Abstract
Background/Objectives
Obesity and low physical fitness are known risk factors for ischemic heart disease (IHD), but their interactive effects are unclear. Elucidation of interactions between these common, modifiable risk factors may help inform more effective preventive strategies. We examined interactive effects of obesity, aerobic fitness, and muscular strength in late adolescence on risk of IHD in adulthood in a large national cohort.
Subjects/Methods
We conducted a national cohort study of all 1,547,407 military conscripts in Sweden during 1969–1997 (97–98% of all 18-year-old males each year). Aerobic fitness, muscular strength, and body mass index (BMI) measurements were examined in relation to IHD identified from outpatient and inpatient diagnoses through 2012 (maximum age 62 years).
Results
There were 38,142 men diagnosed with IHD in 39.7 million person-years of follow-up. High BMI or low aerobic fitness (but not muscular strength) was associated with higher risk of IHD, adjusting for family history and socioeconomic factors. The combination of high BMI (overweight/obese vs. normal) and low aerobic fitness (lowest vs. highest tertile) was associated with highest IHD risk (incidence rate ratio, 3.11; 95% CI, 2.91–3.31; P<0.001). These exposures had no additive and a negative multiplicative interaction (i.e., their combined effect was less than the product of their separate effects). Low aerobic fitness was a strong risk factor even among those with normal BMI.
Conclusions
In this large cohort study, low aerobic fitness or high BMI at age 18 was associated with higher risk of IHD in adulthood, with a negative multiplicative interaction. Low aerobic fitness appeared to account for a similar number of IHD cases among those with normal vs. high BMI (i.e., no additive interaction). These findings suggest that interventions to prevent IHD should begin early in life and include not only weight control but aerobic fitness, even among persons of normal weight.
INTRODUCTION
Despite improvements in mortality over the last 4 decades, ischemic heart disease (IHD) continues to be the leading cause of death among US adults, causing one-third of all deaths among persons older than 35 years.1 Its overall prevalence among US adults older than 20 years is 6.4%, affecting more than 15 million persons.2 High body mass index (BMI) in childhood,3–5 adolescence,5, 6 or adulthood,7, 8 and low aerobic (cardiorespiratory) fitness in adolescence9–11 or adulthood,12 are known risk factors for IHD later in life. Studies have reported that higher levels of physical activity in adulthood partially offset the increased risks of IHD13, 14 or cardiovascular mortality15 associated with obesity, but without significant interactions between these risk factors. However, these studies had low statistical power to detect interactions, were based on self-reported physical activity rather than objectively measured physical fitness, and examined these exposures only in mid-adulthood. Larger, well-powered studies have not been conducted with objectively measured aerobic fitness and BMI obtained early in life. As a result, the interactive effects of early-life obesity and aerobic fitness on the long-term risk of IHD remain unknown. A better understanding of these effects may help inform early interventions in susceptible subgroups to improve the primary prevention of IHD.
We conducted the largest study to date of the potential interactive effects of physical fitness (including aerobic fitness and muscular strength) and BMI in late adolescence on subsequent risk of IHD in adulthood. Our aim was to examine interactions among these common exposures in relation to the long-term risk of IHD in a large cohort, which may help guide more effective preventive strategies in childhood and adolescence.
METHODS
Study Population
We identified 1,547,478 males (age 18 years) who underwent a military conscription examination in Sweden during 1969–1997.16–18 This examination was compulsory for all 18-year-old males nationwide each year except for 2–3% who either were incarcerated or had severe chronic medical conditions or disabilities documented by a physician. We excluded 71 (<0.01%) individuals who had a prior diagnosis of IHD identified from hospital discharge diagnoses. A total of 1,547,407 (>99.9% of the original cohort) remained for inclusion in the study. This study was approved by the Regional Ethics Committee of Lund University in Sweden.
