Abstract
Background
Globally, the most widely used set of compounds among the internationally regulated drugs is cannabis.
Objective
To review evidence from epidemiological research on cannabis, organized in relation to this field’s five main rubrics: quantity, location, causes, mechanisms, and prevention/control.
Method
The review covers a selection of evidence from standardized population surveys, official statistics, and governmental reports, as well as published articles and books identified via MEDLINE, Web of Science, and Google Scholar as of July 2016.
Results
In relation to quantity, an estimated 3% to 5% of the world population is thought to have tried a cannabis product, with at least one fairly recent use, mainly extra-medical and outside boundaries of prescribed use. Among cannabis users in the United States, roughly one in 7–8 has engaged in medical marijuana use. In relation to location, prevalence proportions reveal important variations across countries and between subgroups within countries. Regarding causes and mechanisms of starting to use cannabis, there is no compelling integrative and replicable conceptual model or theoretical formulation. Most studies of mechanisms have focused upon a ‘gateway sequence’ and person-to-person diffusion, with some recent work on disability-adjusted life years. A brief review of cannabis use consequences, as well as prevention and control strategies is also provided.
Conclusion
At present, we know much about the frequency and occurrence of cannabis use, with too little replicable definitive evidence with respect to the other main rubrics. Given a changing regulatory environment for cannabis products, new institutions such as an independent International Cannabis Products Safety Commission may be required to produce evidence required to weigh benefits versus costs. It is not clear that government sponsored research will be sufficient to meet consumer demand for balanced points of view and truly definitive evidence.
Keywords: Cannabis, marijuana, epidemiology, incidence, prevalence, medical marijuana
Introduction and the Main Rubrics of Epidemiology as Applied to Cannabis Use
This article provides an overview of recent issues in cannabis epidemiology, with coverage of a small but growing number of research contributions on ‘medical marijuana’. The review is organized in relation to five main rubrics of epidemiology.
The article is not a comprehensive review because its intent is to focus attention upon the global and country-specific magnitude of cannabis use in terms of frequency and occurrence. Some attention is paid to cannabis problems such as the cannabis dependence syndrome. As such, the article offers an overview of recent evidence on a subset of the epidemiological topics that deserve more serious investigation when we consider ‘potential futures’ for cannabis research in the domain of pharmaceutical design.
Organization of a subject matter review on cannabis in relation to epidemiology’s five main rubrics must necessarily build from and have some overlap with prior contributions, as described in this paper’s acknowledgments section. Nonetheless, for the most part, the summarized evidence is new, and follows the outline of the five main rubrics:
Quantity: How many? Under this rubric, epidemiologists ask about the world’s population. In the world, how many cannabis users are there? What can we say about either the frequency of cannabis use (i.e., the estimated numbers of recently active users, and associated ‘prevalence proportions’) or the occurrence of cannabis use (i.e., the ‘incidence rates’ for cannabis use, or ‘cumulative incidence proportions’ that convey the rate at which newly incident cannabis use occurs or has occurred in the world population at large?
Location: Where? Where within the world are we more or less likely to find cannabis users as arrayed along dimensions of space and location, and time, and arranged into subgroups of the population on the basis of possible host susceptibility traits?
Causes: Why? Is there a definitive evidence base to answer questions about why some individuals become newly incident cannabis users while others abstain?
Mechanisms: How? As we work backward from cannabis use and its consequences toward prior origins, can we say anything definitive about the sequences of states and processes that lead toward the occurrence of newly incident cannabis use and onward toward what happens after the first occasion of cannabis use?
Prevention and Control: What can we do? What might be done to reduce the prevalence of cannabis use and its consequences, either by preventing onset of use in the first place, or by reducing duration of use once it occurs, or by ameliorating or reducing personal, familial, or social harms attributable to cannabis use?
This review article may be of greatest use to readers interested in the first two of these five rubrics. Readers interested in the other three rubrics are provided with short introductions that highlight other published reviews. As noted above, and in the works cited, some prior contributions are more comprehensive.
A Glossary of Terms
Cannabis
The term ‘cannabis’ is used throughout the article because it is a catch-all scientific term that encompasses the compound as used in its herbal form, in its resin form, and in various derived or synthesized cannabinoid products. A review of the world literature assembled in the Web of Science suggests that ‘cannabis’ is the term used more often outside the United States (US), Canada, and Mexico. In these three countries of the western hemisphere, most contributors to the literature have adopted ‘marijuana’ or ‘marihuana,’ which have historical origins in early attempts by US federal law enforcement officials to demonize the compound, and to portray it as something used by ‘the other’ (i.e., Mexican immigrants or those of Mexican heritage now living in the US). Due to this history, ‘marijuana’ and ‘marihuana’ are not scientific terms. They are US origin slang names that have counterparts in other countries, where the histories of use are not wrapped up in issues of drug law enforcement and attempts to attach stigma or ‘otherness’ to those using cannabis compounds. Other slang names such as ‘bhang,’ ‘charas,’ and ‘ganja’ are mentioned later in the chapter.
Frequency
A defining characteristic of epidemiological research is orientation to a defined population or population subgroup, large or small. The largest human population considered by epidemiologists is that of this Earth, with billions of inhabitants to consider. Often in epidemiological research, the world population is categorized by residence within a specific region (e.g., Oceania) or on a continent (e.g., Africa), and within boundaries of a geopolitical jurisdiction (e.g., US, ‘Europe’ or ‘the Middle East’). In public health practice, the jurisdictions are much smaller and might follow the boundaries of states or sub-state jurisdictions such as a county, or a ‘public health district’ or ‘catchment areas’ as were created for the National Institute on Mental Health’s Epidemiologic Catchment Area Surveys from which the first nation-level estimates of the frequency of cannabis use disorders were derived [1].
Estimates of the ‘frequency’ of a condition or behavior can be expressed in terms of a raw count for a defined population, as a public health officer might provide in reports on how many population members were identified as cases of schizophrenia were treated during a stated calendar year, versus how many non-resident visitors to the population were treated for schizophrenia that same year. Often, the raw frequency is converted into a ‘prevalence proportion,’ with the numerator consisting of the number of population members found to be actively affected by the condition in a given year (or other pre-specified interval of time), and with the denominator consisting of a census count of the pre-defined population size at mid-year (or mid-interval).
In the estimates for prevalence proportions, it is typical to exclude from both the numerator and the denominator the temporary visitors to the jurisdiction, who should be counted in the jurisdictions where they maintain a primary residence. In many respects, public health’s estimates of the raw frequencies and prevalence proportions generally follow the ideas of one of the US founding fathers with respect to the idea that each individual is attached to a given district with agreed upon boundaries and should be counted once and only once. These same ideas have been expressed in other countries. For example, the first published estimates of prevalence proportions for specific categories of mental disorders (e.g., idiotia, melancholia, mania, dementia) were based on surveys of the residents of Norway in the early 19th century, with the boundaries of sub-populations determined by the roster of each national church parish, which listed not only the practitioners in the area but also the non-practitioners [2].
As applied to cannabis use, the most frequently reported raw frequency is the number of recently active cannabis users in a given population. The most frequently reported estimate of the prevalence proportion is formed with the number of recently active users in the numerator, versus a denominator consisting of the number of members of a defined population. Unless stated otherwise, the specification for ‘recently active’ for this review article is ‘use within a 12 month interval of time,’ most often the 12 months just prior to the date of a survey assessment. In some instances, epidemiologists have reported prevalence proportions for recently active cannabis use in a 30-day time interval. Often, these estimated proportions are called estimates of ’30-day prevalence’ and 12-month prevalence.
It does not take a lot of arithmetic to appreciate that these cannabis ‘prevalence proportions’ are determined in large part by two aggregated numbers in the numerator: (1) the newly incident cannabis users who first started to use the drug during the specified interval of time, and (2) the past-onset users who had started in some prior time interval but whose use persisted into the interval of time being considered. In this sense, there is a basic principle of epidemiology that any prevalence proportions should vary as a function of the condition’s incidence rate and its mean duration of the condition. A simplified formula, based on this fundamental principle of epidemiology, is that the prevalence proportion for cannabis users in a given year is the annual incidence rate (for becoming a newly incident cannabis user in a given population during a year) times the mean duration of cannabis use in the population (from start to finish).
In this review article, this distinction between ‘newly incident cannabis users’ and ‘recently active cannabis users’ is crucial. In any aggregation of recently active cannabis users, there will be some who qualify as newly incident users, but depending upon the mean duration of cannabis use in the population, the newly incident users might be a small minority relative to the number of past-onset cannabis users of long duration. Cheng and colleagues [3] have provided a useful illustration of these distinctions in a recent study of underage drinking in the US. They were able to show that a US male excess in the prevalence of underage drinking in mid-adolescence now is largely determined by greater mean duration of drinking for males. At present in the US, during early adolescence, it appears that girls are more likely than boys to become newly incident drinkers (i.e., the risk of becoming an underage drinking is greater for girls than for boys).
Occurrence
Epidemiology’s estimates of ‘frequency’ refer to the number of cases of a disease (or users of a drug) at a given point in time such as the midpoint or end-date of an interval of calendar time. Here, there is no specification for a component of time passing, as is required in the scientific specification for a ‘rate.’ Readers might wish to think back to physics and its concept of the ‘rate’ with an inherent component of time passing. (Once this thought is in mind, it is difficult to listen or read about a ‘prevalence rate’ without cringing, and to feel a bit of wonderment about whether the speaker or author ever studied basic physics, chemistry, or other sciences in which the concept of a rate is tied intimately to passage of time).
