mok SS, Sberna G, Heffernan D, Cappai R, Galatis D, Clarris HJ, Sawyer WH, Beyreuther K, Masters CL, Small DH. Expression and analysis of heparin-binding regions of the amyloid precursor protein of Alzheimer’s disease. FEBS letters. 1997;415:303–307. doi: 10.1016/s0014-5793(97)01146-0. [DOI] [PubMed] [Google Scholar]
- Moreno L, Rose C, Mohanraj A, Allinquant B, Billard JM, Dutar P. sAbetaPPalpha Improves Hippocampal NMDA-Dependent Functional Alterations Linked to Healthy Aging. Journal of Alzheimer’s disease : JAD. 2015;48:927–935. doi: 10.3233/JAD-150297. [DOI] [PubMed] [Google Scholar]
- Muller U, Cristina N, Li ZW, Wolfer DP, Lipp HP, Rulicke T, Brandner S, Aguzzi A, Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Muller UC, Zheng H. Physiological functions of APP family proteins. Cold Spring Harbor perspectives in medicine. 2012;2:a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem cells and development. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya H, Roch JM, Sundsmo MP, Otero DA, Saitoh T. Amino acid sequence RERMS represents the active domain of amyloid beta/A4 protein precursor that promotes fibroblast growth. The Journal of cell biology. 1993;121:879–886. doi: 10.1083/jcb.121.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nature communications. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Takamura C, Kohsaka S. Fibulin-1 binds the amino-terminal head of beta-amyloid precursor protein and modulates its physiological function. Journal of neurochemistry. 2001;76:1411–1420. doi: 10.1046/j.1471-4159.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Takamura C, Morimoto T, Ishiguro M, Kohsaka S. Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. The European journal of neuroscience. 1999;11:1907–1913. doi: 10.1046/j.1460-9568.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Usiak M, Mayeux R, Raskind M, Tourtellotte WW, Younkin SG. Soluble derivatives of the beta amyloid protein precursor in cerebrospinal fluid: alterations in normal aging and in Alzheimer’s disease. Neurology. 1990;40:1028–1034. doi: 10.1212/wnl.40.7.1028. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature neuroscience. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasciuto E, Ahmed T, Wahle T, Gardoni F, D’Andrea L, Pacini L, Jacquemont S, Tassone F, Balschun D, Dotti CG, Callaerts-Vegh Z, D’Hooge R, Muller UC, Di Luca M, De Strooper B, Bagni C. Dysregulated ADAM10-Mediated Processing of APP during a Critical Time Window Leads to Synaptic Deficits in Fragile X Syndrome. Neuron. 2015;87:382–398. doi: 10.1016/j.neuron.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Peduto L. ADAM9 as a potential target molecule in cancer. Current pharmaceutical design. 2009;15:2282–2287. doi: 10.2174/138161209788682415. [DOI] [PubMed] [Google Scholar]
- Perez RG, Zheng H, Van der Ploeg LH, Koo EH. The beta-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:9407–9414. doi: 10.1523/JNEUROSCI.17-24-09407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Guo LH, Kagerbauer SM, Werle L, Kurz A, Martin J, Alexopoulos P. Soluble amyloid precursor protein beta as blood-based biomarker of Alzheimer’s disease. Translational psychiatry. 2013;3:e227. doi: 10.1038/tp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Tsolakidou A, Arnold A, Diehl-Schmid J, Grimmer T, Forstl H, Kurz A, Alexopoulos P. CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology. 2011;77:35–38. doi: 10.1212/WNL.0b013e318221ad47. [DOI] [PubMed] [Google Scholar]
- Peters-Libeu C, Campagna J, Mitsumori M, Poksay KS, Spilman P, Sabogal A, Bredesen DE, John V. sAbetaPPalpha is a Potent Endogenous Inhibitor of BACE1. Journal of Alzheimer’s disease : JAD. 2015;47:545–555. doi: 10.3233/JAD-150282. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Hoffmann J, Stober K, Chen CY, Bauer C, Otero DA, Roch JM, Herzog V. From differentiation to proliferation: the secretory amyloid precursor protein as a local mediator of growth in thyroid epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1770–1775. doi: 10.1073/pnas.95.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. The Journal of biological chemistry. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R, Monning U, Schreiter-Gasser U, Weidemann A, Blennow K, Gottfries CG, Masters CL, Beyreuther K. Quantitative changes in the amyloid beta A4 precursor protein in Alzheimer cerebrospinal fluid. Neuroscience letters. 1991;124:69–73. doi: 10.1016/0304-3940(91)90824-d. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Long JM, Sokol DK, Lahiri DK. Increased secreted amyloid precursor protein-alpha (sAPPalpha) in severe autism: proposal of a specific, anabolic pathway and putative biomarker. PloS one. 2011;6:e20405. doi: 10.1371/journal.pone.0020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Borgers M, David G, De Strooper B. Soluble amyloid-beta precursor protein binds its cell surface receptor in a cooperative fashion with glypican and syndecan proteoglycans. Journal of cell science. 2013;126:4856–4861. doi: 10.1242/jcs.137919. [DOI] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Muller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch JM, Masliah E, Roch-Levecq AC, Sundsmo MP, Otero DA, Veinbergs I, Saitoh T. Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7450–7454. doi: 10.1073/pnas.91.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song Y-Q, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C, Andreasson U, Mattsson N, Marcusson J, Minthon L, Andreasen N, Blennow K, Zetterberg H. Cerebrospinal fluid profiles of amyloid beta-related biomarkers in Alzheimer’s disease. Neuromolecular medicine. 2012;14:65–73. doi: 10.1007/s12017-012-8171-4. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Cappai R, Feil SC, Henry A, McKinstry WJ, Galatis D, Hesse L, Multhaup G, Beyreuther K, Masters CL, Parker MW. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nature structural biology. 1999;6:327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- Saftig P, Reiss K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? European journal of cell biology. 2011;90:527–535. doi: 10.1016/j.ejcb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Sundsmo M, Roch JM, Kimura N, Cole G, Schubert D, Oltersdorf T, Schenk DB. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;58:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Salbaum JM, Ruddle FH. Embryonic expression pattern of amyloid protein precursor suggests a role in differentiation of specific subsets of neurons. The Journal of experimental zoology. 1994;269:116–127. doi: 10.1002/jez.1402690205. [DOI] [PubMed] [Google Scholar]
- Santiago-Garcia J, Mas-Oliva J, Innerarity TL, Pitas RE. Secreted forms of the amyloid-beta precursor protein are ligands for the class A scavenger receptor. The Journal of biological chemistry. 2001;276:30655–30661. doi: 10.1074/jbc.M102879200. [DOI] [PubMed] [Google Scholar]
- Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA, Beyreuther K, Multhaup G. Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. The Journal of biological chemistry. 2001;276:33923–33929. doi: 10.1074/jbc.M105410200. [DOI] [PubMed] [Google Scholar]
- Schonherr C, Bien J, Isbert S, Wichert R, Prox J, Altmeppen H, Kumar S, Walter J, Lichtenthaler SF, Weggen S, Glatzel M, Becker-Pauly C, Pietrzik CU. Generation of aggregation prone N-terminally truncated amyloid beta peptides by meprin beta depends on the sequence specificity at the cleavage site. Molecular neurodegeneration. 2016;11:19. doi: 10.1186/s13024-016-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Selnes P, Blennow K, Zetterberg H, Grambaite R, Rosengren L, Johnsen L, Stenset V, Fladby T. Effects of cerebrovascular disease on amyloid precursor protein metabolites in cerebrospinal fluid. Cerebrospinal fluid research. 2010;7:10. doi: 10.1186/1743-8454-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennvik K, Fastbom J, Blomberg M, Wahlund LO, Winblad B, Benedikz E. Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer’s disease patients. Neuroscience letters. 2000;278:169–172. doi: 10.1016/s0304-3940(99)00929-5. [DOI] [PubMed] [Google Scholar]
- Small DH, Clarris HL, Williamson TG, Reed G, Key B, Mok SS, Beyreuther K, Masters CL, Nurcombe V. Neurite-outgrowth regulating functions of the amyloid protein precursor of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 1999;1:275–285. doi: 10.3233/jad-1999-14-508. [DOI] [PubMed] [Google Scholar]
- Small DH, Nurcombe V, Reed G, Clarris H, Moir R, Beyreuther K, Masters CL. A heparin-binding domain in the amyloid protein precursor of Alzheimer’s disease is involved in the regulation of neurite outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Pettigrew LC, Craddock SD, Culwell AR, Rydel RE, Mattson MP. Secreted forms of beta-amyloid precursor protein protect against ischemic brain injury. Journal of neurochemistry. 1994;63:781–784. doi: 10.1046/j.1471-4159.1994.63020781.x. [DOI] [PubMed] [Google Scholar]
- Spilman PBD, Jagodzinska B, John V. Enhancement of sAPPα as a Therapeutic Strategy for Alzheimer’s and Other Neurodegenerative Diseases. HOSA Journal of Alzheimer’s & Neurodegenerative Diseases. 2015;1:10. [Google Scholar]
