Abstract
Described here is the application of thermodynamic stability measurements to study age-related differences in the protein folding and stability of proteins in a rodent model of ageing. Thermodynamic stability profiles were generated for 809 proteins in brain cell lysates from mice, aged 6- (n=7) and 18-months (n=9) using the Stability of Proteins from Rates of Oxidation (SPROX) technique. The biological variability of the protein stability measurements was low and within the experimental error of SPROX. A total of 83 protein hits were detected with age-related stability differences in the brain samples. Remarkably, the large majority of the brain protein hits were destabilized in the old mice, and the hits were enriched in proteins that have slow turnover rates (p <0.07). Furthermore, 70% of the hits have been previously linked to ageing or age-related diseases. These results help validate the use of thermodynamic stability measurements to capture relevant age-related proteomic changes, and establish a new biophysical link between these proteins and ageing.
Keywords: Mass Spectrometry, iTRAQ, Proteomics, Chemical Denaturation, SPROX, ageing
Graphical abstract
INTRODUCTION
Ageing is phenotypically categorized by a loss of cellular function or an increase in cellular degeneration. Certain biological pathways (e.g. oxidative stress, deamidation, ubiquitination) are known to be critically important in ageing (1–8). Age-related changes in the function of some proteins in these pathways have also been documented (8–12). However, the molecular level understanding of this important biological process is incomplete. Such an understanding is not only of fundamental importance, but it also serves as a starting point for finding connections between seemingly disparate diseases that share age as a major risk factor (e.g., cancer, neurodegenerative disorders).
Several large-scale gene and protein expression level studies have been conducted in rodents and nematodes to better understand the molecular basis of ageing (13–15). Of particular significance to the current work is that two rodent studies identified relatively few age-related differences in gene and protein expression levels(13, 15); perhaps because changes in protein abundance are not always directly tied to changes in protein function. In contrast, the link between protein folding stability and function is more direct (16, 17). Changes in protein folding stability can occur as a result of several biologically significant phenomena such as the mutation, modification, and misfolding of proteins. These phenomena are commonly associated with ageing(1–11, 18). Indeed, the folding properties of a number of proteins with importance in the ageing process and in age-related diseases have been investigated(19–28). However, these investigations have largely involved the targeted analysis of specific purified protein constructs. The global analysis of protein folding and stability in ageing and age-related diseases has the potential to produce a more complete understanding of the functionally relevant proteins and pathways involved in this biological process. Protein folding stability is a biophysical property that can be modulated with small molecule drugs. For example, pharmacological chaperones have been targeted to proteins that play pathogenic roles in various protein misfolding diseases(29–34). Therefore, it is possible that proteins with age-related destabilizations may be useful therapeutic targets to treat the adverse effects of ageing with pharmacological chaperones.
The thermodynamic stability profiles determined here were generated using the Stability of Proteins from Rates of Oxidation (SPROX) technique. The SPROX technique has been used to measure the thermodynamic stability of both purified and unpurified proteins as well as protein ligand complexes(35–40). The technique has not only proven useful for finding the protein targets of small molecule ligands(35, 38, 41), it has also been used to characterize disease states (42). For example, thermodynamic stability profiles generated using a SILAC-SPROX approach successfully differentiated three breast cancer cell lines and identified novel molecular signatures of breast cancer (42).
Described here is the use of SPROX to profile the thermodynamic stability of proteins in mouse tissue lysates from the brains of young and old mice, aged 6- and 18-months, respectively. The thermodynamic stabilities of over 800 proteins in brain cell lysates (n= 7 for young and n=9 for old) were profiled to determine age-related thermodynamic stability differences. A total of 83 protein hits with age-related differences were identified. The protein hits identified included many that have known functions in, and connections to, biological pathways previously linked to ageing. This not only substantiates the results reported here, but it also establishes a biophysical link between these proteins and ageing. This link suggests that pharmacological chaperones designed to rescue the stability of one or more of the protein hits identified here could potentially be used to combat the adverse effects of ageing and age-related diseases.
MATERIALS and METHODS
Organ Tissue Cell Lysate Preparation
The 6-month old female mice (C57BL/6) were purchased from Jackson Laboratory (Mouse 1–7). The 18-month old female mice (C57BL/6) (Mouse 8–16) were obtained from the NIH National Institute on Aging (NIA) ageing mouse colony. Mice were euthanized by cervical dislocation, and the brains used for these studies were harvested immediately and kept at −20°C until tissue cell lysates were prepared.
Immediately prior to lysis, organs were cut into small pieces with a straight razor and combined with 400 µL of 20mM phosphate buffer (pH 7.4) and 4 µL of 100X protease inhibitor cocktail in Lysing Matrix A tubes (MP Biomedicals, LLC Solon, OH). The 100X protease inhibitor cocktail included pepstatin A (0.2 mM), leupeptin (0.4 mM), E-64 (0.3 nM), bestatin (1 mM), and AEBSF (20 mM) protease (all from ThermoFisher Scientific, Waltham, MA). The resulting mixture was added to a MP Fast-Prep-24 Tissue Homogenizer in which the sample was homogenized for 20 seconds at 4m/s, put on ice for 5 minutes, and homogenized again for 20 seconds at 4m/s. The homogenized sample was centrifuged for 90 minutes at 4°C and 14,000 rcf. The supernatant was removed and kept at −20°C until it was subject to an iTRAQ-SPROX analysis as described below.
iTRAQ-SPROX Analyses
iTRAQ-SPROX analyses were performed on the brain tissue cell lysates according to previously established protocols (40). Briefly, ~100 µg of total protein from each lysate was distributed into a series of 8 GdmCl-containing SPROX buffers comprised of 20 mM phosphate buffer, pH 7.4, and increasing concentrations of GdmCl. A concentrated solution of hydrogen peroxide was added into each protein-containing buffer to selectively oxidize solvent exposed methionine residues in each denaturant-containing SPROX buffer. The final GdmCl concentrations in SPROX buffers were 0.8, 1.1, 1.2, 1.4, 1.6, 1.8, 2.0, and 2.5 M. The final hydrogen peroxide concentration (3% v/v) and reaction time (3 min) was the same in each SPROX buffer. The oxidation reaction in each SPROX buffer was quenched with L-methionine, and the protein precipitated with trichloroacetic acid.
The protein pellets from each denaturant-containing buffer were submitted to a standard quantitative, bottom-up proteomics workflow using iTRAQ reagents for quantitation. The workflow included: dissolution of each pellet in 30 µL of 0.5M triethylammonium bicarbonate (TEAB) buffer (pH 7.5) containing 0.1% sodium dodecylsufate (SDS), disulfide bond reduction using 5 mM tris(2-carboxyethyl)phosphine (TCEP) for 1 hour at 60°C, free cysteine modification using 10 mM methylmethanethiosulfonate (MMTS) for 10 minutes at RT, and trypsin digestion using 1 µg of trypsin at 37°C with shaking overnight. The tryptic peptides derived from the proteins in each denaturant-containing SPROX buffer were labeled with iTRAQ reagents from an iTRAQ 8-plex (AB Sciex, Framingham, MA). The protein samples from the 0.8, 1.1, 1.2, 1.4, 1.6, 1.8, 2.0, 2.5 M denaturant concentrations were labeled with the 113, 114, 115, 116, 117, 118, 119, and 121 iTRAQ reagents, respectively. The iTRAQ labeling reaction was performed according to the manufacturer’s protocol, with the exception that 0.5 unit instead of 1 unit of each iTRAQ reagent was used in each labeling reaction.
Ultimately, 50 µL of each iTRAQ-labeled sample in each set of 8 SPROX samples was combined. From the combined sample an aliquot of 120 µL was desalted with a C18 column (Nest Group, Southborough, MA) according to the manufacturer’s protocol, and 240 µL was enriched for methionine-containing peptides using a Pi3™-Methionine Reagent kit (Nest Group, Southborough, MA) according to the manufacturer’s protocol. We have previously demonstrated the efficiency and utility of using the Pi3™-Methionine Reagent kit in SPROX analyses (39). The methionine enriched sample was also desalted using a C18 column (Nest Group, Southborough, MA) according to the manufacturer’s protocol.