Epidemiology’s primary measure of the occurrence of a disease, condition, or behavior is an incidence rate, formed with one of two denominators, and with either an implicit or explicit specification of units of time passing. To illustrate, the annual incidence rate for clinically apparent varicella zoster (‘chickenpox’) in a population has a denominator based on the population under surveillance as time passes during a given year, typically a calendar year. The numerator for the varicella zoster annual incidence rate is the number becoming affected as cases during that year. The denominator might be the mid-year population size for that year times one year. Or, if the goal is to estimate an individual’s chances of becoming a clinical case, the denominator might be re-specified to exclude the time contribution made by all population members who previously had been cases in prior years (because they no longer are ‘at risk’ of becoming newly incident cases during the next year). It should be clear that for rare diseases the annual incidence rate does not depend heavily upon which form of denominator is specified. For more common conditions, particularly when there is active surveillance (e.g., school or population surveys), the incidence rate’s denominator is constructed as a measure of person-time, with past onset conditions excluded from consideration.
In the estimation of incidence rates for ‘becoming’ a case as person-time passes, it is customary to count each affected individual once and only once during a year or other interval of time, but there are some exceptions when the condition is said to be ‘non-absorbing’ and can occur more than one time in the same individual. Influenza rates often count an individual more than once (e.g., when the person is a victim of influenza infection during two ‘flu seasons’ of the same calendar year). Similarly, given recurrences within short intervals of time, the total burden of non-fatal suicide attempts is not captured in the annual incidence rate that restricts the numerator to first-time suicide attempters: a unit is added to the numerator each time a non-fatal suicide attempt is registered.
Epidemiology most often expresses its estimates of occurrence in terms of ‘incidence rates’ defined in relation to a specified interval of calendar time passing (e.g., the ‘annual incidence rate’). Nevertheless, an ‘attack rate’ formulation of the incidence rate sometimes can be useful, most often learned in a basic epidemiology course during a laboratory exercise about cases of a food-borne illness observed during the hours or days after a public luncheon, and the task is to identify the food, foods, or beverages that were the causes of the outbreak. In the workup of the outbreak, the foodborne illness cases come to light over a span of time after the luncheon event, and one of the epidemiological patterns studied is the time from the luncheon until the case status is realized. The result is a frequency distribution for the incubation interval, with hours or days on the x-axis, and with frequency of cases observed as time passes on the y-axis, from which the median incubation period can be derived, and this empirical evidence sometimes is useful in specifying which agent has caused the outbreak. Subsequently, a series of ratios are formed, typically by asking all luncheon attendees to state which foods and beverages they consumed, and then forming denominators as a count of how many consumed each item. This is the denominator for the ‘food-specific attack rate’ calculation, with time implicit in the denominator as (time passing since the luncheon). The food-specific numerator consists of affected cases, food-by-food. That is, this form of incidence rate is estimated, food by food, by multiplying the number of affected cases among luncheon attendees who consumed the food item by the inverse of the number of luncheon attendees who consumed that same food item (both the sick and the well), with the rate’s passage of time implicitly framed as time since the luncheon (or the time of food exposure if that is known to be distributed across many hours).
A note should be made about epidemiological parameter of this type that was introduced roughly 60 years ago by sociologists studying mental illnesses of religious communities in the US. They defined denominators as the population size of each community, and considered a time dimension in relation to the interval from the birth of the individual until the date of the survey looking for affected cases of mental illnesses, much as epidemiologists do when they estimate incidence by counting up the number of affected cases among members who consumed food at a luncheon or social get -together that is distributed over many hours or days. A little thought discloses a potential problem if the conditions under study include fatal outcomes (e.g., death after salmonella infection from eating a raw egg; death from suicide after onset of depression). In the food-borne illness context, these fatal cases typically are not counted in the food-specific incidence rates because it is not possible to interview or survey the dead cases about which food items were eaten. In this instance, the incidence estimate is based on the experience of the survivors. The same was true in the sociological survey of mental illnesses in religious communities; the resulting incidence estimate for the first bout of mental illness occurring since the birth of each population member was based on the experience of the survivors. Regrettably, the sociologists did not appreciate epidemiology’s distinction between ‘frequency’ and ‘occurrence’ of conditions, and did not see that they were estimating the ‘attack rate’ formulation of a mental illness ‘incidence rate’. Instead, they erroneously used the term ‘lifetime prevalence’ to name the ratio they have formed. The error can be seen as a violation of the basic principle of epidemiology that any ‘prevalence’ estimate varies as a function of incidence of a condition and its mean duration.
The ratio they formed is one that depends upon the incidence of the mental illnesses studied, but not at all on the duration of the illnesses. For this reason, Gruenberg’s review of the work called the ‘lifetime prevalence’ concept a ‘gimmick’ that should not be employed by others [4]. More recently, Streiner et al. [5] argued that the ‘lifetime prevalence’ concept should be retired.
In summary, studied in depth, what cannabis researchers call ‘lifetime prevalence’ of cannabis use actually is akin to epidemiology’s ‘attack rate’ formulation for a true incidence rate, and fails to have the lawful properties of true prevalence estimates. Specifically, as an epidemiological parameter to be estimated, the ‘lifetime prevalence’ proportion now has no clear relationship with the mean duration of the condition or behavior under study, whereas one of the defining characteristics of a prevalence estimate is that it is determined by mean duration.
Of what good is the ratio erroneously called ‘lifetime prevalence’? In cannabis epidemiology, this proportion expresses the occurrence of cannabis use in a population, as assessed at a specific time, among survivors who have lived long enough to be identified as cannabis users if they have used (i.e., those who have not died after using cannabis). For this reason, most epidemiologists who seek to have cannabis epidemiology converge with basic concepts of the field do not use the term ‘lifetime prevalence.’ Whereas the term ‘cannabis attack rate’ might be used, it is true that ‘attack rate’ is rarely seen outside of communicable disease epidemiology. Elsewhere in epidemiology, the same concept for measuring occurrence of a condition in a population is called a ‘cumulative incidence proportion’ even though it actually is a ‘cumulative incidence proportion among survivors’. In this review article, the term ‘cumulative incidence proportion’ has been substituted where some original reports erroneously use the term ‘lifetime prevalence’. In time ‘cumulative incidence’ (or ‘attack rate’) might come to displace the ill-conceived gimmicky term ‘lifetime prevalence.’
Medical Marijuana
It is possible that the concept of ‘medical marijuana’ deserves a glossary note because this concept emerged only when the US federal government decided that it should have a monopoly over the possession and use of cannabis-containing products (much as governments seek monopolies over the occurrence of violence-caused deaths). Thereafter, some states and other jurisdictions thought that medical doctors should be able to prescribe cannabis for treatment of disturbances of general medical conditions and/or mental health problems (e.g., post-herpetic neuralgia). In many countries, federal governments do not seek this control with respect to cannabis and the concept of ‘medical marijuana’ is not something deemed worth counting. In the US, many states tally the number of registered ‘medical marijuana’ users and a new tradition of epidemiological research based on these registries has emerged [6].
The Five Main Rubrics of Epidemiology as Applied to Cannabis Use
1. The First Rubric: Quantity (How Many?)
According to most recent United Nations (UN) report on drug use, based on official statistics as reported to the World Health Organization and the UN, and summarized in 2015, there might be as few as 128,480,000 world inhabitants who recently have tried cannabis on at least one occasion, or as many as 232,070,000 who have done so. Expressed as a percentage of the world population, the numbers can be re-expressed in prevalence proportions across the range from about 2.7% of the world population (low end estimate) to about 4.9% of the world population (high end estimate).
It is useful to place these global estimates in a general context. For example, by comparison, the UN report asserts that roughly 13,800,000 to 20,730,000 recently have tried cocaine compounds on at least one occasion, outside the boundaries of prescribed cocaine use (i.e., not counting use in legitimate dental or other surgery). Corresponding estimates for the opium poppy-derived ‘opiates’ such as heroin and morphine are not appreciably different from these global estimates for the cocaine compounds. As such, the numbers of individuals who have used cannabis might be some 10 times greater than the numbers of individuals who have tried cocaine, the opiates, or other internationally regulated drugs.
There is no current global estimate for the number of individuals who have used cannabis in the context of medically prescribed ‘medicinal marijuana use’ and one must presume that most observed cannabis users have tried the drug ‘extra-medically’ (again, ‘outside the boundaries of what was intended by a prescribing clinician’). In the absence of credible epidemiological estimates, it may be necessary to turn to estimates for the United States, which suggest that as many as one in 7–8 cannabis users in the US might be engaged in medical marijuana use [7]. Whiting and colleagues have prepared a useful systematic review of randomized controlled trials on an array of indications and cannabinoid effects as observed under RCT-controlled conditions [8].
For most of human history, self-administration of cannabis has involved smoking of herbal cannabis or consumption of relatively simple derivative plant products known by their slang or colloquial names in English: hashish, bhang, charas, ghee, ganja, marijuana or weed (mainly US slang) and marihuana (widespread slang term in Canada). During the past several decades, there has been an increased use of cannabinoids in the form of more complex derivative products such as ‘resin cannabis’ (often defined to include hashish) as well as synthetic cannabinoids. Schauer and colleagues [9] recently published cannabis patterns for 21st century routes of administration such as ‘vaping’ (inhaling fumes), and these variations in modes of cannabis self-administration almost certainly will become more prominent in the coming decades. It is too early to say anything definitive about global prevalence of use of these compounds and modes of self-administration. Epidemiological surveillance of the use of these compounds is not yet complete nor convincing, due in large part to the untested validity of self-reported use. Recent UN World Drug Reports provide some starting estimates for readers with interest in these developments [10].