Aerobic Fitness, Muscular Strength, and BMI Ascertainment
Aerobic fitness, muscular strength, height, and weight measurements were obtained using the Swedish Military Conscription Registry, which contains information from a 2-day standardized physical and psychological examination required for all conscripts starting in 1969.16–18 Aerobic fitness was measured as the maximal aerobic workload in Watts, using a well-validated electrically-braked stationary bicycle ergometer test, as previously described.19 Maximal aerobic workload is highly correlated with maximal oxygen uptake (VO2 max; correlation ~0.9),20 and its measurement using this bicycle ergometer test is highly reproducible, with a test-retest correlation of 0.95.21 Muscular strength was measured as the weighted sum of maximal knee extension (weighted × 1.3), elbow flexion (weighted × 0.8), and hand grip (weighted × 1.7), each measured in Newtons, using well-validated isometric dynamometer tests.22 Each dynamometer test was performed three times and the maximum value was recorded for analysis, except when the last value was highest, in which case testing was repeated until strength values stopped increasing. BMI was calculated using standardized height and weight measurements as the weight in kilograms divided by the square of height in meters. All testing equipment was calibrated daily.19
In the present study, aerobic fitness and muscular strength were modeled alternatively as continuous variables or categorical variables in tertiles (aerobic fitness in Watts: low [<240], medium [240–288], high [≥289]; muscular strength in Newtons: low [<1900], medium [1900–2170], high [≥2171]).16–18 BMI was modeled alternatively as a continuous or categorical variable using Centers for Disease Control and Prevention (CDC) definitions for children and adolescents aged 2 to 19 years to facilitate comparability with US studies: “overweight or obesity” is defined as ≥85th percentile on the CDC’s 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males.23
IHD Ascertainment
The study cohort was followed up for IHD incidence from the time of the military conscription examination through December 31, 2012. IHD was identified using International Classification of Diseases (ICD) codes for all primary and secondary diagnoses in the Swedish Hospital and Outpatient Registries (codes 410–414 in ICD-8/9, and I20-I25 in ICD-10). The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; and the Swedish Outpatient Registry contains outpatient diagnoses from all specialty clinics nationwide starting in 2001. Diagnoses in the Hospital Registry are currently >99% complete and have a reported positive predictive value of 85–95%.24
Adjustment Variables
Other variables that may be associated with obesity, physical fitness, and IHD were obtained from the Swedish Military Conscription Registry and national census data, which were linked using an anonymous personal identification number.16–18 The following were used as adjustment variables: year of the military conscription examination (included to account for follow-up time, which also corresponds to attained age because baseline age was the same [18 years] for all conscripts; modeled simultaneously as continuous and categorical [1969–1979, 1980–1989, 1990–1997] variables to account for possible linear and non-linear effects); family history of IHD in a parent or sibling (yes or no, identified from diagnoses in the Swedish Hospital Registry during 1964–2012 and the Swedish Outpatient Registry during 2001–2012, using the same diagnosis codes noted above, plus 420 in ICD-7); highest education level attained during the study period (<12, 12–14, ≥15 years); and neighborhood socioeconomic status (SES, included because neighborhood characteristics have been associated with IHD25, 26 and with physical activity and BMI27; composed of an index that includes low education level, low income, unemployment, and social welfare receipt, as previously described28; categorized as low [< −1 SD from the mean], medium [−1 to 1 SD], or high [>1 SD]). As alternatives to BMI, we also examined height and weight simultaneously in a separate model, which were modeled alternatively as continuous or categorical (height: <175, 175–184, ≥185 cm; weight: <60, 60–79, ≥80 kg) variables.
Missing data for each variable were imputed using a standard multiple imputation procedure based on the variable’s relationship with all other covariates and the outcome (IHD).29 Missing data were relatively infrequent for aerobic fitness (5.7%), muscular strength (5.0%), height (7.2%), weight (7.3%), education level (0.4%), and neighborhood SES (9.1%). As an alternative to multiple imputation, sensitivity analyses were performed after restricting to men with complete data for all variables (N=1,361,083; 88.0%).16–18
Statistical Analysis
Poisson regression with robust standard errors was used to compute incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for associations between aerobic fitness, muscular strength, or BMI and subsequent risk of IHD.30 Two different adjusted models were performed: the first was adjusted for year of the military conscription examination (to account for follow-up time and attained age), and the second was additionally adjusted for aerobic fitness, muscular strength, BMI, family history of IHD, education level, and neighborhood SES (each as a categorical variable as defined above). Poisson model goodness-of-fit was assessed using deviance and Pearson chi-squared tests, which showed a good fit in all models.
Interactions among aerobic fitness, muscular strength, and BMI on either the additive or multiplicative scale were examined in relation to IHD risk.16–18 Additive interactions were assessed using the “relative excess risk due to interaction” (RERI), which is computed for binary variables as: RERIIRR = IRR11 − IRR10 − IRR01 + 1.31 Multiplicative interactions were assessed using the ratio of IRRs: IRR11 / (IRR10 × IRR01).