LC-MS/MS Analyses
All samples were analyzed on a Thermo Scientific Q-Exactive Plus high-resolution mass spectrometer with a nanoAcquity UPLC system (Waters Corp, Milford, MA). This instrument also utilized a nano-electrospray ionization source. Sample was trapped on a column (Symmetry C18 300 mm x 180 µm) for 3 minutes at 5 µL/min 0.1% formic acid in water. The sample was then separated using the following gradient: 3% to 30% acetonitrile with 0.1% formic acid over 90 minutes. The column used for separation was 75 µm x 250 mm packed with 1.7 µm Acquity HSST3 C18 stationary phase (Waters Corp). The flow rate used was 0.4 µL/min at 55°C. This mass spectrometer used a full MS scan from m/z 375–1600 with a target AGC value of 1 × 106 ions in profile mode and a resolution of 70,000 (at m/z 200) for data dependent acquisition. This was followed by 20 MS/MS scans at a resolution of 17,500 at m/z of 200 in centroid mode with an AGC target value of 1 × 105. The MS/MS scans also had a normalized collision energy of 30 V and a maximum fill time of 60 msec. Dynamic exclusion of 30 seconds was used to reduce MS/MS oversampling.
Peak lists were extracted from the LC-MS/MS data and were searched against the 24,855 proteins in the SwissProt Mus musculus database version 2016-04-13, downloaded on 05/17/16 using Proteome Discoverer (Version 2.1.0.81). Cysteine residue modification by MMTS was a fixed modification in the search. N-termini and lysine residues modified by iTRAQ 8-plex and oxidized methionine residues and deamidation on asparagine and glutamine were variable modifications in the search. Up to three missed tryptic cleavages after R and K were allowed. The parameters included a 10 ppm mass tolerance window for precursor masses and 0.02 Da for fragment mass tolerance. Only peptide spectra with FDR <1% and iTRAQ reporter ion intensities that summed to >1000 were used in subsequent analyses of the data.
The LC-MS/MS analyses of the iTRAQ-SPROX samples analyzed here included 2 replicate LC-MS/MS runs of each methionine-containing peptide enriched sample and 1 LC-MS/MS run of each non-enriched sample generated for each brain. The number of replicates was chosen to maximize peptide and protein coverage. The mass spectrometry proteomics data collected including the raw data and search outputs have been deposited to the ProteomeXchange Consortium via the Pride (43) partner repository with the data set identifiers PXD005184.
iTRAQ-SPROX Data Analysis
The data was normalized as previously described in reference (40). Briefly, the 8 iTRAQ reporter ion intensities generated in each product ion mass spectra were averaged, and the raw intensity of each reporter ion in the product ion mass spectrum was divided by the average to generate so-called N1-normalized values. All the N1 values for non-methionine containing peptides were averaged for each reporter ion. Summarized in Table S-1 are the set of 8 average values (so-called N2 normalization factors) that were generated in each SPROX analysis. Ultimately, the N1-normalized values generated for the methionine containing peptides were divided by the corresponding N2 normalization factor to determine the final N2-normalized reporter ion intensity. Table S-2 includes a summary of all the N2-normalized iTRAQ reporter ion intensities (i.e., chemical denaturation data sets) generated for all the product ion mass spectra that were successfully identified and quantified in the iTRAQ-SPROX analyses performed on the brain tissue lysates from the 16 mice studied here.
The chemical denaturation data sets were fitted to a four parameter sigmoidal equation, equation 1, using a JAVA-based program (developed in house) that utilized the Nelder and Mead Simplex method for regression analysis(44).
| Equation (1) |
In equation 1, A is the pre-transition baseline, B is the post-transition baseline, C1/2 is the transition midpoint and b is a measure of the steepness of the transition. This program fits each set of data nine times, once with all eight points and then eight more times, each time leaving out a different one of the eight data points. The fit with the highest R2 was chosen as the final output. This is similar to previous data analysis methods for iTRAQ-SPROX that allowed for one data point to be ignored when C1/2 values were assigned by a visual inspection of the data (40). The data output from fitting all the product-ion spectra generated for the methionine-containing peptides can be found in Table S-2. Subsequent analyses of the data only utilized the chemical denaturation data sets that were determined to be high quality (R2 ≥ 0.8) and were generated from product ion mass spectra with low isolation interference (≤ 30%). If a peptide was identified multiple times within the same mouse the N2-normalized iTRAQ reporter ion intensities from the high quality data were averaged together to generate one set of iTRAQ reporter ion intensities at the 8 denaturant concentrations and the averaged data was fit to equation 1 as described above to extract a single C1/2 value (Table S-3).
Hit Identification
A Student’s two-tailed t-test was used to identify significant differences between the assigned C1/2 values in the young and old mice. A value was determined to be significant if the determined t-test p-value was < 0.05.
Quantitation of Thermodynamic Stability Changes
The transition midpoint shifts of the hit peptides were used to calculate free energy (ΔΔG) changes according to equation 2.
| Equation (2) |
In equation 2, ΔΔG is the change in free energy, m is δΔG/δΔC1/2, and ΔC1/2 is the transition midpoint shift. An m-value of 2.6 kcal mol−1M−1 for GdmCl was used for all proteins. This m-value was estimated based upon the average protein domain size of 100 amino acids and the average contribution of 0.026 kcal mol−1 M−1 per amino acid to the m-value of the protein as determined in reference (45).
RESULTS
Experimental Design
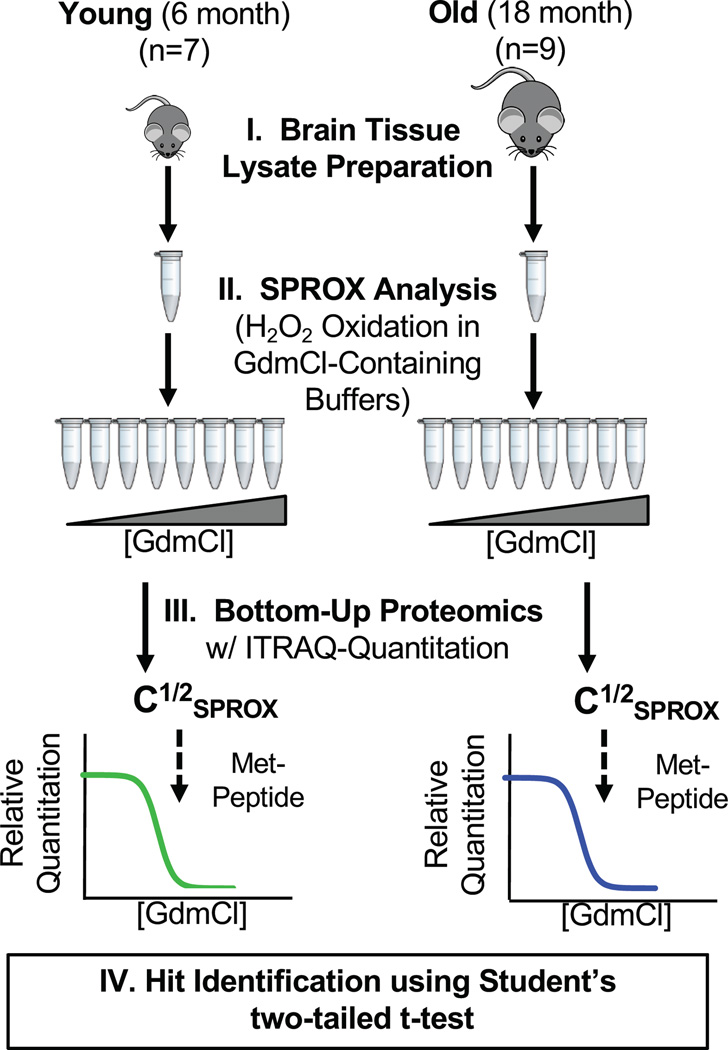
The experimental workflow outlined in Figure 1 was used to characterize the chemical denaturant-induced equilibrium unfolding properties of the proteins in organ tissue cell lysates from a total of 16 mice (n=7 for 6 month age point, n=9 for 18 month age point). The iTRAQ-SPROX technique used to generate the chemical denaturation data sets in this work utilizes the chemical denaturant dependence of a methionine oxidation reaction with H2O2 to report on the thermodynamic stability of proteins. Oxidative stress has long been known to be an ageing factor (46). Therefore, it is possible that some background oxidation may exist in the samples prior to the H2O2 treatment in SPROX. However, such background oxidation will not be denaturant dependent, and thus not compromise the chemical denaturation data generated in SPROX experiment. Furthermore, only those denaturation curves from wild-type methionine residues were considered for this work.
