Abstract
Background and Objective
Accurately recording vaccine lot number, expiration date, and product identifiers, in patient records is an important step in improving supply chain management and patient safety in the event of a recall. These data are being encoded on two-dimensional (2D) barcodes on most vaccine vials and syringes. Using electronic vaccine administration records, we evaluated the accuracy of lot number and expiration date entered using 2D barcode scanning compared to traditional manual or drop-down list entry methods.
Methods
We analyzed 128,573 electronic records of vaccines administered at 32 facilities. We compared the accuracy of records entered using 2D barcode scanning with those entered using traditional methods using chi-square tests and multilevel logistic regression.
Results
When 2D barcodes were scanned, lot number data accuracy was 1.8 percentage points higher (94.3% to 96.1%, P < .001) and expiration date data accuracy was 11 percentage points higher (84.8% to 95.8%, P < .001) compared with traditional methods. In multivariate analysis, lot number was more likely to be accurate (aOR = 1.75; 99% CI, 1.57–1.96) as was expiration date (aOR = 2.39; 99% CI, 2.12–2.68). When controlling for scanning and other factors, manufacturer, month vaccine was administered, and vaccine type were associated with variation in accuracy for both lot number and expiration date.
Conclusion
Two-dimensional barcode scanning shows promise for improving data accuracy of vaccine lot number and expiration date records. Adapting systems to further integrate with 2D barcoding could help increase adoption of 2D barcode scanning technology.
Keywords: vaccines, immunization, automatic data processing, 2D barcode technology, electronic health record, technology adoption
Background and Objectives
The National Childhood Vaccine Injury Act (NCVIA), passed in 1986, requires that healthcare providers record certain data, including lot number, for all vaccines administered.[1] The American Academy of Pediatrics and the Centers for Disease Control and Prevention (CDC) recommend also recording additional data elements, including expiration date.[2,3] Despite these requirements and recommendations, in 2011 only 60% of Immunization Information System (IIS) vaccination records for children younger than six years of age included the vaccine lot number.[4] Evidence suggests that vaccine data elements recorded in EMRs are not always recorded accurately.[5–10] Missing and inaccurate data may be due in part to the fact that providers read and interpret printed lot number and expiration date on vaccine vials and then enter data manually into the electronic medical record (EMR).
Consistent and accurate documentation of vaccine lot number and expiration date in patient records is a first step in determining whether recalled or expired vaccines have been administered, identifying lots associated with adverse events for possible recall, and facilitating efficient and effective vaccine inventory management. In 2004, the U.S. Food and Drug Administration (FDA) passed a labeling rule requiring the placement of a machine-readable linear barcode containing the National Drug Code (NDC) on certain human drug and biological products, including vaccines, to reduce medication errors.[11] The NDC can be used to identify manufacturer (or labeler), vaccine product, and packaging; however, it cannot be used to determine lot number or expiration date.[12] Due to the importance of these additional data elements, the FDA published guidance in 2011 permitting vaccine manufacturers to request a waiver and replace linear barcodes with “alternative technology such as two dimensional symbology” capable of capturing additional data elements (Figure 1).[13] Two-dimensional vaccine barcodes encode NDC, lot number and expiration date, and as of July 2016, there were over 90 2D barcoded vaccines.[14]
Figure 1. Components of Linear Barcode and 2D Barcode on Vaccine Vials.