2. The Second Rubric: Location (Where?)
2.1 Location in terms of the time dimension
Deliberate cultivation of cannabis hemp to derive clothing and rope had its origins as early as the Neolithic era. Some pre-historic sites suggest cannabis burning and possible inhalation of cannabis fumes, possibly in entheogenic rituals or spiritual practices. As early as 3000–4000 years ago, cannabis self-administration and human contact with spirits, demons, and deities begin to appear in literature of the ‘Orient’ (e.g., in the Vedas of India; ancient medical texts from sites in contemporary China). More recent entries are found in reports on cannabis used in Africa and the Americas. As for what is now Western Europe, traces of deliberate cultivation of cannabis as hemp (e.g., for rope and ship-rigging) have been found in or near Anglo-Saxon settlements and other sites of Roman conquest throughout the modern era, but there is no definitive evidence of cannabis self-administration in European countries before the 15th century [11].
Across the years since that time, one can find merchant and adventure traveler reports of what might well be allusions to cannabis self-administration for intoxicating purposes. Uncertainty exists, in part due to the unknown identity of the plants involved. It was not until the early 1700s that Linnaeus formalized ‘cannabis sativa’ as the species’ name in his botanical taxonomy.
The most widely distributed 19th century description of the intoxicating effects of cannabis most likely was Moreau’s 1845 characterization [12]. Green [13], writing in The Guardian, has provided an account of social cannabis use during convivial gatherings of French authors and philosophers during the subsequent decade (https://www.theguardian.com/books/2002/oct/12/featuresreviews.guardianreview34, last accessed 20 April 2016). Green’s book on cannabis in general provides a more complete history and popular account of cannabis along the time dimension [14], complementing prior scholarly contributions by Abel [11] and Musto [15]. Some readers might be interested in a 19th century description of several cases and instances of cannabis intoxication under medically supervised conditions, as compiled by a University of Pennsylvania professor of botany; these experiences were intended to guide decision-making about inclusion of medicinal cannabis in the US Pharmacopeia of the 19th century [16].
Origins of modern detailed epidemiological studies of cannabis self-administration can be seen in summary reports and background documents written by a series of governmental commissions, the most prominent of which are: (1) the Indian Hemp Drug Commission of 1893–94, which Shamir and Hacker characterized as a “quasi-judicial and quasi–scientific commission” organized by Great Britain during a colonial effort to apply the British alcohol temperance (prohibition) model to cannabis use in its colony [17, 18]; (2) the New York City La Guardia Committee of 1944 [19]; (3) the Canadian LeDain Commission of 1969–1973 [20]; (4) the United States Commission on Marihuana and Drug Abuse of 1972 [21]. Each of these reports deserves study for its wealth of information about the history of cannabis use, frequency and occurrence of use at the time of each inquiry, and a summary evaluation that included expressions of concern that cannabis smoking, in general, carries some tangible risks of harm, but might not be as dangerous as had been supposed by drug law enforcement authorities in government.
The overall sentiment of the US Commission on Marihuana and Drug Abuse is expressed in the title of its interim report: “Marihuana: A Signal of Misunderstanding.” That commission’s widely ignored general conclusion was that the US regulatory approach toward simple cannabis possession and use should be one without criminal penalties but with general discouragement of use, and a note about particular attention to vulnerable subgroups such as adolescents under age 18 years.
It is remarkable that this commission conclusion and a similar conclusion of Canada’s LeDain Commission, written as North America faced peak years of its mid-20th century epidemic of cannabis smoking, generally were ignored by government and policymakers, and the criminal justice approach was maintained. For a variety of reasons, the numbers of newly incident users of cannabis in the US started to decline roughly 5–10 years after these reports were published, concurrent with what appear to have been trends of increasing use in the countries of western Europe and certain countries of the western hemisphere (e.g., Brazil). Subsequent trends observed during the 1990s and first decade of the 21st century turned upward or remained relatively stable, depending upon the country under study. Recent time trends over the past several decades appear to be relatively flat with respect to incidence of smoking cannabis herb, but there is some evidence of increasing incidence in the most recent years of the 21st century [10, 22].
2.2. Location in Terms of Geography, Space, and Place
Estimated on the basis of standardized population surveys or on the basis of official statistics reported to the UN and WHO, there are several countries that stand out with top-ranked prevalence and numbers of cannabis users. This top rank includes the United States and Canada, as well as Australia and New Zealand. Work of the World Health Organization World Mental Health Surveys Consortium has provided some approximations for these countries based on retrospective histories elicited from cross-sectional samples in each of the participating countries, including estimates of cumulative incidence proportions as defined in the Glossary section of this article [23].
Taking the United States as an example, virtually all cannabis use in the US starts during adolescence and young adulthood, and the annual incidence rate for starting to smoke cannabis now is roughly six percent per year. For example, consider 100 young people in the US who at the start of a calendar year have never used cannabis. According to this 6% estimate, by the end of the year, an estimated six of this 100 will have started to use. Nevertheless, there is some variation across states of the US, and across regions. To illustrate, the state with what is apparently the largest cannabis incidence rate is Vermont, where an annual incidence rate of nine percent per year has been estimated (95% confidence interval, CI = 8, 10). The state of Utah is at the other extreme, with an incidence rate estimate of just above 3% and a 95% CI that ranges from 3% to 4% [24].
It is not at all clear that there is any other country with annual incidence rates as large as those seen in the United States. One difficulty is that few countries routinely produce annual incidence rates for nationally representative samples of young people in the community. Instead, they report cumulative incidence proportions for school-attending adolescents, as is the case for most European countries. To illustrate, in the United States, roughly 30% of 15–16 year olds have tried cannabis (typically cannabis herb) - i.e., by age 15–16 years. The corresponding estimate based on recent surveys completed in more than 25 European countries is close to 16% (i.e., roughly one-half the estimate for the US), although there is marked country-by-country variation from low range values in many of the former countries of the Union of the Soviet Socialist Republics (USSR; e.g., 5% in Republic of Moldova), values of about 5%–9% in Sweden, Norway, and Greece, midrange values in many countries (e.g., Finland, 11%; Germany, 19%; United Kingdom, 25%; Netherlands, 27%), and higher-than-US-values in several countries (e.g., Monaco, 37%; France, 39%), including one of the former USSR jurisdictions: Czech Republic, 42% [25].
Whereas these annual incidence rates and cumulative incidence proportions refer to the occurrence of newly incident cannabis users, the prevalence proportion is a much more commonly seen epidemiological parameter - i.e., the parameter that combines incidence with duration in a count of the number of recently active users. To illustrate, in the European countries just mentioned, roughly 13% of the 15-to-16-year olds school-attending adolescents qualified as recently active users because they had consumed cannabis in the 12 months prior to the survey assessment date. Estimates for Monaco and France were roughly 20%; estimates for five countries (including Norway) were under 3 percent [25]. Corresponding estimates for the US are closer to 30% for students of a similar age, as estimated mid-way through the secondary school years [26].
Given potentially greater vulnerability of adolescents and young adults to noxious effects of cannabis exposures, most of the available estimates are for young persons. Nonetheless, there is some interest in frequency of cannabis use in older populations. The World Mental Health Surveys Consortium, mentioned above, is one of the most recent sources of evidence on adults age 15 years and older and on adults age 15-to-middle-age-years in multiple countries.
Based on these estimates [23], the US and New Zealand are in the top rank for cumulative incidence proportions (i.e., the proportion of adults who had become newly incident cannabis users by the age when assessed), with estimates just above 40%. In Mexico and Columbia, the estimates were 7.8% and 10.8%, respectively (based primarily upon city-dweller surveys). Estimates below 2% were obtained in the China and Japan surveys. Estimates for the European countries ranged from about 6% (e.g., Italy, Ukraine) to about 18%–20% (France, Germany, Netherlands). Of four countries surveyed in the Middle East and Africa, Israel and South Africa had larger estimates (11.5% and 8.4%, respectively), while Lebanon (4.6%) and Nigeria (2.7%) had lower estimates.
The prevalence proportions and numbers of recently active cannabis users in various regions of the world also might be of interest to those seeking to estimate potential needs for intervention services and potential markets for pharmaceutical design of future cannabis-related products or alternatives to cannabinoids. As shown in Table 1, based on official statistics compiled by the United Nations and reported in 2015, the region with the largest number of the world’s estimated 182 million recently active cannabis users is Asia, with 55.5 million recently active users, with the Americas (54.2 million) and Africa (45.8 million) in second place and third place, respectively. Most of the rest of the cannabis users are in Western Europe and the former USSR (outside of Asia): 23.7 million. Oceania (including Australia and New Zealand) is the region with the smallest number: 2.65 million [10].
Table 1.