Figure 1.
Schematic representation of the experimental workflow used in this work. See text for details.
The SPROX technique relies on the detection and quantitation of methionine-containing peptides in the bottom-up proteomics readout to report on the folding stability of the protein folding domains to which they map. Therefore, the proteomic coverage in iTRAQ-SPROX experiments is restricted to those proteins that can be identified with methionine-containing peptides. The frequency of methionine residues in protein sequences is relatively low, ~2.5%. However, based on this frequency, a typical protein contains a number of methionine residues. In theory, one methionine-containing peptide probe can report on age-related thermodynamic stability differences occurring anywhere in the protein folding domain to which the peptide probe maps. Thus, the cause of the stability change (e.g., mutation, post-translational modification, and/or altered binding interaction) need not directly involve the methionine-containing peptide probe for the stability change to be successfully detected in the iTRAQ-SPROX experiment.
Proteomic Coverage
The proteomic coverage in the iTRAQ-SPROX experiments conducted on the sixteen mice brain samples averaged about 1200 methionine-containing peptides from about 675 proteins per mouse. The exact coverage obtained for each mouse is summarized in Table 1. To be included in the peptide coverage reported in Table 1, a methionine-containing peptide must have been identified with Isolation Interference ≤ 30% and the chemical denaturation curve fitted to equation 1 with an R2 ≥ 0.8. In total, the young mouse data set (mice 1 to 7) was comprised of 3260 unique peptide identifications from 1448 proteins and the old mouse data set (mice 8 to 16 was comprised of 3394 unique peptide identifications from 1506 proteins (Table 2). The Student’s two-tailed t-test requires that a peptide be identified in at least two mice in each age group. Overall, 1630 unique peptides from 809 proteins were assayed in the t-test analysis and used for further age-related difference analyses (Table S-4).
Table 1.
Summary of the proteomic data obtained in the iTRAQ-SPROX experiments on each mouse brain tissue lysate.
| Mouse Age | Mouse Identifier | Assayed Peptides (Proteins) |
|---|---|---|
| Young (6 months) | 1 | 1342 (753) |
| 2 | 1660 (897) | |
| 3 | 586 (381) | |
| 4 | 1315 (752) | |
| 5 | 1595 (835) | |
| 6 | 670 (426) | |
| 7 | 1559 (838) | |
| Old (18 months) | 8 | 1593 (887) |
| 9 | 1245 (721) | |
| 10 | 1861 (982) | |
| 11 | 857 (516) | |
| 12 | 340 (232) | |
| 13 | 832 (496) | |
| 14 | 1617 (855) | |
| 15 | 829 (538) | |
| 16 | 1252 (674) |
Table 2.
Summary of the proteomic data obtained in the iTRAQ-SPROX experiments used to determine age-related protein folding and stability differences.
| Mouse Age | Assayed Peptides (Proteins) |
Included in T.Test Peptides (Proteins) |
Hit Peptides (Proteins) |
|---|---|---|---|
| Young (6 months) | 3260 (1448) | 1630 (809) | 89 (83) |
| Old (18 months) | 3394 (1506) |
Biological Variability
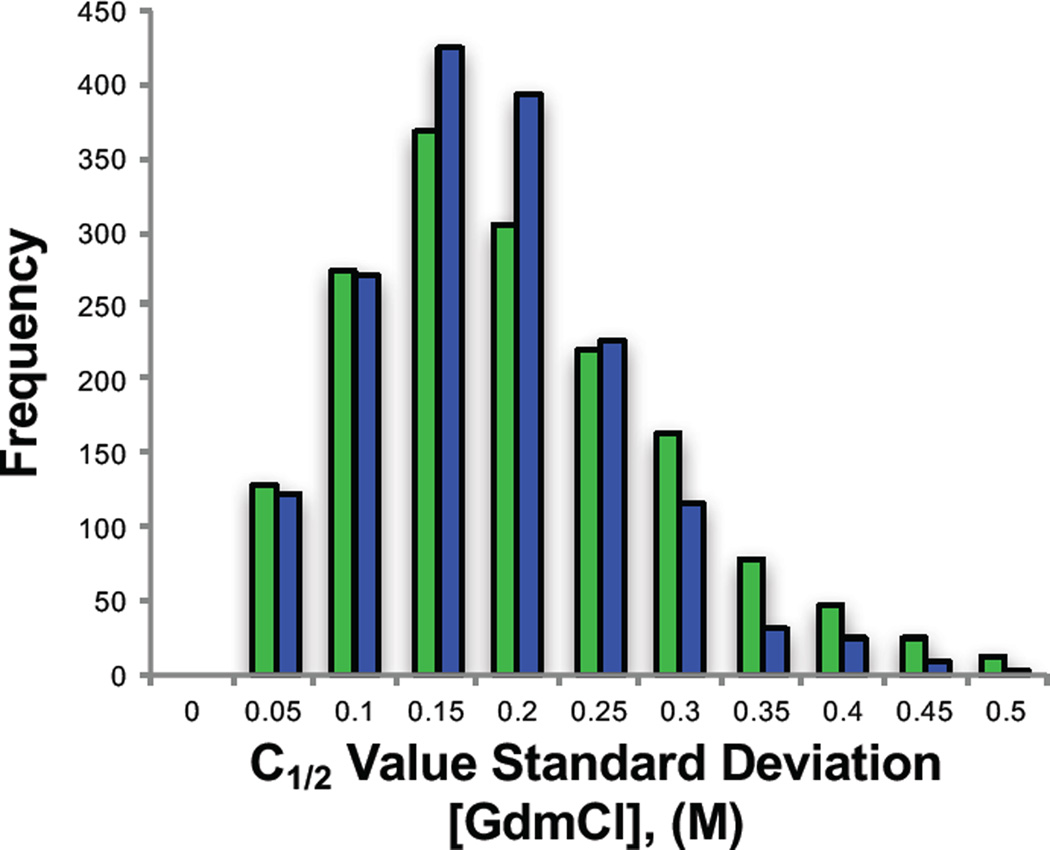
The experiments described here are the first instance in which the iTRAQ-SPROX methodology was used to study the thermodynamic stability of proteins from a rodent model. Previous SPROX experiments have been conducted on yeast and human cell lines (34–42). The increase in biological relevancy that comes with a rodent model also increases the potential for biological variability. The averages and standard deviations of the C1/2 values assigned for a given methionine-containing peptide within the old and young mouse age group samples were considered in order to assess the biological variability of the C1/2 value measurements. The distributions of the standard deviations associated with the C1/2 value assignments for a given methioinine-containing peptide in the old and young mice samples were similar (Figure 2). The median value in both cases was 0.15M GdmCl, which was also very close to the pooled standard deviation (spooled) of 0.18M GdmCl calculated using all the peptide C1/2 values in the young and old data sets. These values are consistent with that expected based on the denaturant concentration spacing of the data points in the experiment, which was 0.2M GdmCl for the majority of the denaturant curve. The C1/2 assignments in this experiment were consistent between mice to less than the spacing between data points. Thus, it appears that any biological variability in the C1/2 values reported here is generally small and within the technical error of the measurement.
Figure 2.
Frequency distribution of standard deviations associated with the C1/2 values determined for the assayed peptides in the different young (green) and old (blue) mice. Each bar represents the number of total peptides within each standard deviation bin. The high limit of each bin is listed on the x-axis.