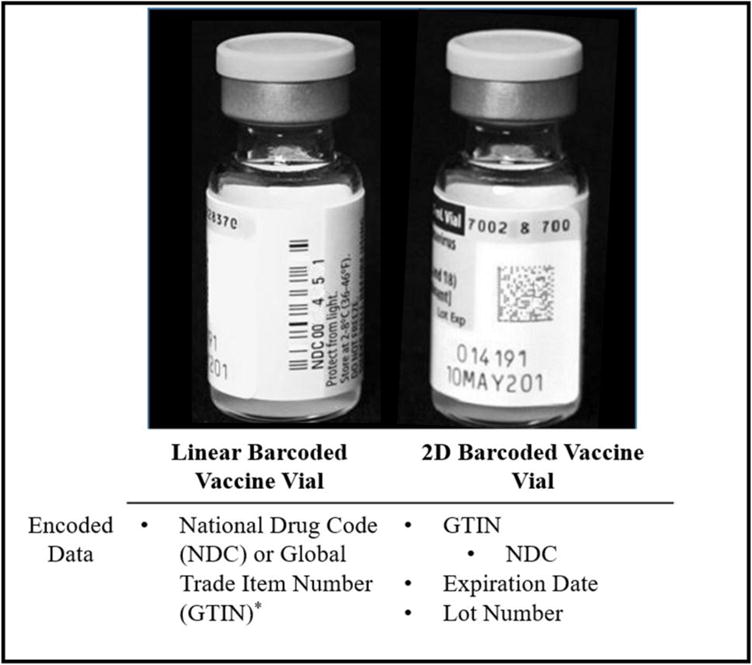
Either GTIN or NDC is encoded in a linear barcode. NDC is a unique 10-digit, 3-segment number that identifies labeler, product, and trade package size. GTIN (Global Trade Item Number) is a global product identification standard in which the NDC is encoded.
Theoretically, scanning 2D barcoded vaccines should increase the accuracy of vaccination records in EMRs, compared with manual data entry. However, the effect of 2D barcode scanning on vaccine data accuracy has not been widely studied.[10,15–16] In this evaluation, we assess the accuracy of lot number and expiration date in records entered via 2D barcode scanning compared with traditional data entry methods (e.g., drop down menu with jump capability, manual typing, or a combination of the two).
Methods
To recruit facilities, we contacted facilities that had expressed interest but were not enrolled in a previous CDC 2D vaccine barcode evaluation.[17] Additionally, we conducted targeted recruitment of other types of facilities (e.g., community vaccinators and pharmacies). We included facilities that: (1) were likely to use vaccines with 2D barcodes scheduled for distribution during the project period; (2) volunteered to scan 2D barcoded vaccines administered into their EMRs; (3) agreed to report de-identified EMR vaccine administration data for this evaluation; (4) used an EMR system to capture vaccine administration data that could be configured to input data using a 2D barcode scanner; and (5) agreed to use technology that allowed us to determine if the lot number and expiration date for a given vaccine administration was entered into the system with a 2D barcode scanner.
For each participating facility, we provided, installed, and configured corded, handheld image scanners with USB interface (i.e., 2D barcode scanners) to scan 2D barcoded vaccines to enter lot number and expiration date into EMRs. We conducted in-person staff training on use of scanners. Staff entered lot number and expiration date directly into EMRs using either 2D barcode scanning or traditional methods, depending on presence or absence of a 2D barcode; staff were encouraged, not required, to scan a 2D barcode if one was available. This evaluation was deemed to be public health practice and did not require IRB review.
Data Sources
We collected EMR data for all linear and 2D barcoded vaccines administered between July 1, 2014 and January 31, 2015. To determine whether scanning took place, we used a flag on records (native scan logs) or supporting text files (installed scan logs)1. Some facilities employed an EMR system capable of tracking which vaccine records were entered into the system via a 2D barcode scanner (native scan log). Other facilities allowed us to install software to record data from the scanner into a separate text file to indicate whether a 2D barcode was scanned (installed scan log); to be considered scanned, the text file had to match an EMR record on lot number, time recorded, and date recorded. EMR vaccine administration records that did not match the text file across all three data points were considered entered via “traditional methods.”
We determined whether lot number and expiration date entries were accurate by comparing data in the EMRs with a reference file containing 31,441 unique lot numbers. Lot numbers were compared to the reference file and considered accurate if a valid match was identified. The reference file was created from five sources: files from three manufacturers of 2D barcoded vaccines; records from the Vaccine for Children’s (VFC) program; facility shipping manifests; facility inventory records; and Vaccine Adverse Events Reporting System (VAERS) data.[18] Manufacturer files and VFC data were supplemented by the other sources only if certain criteria were met (e.g., previously unidentified lot numbers had appeared at least three times in VAERS). Four of the five reference file sources included information from one year before start of this evaluation (July 2013) through January 2015; VFC records contained vaccines shipped from May 2014 to January 2015.