Region-Specific Estimates for Prevalence (%) and Number of Recently Active (Past Year) Cannabis Users, 2015. (Source: United Nations. Office of Drugs and Crime. World Drug Report 2009. United Nations Publication, Sales No. E.15.XI.6, 2015).
| Region | Rank* | Recently Active Cannabis Users | |||
|---|---|---|---|---|---|
| Estimated Prevalence (%) & Number in Millions | Lower Bound Estimate (in millions) | Upper Bound Estimate (in millions) | |||
| Oceania | 5 | 10.70% | 2.65 | 2.22 | 3.57 |
| Africa | 3 | 7.50% | 45.8 | 20.38 | 59.12 |
| Americas | 2 | 8.40% | 54.2 | 53.51 | 55.62 |
| -North America | 11.60% | 36.7 | 36.47 | 38.87 | |
| -South America | 5.90% | 16.1 | 16.01 | 16.05 | |
| -Rest of Americas | 2.50% | 1.5 | 1.03 | 2.70 | |
| Europe | 4 | 4.30% | 23.7 | 22.98 | 24.38 |
| -West/Central Europe | 5.70% | 18.4 | 18.33 | 18.46 | |
| -East/SE Europe | 2.30% | 5.3 | 4.65 | 5.92 | |
| Asia | 1 | 1.90% | 55.5 | 29.39 | 89.38 |
| GLOBAL | NA | 3.90% | 181.8 | 128.48 | 232.07 |
Rank of the overall region in terms of numbers of recently active cannabis users.
As for the western hemisphere, about 36.7 million recently active cannabis users are found in North America. An estimated 16.1 million are found in South America. The rest of the Americas have 1.5 million. Within Europe and non-Asia former USSR jurisdictions, Western Europe has an estimated 23.7 million recently active cannabis users, while East/Southeast Europe has 5.3 millions [10].
Corresponding prevalence proportions for these regions also may be of interest, and these estimates also are shown in Table 1. In Oceania, the estimated prevalence of recently active cannabis use is 10.7%. In Africa, it is 7.5%. Prevalence in North America (11.6%) exceeds the estimate for the South American countries (5.9%), and there is a 2.5% estimate for the rest of the Americas. Prevalence in West/Central Europe is 5.7%, and in South/Southeast Europe it is 2.3%. The estimate for Africa is 7.5%, and that for Asia is 1.9%. Globally, the estimated proportion with recently active cannabis smoking is 3.9% [10].
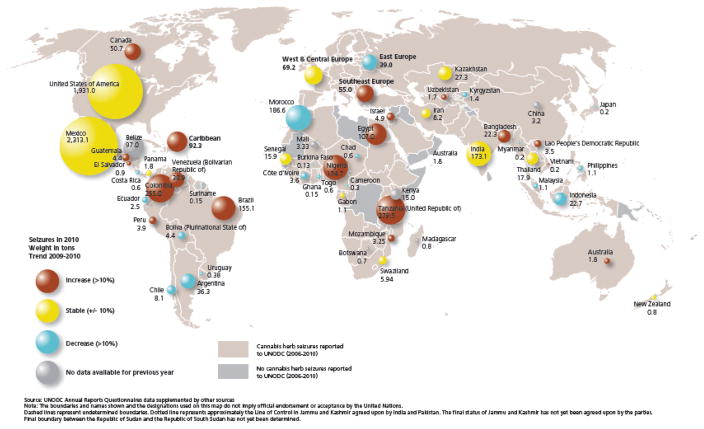
It sometimes is thought that the size of markets for cannabis and other drugs now regulated under the international psychotropic drugs conventions can be gauged in relation to drug law enforcement statistics, which are more readily obtained as ‘official statistics’ than is the case for estimates from population surveys. Fig. (1) suggest otherwise, although its relatively ranking of regions has some congruence with the estimated numbers and prevalence just summarized. For the time being, population surveys seem to provide a more complete and accurate reflection of drug demand, even though there clearly are issues of under-reporting and inaccuracy in the survey-based estimates [27–29].
Figure 1.
Distribution of and trends in official statistics about law enforcement seizures of cannabis (herb product) by country of the world. (Source: United Nations. Office of Drugs and Crime. World Drug Report 2012. United Nations Publication, https://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf)
2.3 Location in terms of characteristics of the susceptible host (i.e., ‘person’ characteristics’)
Age
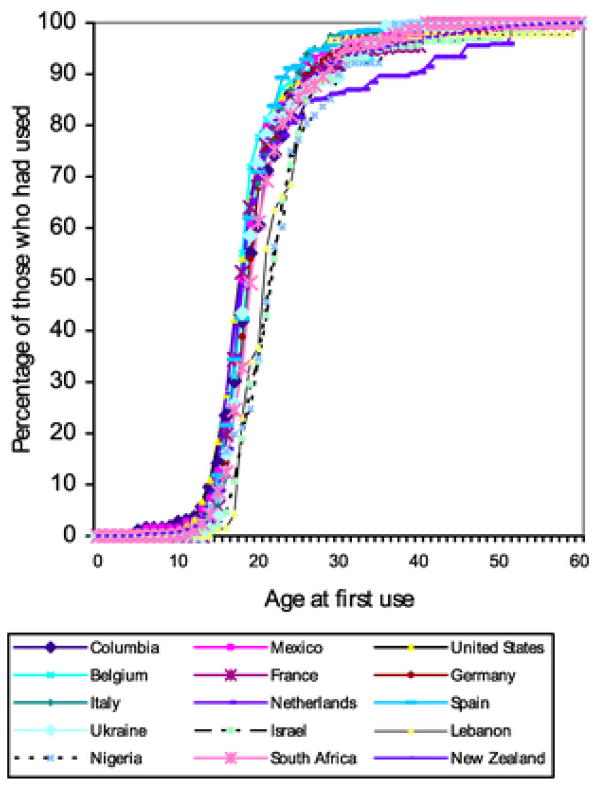
Age in years (or alternately, one’s birth year) was discussed in Section 4.2 as a major correlate of cannabis smoking. Fig. (2), from Degenhardt et al. [23], age-specific estimates from multiple countries surveys by the World Mental Health Surveys Consortium. It seems that countries vary somewhat in how early in life cannabis use begins (typically in adolescent years), as well as the fact that few newly incident cannabis users are found after age 35 years.
Figure 2.
Estimated age of onset distributions for new initiates of cannabis use, by countries participating in the World Mental Health Surveys project. (Source: Degenhardt et al., 2008).
Sex
Concurrent with a narrowing of male-female differences in the frequency and occurrence of alcohol drinking and tobacco smoking in many countries of the world, in recent decades there has been a reduction of the traditional male excess in cannabis smoking. Nevertheless, in virtually every country surveyed in adolescence or the young adult years, there continues to a male excess in both incidence and prevalence of cannabis smoking. An exception is the United States, where there appears to be male-female parity during the adolescent years, at least with respect to becoming a newly incident cannabis user in community samples of adolescents [30]. The school survey data from the US and Europe continue to show male excess in frequency and occurrence of cannabis smoking at mid-adolescence [22, 26].
Ethnicity
Cannabis use patterns can be manifestations of culture and ethnicity within countries. For example, early in the 20th century, Hispanics in the US were over-represented among cannabis smokers, but this ethnicity pattern no longer seems to be the case. Routinely, non-Hispanic Whites are found to have larger prevalence proportions and incidence estimates, as compared with other ethnicity subgroups within the US [31, 32]. Of course in some places, ethnicity, religion, and cultural practices are tightly intertwined. To illustrate, in Jamaica, there is a tight connection between cannabis smoking and being Rastafarian [33].
Other Host Characteristics
In many parts of the world, one of the most ‘sturdy’ correlates and predictors of cannabis use is a childhood history of socially maladaptive behavior, sometimes with serious conduct problems, defined to encompass not living up to the social role expectations of others, such as family members, teachers, the police, and society at large. Originally observed as a cross-sectional relationship with uncertainty about temporal sequencing, this association now has more than a half-century of supportive evidence from longitudinal and prospective studies [34], as well as especially important implicative evidence from randomized prevention trials in which the intervention seeks to reduce early social maladaptation [35, 36].
One unexplored explanation for this association involves its potential mediation via person-to-person spread or diffusion of cannabis exposure opportunities within clusters of socially maladaptive peers (i.e., the ‘birds of a feather flocking together’ phenomenon). In cannabis epidemiology, ‘exposure opportunity’ or the timing of the first chance to try cannabis is a necessary but not sufficient condition for cannabis use [37], and it is one that does not occur at random within populations of the world. Wagner & Anthony [38] studied conditions and processes leading to cannabis exposure opportunities and subsequent cannabis use, and learned that users of alcohol and tobacco are more likely to have a chance to try cannabis, which helps explain a so-called ‘gateway’ sequence in which use of internationally regulated psychoactive drugs is preceded by use of psychoactive drug compounds not regulated by international treaty agreements. Wells and colleagues [39] summarized evidence on the possibility that male-female differences in the occurrence of cannabis use actually can be traced back to male-female differences in cannabis exposure opportunities, age by age, with some supportive evidence. They also found some inconsistencies, which might have to do with country-specific circumstances.
The research on ‘exposure opportunities’ intersects with a longer tradition of research on peer and familial influences on cannabis use. The most recent empirical evidence on these topics suggests that inept parental monitoring and supervision might foster affiliation with deviant and drug-using peers, through which cannabis ‘exposure opportunities’ may occur, followed by newly incident cannabis use, or by refusals [40,41]. Of course, peer influence also may be influential in the process of persuading a young person to try cannabis, and in providing social reinforcers that increase the probability of repetitive cannabis use as well.
The degree to which international, federal, state, and local laws and regulations shape the incidence or prevalence of cannabis use is subject to debate. Within the US, there are reports of increasing prevalence after cannabis laws at the state level have been liberalized, as well as contradictory reports of no change [42–44]. Under these circumstances, no clear conclusion is possible at present [45, 46].
An intriguing new report about trends of perceived harmfulness of cannabis use deserves comment. It provides epidemiological evidence that young people’s sense of cannabis harmfulness started to decline as early as 1991, well before the vanguard medical marijuana (MM) states started to amend their state laws and to allow medical marijuana use. For example, California was a leading vanguard medical marijuana state, and its proposition to enable MM was enacted in 1996, well after the trend line for decline in perceived harmfulness had started [47].