Age Related Thermodynamic Stability Differences
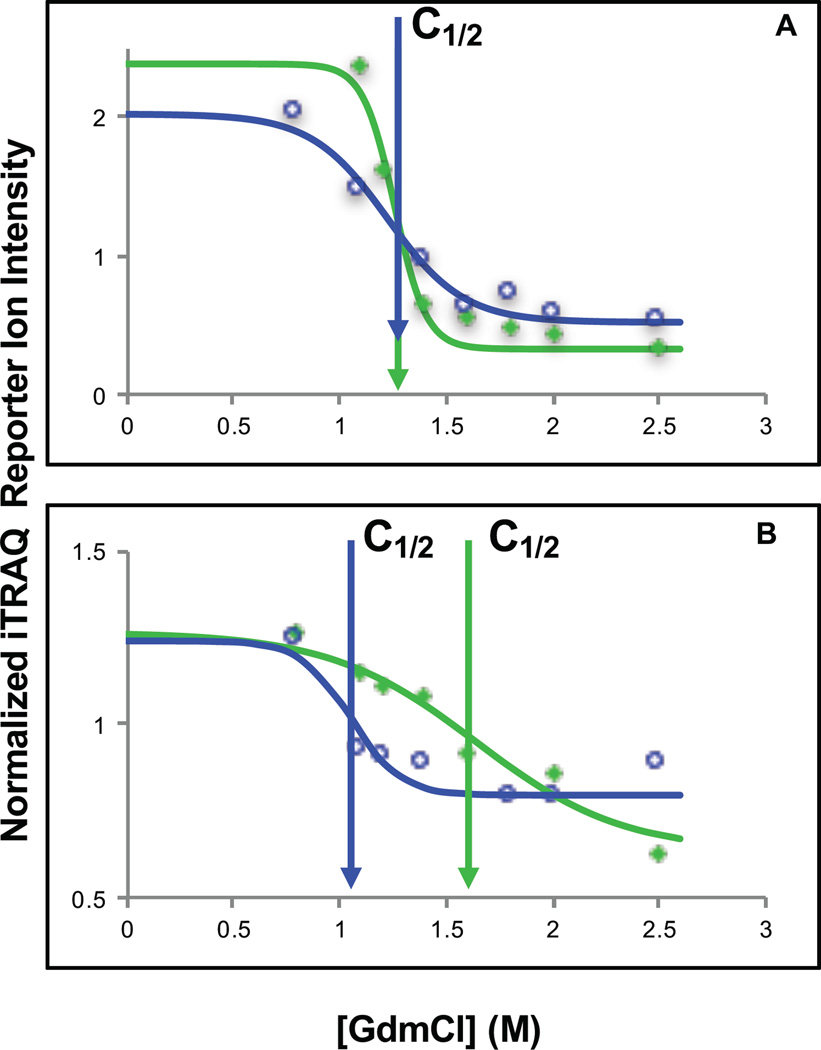
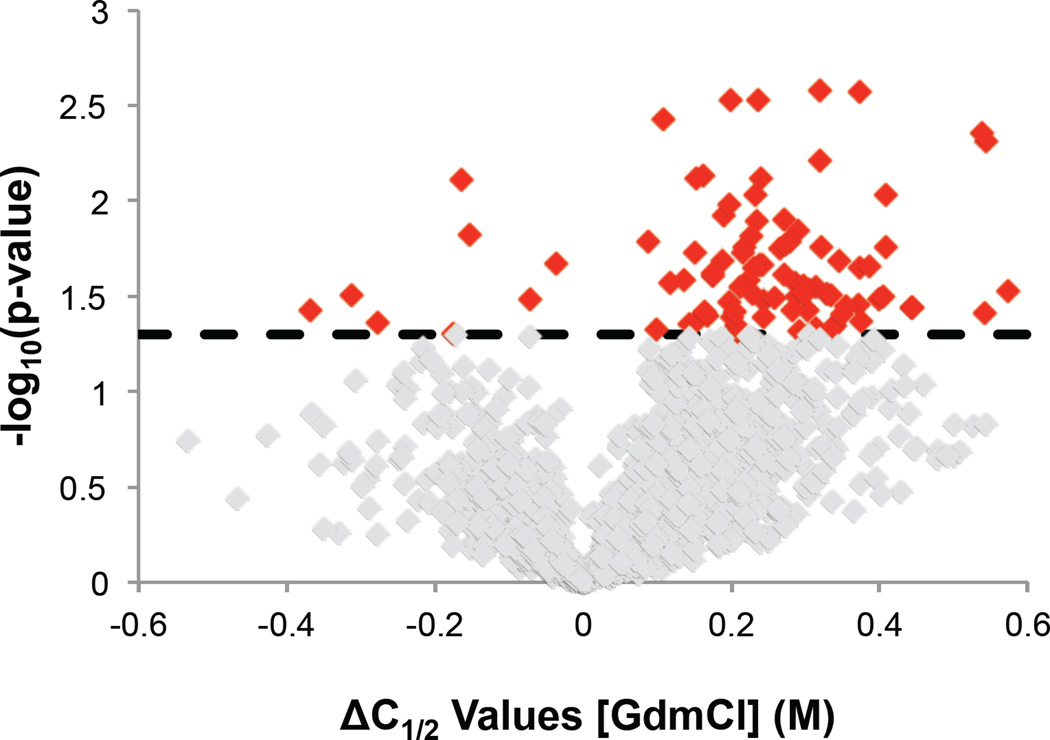
A Student’s two-tailed t-test was used to identify age-related thermodynamic stability differences that were consistent within an age group and different between young and old mice. To be considered in this analysis a peptide had to be identified in at least two mice in each age group (four mice total). In total this analysis included 1630 peptides from 809 proteins, and it identified 89 peptides from 83 proteins as hits. Data representative of a non-hit peptide identification and a hit peptide identification is shown in Figure 3. A volcano plot showing the distribution of ΔC1/2 values and associated p-values (Figure 4) reveals that the protein hits were largely destabilized in the old mouse brain. In fact, over 90% of the 89 peptide hits were the result of a protein destabilization in the old mouse brain. This is particularly remarkable given that the differentially stabilized protein hits previously identified in an earlier disease state analysis involving cell culture models of breast cancer was almost equally split between stabilizations and destabilizations (41). The ΔC1/2 values associated with these destabilizations ranged from 0.1 to 0.7 M, which correspond to destabilizations on the order of 0.3 to 1.8 kcal/mol. Two-thirds of the ΔC1/2 values were in the range of 0.2 to 0.4 M, corresponding to destabilizations of 0.5 to 1.0 kcal/mol.
Figure 3.
Representative iTRAQ-SPROX data for non-hit and hit peptides. (A) Shown are the chemical denaturation data sets and fitted curve obtained for the non-hit peptide AGAGSATLSMAYAGAR from the protein malate dehydrogenase when it was analyzed in young mouse 3 (green filled diamonds and green curve) and in old mouse 14 (blue open circles and blue curve). (B) Shown are the chemical denaturation data sets and fitted curves obtained for the hit peptide SVMDQANLQR from the protein heat shock 70 kDa 4L when it was analyzed in young mouse 2 (green filled diamonds and green curve) and in old mouse 16 (blue open circles and blue curve). The data shown in (A) and (B) represent the average data generated from all the product ion spectra generated for each peptide within the stated mouse experiment. The solid curve represents the best fit of the data to equation 1. The vertical arrows indicate the C1/2 value of each curve.
Figure 4.
Volcano plot showing the statistical significance of the ΔC1/2 values generated for the peptides in the young versus old mice comparison. The ΔC1/2 values were determined by subtracting the old mouse C1/2 value from the young mouse C1/2 value. A positive ΔC1/2 indicates a peptide that is more stable in the young mice than the old mice. The reported p-value was determined from a Student’s two-tailed t-test performed on the young and old mouse data sets. Non-hit peptide identifications are shown in grey. Hit peptide identifications are shown in red. The dotted line indicates the p-value cutoff (< 0.05) used to identify hits.