A sensitivity analysis was performed to evaluate the effect of unmeasured confounding due to smoking using external adjustment.32 In this analysis, we performed 10,000 model simulations assuming two uniform independent distributions for smoking prevalences among exposed and unexposed between 0.2 and 0.4,33 and a lognormal distribution for the smoking-IHD relative risk that implies a mean IRR of 2.0 and SD of 0.3.34, 35 All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 14.1.36
RESULTS
Among the 1,547,407 men in this cohort, 38,142 (2.5%) were diagnosed with IHD in 39.7 million person-years of follow-up (mean follow-up, 25.7 years). The median age at the end of follow-up was 46.1 years (mean 45.9, SD 8.9, range 18.0 to 62.0). The median age at diagnosis with IHD was 48.2 years (mean 44.7, SD 7.3, range 19.0 to 62.0). Table 1 shows other characteristics among men who were or were not subsequently diagnosed with IHD.
Table 1.
Characteristics of 18-year-old men who were or were not subsequently diagnosed with IHD.
| IHD (N=38,142) | No IHD (N=1,509,265) | |||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Year of military conscription | 1975 (4.7) | 1982 (7.7) | ||
| 1969–1979 | 32,014 (83.9) | 585,119 (38.8) | ||
| 1980–1989 | 5,522 (14.5) | 545,386 (36.1) | ||
| 1990–1997 | 606 (1.6) | 378,760 (25.1) | ||
| Aerobic fitness (Watts) | 231.9 (43.0) | 267.8 (54.4) | ||
| Lowest tertile | 23,408 (61.4) | 487,930 (32.3) | ||
| Middle tertile | 11,528 (30.2) | 508,994 (33.7) | ||
| Highest tertile | 3,206 (8.4) | 512,341 (34.0) | ||
| Muscular strength (Newtons) | 2016 (312) | 1985 (456) | ||
| Lowest tertile | 12,068 (31.6) | 498,830 (33.1) | ||
| Middle tertile | 15,632 (41.0) | 507,899 (33.6) | ||
| Highest tertile | 10,442 (27.4) | 502,536 (33.3) | ||
| BMIa | 21.8 (3.0) | 21.6 (2.8) | ||
| Normal | 34,396 (90.2) | 1,393,055 (92.3) | ||
| Overweight or obese | 3,746 (9.8) | 116,210 (7.7) | ||
| Height (cm) | 165.7 (44.7) | 175.3 (26.5) | ||
| <175 (5 ft. 9 in.) | 10,053 (27.3) | 338,542 (22.7) | ||
| 175–184 | 22,380 (60.7) | 871,233 (58.4) | ||
| ≥185 (6 ft. 1 in.) | 4,412 (12.0) | 282,223 (18.9) | ||
| Weight (kg) | 65.1 (21.3) | 68.3 (14.5) | ||
| <60 (132 lbs.) | 5,639 (15.3) | 193,728 (13.0) | ||
| 60–79 | 26,196 (71.1) | 1,096,366 (73.5) | ||
| ≥80 (176 lbs.) | 5,010 (13.6) | 201,904 (13.5) | ||
| Family history of IHD | ||||
| No | 12,452 (32.6) | 945,423 (62.6) | ||
| Yes | 25,690 (67.4) | 563,842 (37.4) | ||
| Education (years) | ||||
| <12 | 10,229 (26.8) | 226,604 (15.0) | ||
| 12–14 | 16,270 (42.7) | 667,319 (44.2) | ||
| ≥15 | 11,643 (30.5) | 615,342 (40.8) | ||
| Neighborhood SES | ||||
| Low | 6,962 (18.2) | 232,417 (15.4) | ||
| Medium | 27,190 (71.3) | 995,002 (65.9) | ||
| High | 3,990 (10.5) | 281,846 (18.7) | ||
BMI was categorized using CDC definitions for children and adolescents aged 2 to 19 years: “overweight or obese” is defined as ≥ 85th percentile from the CDC’s 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥ 25.6 for 18-year-old males.
BMI = body mass index, IHD = ischemic heart disease, SES = socioeconomic status.
Main Effects of Aerobic Fitness, Muscular Strength, and BMI
Low aerobic fitness was strongly associated with subsequent increased risk of IHD, after adjusting for BMI and other factors (Table 2, adjusted model 2: IRR for lowest vs. highest tertile, 1.80; 95% CI, 1.73–1.88; P<0.001), including a strong inverse trend across the full range of aerobic fitness (adjusted model 2: IRR for trend test per 100 Watts, 0.64; 95% CI, 0.63–0.66; P<0.001). Muscular strength was not clearly associated with risk of IHD (adjusted model 2: IRR for trend test per 1000 Newtons, 1.01; 95% CI, 0.98–1.05; P=0.37).
Table 2.