Another interesting observation from that same study of trends is something that might have been predicted with ease. Namely, when penalties for simple possession and use of cannabis are relaxed at the state level, and allowances for ‘legal’ use of cannabis are made (e.g., in the form of medical marijuana), it stands to reason that one of the major sources of harm from using cannabis once or twice, or even regularly, is diminished - i.e., a source in the form of criminal arrest, prosecution, and sometimes harsh penalties that thwart educational attainment and employment [47]. Accordingly, it is not terribly surprising to see appreciable downward trends in perceived harmfulness of cannabis use in the states that have liberalized their cannabis policies, with removal of criminal penalties and reduced law enforcement pressure on users not engaged in cannabis supply and distribution to others, and with medical marijuana possibilities created when previously there had been none.
Review
All of the hypothesized correlates, predictors, and explanatory variables can be sorted along an ecological scale that runs from the international regulations at the macroscopic level down to the possible influence of genetic susceptibility traits observable at the microscopic level, with additional meso-level conditions and processes in the form of interpersonal relationships between peers and within families (e.g., with respect to adept parenting). It now is possible to catalog these suspected determinants, but there now is no over-arching integrated theory or synthesis as would be needed to specify theoretical propositions or to guide lines of epidemiological research intended to distinguish what is a mere correlate or predictor of cannabis use from what qualifies as a truly causal influence, or as what might convey protection. For this reason, the next sections of this review articles are relatively concise.
3. The Third Rubric: Causes (Why?)
All of the above-listed dimensions or facets of time, place, and personal characteristics are entertained as potential causal or protective influences with respect to explaining what accounts for variation in incidence rates for cannabis use, and why some individuals in the population are becoming newly incident users while others never start. That is to say, all truly protective factors, if they convey protection, deserve to be counted in the array of causal influences. By definition, protective factors cause a reduced risk of becoming a newly incident cannabis user. According to general principles of epidemiology [48], if we were to take them away, or nullify their effects, then population incidence rates of cannabis use should increase.
The most strongly implicated and sturdily replicated causal influence already has been mentioned - namely, the early characteristic of socially maladaptive behavior or conduct problems manifest in childhood, well before onset of cannabis or other drug use. The definitiveness of the evidence on this hypothesized causal influence is enhanced by a series of prevention experiments in which experimental manipulations to promote socially adaptive behavior and to discourage social maladaptation during the primary school years have been followed by sustained reductions in incidence and persistence of cannabis use [34, 35].
Cannabis ‘exposure opportunities’ have been mentioned as necessary but not sufficient pre-conditions for occurrence of newly incident cannabis use. No research is needed to substantiate the idea that cannabis must be available in the environment; all individuals must have a cannabis exposure opportunity before cannabis use can occur [37].
Otherwise, the literature under this rubric is patchy with insufficient replication evidence to merit detailed coverage. There is a handful of longitudinal and prospective studies of newly incident cannabis users, but the guiding conceptual models generally do not have much overlap, and the literature contains too few estimates from comparable studies for systematic review or a basic meta-analysis. That is to say, there are many different kinds of studies, with a range from convenience samples to school survey samples to properly conducted studies of all persons in probability samples of a pre-defined and epidemiologically credible population, with designs of the case-control variety, cross-sectional surveys, longitudinal follow-up studies, and even prevention experiments. But the heterogeneity of populations and study designs makes it difficult to conduct proper systematic reviews or meta-analyses at this point in time. In one of its main conclusions, a just-completed and very ambitious systematic review on the topic of cannabis use and cannabis dependence illustrates the complex challenges faced when trying to forge chains of inference from heterogeneous sources of evidence: “…, the link between cannabis dependence and predisposing factors could not be resolved convincingly by most studies due to methodological weaknesses regarding dependence criteria [49].
As it happens, most studies seeking causes have focused attention on prevalence of cannabis use. Due to failure to separate out the force of incidence from duration and prevalence, studies of cannabis prevalence do not have the resolving power to discriminate conditions that cause cannabis use to start in the first place from the conditions that foster persistence of cannabis use, once it starts.
In this context, it is noteworthy that the concentration of delta-9 tetrahydrocannabinol in a cannabis preparation, or the relative balance of THC versus other cannabinoids, might well be an important determinant of whether cannabis use persists once it starts. This proposition is supported by evidence that THC effects can be modulated by cannabinoids and possibly other chemicals in the cannabis preparation [50]. Whereas facets of cannabis compounds are likely to be influential in whether a newly incident cannabis user persists in use of the drug, they are not central in determining whether someone becomes a first-time user of the drug.
Much the same can be said for route of administration, except in an indirect sense that individuals who tell their peers that they do not like to smoke tobacco because it makes them cough too much or because they have respiratory problems might never be presented with a chance to try cannabis in the smoked form. Increased availability of oral dosage forms (e.g., candies, cakes containing cannabinoids) might be followed by increased incidence rates of cannabis products in this form, and will become deserving of scrutiny as a causal influence with respect to population-level incidence rates.
The present state of evidence about causes of becoming a newly incident cannabis user lags behind what has been discovered about causes of becoming a newly incident tobacco cigarette smoker or the causes of becoming a newly incident drinker of alcoholic beverages. At present, there is no compelling integrative conceptual model or theoretical formulation as might serve to create a systematically applied template in a series of studies that shed light on the issue of replicability.
One of the complexities faced when attempting to study the causes of cannabis use is that virtually all cannabis smokers (the most prevalent form of cannabis use to date) also are tobacco smokers, with tobacco onsets either prior to or concurrent with cannabis onsets, although there are some exceptions [51]. In consequence, the causal determinants of cannabis smoking have been bundled tightly with the causal determinants of tobacco smoking, and the world literature on newly incident cannabis smoking does not yet include many new initiates who have no past history of tobacco smoking, for whom the causal determinants might be different. This situation may change if there are continuing reductions in tobacco smoking, if worldwide use of cannabis shifts away from the practice of combining tobacco with cannabis herb or resin while smoking both compounds concurrently, and if non-smoking products are introduced and adopted with greater frequency.
There is a growing body of evidence on the occurrence of cannabis dependence, for which cannabis use is a necessary cause, as well as suspected consequences of cannabis use such as psychoses of otherwise unknown etiology and car crashes. In writing of this article, a decision was made to consider this material under the fourth rubric, mechanisms, in order to focus the rubric of causes on the main topic under study - namely, cannabis use. The placement of this material under the rubric of mechanisms can be justified in relation to the conceptual model just outlined, with a longitudinal sequence leading from the first cannabis exposure opportunity to first cannabis use, and then onward longitudinally toward identified (but not inevitable) causal consequences such as cannabis dependence, as well as suspected consequences about which we do not yet have definitive evidence (e.g., idiopathic psychosis experiences, car crashes). A comprehensive understanding of the causes of cannabis dependence, psychoses attributed to cannabis use, car crashes attributed to cannabis use, and the like, will require attention to the temporally upstream questions about causes of cannabis use. Otherwise, the evidence will fall short of a definitive quality. For example, it is quite plausible that the same conditions and processes that influence timing of the first cannabis exposure opportunity also influence whether a cannabis-associated car crash occurs (e.g., higher levels of openness to experience traits coupled with low levels of harm avoidance). Similarly, in virtually all of the published evidence on the suspected causal linkage from cannabis use to otherwise idiopathic psychoses, there have been unanswered questions about why the psychosis-affected case was using cannabis in the first place, and whether the explanation for cannabis use also might explain the appearance of a psychosis experience. If so, the explanation for the cannabis use qualifies as an uncontrolled confounding variable that actually might be the determinant of the otherwise unexplained psychosis experience. These complexities involve longitudinal sequences of states and processes that unfold over time, as is true for ‘mechanisms’ in epidemiology, and definitive evidence about these sequences cannot be produced by epidemiology’s standard case-control or prospective cohort research designs for simple cause-effect X→Y relationships. We are not the first, and most likely will not be the last, to criticize the body of evidence on cannabis consequences [52].
4. The Fourth Rubric: Mechanisms (How?)
The topic of mechanisms leading to and beyond newly incident cannabis use already has been mentioned in the context of the second rubric and its evidence on the ‘gateway sequence’ as well as the fact that, by logic, a chance to try cannabis must occur before first-time use of cannabis can start. Epidemiology’s work under the rubric of mechanisms for any condition tends to start with the condition, and then attempts are made to work backward toward precursor conditions and processes that qualify as facets of mechanism, as well as forward in time from precursors or predictors to cannabis use.
In that the study of mechanisms in epidemiology also includes secondary consequences, comorbidities, and residual disabilities or terminal outcomes, this rubric encompasses hypotheses about whether cannabis use or any of its consequences is genetically mediated. Work under this rubric often is longitudinal because the goal includes tracing the pathways, direct and indirect, through which cannabis use starts to occur and then cascades into later sequelae.
Before turning to consequences, disability, and terminal outcomes such as premature death, a note about contagion and person-to-person diffusion of cannabis use should be made. Given research on peer influence on starting to use cannabis, one might think that there would be a strong tradition of epidemiological research on contagion processes and person-to-person diffusion. In fact, there are a few studies in this arena, all supportive of the idea that newly incident cannabis use occurs in local area clusters [53, 54], but too few to provide a systematic review. Bobashev & Anthony [53] raised the possibility that local area clustering of perceptions about harmfulness of cannabis use might foster a sociocultural neighborhood environment that is conducive to higher or lower incidence rates for cannabis use (i.e., higher if it is judged to be not risky; lower if it is judged to be quite risky). Parker & Anthony [54] have taken that work a step forward, and evaluated the degree to which cannabis risk perceptions among high school seniors in one calendar year’s graduating class might influence the clustering and occurrence of newly incident cannabis use in the next calendar year’s graduating class. They found evidence of clustering of newly incident cannabis use within schools, albeit at a modest level, and they also found evidence that occurrence of newly incident cannabis use depends upon and might be caused by the cannabis risk perceptions of the graduating class of the prior year.