DISCUSSION
Classification of Age Related Protein Hits
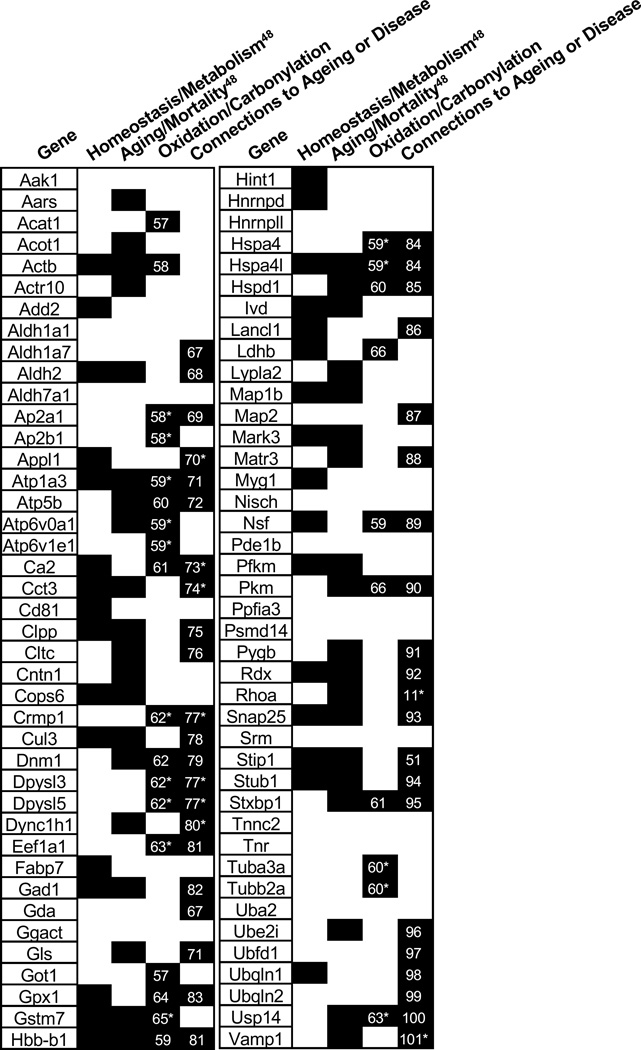
The age-related thermodynamic stability changes detected here were generally small and destabilizing in the old mice. This is in contrast to an earlier study of the thermodynamic stability changes of proteins in cell culture models of breast cancer in which the changes were not only larger (from 1.7 to 6 kcal/mol) but also equally distributed between stabilizing and destabilizing (41). A number of different biologically significant phenomena (e.g., point mutations and post-translational modifications) can change the folding stability of a protein. Therefore, the exact cause of the thermodynamic stability changes observed here cannot be determined from the data collected in this work. However, one explanation for the destabilizations is that they result from age-related post-translational modifications (PTM) (e.g., protein carbonylation events that are known to occur during ageing) of the protein hits or of other proteins that interact with the protein hits. Such PTMs have been theorized to destabilize proteins (47). Indeed, a significant fraction of the peptide hits (32 of 89) map to proteins known to have post-translational modifications, specifically carbonylation or oxidation, related to ageing or a neurodegenerative disease with ageing as a risk factor (Figure 5). However, we note that the molecular basis for the detected stability changes in hit peptides (proteins) cannot be directly determined from the data generated here. Additional studies on the protein hits are needed to uncover the biophysical phenomena responsible for the destabilizations. For example, SPROX experiments on the protein targets after purification from the lysates could be used to determine if the destabilization was the direct result of a mutation or PTM. Pull-down experiments using antibodies to the hit protein could also be used to better understand the network of protein-protein interactions that might be altered in aging.
Figure 5.
Summary of the hit proteins and corresponding classification. A (*) indicates that the protein identified as a hit was a different isoform, subunit or closely related protein to the protein linked to ageing or age-related diseases. The number inside of the box corresponds to the reference for the experimental connection.
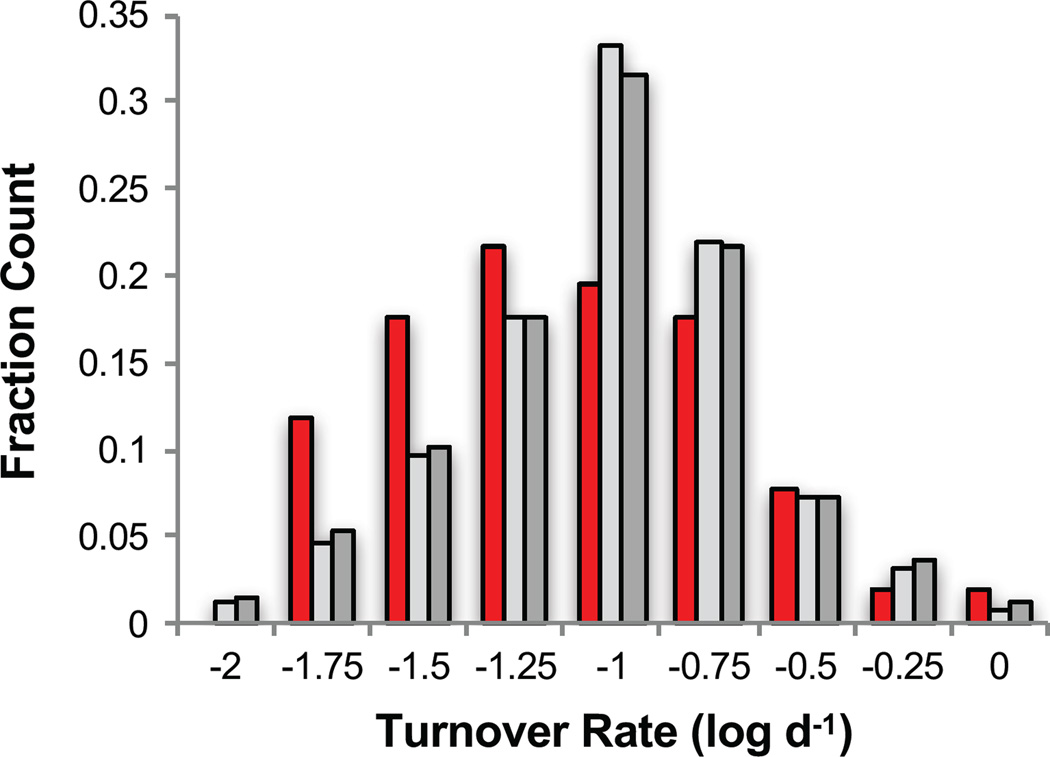
It has been shown that brain proteins have lifetimes that average about 9 days, with a majority of brain proteins having lifetimes between 1 and 100 days (48). An analysis of the turnover rates available for 51 of the 89 brain protein hits identified here revealed a distribution that was shifted toward longer lived proteins when compared to all the brain proteins assayed in this work and all of the proteins characterized in reference (48) (Figure 6). A Student’s two-tailed t-test between the turnover rates for the hit proteins determined in this work and all turnover rates for the 1007 proteins assayed by Price et. Al. indicated a p-value of 0.07. The turnover rates used to construct the distributions were those measured in relatively young (~4 month old) mice (48). Protein turnover rates may be age-dependent and different proteins may have different age-dependencies to their turnover rates. A longer-lived protein has more time in the organism before being turned over and therefore more time to experience an aberrant PTM or protein-protein interaction that could lead to its inability to function properly or be degraded appropriately. For example, proteins that accumulate aberrant and destabilizing post-translational modifications may overburden and escape the proteostasis network and biological pathways that regulate their synthesis and degradation.
Figure 6.
Bar graph showing the distribution of protein turnover rates previously measured in reference 44 for 51 of the brain protein hits identified here (red shaded bars), for 486 of the brain assayed proteins identified in this work (light grey shaded bars) and for all of the 1007 brain proteins analyzed in reference 44 (dark grey shaded bars). Each bar represents the fraction of the total protein population within each rate bin. The high limit of each bin is listed on the x-axis.
It has been well documented that there is an increase in oxidation with ageing. However, the exact impact oxidation has on ageing cells is still not well-understood. Recently it was theorized that highly charged proteins may be more susceptible to age dependent oxidation and thus a change in stability and functionality(47). The addition of an oxidation event on the side chain of a protein has the potential to destabilize that protein and cause functional deficits throughout the organism. In order to explore this potential connection further, net charges for all 809 proteins that were assayed in this work were calculated as described previously (47). A global trend in the hits to be more or less enriched in highly charged proteins was not observed (Table S-4). However, there were a small number of the hits that have a high charge to length ratio (Table S-4), which makes a compelling argument for the age-dependent thermodynamic stability loss of those proteins being due to age-related oxidations.
The phenotypic effects of the hit proteins identified here were also investigated. The Mouse Genome Database (49) was used to identify if a protein has been linked to one of two specific phenotypes of particular interest to the study of ageing: homeostasis/metabolism and mortality/ageing. A total of 33 of the 83 protein hits identified in this work induced an alteration in the homeostasis/metabolism phenotype (Figure 5). An even larger fraction of the protein hits (41 of the 83) were implicated in a mortality/ageing phenotype (Figure 5). These enrichments provide support for the use of this experimental setup for determining proteins with age-related functionality differences. The work here suggests a biophysical mechanism for why the known proteins are important in ageing and added a list of novel age-related proteins for future study.