All facilities had EMR systems that automatically populated vaccine lot number by scanning the 2D barcode; however, not all EMRs could capture expiration date from the scan. When expiration date was populated from an inventory system or a prepopulated table, records were excluded from the analysis of expiration date. Expiration dates were considered accurate if the expiration date in the EMR was the same as the expiration date associated with the lot number in the reference file. Records were excluded from expiration date analysis if lot number was not accurate because expiration date could not then be verified or if the reference file did not include expiration date for that vaccine lot.
Data Analysis
We performed chi-square analysis to compare the frequency of accurate lot numbers and expiration dates entered by 2D barcode scanning with the frequency of accurate data entered via traditional methods. To account for potential confounders, we created one multilevel logistic regression model for each of the dependent variables of interest—lot number accuracy and expiration date accuracy. The independent variable of interest was data entry method (2D barcode scan or traditional methods). We selected independent variables for inclusion in the final models through a backward elimination process that accounted for multicollinearity and model fit assessed by maximum likelihood estimation. We tested the following independent variables for inclusion: indicator that a vaccine was scanned, type of scan log (native versus installed scan log), state, facility specialty (pediatric versus all other), EMR type, manufacturer (A, B, C and all others combined), month the vaccine was administered (July 2014 through January 2015), facility type (privately-funded, publicly-funded, or mass vaccinator as self-identified), type of barcode (2D versus linear), facility vaccination volume (high > 400 per week; low ≤ 400 per week), and vaccine type (influenza vaccine versus non-influenza). Final models included site-specific random intercepts; other potential confounding factors (manufacturer, scan log type, facility type, facility vaccine volume, month vaccine administered, vaccine type, facility specialty) were included as fixed effects. We calculated unadjusted and adjusted odds ratios to examine the impact of each variable on lot number and expiration date accuracy in the presence and absence of potential confounding factors. We attempted to include all independent variables in both models, but the inclusion of facility funding type and vaccine volume failed to find a solution in the expiration date model; therefore, these variables were only included in the final lot number model.
Given the large sample size, we determined 0.001 to be the appropriate alpha for determining statistical significance using a Bonferroni adjustment, which corresponds to a 99% confidence interval.[19] Statistical models were run using SAS 9.2 (Carey, NC).
Results
Thirty-four facilities participated. Two facilities were unable to provide vaccination records at the end of the project; therefore, we analyzed records from 32 (94%) facilities. Among the facilities included in the analysis, 22 (69%) had native scan logs and ten (31%) had scan logs installed. We collected 128,573 vaccination administration records from six different EMR types. We excluded 11,145 expiration date records (as described in the methods); 117,428 records were available for expiration date analysis (Table 1). Facilities focused on pediatrics (n = 13) or other specialties (n = 19). Facilities were either privately-funded (n = 19), publicly-funded (n = 8) or mass vaccinators (n = 5). For both lot number and expiration date, the majority of all records scanned (77.7% lot number; 92.8% expiration date) and entered via traditional methods (96.9% lot number; 98.8% expiration date) came from privately-funded vaccinators.
Table 1.