Adjusted incidence rate ratios for associations between physical fitness, BMI, or other factors and risk of IHD.
| Adjusted Model 1a | Adjusted Model 2b | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| IRR | 95% CI | P | IRR | 95% CI | P | |
| Aerobic fitness (tertiles) | ||||||
| Low | 2.04 | 1.96, 2.13 | <0.001 | 1.80 | 1.73, 1.88 | <0.001 |
| Medium | 1.49 | 1.43, 1.55 | <0.001 | 1.37 | 1.32, 1.43 | <0.001 |
| High | 1.00 | 1.00 | ||||
| Per 100 Watts (trend test) | 0.63 | 0.62, 0.65 | <0.001 | 0.64 | 0.63, 0.66 | <0.001 |
| Muscular strength (tertiles) | ||||||
| Low | 1.05 | 1.02, 1.08 | <0.001 | 0.98 | 0.95, 1.00 | 0.11 |
| Medium | 1.13 | 1.11, 1.16 | <0.001 | 1.06 | 1.03, 1.08 | <0.001 |
| High | 1.00 | 1.00 | ||||
| Per 1000 Newtons (trend test) | 0.92 | 0.89, 0.95 | <0.001 | 1.01 | 0.98, 1.05 | 0.37 |
| BMI | ||||||
| Normal | 1.00 | 1.00 | ||||
| Overweight or obese | 1.79 | 1.74, 1.85 | <0.001 | 1.81 | 1.75, 1.88 | <0.001 |
| Per 1 BMI unit (trend test) | 1.06 | 1.05, 1.06 | <0.001 | 1.07 | 1.06, 1.07 | <0.001 |
| Height (cm) | ||||||
| <175 (5 ft. 9 in.) | 1.13 | 1.10, 1.15 | <0.001 | 1.20 | 1.18, 1.23 | <0.001 |
| 175–184 | 1.00 | 1.00 | ||||
| ≥185 (6 ft. 1 in.) | 0.68 | 0.65, 0.70 | <0.001 | 0.70 | 0.68, 0.73 | <0.001 |
| Per 5 cm (trend test) | 0.88 | 0.87, 0.89 | <0.001 | 0.87 | 0.86, 0.88 | <0.001 |
| Weight (kg) | ||||||
| <60 (132 lbs.) | 0.93 | 0.90, 0.96 | <0.001 | 0.75 | 0.73, 0.77 | <0.001 |
| 60–79 | 1.00 | 1.00 | ||||
| ≥80 (176 lbs.) | 1.32 | 1.29, 1.36 | <0.001 | 1.62 | 1.57, 1.67 | <0.001 |
| Per 5 kg (trend test) | 1.07 | 1.06, 1.08 | <0.001 | 1.13 | 1.12, 1.13 | <0.001 |
| Family history of IHD | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.13 | 2.08, 2.18 | <0.001 | 2.05 | 2.00, 2.09 | <0.001 |
| Education (years) | ||||||
| <12 | 1.14 | 1.11, 1.17 | <0.001 | 1.09 | 1.06, 1.11 | <0.001 |
| 12–14 | 1.00 | 1.00 | ||||
| ≥15 | 0.70 | 0.68, 0.72 | <0.001 | 0.77 | 0.75, 0.79 | <0.001 |
| Per higher category (trend) | 0.78 | 0.77, 0.79 | <0.001 | 0.84 | 0.83, 0.85 | <0.001 |
| Neighborhood SES | ||||||
| Low | 1.04 | 1.01, 1.07 | 0.004 | 1.03 | 1.00, 1.05 | 0.04 |
| Medium | 1.00 | 1.00 | ||||
| High | 0.70 | 0.68, 0.72 | <0.001 | 0.81 | 0.78, 0.83 | <0.001 |
| Per higher category (trend) | 0.85 | 0.83, 0.86 | <0.001 | 0.90 | 0.89, 0.92 | <0.001 |
Adjusted for year of military conscription examination.
Adjusted for year of military conscription examination, aerobic fitness, muscular strength, BMI, family history of IHD, education level, and neighborhood SES. Height and weight were modeled simultaneously as an alternative to BMI in a separate model. The reference category for all variables is indicated by an IRR of 1.00.
BMI = body mass index, IHD = ischemic heart disease, IRR = incidence rate ratio, SES = socioeconomic status.
High BMI also was a strong risk factor for IHD. Overweight or obese men (≥85th percentile on the CDC’s 2000 sex-specific BMI-for-age growth chart) had a ~1.8-fold risk of IHD relative to those with normal BMI (Table 2, adjusted model 2). When both height and weight were included in the model as an alternative to BMI, low height and high weight were each associated with increased IHD risk (see Table 2, adjusted model 2), although high weight was the stronger risk factor (Pheterogeneity<0.001).