Correlations Between Different Stabilities and Altered Functions
In total 58 of the 83 protein hits have been previously associated with ageing or age-related neurodegenerative diseases (Figure 5). For example, gene-knockout studies in mice have shown that dynamin 1 is important in memory development (50) and a knock-out of the stress induced-phosphoprotein 1 gene in nematode shortened the lifespan by 40% (51). Our results suggest that the important age-related functions of dynamin 1 and stress-induced-phosphoprotein 1 may be compromised in old mice because the proteins are destabilized.
Stress-induced phosphoprotein 1 has been shown to coordinate the functional interaction between HSP70 and HSP90 (52) two well-known chaperone proteins necessary to aid in the proper folding of proteins. It is also interesting to note that there are 6 other protein hits that are heat shock proteins or associated with the functionality of heat shock proteins (Clpp, Stub1, Hspa4, Hspa4L, Hspd1, Cct3). Furthermore, experiments conducted in C. elegans have indicated an increase in organism longevity upon overexpression of chaperone proteins (53, 54). Even more relevant to the work shown here, a study of an Hsp70 chaperone protein (Stub1), a protein hit in this work, indicated that lower levels of this protein decreased the longevity of a mouse model (55). These studies and our protein hits together could be an interesting insight into the down stream effects caused by an age-related loss of functionality in the identified protein hits. Our work suggests that the specific age-related functions of these proteins may be compromised with age due to a loss of thermodynamic stability.
The protein hits involved in the ubiquitin proteasome pathway are also of interest due to the importance of the proteasome in the progression of ageing. The ubiquitin proteasome pathway is the major mode of protein degradation in the cell and has been shown to have a decrease in activity with ageing leading to a loss of cellular proteostasis and increase in aggregation and dysfunction (56). Ten of the protein hits described here (Ubfd1, Psdm14, Usp14, Ubqln2, Cops6, Cul3, Uba2, Ubei2, Stub1, Cct3) are annotated with association to the ubiquitin or proteasome pathways. These proteins are involved in many different stages of the proteasome pathway. A small change in the protein thermodynamic stability, and thus functionality, at any point could lead to a breakdown of the pathway.
Our results suggest that altered thermodynamic stability may be a biophysical reason for the loss of functionality of these proteins in ageing systems. Furthermore, this provides validity to the use of thermodynamic stability measurements to capture age-related changes in the proteome. Although small, the stability differences detected here still have the potential to impair the ability of cells to function properly (i.e., through affecting the degradation of improperly folded proteins via the ubiquitin pathway or through affecting the ability for proteins to properly fold via chaperones) and lead to poor conditions for cell homeostasis.
CONCLUSIONS
This study is the first proteome-wide screen of the effect of ageing on protein thermodynamic stability. Identified here were 89 peptide probes from 83 proteins that that were differentially stabilized in the old and young mice populations. The protein hit rate observed here, ~12%, is significantly higher than that previously reported for age-related protein expression level changes (<1%) in a similar mouse model of ageing(13). Our results suggest that thermodynamic stability measurements may provide a better means than protein expression levels to study the effects of ageing. The thermodynamic stability differences detected in the protein hits provide an important first step in understanding the role that proteins play in the progression of ageing. The age-related thermodynamic stability changes detected here were generally small and destabilizing in old mice. This has important implications for the development of longevity drugs. For example, small molecule ligands that stabilize protein folds (i.e., pharmacological chaperones) could be pursued to rescue a loss of protein thermodynamic stability and treat the adverse effects of ageing. Such an approach has shown promise for the treatment of specific protein misfolding diseases. Because ageing is a complex process, it is likely that the use of pharmacological chaperones to reverse or slow the effects of ageing will require targeting multiple proteins. This work helps identify some proteins that may be useful targets for such a strategy.
Supplementary Material
Acknowledgments
The authors thank the Duke Proteomics Facility for collecting the LC-MS/MS data. J.H.R. is also grateful for support from a US Department of Education GAANN Fellowship (Award Number: P200A150114).
*This work was supported by a grant from the National Institutes of Health Grants 2R01GM08174 and S10RR027746 to M.C.F.
Footnotes
**This article contains the following Supporting Information:
The Supporting Information Includes:
Table S-1: Summary of the N2 normalization factors for the iTRAQ-SPROX experiments performed in this study.
Table S-2: Fitted chemical denaturation data sets obtained on all the wildtype methionine containing peptides identified in the brain tissue cell lysates.
Table S-3: Averaged and fitted data for all young and old mice used for the t-test analysis
Table S-4: (A) t-test output, (B) annotated hit list, (C) calculated net charges and (D) protein turn-over rates for all assayed proteins.
Figure S-1: Fitted chemical denaturation curves generated for all hit peptides.
REFERENCES
- 1.Lindner H, Helliger W. Age-dependent deamidation of asparagine residues in proteins. Exp. Gerontol. 2001;36(9):1551–1563. doi: 10.1016/s0531-5565(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 2.Gaczynska M, Osmulski PA, Ward WF. Caretaker or undertaker? The role of the proteasome in aging. Mech. Ageing Dev. 2001;122(3):235–254. doi: 10.1016/s0047-6374(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 3.Friguet B, Bulteau A-L, Chondrogianni N, Conconi M, Petropoulos I. Protein Degradation by the Proteasome and Its Implications in Aging. Ann. N. Y. Acad. Sci. 2000;908(1):143–154. doi: 10.1111/j.1749-6632.2000.tb06643.x. [DOI] [PubMed] [Google Scholar]
- 4.Chondrogianni N, Gonos ES. Proteasome dysfunction in mammalian aging: Steps and factors involved. Exp. Gerontol. 2005;40(12):931–938. doi: 10.1016/j.exger.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Carrard G, Bulteau A-L, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int. J. Biochem. Cell B. 2002;34(11):1461–1474. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 6.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21(10):569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaban RS, Nemoto S, Finkel T. Mitochondria, Oxidants, and Aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Reeg S, Grune T. Protein oxidation in aging: Does it play a role in aging progression? Antioxid. Redox Sign. 2015;23(3):239–255. doi: 10.1089/ars.2014.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraibar MA, Friguet B. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp.Gerontol. 2013;48(7):620–625. doi: 10.1016/j.exger.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J. Proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 11.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8(8):1925. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22(11):1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walther DM, Mann M. Accurate Quantification of More Than 4000 Mouse Tissue Proteins Reveals Minimal Proteome Changes During Aging. Mol. Cell. Proteomics. 2011;10(2):1–7. doi: 10.1074/mcp.M110.004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther Dirk M, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto Richard I, Dobson Christopher M, Vendruscolo M, Mann M, Hartl FU. Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell. 2015;161(4):919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ori A, Toyama BH, Harris MS, Bock T, Iskar M, Bork P, Ingolia NT, Hetzer MW, Beck M. Integrated Transcriptome and Proteome Analyses Reveal Organ-Specific Proteome Deterioration in Old Rats. Cell Systems. 2015;1(3):224–237. doi: 10.1016/j.cels.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyman J, Gill SJ. Binding and linkage: functional chemistry of biological macromolecules. University Science Books; 1990. [Google Scholar]
- 17.Schellman JA. Macromolecular binding. Biopolymers. 1975;14(5):999–1018. [Google Scholar]
- 18.Baraibar MA, Liu L, Ahmed EK, Friguet B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid. Med. Cell Longev. 2012 doi: 10.1155/2012/919832. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apetri AC, Surewicz K, Surewicz WK. The effect of disease-associated mutations on the folding pathway of human prion protein. J. Biol. Chem. 2004;279(17):18008–18014. doi: 10.1074/jbc.M313581200. [DOI] [PubMed] [Google Scholar]