When we turn from the precursors and beginnings that lead toward cannabis use in the direction of its consequences, we first must consider a consequence for which cannabis use is a necessary cause -- namely, the cannabis dependence syndrome and allied cannabis use disorders. With origins in the early-mid-1980s when the first epidemiological study on this topic was completed [1], the accumulated body of evidence from this line of research is surprisingly modest. Since the mid-1980s, we have no more than three large sample epidemiological studies have produced 360-degree estimates on the transition probability for becoming cannabis dependent once cannabis use starts. Two studies of nationally representative US samples found that roughly one in 9–11 cannabis users develop the disorder after onset of use [55, 56]. In the third, a European study of cannabis users in a region of Germany, there is a suggestion of a slightly lower transition probability, but there are methodological concerns to make interpretation of this estimate somewhat problematic, such as focus on a specific cohort in a specific region, rather than national representation across a broad range of cohorts [57]. The transition probability in Australia also might be greater than is observed in the US, but the available evidence from Australia now speaks only to recently active users with no coverage of past histories [58], as was the case in the 1991–92 US National Longitudinal Alcohol Epidemiologic Survey and the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions [59].
An important issue of polydrug use recently surfaced in relation to these published estimates on cannabis dependence transition probabilities. Namely, in a new analysis of data from prior US national samples, there is a suggestion that the risk of becoming cannabis dependent is markedly lower when there is no concurrent or subsequent use of other internationally regulated drugs such as heroin, with a cumulative incidence proportion perhaps as low as two percent when ‘cannabis only’ users are studied [60].
Drawing analogies with study of the median incubation period after effective contact with a communicable disease agent, drug epidemiologists have investigated how quickly the dependence syndrome emerges after first use of each drug compound. For example, the transition from initial cocaine use to onset of cocaine dependence occurs rapidly such that about 5%–6% become cocaine dependent within 1–2 years after first cocaine use; then risk subsides. In contrast, this transition from cannabis use to dependence does not have peak risk during the first 1–2 years after first use. Rather, the risk estimates for Years 1–2 after first use of cannabis appear to be at 1%–2% or a somewhat smaller value, with a peak reached some 3–5 years after initial use [37]. [The estimate of one case for every 9–10 cannabis users is a cumulative incidence proportion that is built up over time, during each passing year after first cannabis use, among those with persistent cannabis use beyond the initial year, and often with concomitant use of other internationally regulated drugs [37,55,56,60].
Other consequences of cannabis use touched on in multiple papers include general medical conditions and mental health outcomes, recently reviewed by Hall [61]. There is a general acknowledgment that risk of an acute panic attack or anxiety state might be elevated soon after onset of first cannabis use, but with little evidence that cannabis use causes panic disorder or other anxiety disorders [62]. There is some equivocation about whether depressed mood precedes or follows cannabis use [63], as well as mixed evidence on the possibility that cannabis use might be causing schizophrenia or idiopathic psychosis (IP) cases that otherwise would not occur [64,65].
At present, three main camps or points of view have emerged in relation to these cannabis-schizophrenia and cannabis-IP linkages. For the first camp, the jury is still out, and there is insufficient evidence to be confident that cannabis use is causing schizophrenia or IP to occur - due primarily to an incapacity to be confident that there has been a thorough rule-out of the previously mentioned confounding (shared) determinants, as well as pre-existing schizophrenia or IP proneness. For the second camp, the judgment is one of guilt, and there is a firm belief that cannabis does cause severely disabling cases of schizophrenia or IP to occur that otherwise would not occur. The third camp declines to make a causal inference based on the evidence, but holds that the possibility and plausibility of a cannabis-schizophrenia or cannabis-IP link are reason enough to maintain the current regulatory scheme, with criminal penalties used to discourage cannabis use until and unless uncertainties about toxicity can be cleared up.
There is a growing body of literature on cannabis use as a contributor to the global burden of disease and disabilities (e.g., as a cause of car crashes, ibid. 60), but in many respects the estimates rest upon unstable footing, and are being improved upon in ways that help isolate effects of cannabis [66]. For this reason, this review article will not provide a summary of the available estimates, and instead will refer readers to the primary articles where the caveats are stated clearly [66–72]. One fairly robust conclusion from these estimates is that cannabis use is a relatively trivial contributor to the global burden of disease and disability, as compared to use of tobacco and use of alcoholic beverages [66].
5. The Fifth Rubric: Prevention and control (What Can We Do?)
Separate review articles in this volume are dedicated to prevention and control of cannabis use and its consequences in the form of cannabis dependence and related syndromes. Nevertheless, several recent contributions of empirical evidence stand out and deserve special attention. For example, the promise of university-community partnerships that build from more than 100 years of experience with US land grant ‘extension services’ now is being realized in the domain of cannabis use prevention - i.e., with experimentally induced reduced risks of becoming newly incident cannabis users during adolescence [73,74]. These new directions for prevention of cannabis use in adolescence assume greater importance when viewed in light of generally weak effects of traditional models of school-based prevention programming (without involvement of community and families), as disclosed in recent systematic reviews of prevention research [75].
‘Control’ in the public health context encompasses systematic activities such as disruption of network spread of health problems and behavior that otherwise multiply as ‘after-effects of prior effects’ - i.e., as in behavioral contagion [76]. Whereas there are few school-based programs that can claim efficacy or effectiveness with respect to these network disruptions and subsequent reduced cannabis incidence rates, there are observational studies that suggest some promising new directions for public health work along these lines [40].
‘Control’ also encompasses public health outreach and early intervention efforts intended to reduce the duration of cannabis dependence and related problems, even when progression from initial use toward these problems has not been thwarted. For many years, progress in the field of treating cannabis dependence was retarded by a widespread belief that ‘cannabis dependence’ syndromes did not exist. This belief continues to be challenged by emerging evidence on the coherence of the dependence syndrome and related conditions such as ‘cannabis use disorder’, the biological plausibility of these conditions, and their clinical implications [77–85]. Early forms of cannabis dependence treatment were introduced, as described in a review article by Budney and colleagues [86], published more than a decade ago. More recent refinements have produced some successes, as well as some noteworthy disappointments [87–89]. Clearly, more research in this domain is needed, as illustrated in a just-published study on computer-assisted behavioral therapy and contingency management approaches in efforts to reduce duration and to promote the amelioration of cannabis use disorders [90].
This overview of prevention and control methods as applied to cannabis would be incomplete without mention of ‘supply reduction’ initiatives, often with larger shares of national budgets than has been the case for ‘demand reduction’ initiatives of the type just mentioned. Against a backdrop of uncertainty about the relative effects of attempts to control supply versus attempts to achieve demand reduction [91], there are strongly held views about the importance of sustained efforts to eradicate or substitute crops in place of cannabis, to control the borders in order to reduce clandestine importation, and to restrict supplies while increasing costs of herbal cannabis, cannabis resin, and an increasing number of synthetic cannabinoids. Efforts to block or to change medical marijuana laws or to retard public enthusiasm for liberalized cannabis policies also can be understood as a manifestation of continuing concern about externalities to be faced if liberalized policies are followed by increases in incidence rates for cannabis use and dependence, duration of these conditions, and other challenges such as possibly increased numbers with early-onset adolescent cannabis involvement. There is much room for debate, but meagre evidence for policy analysis, as discussed in Section 4.2.
Conclusions
One conclusion from this selective review of epidemiological evidence on cannabis is the same conclusion one would draw from a more comprehensive review. Given the current regulatory environment coupled with what often are highly polarized viewpoints about cannabis policy, our governmental priorities for cannabis research often have been oriented toward discovery of initial threads of evidence about hazards of cannabis use, and toward production of statistical ‘report cards’ on how many people are using cannabis, issued every year or so. Especially during difficult economic times with belt-tightening for science funds, there has been considerable reluctance to spend tax revenues on in-depth studies of potential medical benefits of cannabinoids, or to insist upon an even-handed and thorough work-up of each hint that cannabis might be causing serious harm, with a systematic approach to reproducibility and replication, and with remedies for deficiencies already noted [52]. A widely held point of view is that we do not need more research on a drug we already consider to be harmful (or harmless).
Meanwhile, the stream of cannabinoid product innovations from research and development activities of an increasingly entrepreneurial private sector will not stop any time soon. Since government cannot be counted upon to satisfy consumer demand for balanced points of view and truly definitive evidence on these new cannabinoid products (nor the old products), with respect to both benefits and harms, it may be time to look toward creation of new institutions such as an independent International Cannabis Products Safety Commission. This conclusion seems self-evident, given the political realities just described, and given a realization that members of the 21st century cannabis-consuming public do not trust what government or government-funded agencies say about these products. With respect to evidence from government-funded research on cannabis, many cannabis consumers apparently think that ‘the system is rigged.’