Characteristics of Vaccine Administration Record Data and the Administration Records were 2D Barcode Scanned or Entered Using Traditional Methods for Lot Number and Expiration Date
| Characteristics | Facilities (N = 32) |
Lot number (N = 128,573) |
Expiration date (N = 117,428) |
||
|---|---|---|---|---|---|
| Records scanned | Records entered using traditional method | Records scanned | Records entered using traditional method | ||
| (n = 15,496) | (n = 113,077) | (n = 12,423) | (n = 105,005) | ||
| Scan Log Typea | |||||
| Installed | 11 (34) | 2,076 (13.4) | 6,782 (6.0) | 1,882 (15.1) | 4,331 (3.7) |
|
| |||||
| Native | 21 (66) | 13,420 (86.6) | 106,295 (94.0) | 10,541 (74.9) | 100,674 (85.7) |
|
| |||||
| Facility Type | |||||
| Privately Funded | 19 (59) | 12,043 (77.7) | 109,617 (96.9) | 11,532 (92.8) | 103,753 (98.8) |
|
| |||||
| Publicly Funded | 8 (25) | 1,082 (7.0) | 3,460 (3.1) | 891 (7.2) | 1,252 (1.2) |
|
| |||||
| Mass Vaccinator | 5 (16) | 2,371 (15.3) | – | – | – |
|
| |||||
| Facility Focus | |||||
| Private Practice Pediatrics | 13 (41) | 9,106 (58.8) | 83,564 (73.9) | 8,773 (70.6) | 79,145 (75.3) |
|
| |||||
| Remaining Specialtiesb | 19 (59) | 6,390 (41.2) | 29,513 (26.1) | 3,650 (29.4) | 25,860 (24.6) |
|
| |||||
| Manufacturer | |||||
| Manufacturer A | 1,961 (12.6) | 34,202 (30.2) | 1,958 (15.8) | 33,066 (31.5) | |
|
| |||||
| Manufacturer B | 2,040 (13.2) | 8, 690 (7.7) | 2,014 (16.2) | 7,951 (7.6) | |
|
| |||||
| Manufacturer C | 9,108 (58.8) | 55,947 (49.5) | 8,451 (68.0) | 50,407 (48.0) | |
|
| |||||
| Other Manufacturers | 2,387 (15.4) | 14,238 (12.6) | – | 13,581 (12.9) | |
|
| |||||
| Month Administered | |||||
| July 2014 | 888 (5.7) | 8,650 (7.6) | 807 (6.5) | 8,089 (7.7) | |
|
| |||||
| August 2014 | 1,435 (9.3) | 11,412 (10.1) | 1,329 (10.7) | 10,645 (10.1) | |
|
| |||||
| September 2014 | 3,367 (21.7) | 17,992 (15.9) | 2,047 (16.5) | 17,258 (16.4) | |
|
| |||||
| October 2014 | 3,942 (25.4) | 23,326 (20.6) | 2,649 (21.3) | 21,385 (20.4) | |
|
| |||||
| November 2014 | 2,332 (15.0) | 17,738 (15.7) | 2,241 (18.0) | 16,070 (15.3) | |
|
| |||||
| December 2014 | 1,864 (12.2) | 17,495 (15.5) | 1,798 (14.5) | 16,274 (15.5) | |
|
| |||||
| January 2015 | 1,668 (10.8) | 16,464 (14.6) | 1,552 (12.5) | 15,284 (14.6) | |
|
| |||||
| Facility Vaccine Volumec | |||||
| High Volume | 16 (50) | 7,003 (45.2) | 71,021 (62.8) | 4,846 (39.0) | 66,771 (63.6) |
|
| |||||
| Low Volume | 16 (50) | 8,493 (54.8) | 42,056 (37.2) | 7,577 (61.0) | 38,234 (34.4) |
|
| |||||
| Vaccine Type | |||||
| Flu Vaccine | 5,497 (35.5) | 36,106 (31.9) | 5,333 (42.9) | 33,210 (31.6) | |
|
| |||||
| Other Vaccine Typesd | 9,999 (64.5) | 76,971 (68.1) | 7,090 (57.1) | 71,795 (68.4) | |
Data presented as N (%).
Native scan logs came from systems that previously existed at the facility versus installed.
Health department (n=1), Federally Qualified Health Centers (n=3), family medicine (n=5), clinics (n=10)
High volume facilities administered > 400 vaccines/week; low volume facilities administered ≤ 400 vaccines/week.