A first-degree family history of IHD was associated with a ~2-fold risk of IHD (Table 2). Education level and neighborhood SES were inversely related to IHD risk (i.e., high education level and high neighborhood SES were modestly protective) (Table 2; Ptrend<0.001). In sensitivity analyses that were restricted to men without any missing data, all risk estimates were very similar to the main results (data not shown).
Interactions Among Aerobic Fitness, Muscular Strength, and BMI
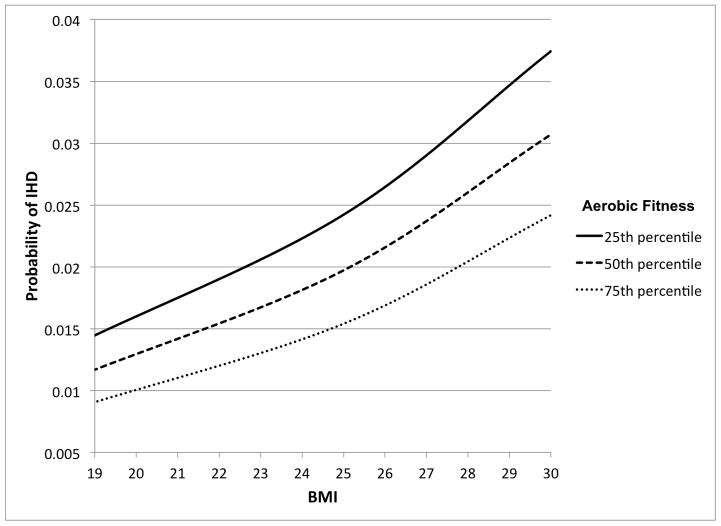
The interactive effects of aerobic fitness and BMI on risk of IHD are shown in Table 3. Low aerobic fitness was associated with increased IHD risk among men with either normal or high BMI (IRRs, 1.87 and 1.45, respectively; Table 3, right-most column). The combination of low aerobic fitness and high BMI was associated with the highest risk of IHD, which was more than 3-fold relative to the reference group of those with high aerobic fitness and normal BMI. Low aerobic fitness and high BMI had a negative interaction on the multiplicative scale (Pinteraction<0.001) (i.e., their combined effect was less than the product of their separate effects), and no interaction on the additive scale (Pinteraction=0.40). Figure 1 shows the probability of IHD for the 25th, 50th, and 75th percentiles of aerobic fitness across the full distribution of BMI, from the fully adjusted model.
Table 3.
Interactions between aerobic fitness and BMI among 18-year-old men in relation to subsequent risk of IHD.a
| Aerobic fitness (tertiles) | IRRs for medium aerobic fitness within strata of BMI | IRRs for low aerobic fitness within strata of BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||
|
| ||||||||
| No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | No. cases/total | IRR (95% CI) | |||
| BMI | ||||||||
| Normal | 2,567/457,955 | 1.00 | 9,887/479,766 | 1.41 (1.34, 1.47); P<0.001 | 21,942/489,730 | 1.87 (1.79, 1.96); P<0.001 | 1.41 (1.34, 1.47); P<0.001 | 1.87 (1.79, 1.96); P<0.001 |
| Overweight or obese | 639/57,592 | 2.14 (1.96, 2.33); P<0.001 | 1,641/40,756 | 2.64 (2.48, 2.80); P<0.001 | 1,466/21,608 | 3.11 (2.91, 3.31); P<0.001 | 1.23 (1.12, 1.34); P<0.001 | 1.45 (1.32, 1.59); P<0.001 |
| IRRs for high BMI within strata of aerobic fitness | 2.14 (1.96, 2.33); P<0.001 | 1.87 (1.78, 1.97); P<0.001 | 1.66 (1.58, 1.74); P<0.001 | |||||
| Interaction on additive scale: RERI (95% CI) | 0.09 (−0.12, 0.30); P=0.40 | 0.10 (−0.13, 0.32); P=0.40 | ||||||
| Interaction on multiplicative scale: IRR ratio (95% CI) | 0.88 (0.79, 0.96); P=0.005 | 0.78 (0.70, 0.85); P<0.001 | ||||||
IRRs are adjusted for year of military conscription examination, muscular strength, family history of IHD, education level, and neighborhood socioeconomic status.
BMI = body mass index, IHD = ischemic heart disease, IRR = incidence rate ratio, RERI = relative excess risk due to interaction
Figure 1.