It is beyond the scope of this review article to provide anything more than the germ of an idea for an International Cannabis Products Safety Commission that will pay attention to fundamental principles of design, research, and development in the pharmaceutical sciences. When electricity was being harnessed, and electricity-powered machinery and consumer products were being developed, the independent not-for-profit Underwriters’ Laboratories (UL) and allied institutions were organized to evaluate product hazards, to furnish information about these hazards, and to protect buyers as well as the public at large and the insurance companies liable for coverage of damages due to electricity-attributable fires and product defects. The UL model, or one of the more recently developed social enterprise models such as the benefit corporation, might be useful in creation of a cannabis safety product safety commission that has a legal obligation to promote the public interest in these matters [92, 93]. With or without change in the current traditions of government-supported public health research on cannabis products, ideas for non-governmental organizations along these lines deserve consideration.
Acknowledgments
Support, and Credits: The authors wish to acknowledge support from the Michigan State University and the National Institutes of Health for preparation of this manuscript. JCA’s work on the article has been supported by an NIH/National Institute on Drug Abuse Senior Scientist and Mentorship Award (K05DA01579), while the NIH/NIDA support of OA and CLQ has been through an institutional research training program award (T32DA021129). In addition, during completion of the article, OA received a K99 career development award from the NIH National Center for Complementary and Integrative Health (K99AT009156). Finally, we note that some of the ideas and material covered in this article have appeared in prior articles and chapters, especially the concept of the ‘rubrics’ organization of epidemiological evidence, as well as some of the thoughts specific to cannabis epidemiology [e.g., as in Anthony JC, Alshaarawy O, Lopez-Quintero C. International Trends in Cannabis Use. In: Winters KC & Sabet KA (Editors), Contemporary Health Issues On Marijuana. New York: Oxford University Press. In press.] Necessarily, this article and any of our chapters on the topic of international trends in epidemiological evidence on cannabis use will include similar ideas. Nonetheless, the expression of the ideas will be seen to be different across these contributions, different words are being used to express similar thoughts, and new ideas have been added to this article.
References
- 1.Anthony J, Helzer J. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York: Free Press; 1991. pp. 116–54. [Google Scholar]
- 2.Holst P. On the Statistics of the Insane, Blind, Deaf and Dumb, and Lepers, of Norway. Journal of the Statistical Society of London. 1852;15:250–6. [Google Scholar]
- 3.Cheng HG, Cantave MD, Anthony JC. Taking the first full drink: Epidemiological evidence on male-female differences in the United States. Alcohol Clin Exp Res. 2016;40:816–25. doi: 10.1111/acer.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruenberg EM. A review of mental health in the metropolis: The Midtown Manhattan Study. Milbank Memorial Fund Quarterly. 1963;16:77–93. [PubMed] [Google Scholar]
- 5.Streiner DL, Patten SB, Anthony JC, Cairney J. Has ‘lifetime prevalence ‘reached the end of its life? An examination of the concept. International Journal of Methods in Psychiatric Research. 2009;18:221–8. doi: 10.1002/mpr.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairman BJ. Trends in registered medical marijuana participation across 13 states and District of Columbia. Drug Alcohol Depend. 2016;159:72–79. doi: 10.1016/j.drugalcdep.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin LA, Ilgen MA, Jannausch M, Bohnert KM. Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addict Behav. 2016;61:99–103. doi: 10.1016/j.addbeh.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S. Cannabinoids for medical use: a systematic review and meta-analysis. Jama. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 9.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, US, 2014. American journal of preventive medicine. 2016;50:1–8. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 10.United Nations Office on Drugs and Crime. World drug report 2015. United Nations Publications. Vienna: UNODC; 2015. [Google Scholar]
- 11.Abel EL. Marihuana: the first twelve thousand years. New York: Plenum Press; 1980. [Google Scholar]
- 12.Moreau JJ. Du hachisch et de l’aliénation mentale: études psychologiques. Librairie de Fortin; Masson et C: 1845. [Google Scholar]
- 13.Green J. Spoonful of Paradise. The Guardian. 2002 Oct 11; Available from: www.theguardian.com/books/2002/oct/12/featuresreviews.guardianreview34.
- 14.Green J. Cannabis. New York: Thunders Mouth Press; 2002. [Google Scholar]
- 15.Musto DF. Opium, cocaine and marijuana in American history. Scientific American. 1991;265:40–47. doi: 10.1038/scientificamerican0791-40. [DOI] [PubMed] [Google Scholar]
- 16.Wood HC. On the medical activity of the hemp plant, as grown in North America. Proceedings of the American Philosophical Society. 1869;11:226–232. [Google Scholar]
- 17.Republic of India Indian Hemp Drug Commission. Report of the Indian Hemp Drug Commission 1893–1894. New York: Johnson Reprint Corporation; Reprint. (Originally issued by the Government of India Central Printing Office in Simla) [Google Scholar]
- 18.Shamir R, Hacker D. Colonialism’s civilizing mission: the case of the Indian Hemp drug commission. Law & Social Inquiry. 2001;26:435–461. [Google Scholar]
- 19.Wallace GB, Cunningham EV. The Marihuana Problem in the City of New York; Sociological, Medical and Pharmacological Studies. Lancaster, Pennsylvania: The Jacques Cattell Press; 1944. [Google Scholar]
- 20.Le Dain Commission. Final Report of the Commission of Inquiry into the Non-Medical Use of Drugs. Ottawa: Information Canada; 1973. [Google Scholar]
- 21.Shafer A, Marihuana RPa. United States. Commission on Marihuana and Drug. Signal of Misunderstanding; First Report of the Commission, March 1972. US Government Printing Office; 1972. [Google Scholar]
- 22.Anthony JC, Alshaarawy OS, Lopez-Quintero C. International Trends in Cannabis Use. In: Winters KC, Sabet KA, editors. Contemporary Health Issues on Marijuana. New York: Oxford University Press; In press. [Google Scholar]
- 23.Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leinweber JP, Cheng HG, Lopez-Quintero C, Anthony JC. Regional and State Variations of Newly Incident Cannabis Use in the United States, 2002–2011. doi: 10.7717/peerj.3616. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibell B, Guttormsson U, Ahlström S, et al. Substance use among students in 36 European Countries. The Swedish Council for Information on Alcohol and other Drugs (CAN), The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Council of Europe, Co-operation Group to Combat Drug Abuse and Illicit Trafficking in Drugs (Pompidou Group); 2012. The 2011 ESPAD report. [Google Scholar]
- 26.Johnston LD, O’Malley PM, Miech RA, Bachman J, Schulenberg JE. Monitoring the Future national survey results on drug use. Ann Arbor: Institute for Social Research, The University of Michigan; http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. [Google Scholar]
- 27.Hickman M, Taylor C, Chatterjee A, et al. Estimating drug prevalence: review of methods with special reference to developing countries. UN Bulletin of Narcotics. 2003;54:15–32. [Google Scholar]
- 28.Degenhardt L, Cheng H, Anthony JC. Assessing cannabis dependence in community surveys: methodological issues. Int J Methods Psychiatr. 2007;16:43–51. doi: 10.1002/mpr.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibell B, Molinaro S, Siciliano V, Kraus L. The ESPAD Validity Study in four countries in 2013. Available from: www.espad.org/Uploads/ESPAD_reports/2015/ESPAD%20Validity%20Study.PDF.