17 other vaccine types
A higher percentage of lot numbers were accurate when entered via 2D barcode scanning (96.1%) compared with traditional methods (94.3%) (X2 = 84.4682, P < .001) (Figure 2). A higher percentage of expiration dates were accurate when entered via 2D barcode scanning (95.8%) compared with traditional methods (84.8%) (X2 = 1,113.55, P < .001).
Figure 2. Accuracy of Lot Number and Expiration Date When Entered via Traditional Methods or 2D Barcode Scanning.
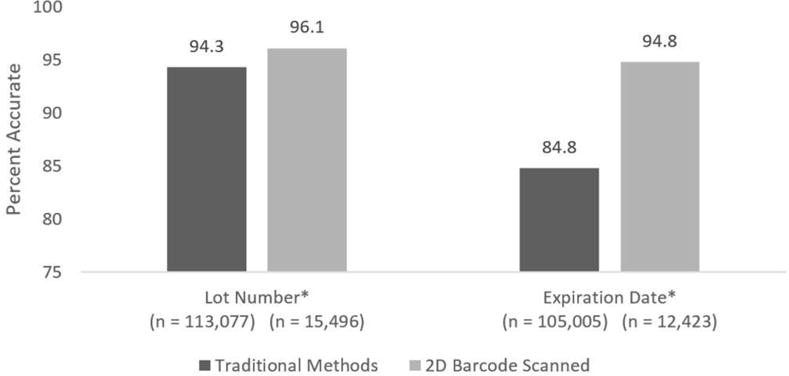
Using chi-square tests the differences between the percentage of accurate data using traditional methods and 2D barcode scanning is statistically significant at the P < .001 level.
When data were entered via 2D barcode scan, lot number was 1.48 (99% CI, 1.34–1.64) times more likely to be accurate and expiration date was 4.09 (99% CI, 3.68–4.55) times more likely to be accurate compared with traditional methods. When controlling for other potential confounders, lot number was 1.75 (99% CI, 1.57–1.96) (Table 2) times more likely to be accurate and expiration date was 2.39 (99% CI, 2.12–2.68) (Table 3) times more likely to be accurate when scanned compared with traditional methods of data entry.
Table 2.
Unadjusted and Adjusted Odds Ratio of Lot Number Accuracy on Key Explanatory Variables
| ORa (99% CI)
|
||
|---|---|---|
| Unadjusted | Adjusted | |
| Data Entry Method | ||
| 2D Barcode Scanned | 1.48 (1.34 – 1.64) | 1.75 (1.57 – 1.96) |
|
| ||
| Traditional Entry | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Scan Log Typeb | ||
| Installed Scan Log | 1.06 (0.95 – 1.18) | 1.14 (0.42 – 3.10) |
|
| ||
| Native Scan Log | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Manufacturer | ||
| Manufacturer A | 1.91 (1.69 – 2.15) | 2.94 (2.57 – 3.36) |
|
| ||
| Manufacturer B | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Manufacturer C | 0.60 (0.54 – 0.66) | 0.48 (0.42 – 0.54) |
|
| ||
| Other Manufacturers | 1.41 (1.23–1.62) | 2.34 (2.01–2.72) |
|
| ||
| Month Administered | ||
| July 2014 | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| August 2014 | 0.94 (0.83 – 1.06) | 0.93 (0.82 – 1.06) |
|
| ||
| September 2014 | 1.66 (1.47 – 1.88) | 1.41 (1.24 – 1.61) |
|
| ||
| October 2014 | 1.39 (1.25 – 1.56) | 1.09 (0.96 – 1.23) |
|
| ||
| November 2014 | 1.23 (1.10 – 1.39) | 1.03 (0.91 – 1.17) |
|
| ||
| December 2014 | 1.17 (1.04 – 1.32) | 1.07 (0.95 – 1.22) |
|
| ||
| January 2015 | 1.08 (0.96 – 1.21) | 1.00 (0.88 – 1.13) |
|
| ||
| Facility Vaccine Volumec | ||
| High | 0.61 (0.57 – 0.65) | 0.59 (0.29 – 1.18) |
|
| ||
| Low | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Facility Funding Type | ||
| Publicly Funded | 1.11 (0.94 – 1.30) | 0.30 (0.05 – 1.66) |
|
| ||
| Privately Funded | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Facility Specialty | ||
| Pediatrics Specialty | 0.90 (0.84 – 0.96) | 1.17 (0.53 – 2.62) |
|
| ||
| All Other Specialties | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Vaccine Type | ||
| Flu Vaccine | 1.87 (1.75 – 2.01) | 4.07 (3.77 – 4.40) |
|
| ||
| Other Vaccine Types | 1.00 [Reference] | 1.00 [Reference] |
OR is odds ratio.