- 20.Chiti F, Taddei N, Bucciantini M, White P, Ramponi G, Dobson CM. Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J.l. 2000;19(7):1441–1449. doi: 10.1093/emboj/19.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liemann S, Glockshuber R. Influence of amino acid substitutions related to inherited human prion diseases on the thermodynamic stability of the cellular prion protein. Biochemistry. 1999;38(11):3258–3267. doi: 10.1021/bi982714g. [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Wollmann R, Lindquist S. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science. 2002;298(5599):1781–1785. doi: 10.1126/science.1073725. [DOI] [PubMed] [Google Scholar]
- 23.Qu B-H, Thomas PJ. Alteration of the Cystic Fibrosis Transmembrane Conductance Regulator Folding Pathway Effects of the ΔF508 Mutation on the Thermodynamic Stability and Folding Yeild of NBD1. J. Biol. Chem. 1996;271(13):7261–7264. doi: 10.1074/jbc.271.13.7261. [DOI] [PubMed] [Google Scholar]
- 24.Varani L, Hasegawa M, Spillantini MG, Smith MJ, Murrell JR, Ghetti B, Klug A, Goedert M, Varani G. Structure of tau exon 10 splicing regulatory element RNA and destabilization by mutations of frontotemporal dementia and parkinsonism linked to chromosome 17. P. Natl. Acad. Sci.U.S.A. 1999;96(14):8229–8234. doi: 10.1073/pnas.96.14.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR, Lane DP, Fersht AR. Thermodynamic stability of wild-type and mutant p53 core domain. P. Natl. Acad. Sci.U.S.A. 1997;94(26):14338–14342. doi: 10.1073/pnas.94.26.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer S, Rüdiger S, Ang HC, Joerger AC, Fersht AR. Correlation of levels of folded recombinant p53 in Escherichia coli with thermodynamic stability in vitro. J. Mol. Biol. 2007;372(1):268–276. doi: 10.1016/j.jmb.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 27.György B, Tóth E, Tarcsa E, Falus A, Buzás EI. Citrullination: a posttranslational modification in health and disease. Int. J. Biochem. Cell B. 2006;38(10):1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Proctor EA, Ding F, Dokholyan NV. Structural and thermodynamic effects of post-translational modifications in mutant and wild type Cu, Zn superoxide dismutase. J. Mol. Biol. 2011;408(3):555–567. doi: 10.1016/j.jmb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999;5(1):112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 30.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299(5607):713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 31.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease. P. Natl. Acad. Sci. U. S. A. 2002;99(24):15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb. Perspect. Biol. 2011;3:12. doi: 10.1101/cshperspect.a004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 34.Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014;10(6):443–449. doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West GM, Tucker CL, Xu T, Park SK, Han X, Yates JR, Fitzgerald MC. Quantitative proteomics approach for identifying protein-drug interactions in complex mixtures using protein stability measurements. P. Natl. Acad. Sci. U. S. A. 2010;107(20):9078–9082. doi: 10.1073/pnas.1000148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West GM, Thompson JW, Soderblom EJ, Dubois LG, DeArmond PD, Moseley MA, Fitzgerald MC. Mass Spectrometry-Based Thermal Shift Assay for Protein- Ligand Binding Analysis. Anal. Chem. 2010;82(13):5573–5581. doi: 10.1021/ac100465a. [DOI] [PubMed] [Google Scholar]
- 37.West GM, Tang L, Fitzgerald MC. Thermodynamic analysis of protein stability and ligand binding using a chemical modification-and mass spectrometry-based strategy. Anal. Chem. 2008;80(11):4175–4185. doi: 10.1021/ac702610a. [DOI] [PubMed] [Google Scholar]
- 38.Tran DT, Adhikari J, Fitzgerald MC. SILAC-based strategy for proteome-wide thermodynamic analysis of protein-ligand binding interactions. Mol. Cell. Proteomics. 2014;13(7):1800–1813. doi: 10.1074/mcp.M113.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeArmond PD, Xu Y, Strickland EC, Daniels KG, Fitzgerald MC. Thermodynamic analysis of protein-ligand interactions in complex biological mixtures using a shotgun proteomics approach. J. Proteome Res. 2011;10(11):4948–4958. doi: 10.1021/pr200403c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strickland EC, Geer MA, Tran DT, Adhikari J, West GM, DeArmond PD, Xu Y, Fitzgerald MC. Thermodynamic analysis of protein-ligand binding interactions in complex biological mixtures using the stability of proteins from rates of oxidation. Nat. Protoc. 2013;8(1):148–161. doi: 10.1038/nprot.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geer MA, Fitzgerald MC. Characterization of the Saccharomyces cerevisiae ATP-Interactome using the iTRAQ-SPROX Technique. J Am Soc Mass Spectrom. 2016;27(2):233–243. doi: 10.1007/s13361-015-1290-z. [DOI] [PubMed] [Google Scholar]
- 42.Adhikari J, West GM, Fitzgerald MC. Global Analysis of Protein Folding Thermodynamics for Disease State Characterization. J. Proteome Res. 2015;14(5):2287–2297. doi: 10.1021/acs.jproteome.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vizcaino JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Rios D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolome S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nature Biotechnol. 2014;32(3):223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelder JA, Mead R. A Simplex Method for Function Minimization. The Comput. J. 1965;7(4):308–313. [Google Scholar]
- 45.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Prot. Sci. 1995;4(10):2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 47.de Graff Adam MR, Hazoglou Michael J, Dill Ken A. Highly Charged Proteins: The Achilles’ Heel of Aging Proteomes. Structure. 24(2):329–336. doi: 10.1016/j.str.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Price JC, Guan S, Burlingame A, Prusiner SB, Ghaemmaghami S. Analysis of proteome dynamics in the mouse brain. P. Natl. Acad. Sci. U. S. A. 2010;107(32):14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE The Mouse Genome Database, G. The Mouse Genome Database (MGD): facilitating mouse as a model for human biology and disease. Nucleic Acids Res. 2015;43(Database issue):D726–D736. doi: 10.1093/nar/gku967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fà M, Staniszewski A, Saeed F, Francis YI, Arancio O. Dynamin 1 Is Required for Memory Formation. PLoS ONE. 2014;9(3):e91954. doi: 10.1371/journal.pone.0091954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song H-O, Lee W, An K, Lee H-s, Cho JH, Park Z-Y, Ahnn J. C. elegans STI-1, the Homolog of Sti1/Hop, Is Involved in Aging and Stress Response. J. Mol. Biol. 2009;390(4):604–617. doi: 10.1016/j.jmb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, Masison DC. Independent Regulation of Hsp70 and Hsp90 Chaperones by Hsp70/Hsp90-organizing Protein Sti1 (Hop1) J. Biol. Chem. 2005;280(40):34178–34185. doi: 10.1074/jbc.M505420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu A-L, Murphy CT, Kenyon C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science. 2003;300(5622):1142. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz MJ. Longevity and heat stress regulation in Caenorhabditis elegans. Mech. Ageing Dev. 2003;124(1):43–48. doi: 10.1016/s0047-6374(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 55.Min J-N, Whaley RA, Sharpless NE, Lockyer P, Portbury AL, Patterson C. CHIP Deficiency Decreases Longevity, with Accelerated Aging Phenotypes Accompanied by Altered Protein Quality Control. Mol. Cell. Biol. 2008;28(12):4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saez I, Vilchez D. The Mechanistic Links Between Proteasome Activity, Aging and Age-related Diseases. Curr. Genomics. 2014;15(1):38–51. doi: 10.2174/138920291501140306113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meany DL, Xie H, Thompson LV, Arriaga EA, Griffin TJ. Identification of carbonylated proteins from enriched rat skeletal muscle mitochondria using affinity chromatography-stable isotope labeling and tandem mass spectrometry. Proteomics. 2007;7(7):1150–1163. doi: 10.1002/pmic.200600450. [DOI] [PubMed] [Google Scholar]