Probability of IHD by aerobic fitness and BMI in 18-year-old men with mean follow-up of 25.7 years (maximum age 62 years).
In contrast, muscular strength had little effect on IHD risk, irrespective of BMI (Supplemental Table 1) or aerobic fitness level (Supplemental Table 2). We found no interactions between muscular strength and BMI in relation to IHD risk on either the additive or multiplicative scale (Supplemental Table 1), nor between muscular strength and aerobic fitness (Supplemental Table 2). External adjustment for smoking yielded IRRs for associations between the study exposures and IHD risk that were only 4% lower and remained highly significant (P<0.001), suggesting that unmeasured confounding had little influence on our main findings.
DISCUSSION
In this large cohort study, we found that high BMI or low aerobic fitness (but not muscular strength) was strongly associated with higher risk of IHD, after adjusting for family history and socioeconomic factors. The combination of high BMI and low aerobic fitness was associated with highest IHD risk (more than 3-fold), but with a negative multiplicative interaction (i.e., their combined effect was less than the product of their separate effects). Low aerobic fitness was a strong risk factor even among those with normal BMI. If these associations are causal, they suggest that interventions to prevent IHD should begin early in life and include both weight control and aerobic fitness, even among those of normal weight.16–18 Such interventions are particularly relevant because of current global trends for obesity and low fitness, which are increasing in prevalence and may contribute to even more preventable cases of IHD in the future.37–39
To our knowledge, this is the first study to examine not only the independent effects of BMI and physical fitness on IHD risk, but potential multiplicative and additive interactions. A better understanding of their interactive effects can potentially help target more effective interventions in susceptible subgroups. Additive interactions are often unexamined despite being more informative about public health impact, by indicating in which subgroup an intervention would prevent the most cases.40, 41 Previous studies have reported similar main effects of BMI3–8 or aerobic fitness9–12 on IHD risk. Other studies of self-reported physical activity (rather than objectively measured physical fitness) have suggested that higher levels of physical activity in adulthood may partially offset the increased risks of IHD13, 14 or cardiovascular mortality15 associated with obesity. In the present study, we found that the combination of high BMI and low aerobic fitness in late adolescence was associated with highest risk of IHD in adulthood, with a negative multiplicative and no additive interaction. The negative multiplicative interaction does not necessarily imply a true mechanistic interaction between BMI and aerobic fitness. However, it may reflect some redundancy of their effects on IHD risk, with low aerobic fitness acting partially through its contribution to higher BMI. The absence of additive interaction suggests that low aerobic fitness accounts for a similar number of IHD cases among those with normal compared with high BMI.40, 41 If the observed associations are causal, interventions to improve aerobic fitness would be expected to have a similar public health impact on IHD prevention among those with normal vs. high BMI (or vice versa, improvements in BMI would have a similar impact among those with high vs. low aerobic fitness).
These findings provide further evidence that both obesity and fitness are important factors affecting the long-term risk of IHD. Improvements in either BMI or aerobic fitness may reduce the future risk of developing IHD. The risk estimate that we observed for BMI (a ~1.8-fold risk of IHD among overweight or obese men) is consistent with most previously reported estimates,5–8 though in contrast to a smaller cohort study that reported an association between high BMI in mid-adulthood but not early adulthood and later IHD risk.42 Importantly, we found that low aerobic fitness was associated with substantially increased (~1.8-fold) IHD risk even among those with normal BMI, as previously reported.9 This suggests that aerobic fitness early in life may have important long-term health benefits even among persons of normal weight.16–18 In contrast, low muscular strength was not associated with increased risk of IHD, despite previously reported associations between low muscular strength and type 2 diabetes43 or metabolic syndrome,44, 45 known risk factors for IHD.
The mechanisms by which obesity and low aerobic fitness increase the risk of IHD are multifactorial and include common intermediates. Both increased adiposity and low aerobic fitness are known to have adverse effects on insulin sensitivity, lipid metabolism, autonomic tone, fibrinolysis, and inflammation, which contribute to endothelial dysfunction and atherosclerosis.46, 47 A US cross-sectional study of 2,112 young adults found that both high BMI and (less strongly) low aerobic fitness were associated with multiple cardiovascular risk factors including insulin resistance, elevated C-reactive protein level, and dyslipidemia.48 Physical inactivity also is associated with increased adiposity-related inflammation.49 Physically inactive persons with elevated waist circumference were found to have increased inflammatory markers (e.g., C-reactive protein and fibrinogen) that are associated with IHD risk,50 possibly mediated by oxidative stress and endothelial dysfunction leading to atherosclerosis.46
The present study has several strengths, including its large national cohort with prospective ascertainment of the study exposures and IHD. The national cohort design prevented selection bias, and the use of registry data with prospectively measured exposures prevented bias that may result from self-reporting.16–18 We examined objectively measured aerobic fitness and muscular strength, which are likely better indicators of habitual physical activity than self-reported activity.51 We adjusted for several other known risk factors for IHD, including family history and socioeconomic factors, which also were prospectively ascertained and not self-reported.