Indicator for records that were scanned. Tracking software was installed or native to the EMR.
High volume >400 vaccines administered/week; low ≤400/week.
Table 3.
Unadjusted and Adjusted Odds Ratio of Expiration Date Accuracy on Key Explanatory Variables
| ORa (99% CI)
|
||
|---|---|---|
| Unadjusted | Adjusted | |
| Data Entry Method | ||
| 2D Barcode Scanned | 4.09 (3.68 – 4.55) | 2.39 (2.12 – 2.68) |
|
| ||
| Traditional Entry | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Scan Log Typeb | ||
| Installed Scan Log | 1.74 (1.58 – 1.93) | 1.80 (0.59 – 5.48) |
|
| ||
| Native Scan Log | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Manufacturer | ||
| Manufacturer A | 0.43 (0.38 – 0.50) | 1.00 (0.86 – 1.16) |
|
| ||
| Manufacturer B | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Manufacturer C | 0.32 (0.28 – 0.37) | 0.41 (0.35 – 0.47) |
|
| ||
| Other Manufacturers | 0.03 (0.03 – 0.03) | 0.04 (0.03 – 0.04) |
|
| ||
| Month Administered | ||
| July 2014 | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| August 2014 | 0.65 (0.60 – 0.71) | 0.49 (0.44 – 0.54) |
|
| ||
| September 2014 | 0.96 (0.88 – 1.03) | 0.53 (0.48 – 0.59) |
|
| ||
| October 2014 | 1.73 (1.60 – 1.88) | 1.16 (1.05 – 1.28) |
|
| ||
| November 2014 | 2.12 (1.94 – 2.32) | 1.59 (1.43 – 1.78) |
|
| ||
| December 2014 | 1.51 (1.39 – 1.65) | 1.28 (1.15 – 1.42) |
|
| ||
| January 2015 | 1.42 (1.31 – 1.55) | 1.29 (1.16 – 1.43) |
|
| ||
| Facility Specialty | ||
| Pediatrics Specialty | 0.47 (0.44 – 0.49) | 0.74 (0.27 – 2.03) |
|
| ||
| All Other Specialties | 1.00 [Reference] | 1.00 [Reference] |
|
| ||
| Vaccine Type | ||
| Flu Vaccine | 3.30 (3.13 – 3.48) | 2.38 (2.21 – 2.55) |
|
| ||
| Other Vaccines | 1.00 [Reference] | 1.00 [Reference] |
OR is odds ratio.
Indicator for records were scanned. Tracking software was installed or native to the EMR.