- 58.Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296(1):L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- 59.Martínez A, Portero-Otin M, Pamplona R, Ferrer I. Protein Targets of Oxidative Damage in Human Neurodegenerative Diseases with Abnormal Protein Aggregates. Brain Pathol. 2010;20(2):281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pamplona R, Dalfó E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otín M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation Effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005;280(22):21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- 61.Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R, Butterfield DA. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxid. Redox Signal. 2010;12(3):327–336. doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poon HF, Vaishnav RA, Getchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol. Aging. 2006;27(7):1010–1019. doi: 10.1016/j.neurobiolaging.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radical Biol. Med. 2011;51(7):1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 64.Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radical Biol. Med. 2008;45(5):667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech. Ageing Dev. 2006;127(11):849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol. Dis. 2008;30(1):107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19(9):1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Münzel T, Bürkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res. 2008;80(2):280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waldera-Lupa DM, Kalfalah F, Florea A-M, Sass S, Kruse F, Rieder V, Tigges J, Fritsche E, Krutmann J, Busch H, Boerries M, Meyer HE, Boege F, Theis F, Reifenberger G, Stuhler K. Proteome-wide analysis reveals an age-associated cellular phenotype of in situ aged human fibroblasts. Aging (Albany NY) 2014;6(10):856–872. doi: 10.18632/aging.100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu Y, Zhao D, Pan B, Wang J, Cui Y, Shi F, Wang C, Yin X, Zhou X, Yang L. Proteomic Analysis of Protein Expression Throughout Disease Progression in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015;47:915–926. doi: 10.3233/JAD-150312. [DOI] [PubMed] [Google Scholar]
- 71.Fu Y-J, Xiong S, Lovell MA, Lynn BC. Quantitative Proteomic Analysis of Mitochondria in Aging PS-1 Transgenic Mice. Cell. Mol. Neurobiol. 2009;29(5):649–664. doi: 10.1007/s10571-009-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol. Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 73.Cabiscol E, Levine RL. Carbonic Anhydrase III. OXIDATIVE MODIFICATION IN VIVO AND LOSS OF PHOSPHATASE ACTIVITY DURING AGING. J. Biol. Chem. 1995;270(24):14742–14747. doi: 10.1074/jbc.270.24.14742. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Goodlett DR, Peskind ER, Quinn JF, Zhou Y, Wang Q, Pan C, Yi E, Eng J, Aebersold RH, Montine TJ. Quantitative proteomic analysis of age-related changes in human cerebrospinal fluid. Neurobiol. Aging. 2005;26(2):207–227. doi: 10.1016/j.neurobiolaging.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Bota DA, Davies KJA. Protein degradation in mitochondria: implications for oxidative stress, aging and disease:: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. 2001;1(1):33–49. doi: 10.1016/s1567-7249(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 76.Dencher NA, Frenzel M, Reifschneider NH, Sugawa M, Krause F. Proteome Alterations in Rat Mitochondria Caused by Aging. Ann. N. Y. Acad. Sci. 2007;1100(1):291–298. doi: 10.1196/annals.1395.030. [DOI] [PubMed] [Google Scholar]
- 77.Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, α-enolase and heat shock cognate 71. J. Neurochem. 2002;82(6):1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 78.Chapple SJ, Siow RCM, Mann GE. Crosstalk between Nrf2 and the proteasome: Therapeutic potential of Nrf2 inducers in vascular disease and aging. Int. J. Biochem. Cell Biol. 2012;44(8):1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 79.Lee CH, Won M-H. Increased Dynamin-1 and −2 Protein Expression in the Aged Gerbil Hippocampus. Cell. Mol. Neurobiol. 2014;34(6):791–796. doi: 10.1007/s10571-014-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura N, Imamura O, Ono F, Terao K. Aging attenuates dynactin-dynein interaction: Down-regulation of dynein causes accumulation of endogenous tau and amyloid precursor protein in human neuroblastoma cells. J. Neurosci. Res. 2007;85(13):2909–2916. doi: 10.1002/jnr.21408. [DOI] [PubMed] [Google Scholar]
- 81.Shephard F, Greville-Heygate O, Marsh O, Anderson S, Chakrabarti L. A mitochondrial location for haemoglobins—Dynamic distribution in ageing and Parkinson’s disease. Mitochondrion. 2014;14:64–72. doi: 10.1016/j.mito.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004;89(1):204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 83.Oelze M, Kröller-Schön S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Münzel T, Daiber A. Glutathione Peroxidase-1 Deficiency Potentiates Dysregulatory Modifications of Endothelial Nitric Oxide Synthase and Vascular Dysfunction in Aging Novelty and Significance. Hypertension. 2014;63(2):390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 84.Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol. Cell. Biol. 1993;13(5):2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radical Res. 2006;40(12):1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- 86.Hondius DC, van Nierop P, Li KW, Hoozemans JJM, van der Schors RC, van Haastert ES, van der Vies SM, Rozemuller AJM, Smit AB. Profiling the human hippocampal proteome at all pathologic stages of Alzheimer’s disease. Alzheimers Dement. 2016;12(6):654–668. doi: 10.1016/j.jalz.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 88.Johnson JO, Pioro EP, Boehringer A, Chia R, Feit H, Renton AE, Pliner HA, Abramzon Y, Marangi G, Winborn BJ, Gibbs JR, Nalls MA, Morgan S, Shoai M, Hardy J, Pittman A, Orrell RW, Malaspina A, Sidle KC, Fratta P, Harms MB, Baloh RH, Pestronk A, Weihl CC, Rogaeva E, Zinman L, Drory VE, Borghero G, Mora G, Calvo A, Rothstein JD, Italsgen Drepper C, Sendtner M, Singleton AB, Taylor JP, Cookson MR, Restagno G, Sabatelli M, Bowser R, Chiò A, Traynor BJ. Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 2014;17(5):664–666. doi: 10.1038/nn.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perluigi M, Di Domenico F, Giorgi A, Schininà ME, Coccia R, Cini C, Bellia F, Cambria MT, Cornelius C, Butterfield DA, Calabrese V. Redox proteomics in aging rat brain: Involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J. Neurosci. Res. 2010;88(16):3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 90.Ortega-Molina A, Serrano M. PTEN in cancer, metabolism, and aging. Trends Endocrinol. Metab. 2013;24(4):184–189. doi: 10.1016/j.tem.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Favre C, Aguilar PS, Carrillo MC. Oxidative stress and chronological aging in glycogen-phosphorylase-deleted yeast. Free Radical Biol. Med. 2008;45(10):1446–1456. doi: 10.1016/j.freeradbiomed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Parisiadou L, Xie C, Cho HJ, Lin X, Gu X-L, Long C-X, Lobbestael E, Baekelandt V, Taymans J-M, Sun L, Cai H. Phosphorylation of ERM Proteins by LRRK2 Promotes the Rearrangement of Actin Cytoskeleton in Neuronal Morphogenesis. J. Neurosci. 2009;29(44):13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimohama S, Fujimoto S, Sumida Y, Akagawa K, Shirao T, Matsuoka Y, Taniguchi T. Differential Expression of Rat Brain Synaptic Proteins in Development and Aging. Biochem. Biophys. Res. Commun. 1998;251(1):394–398. doi: 10.1006/bbrc.1998.9480. [DOI] [PubMed] [Google Scholar]
- 94.Zhang G-R, Cheng X-R, Zhou W-X, Zhang Y-X. Age-related expression of STUB1 in senescence-accelerated mice and its response to anti-Alzheimer’s disease traditional Chinese medicine. Neurosci. Lett. 2008;438(3):371–375. doi: 10.1016/j.neulet.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 95.Boyd-Kimball D, Sultana R, Poon HF, Lynn B, Casamenti F, Pepeu G, Klein J, Butterfield D. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid β-peptide (1–42) into rat brain: implications for Alzheimer’s disease. Neuroscience. 2005;132(2):313–324. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 96.Sarge KD, Park-Sarge O-K. Sumoylation and human disease pathogenesis. Trends Biochem. Sci. 2009;34(4):200–205. doi: 10.1016/j.tibs.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carrano AC, Liu Z, Dillin A, Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460(7253):396–399. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haapasalo A, Viswanathan J, Bertram L, Soininen H, Tanzi Rudolph E, Hiltunen M. Emerging role of Alzheimer’s disease-associated ubiquilin-1 in protein aggregation. Biochem. Soc. Trans. 2010;38(1):150–155. doi: 10.1042/BST0380150. [DOI] [PubMed] [Google Scholar]
- 99.Zhang KY, Yang S, Warraich ST, Blair IP. Ubiquilin 2: A component of the ubiquitin-proteasome system with an emerging role in neurodegeneration. Int. J. Biochem. Cell Biol. 2014;50:123–126. doi: 10.1016/j.biocel.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 100.Gong B. Leznik, Elena, The Role of Ubiquitin C-Terminal Hydrolase L1 in Neurodegenerative Disorders. Drug News Perspect. 2007;20(6):365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 101.Anagnostou G, Akbar MT, Paul P, Angelinetta C, Steiner TJ, de Belleroche J. Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol. Aging. 2010;31(6):969–985. doi: 10.1016/j.neurobiolaging.2008.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.