Limitations include a lack of information on certain other risk factors, such as smoking and diet.16–18 Sensitivity analyses suggested that unmeasured confounding due to smoking had little effect on our observed associations between low aerobic fitness or obesity and IHD risk. Aerobic fitness, muscular strength, and BMI were measured at only one age (18 years), and hence we were unable to examine changes in these factors over time. This study also did not include women, because they were not subject to military conscription in Sweden. However, other studies have reported similar main effects for high BMI8 or low aerobic fitness52 in relation to IHD among women. This study primarily examined premature IHD (median age at diagnosis 48.2 years, maximum 62.0). Additional follow-up will be needed to examine these relationships at older ages when IHD is more common, and in ethnically diverse populations to explore for potential ethnic differences in our findings.
In summary, this large national cohort study found that low aerobic fitness or high BMI at age 18 was associated with higher risk of IHD in adulthood, with a negative multiplicative interaction. Low aerobic fitness appeared to account for a similar number of IHD cases among those with normal vs. high BMI. These findings suggest that early-life interventions may help reduce the risk of IHD in adulthood, and should include not only weight control but aerobic fitness, even among persons of normal weight.
Supplementary Material
Acknowledgments
Grant support: National Heart, Lung, and Blood Institute at the NIH (R01 HL116381); Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden.
FUNDING
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [R01 HL116381 to K.S.]; the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. There were no conflicts of interest.
Author Contributions: Dr. Jan Sundquist had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Crump, J. Sundquist, Winkleby, K. Sundquist.
Acquisition of data: J. Sundquist, K. Sundquist.
Analysis and interpretation of data: Crump, J. Sundquist, Winkleby, K. Sundquist.
Drafting of the manuscript: Crump.
Critical revision of the manuscript for important intellectual content: Crump, J. Sundquist, Winkleby, K. Sundquist.
Statistical analysis: Crump, J. Sundquist.
Obtained funding: J. Sundquist, K. Sundquist.
Footnotes
Conflicts of interest: None.
Supplementary information is available at the International Journal of Obesity’s website.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LG, Angquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5(11):e14126. doi: 10.1371/journal.pone.0014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 2009;33(8):866–77. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkstedt D, Hemmingsson T, Rasmussen F, Lundberg I. Body mass index in late adolescence and its association with coronary heart disease and stroke in middle age among Swedish men. Int J Obes (Lond) 2007;31(5):777–83. doi: 10.1038/sj.ijo.0803480. [DOI] [PubMed] [Google Scholar]
- 7.Osler M, Lund R, Kriegbaum M, Andersen AM. The influence of birth weight and body mass in early adulthood on early coronary heart disease risk among Danish men born in 1953. Eur J Epidemiol. 2009;24(1):57–61. doi: 10.1007/s10654-008-9301-z. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Kuper H, Weiderpass E. Anthropometric characteristics as predictors of coronary heart disease in women. J Intern Med. 2008;264(1):39–49. doi: 10.1111/j.1365-2796.2007.01907.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergh C, Udumyan R, Fall K, Almroth H, Montgomery S. Stress resilience and physical fitness in adolescence and risk of coronary heart disease in middle age. Heart. 2015;101(8):623–9. doi: 10.1136/heartjnl-2014-306703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogstrom G, Nordstrom A, Nordstrom P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. Eur Heart J. 2014;35(44):3133–40. doi: 10.1093/eurheartj/eht527. [DOI] [PubMed] [Google Scholar]
- 11.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundstrom J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1. 1 million young Swedish men: cohort study. BMJ. 2015;351:h4543. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 13.Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113(4):499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein AR, Sesso HD, Lee IM, Rexrode KM, Cook NR, Manson JE, et al. The joint effects of physical activity and body mass index on coronary heart disease risk in women. Arch Intern Med. 2008;168(8):884–90. doi: 10.1001/archinte.168.8.884. [DOI] [PubMed] [Google Scholar]
- 15.Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes (Lond) 2005;29(8):894–902. doi: 10.1038/sj.ijo.0802870. [DOI] [PubMed] [Google Scholar]