- 30.Seedall RB, Anthony JC. Risk estimates for starting tobacco, alcohol, and other drug use in the United States: male-female differences and the possibility that ‘limiting time with friends’ is protective. Drug Alcohol Depend. 2013;133:751–3. doi: 10.1016/j.drugalcdep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United States, 2014, Department of Health and Human Services; Center for Behavioral Health Statistics and Quality, editor. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Substance Abuse and Mental Health Services Administration; Rockville: 2014. [Google Scholar]
- 32.Lopez-Quintero C, Xue W, Fairman B, Cheng H, Brossig R, Anthony JC. Born in the USA: Estimated Annual Incidence Rates for Use of Cannabis, Alcohol and Tobacco in the 21st Century. in preparation. [Google Scholar]
- 33.Hamid A. The Ganja Complex: Rastafari and Marijuana. Lanham: Lexington Books; 2002. [Google Scholar]
- 34.Robins LN. The natural history of drug abuse. Acta Psychiatrica Scandinavica. 1980;62:7–20. doi: 10.1111/j.1600-0447.1980.tb10426.x. [DOI] [PubMed] [Google Scholar]
- 35.Storr CL, Ialongo NS, Kellam SG, Anthony JC. A randomized controlled trial of two primary school intervention strategies to prevent early onset tobacco smoking. Drug and alcohol dependence. 2002;66:51–60. doi: 10.1016/s0376-8716(01)00184-3. [DOI] [PubMed] [Google Scholar]
- 36.Furr-Holden CD, Ialongo NS, Anthony JC, Petras H, Kellam SG. Developmentally inspired drug prevention: middle school outcomes in a school-based randomized prevention trial. Drug Alcohol Depend. 2004;73:149–58. doi: 10.1016/j.drugalcdep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Van Etten ML, Anthony JC. Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. Journal of women’s health & gender-based medicine. 2001;10:797–804. doi: 10.1089/15246090152636550. [DOI] [PubMed] [Google Scholar]
- 38.Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. American Journal of Epidemiology. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- 39.Wells JE, Haro JM, Karam E, et al. Cross-national comparisons of sex differences in opportunities to use alcohol or drugs, and the transitions to use. Substance use & misuse. 2011;46:1169–1178. doi: 10.3109/10826084.2011.553659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdzovic Andreas J, Pape H. Who receives cannabis use offers: A general population study of adolescents. Drug Alcohol Depend. 2015;156:150–6. doi: 10.1016/j.drugalcdep.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Burdzovic Andreas J, Pape H, Bretteville-Jensen AL. Who are the adolescents saying “No” to cannabis offers. Drug Alcohol Depend. 2016;163:64–70. doi: 10.1016/j.drugalcdep.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Wall MM, Poh E, Cerdá M, Keyes KM, Galea S, Hasin DS. Adolescent marijuana use from 2002 to 2008: higher in states with medical marijuana laws, cause still unclear. Annals of epidemiology. 2011;21:714–716. doi: 10.1016/j.annepidem.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynn-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. Am J Public Health. 2013;103:1500–6. doi: 10.2105/AJPH.2012.301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasin DS, Wall M, Keyes KM, et al. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry. 2015;2:601–8. doi: 10.1016/S2215-0366(15)00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacula RL, Powell D, Heaton P, Sevigny EL. National Bureau of Economic Research, editor. Assessing the effects of medical marijuana laws on marijuana and alcohol use: The devil is in the details. 2013. [Google Scholar]
- 46.Wall MM, Mauro C, Hasin DS, et al. Prevalence of marijuana use does not differentially increase among youth after states pass medical marijuana laws: commentary on and reanalysis of US National Survey on Drug Use in Households data 2002–2011. International Journal of Drug Policy. 2016;29:9–13. doi: 10.1016/j.drugpo.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyes KM, Wall M, Cerdá M, et al. How does state marijuana policy affect U.S. youth? Medical marijuana laws, marijuana use and perceived harmfulness: 1991–2014. Addiction. 2016 doi: 10.1111/add.13523. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthony JC, Van Etten ML. Epidemiology and its Rubrics. In: Bellack A, Hersen M, editors. Comprehensive Clinical Psychology. Oxford: Elsevier Science Publications; 1998. pp. 255–380. [Google Scholar]
- 49.Schlossarek S, Kempkensteffen J, Reimer J, Verthein U. Psychosocial determinants of cannabis dependence: a systematic review of the literature. European addiction research. 2015;22:131–144. doi: 10.1159/000441777. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–74. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temple EC, Brown RF, Hine DW. The ‘grass ceiling’: limitations in the literature hinder our understanding of cannabis use and its consequences. Addiction. 2011;106(2):238–44. doi: 10.1111/j.1360-0443.2010.03139.x. [DOI] [PubMed] [Google Scholar]
- 53.Bobashev GV, Anthony JC. Clusters of marijuana use in the United States. Am J Epidemiol. 1998;148:1168–74. doi: 10.1093/oxfordjournals.aje.a009605. [DOI] [PubMed] [Google Scholar]
- 54.Parker M, Anthony JC. Cannabis Smoking Clusters within Secondary Schools: Results from the United States Monitoring the Future Study 1976–2013. In preparation. [Google Scholar]
- 55.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–68. [Google Scholar]
- 56.Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkonigg A, Goodwin RD, Fiedler A, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 58.McBride O, Teesson M, Slade T, Hasin D, Degenhardt L, Baillie A. Further evidence of differences in substance use and dependence between Australia and the United States. Drug and Alcohol Dependence. 2009;100:258–264. doi: 10.1016/j.drugalcdep.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291(17):2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Quintero C, Anthony JC. Drug use disorders in the polydrug context: new epidemiological evidence from a foodborne outbreak approach. Ann N Y Acad Sci. 2015;1349:119–26. doi: 10.1111/nyas.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 62.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: Results from a population-based sample. Eur Neuropsychopharmacol. 2016;26:493–505. doi: 10.1016/j.euroneuro.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 63.Fairman BJ, Anthony JC. Are early-onset cannabis smokers at an increased risk of depression spells? J Affect Disord. 2012;138:54–62. doi: 10.1016/j.jad.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. The Journal of nervous and mental disease. 1990;178:473–480. [PubMed] [Google Scholar]
- 65.Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68–71. doi: 10.1002/j.2051-5545.2008.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degenhardt L, Ferrari AJ, Calabria B, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8(10):e76635. doi: 10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry. 2016;3:251–64. doi: 10.1016/S2215-0366(15)00508-8. [DOI] [PubMed] [Google Scholar]
- 68.Erskine HE, Moffitt TE, Copeland WE, et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med. 2015;45:1551–63. doi: 10.1017/S0033291714002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study. The Lancet. 2013;387:2383–401. doi: 10.1016/S0140-6736(16)00648-6. [DOI] [PubMed] [Google Scholar]
- 70.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the global burden of disease study 2010. PLoS One. 2015;10:e0116820. doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whiteford H, Ferrari A, Degenhardt L. Global Burden Of Disease Studies: Implications For Mental And Substance Use Disorders. Health Affairs. 2016;35:1114–1120. doi: 10.1377/hlthaff.2016.0082. [DOI] [PubMed] [Google Scholar]
- 72.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. Global Burden of Mental, Neurological, and Substance Use Disorders: An Analysis from the Global Burden of Disease Study 2010. Chapter 2. In: Patel V, Chisholm D, Dua T, Laxminarayan R, Vos T, editors. Disease Control Priorities, (Volume 4): Mental, Neurological, and Substance Use Disorders. World Bank Publications; 2016. [PubMed] [Google Scholar]
- 73.Spoth R, Redmond C, Shin C, Greenberg M, Clair S, Feinberg M. Substance-use outcomes at 18 months past baseline: The PROSPER community–university partnership trial. American journal of preventive medicine. 2007;32:395–402. doi: 10.1016/j.amepre.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spoth R, Redmond C, Shin C, Greenberg M, Feinberg M, Schainker L. PROSPER community–university partnership delivery system effects on substance misuse through 6 1/2years past baseline from a cluster randomized controlled intervention trial. Preventive medicine. 2013;56:190–196. doi: 10.1016/j.ypmed.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faggiano F, Minozzi S, Versino E, Buscemi D. Universal school-based prevention for illicit drug use. Cochrane Database Syst Rev. 2014;(12):CD003020. doi: 10.1002/14651858.CD003020.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anthony JC. Deviant Peer Effects: Perspectives of an Epidemiologist. In: Dodge KA, Dishion TJ, Lansford JE, editors. Deviant Peer Influences in Programs for Youth: Problems and Solutions. New York: Guilford Press; 2006. pp. 44–66. [Google Scholar]
- 77.Beseler CL, Hasin DS. Cannabis dimensionality: dependence, abuse and consumption. Addict Behav. 2010;35:961–9. doi: 10.1016/j.addbeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Budney AJ. Are specific dependence criteria necessary for different substances: how can research on cannabis inform this issue? Addiction. 2006;(Suppl 1):125–33. doi: 10.1111/j.1360-0443.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 79.Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug Alcohol Depend. 2005;79:11–22. doi: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 80.Compton DR, Dewey WL, Martin BR. Cannabis dependence and tolerance production. Adv Alcohol Subst Abuse. 1990;9:129–47. doi: 10.1300/J251v09n01_08. [DOI] [PubMed] [Google Scholar]
- 81.Denson TF, Earleywine M. Pothead or pot smoker? A taxometric investigation of cannabis dependence. Subst Abuse Treat Prev Policy. 2006;1:22. doi: 10.1186/1747-597X-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillespie NA, Kendler KS, Neale MC. Psychometric modeling of cannabis initiation and use and the symptoms of cannabis abuse, dependence and withdrawal in a sample of male and female twins. Drug Alcohol Depend. 2011;118:166–72. doi: 10.1016/j.drugalcdep.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenberg MF, Anthony JC. Early clinical manifestations of cannabis dependence in a community sample. Drug Alcohol Dep. 2001;64:123–131. doi: 10.1016/s0376-8716(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 84.Vsevolozhskaya OA, Anthony JC. Transitioning from first drug use to dependence onset: Illustration of a multiparametric approach for comparative epidemiology. Neuropsychopharmacology. 2016;41:869–876. doi: 10.1038/npp.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vsevolozhskaya OA, Anthony JC. Estimated probability of becoming a case of drug dependence in relation to duration of drug-taking experience: a functional analysis approach. International Journal of Methods in Psychiatric Research. 2016 doi: 10.1002/mpr.1513. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Babbin SF, Stanger C, Scherer EA, Budney AJ. Identifying treatment response subgroups for adolescent cannabis use. Addict Behav. 2016;59:72–9. doi: 10.1016/j.addbeh.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Budney AJ, Stanger C, Tilford JM, Scherer EB, Brown PC, Li Z, Li Z, Walker DD. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol Addict Behav. 2015;29:501–11. doi: 10.1037/adb0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simpson AK, Magid V. Cannabis Use Disorder in Adolescence. Child and Adolescent Psychiatric Clinics of North America. 2016;25:431–443. doi: 10.1016/j.chc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Walker DD, Stephens RS, Towe S, Banes K, Roffman R. Maintenance check-ups following treatment for cannabis dependence. Journal of Substance Abuse Treatment. 2015;56:11–15. doi: 10.1016/j.jsat.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manski CF, Pepper JV, Petrie DV, editors. Informing America’s Policy on Illegal Drugs: What We Don’t Know Keeps Hurting Us. Washington, D.C: National Academies Press; 2001. [Google Scholar]
- 92.Peaslee WDA. The Standardization Activities of Underwriters’ Laboratories. The Annals of the American Academy of Political and Social Science. 1928;137:60–65. Retrieved from http://www.jstor.org/stable/1016768. [Google Scholar]
- 93.Cummings B. Benefit Corporations: How To Enforce A Mandate To Promote The Public Interest. Columbia Law Review. 2012;112(3):578–627. Retrieved from http://www.jstor.org/stable/23238440. [Google Scholar]