- Spilman P, Descamps O, Gorostiza O, Peters-Libeu C, Poksay KS, Matalis A, Campagna J, Patent A, Rao R, John V, Bredesen DE. The multi-functional drug tropisetron binds APP and normalizes cognition in a murine Alzheimer’s model. Brain research. 2014;1551:25–44. doi: 10.1016/j.brainres.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker P, Fang L, Kuhle J, Petzold A, Tumani H, Ludolph AC, Otto M, Brettschneider J. Soluble beta-amyloid precursor protein is related to disease progression in amyotrophic lateral sclerosis. PloS one. 2011;6:e23600. doi: 10.1371/journal.pone.0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck SE, Meister S, Nahrath J, Meissner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I, Korth C, Weggen S, Heimann A, Schwaninger M, Bayer TA, Pietrzik CU. Endothelial LRP1 transports amyloid-beta1–42 across the blood-brain barrier. The Journal of clinical investigation. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Bonventre JV, Uliasz TF, Hewett JA, Hewett SJ. Cytosolic phospholipase A2 alpha inhibition prevents neuronal NMDA receptor-stimulated arachidonic acid mobilization and prostaglandin production but not subsequent cell death. Journal of neurochemistry. 2008;106:1828–1840. doi: 10.1111/j.1471-4159.2008.05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain research. 2006;1094:38–46. doi: 10.1016/j.brainres.2006.03.107. [DOI] [PubMed] [Google Scholar]
- Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Progress in neurobiology. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Shankle WR, Farrow JS, Dick M, Rozemuller JM, Kuiper MA, Wolters EC, Zimmerman J, Cotman CW, et al. Decreased levels of soluble amyloid beta-protein precursor in cerebrospinal fluid of live Alzheimer disease patients. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2551–2555. doi: 10.1073/pnas.89.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent B, Govitrapong P. Activation of the alpha-secretase processing of AbetaPP as a therapeutic approach in Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2011;24(Suppl 2):75–94. doi: 10.3233/JAD-2011-110218. [DOI] [PubMed] [Google Scholar]
- Vincent B, Smith JD. Astrocytes down-regulate neuronal beta-amyloid precursor protein expression and modify its processing in an apolipoprotein E isoform-specific manner. The European journal of neuroscience. 2001;14:256–266. doi: 10.1046/j.0953-816x.2001.01643.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ha Y. The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain. Molecular cell. 2004;15:343–353. doi: 10.1016/j.molcel.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Weyer SW, Klevanski M, Delekate A, Voikar V, Aydin D, Hick M, Filippov M, Drost N, Schaller KL, Saar M, Vogt MA, Gass P, Samanta A, Jaschke A, Korte M, Wolfer DP, Caldwell JH, Muller UC. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. The EMBO journal. 2011;30:2266–2280. doi: 10.1038/emboj.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson JS, Glabe CG, Shintani E, Abcar A, Cotman CW. Beta-amyloid protein promotes neuritic branching in hippocampal cultures. Neuroscience letters. 1990;110:319–324. doi: 10.1016/0304-3940(90)90867-9. [DOI] [PubMed] [Google Scholar]
- Williamson TG, Mok SS, Henry A, Cappai R, Lander AD, Nurcombe V, Beyreuther K, Masters CL, Small DH. Secreted glypican binds to the amyloid precursor protein of Alzheimer’s disease (APP) and inhibits APP-induced neurite outgrowth. The Journal of biological chemistry. 1996;271:31215–31221. doi: 10.1074/jbc.271.49.31215. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48:2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Yang X, Sheng W, Ridgley DM, Haidekker MA, Sun GY, Lee JC. Astrocytes regulate alpha-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2. Neuroscience. 2015;300:508–517. doi: 10.1016/j.neuroscience.2015.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural development. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin SG. Evidence that Aβ42 is the real culprit in Alzheimer’s disease. Annals of neurology. 1995;37:287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K. Biological CSF markers of Alzheimer’s disease. Handbook of clinical neurology. 2008;89:261–268. doi: 10.1016/S0072-9752(07)01224-9. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Hopkins R, Sirinathsinghji DJ, Stevens KA, Conner MW, Slunt HH, Sisodia SS, Chen HY, Van der Ploeg LH. Mice deficient for the amyloid precursor protein gene. Annals of the New York Academy of Sciences. 1996;777:421–426. doi: 10.1111/j.1749-6632.1996.tb34456.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisoda SS, Chen HY, Van der Ploeg LH. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368:635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]