- 16.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive Effects of Physical Fitness and Body Mass Index on the Risk of Hypertension. JAMA Intern Med. 2016;176(2):210–6. doi: 10.1001/jamainternmed.2015.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crump C, Sundquist J, Winkleby MA, Sieh W, Sundquist K. Physical Fitness Among Swedish Military Conscripts and Long-Term Risk for Type 2 Diabetes Mellitus: A Cohort Study. Ann Intern Med. 2016;164(9):577–84. doi: 10.7326/M15-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on risk of stroke: A national cohort study. Int J Stroke. 2016;11(6):683–94. doi: 10.1177/1747493016641961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordesjo L, Schele R. Validity of an ergometer cycle test and measures of isometric muscle strength when predicting some aspects of military performance. Swedish J Defence Med. 1974;10:11–23. [Google Scholar]
- 20.Patton JF, Vogel JA, Mello RP. Evaluation of a maximal predictive cycle ergometer test of aerobic power. European journal of applied physiology and occupational physiology. 1982;49(1):131–140. doi: 10.1007/BF00428971. [DOI] [PubMed] [Google Scholar]
- 21.Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports. 1995;5(3):143–6. doi: 10.1111/j.1600-0838.1995.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 22.Hook O, Tornvall G. Apparatus and method for determination of isometric muscle strength in man. Scand J Rehabil Med. 1969;1:139–142. [PubMed] [Google Scholar]
- 23.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;(25):1–5. [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 26.Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32(2):97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoddard PJ, Laraia BA, Warton EM, Moffet HH, Adler NE, Schillinger D, et al. Neighborhood deprivation and change in BMI among adults with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36(5):1200–8. doi: 10.2337/dc11-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crump C, Sundquist K, Sundquist J, Winkleby MA. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol. 2011;21(4):231–7. doi: 10.1016/j.annepidem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 30.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–36. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Orsini N, Bellocco R, Bottai M, Wolk A, Greenland S. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. The Stata Journal. 2008;8(1):29–48. [Google Scholar]
- 33.Furberg H, Lichtenstein P, Pedersen NL, Bulik C, Sullivan PF. Cigarettes and oral snuff use in Sweden: Prevalence and transitions. Addiction. 2006;101(10):1509–15. doi: 10.1111/j.1360-0443.2006.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation. 1996;93(3):450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 35.Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316(7137):1043–7. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.StataCorp. Stata Statistical Software: Release 14. StataCorp LP; College Station, TX: 2014. [Google Scholar]
- 37.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 39.Global Burden of Disease and Risk Factors Collaborators. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knol MJ, Egger M, Scott P, Geerlings MI, Vandenbroucke JP. When one depends on the other: reporting of interaction in case-control and cohort studies. Epidemiology. 2009;20(2):161–6. doi: 10.1097/EDE.0b013e31818f6651. [DOI] [PubMed] [Google Scholar]
- 41.Greenland S. Interactions in epidemiology: relevance, identification, and estimation. Epidemiology. 2009;20(1):14–7. doi: 10.1097/EDE.0b013e318193e7b5. [DOI] [PubMed] [Google Scholar]
- 42.Owen CG, Kapetanakis VV, Rudnicka AR, Wathern AK, Lennon L, Papacosta O, et al. Body mass index in early and middle adult life: prospective associations with myocardial infarction, stroke and diabetes over a 30-year period: the British Regional Heart Study. BMJ Open. 2015;5(9):e008105. doi: 10.1136/bmjopen-2015-008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crump C, Sundquist J, Winkleby MA, Sieh W, Sundquist K. Physical Fitness Among Swedish Military Conscripts and Long-Term Risk for Type 2 Diabetes Mellitus: A Cohort Study. Ann Intern Med. 2016 doi: 10.7326/M15-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37(11):1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 45.Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39(2):233–40. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 46.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 47.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328(8):533–7. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 48.Diaz VA, Player MS, Mainous AG, 3rd, Carek PJ, Geesey ME. Competing impact of excess weight versus cardiorespiratory fitness on cardiovascular risk. Am J Cardiol. 2006;98(11):1468–71. doi: 10.1016/j.amjcard.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 49.Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation: the Multi-Ethnic Study of Atherosclerosis. Am J Prev Med. 2012;42(1):8–13. doi: 10.1016/j.amepre.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rana JS, Arsenault BJ, Despres JP, Cote M, Talmud PJ, Ninio E, et al. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J. 2011;32(3):336–44. doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 51.Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O’Keefe JH, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77(2):281–92. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413–23. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.