The multilevel logistic regression models showed that factors other than 2D barcode scanning were associated with lot number and expiration date accuracy. Parameter estimates for vaccine manufacturer, month of vaccine administration, and vaccine type were associated with accuracy of both lot number and expiration date. Compared with Manufacturer B, records for vaccines from Manufacturer A (adjusted odds ratio [aOR] = 2.94; 99% CI, 2.57–3.36) and other manufacturers (aOR = 2.34; 99% CI, 2.01–2.72) were more likely to have accurate lot numbers, whereas Manufacturer C (aOR = 0.48; 99% CI, 0.42–0.54) was less likely to have accurate lot numbers. For expiration date, data from Manufacturers C (aOR = 0.41; 99% CI, 0.35–0.47) and other manufacturers (aOR = 0.04; 99% CI, 0.03–0.04) were less likely to be accurate than Manufacturer B. Lot number data were more likely to be accurate when recorded between September and December 2014 than in July 2014 (Table 2). However, compared with July 2014, expiration date was more likely to be accurate between October 2014 and January 2015 (Table 2) and less likely to be accurate in August (aOR = 0.49; 99% CI, 0.44–0.54) and September 2014 (aOR = 0.53; 99% CI, 0.48–0.59). Data for both lot number (aOR = 4.07; 99% CI, 3.77–4.40) and expiration date (aOR = 2.38; 99% CI, 2.21–2.55) were more likely to be accurate when an influenza vaccine was recorded compared to all other types of vaccines combined.
Discussion
This is the first published evaluation of the impact of 2D vaccine barcode use on data accuracy in the United States. Overall, lot number and expiration date in vaccine administration records were more accurate when data were entered via 2D barcode scanning compared with traditional methods, even after controlling for potential confounders. Lot number accuracy was 1.8 percentage points higher and expiration date accuracy was 11 percentage points higher when entered by 2D scanning compared with traditional methods. Two considerations should be made regarding the difference in lot number accuracy compared with the difference in expiration date accuracy. First, there may be more attention paid to recording lot number accurately when using traditional methods since it is required.[1] Second, there is less room for positive change given the high lot number accuracy using traditional methods.
Manufacturer, month of vaccine administration, and vaccine type were associated with variation in accuracy of lot number and expiration date. Variation of accuracy by manufacturer may be due to factors such as curvature of the barcode on the vaccine vial, location of the barcode on the vial, vial size, or print quality of the barcodes or human-readable labels.[15] We hypothesized that variation in accuracy by month could be due to increase in vaccination activity in September and October when children begin the school year.[20,21] However, in some busy vaccination months data quality were more accurate. This evaluation was conducted over seven months and associations between accuracy and month of vaccination might change if conducted over an entire year. Influenza vaccine data were more likely to be accurate compared with all other vaccines combined. There may be something unique about the influenza vaccine administration process that is driving this variation. Additionally, facility funding type and vaccine volume may be related to expiration date quality; however, since these variables were unable to be included in the model, no determination can be made about impact of these variables on expiration date accuracy. The reasons why these variables are associated with vaccination data accuracy have not been fully explored and additional research is suggested to better understand how to improve vaccine administration data.
Although not captured in our analyses, we observed other factors that affect data quality and may warrant further investigation. Manufacturer name is a data element required by the NCVIA and is derived from the product NDC, which is embedded in the Global Trade Item Number (GTIN).[1,22] To record the manufacturer name, EMRs have to interpret the vaccine’s GTIN and then interpret the NDC to capture manufacturer name. We had planned to examine the association between accuracy of manufacturer name and 2D barcode scanning; however, most EMR systems were unable to interpret the NDC. CDC maintains guidance on functional capabilities for EMR software solutions to capture and process 2D barcoded vaccine data.[23]
Given the improvement in data accuracy associated with 2D barcode scanning demonstrated in this evaluation, increased adoption of 2D barcode scanning technology by vaccine providers and enhancement of EMR systems to capture scanned 2D barcoded information is likely to improve vaccine administration data quality. EMR enhancements should include the ability to readily interpret NDCs and populate vaccine manufacturer, vaccine name, and dosage upon scanning. Vaccination providers can work with their EMR vendors to create alternatives for recording vaccine information that is currently being appended to lot number, vaccine name, and manufacturer, such as vaccine funding source. Further integration of EMR and 2D barcode scanning has the potential to improve quality control and reduce medical errors. For example, EMRs could be programed to alert users if the 2D barcode scanned is for the wrong vaccine or dosage or if the vaccine has expired or been recalled.
The findings from this evaluation should be interpreted with several methodological limitations in mind. First, enrollment was voluntary, which may have resulted in selection bias; participating facilities may not be representative of all vaccinating facilities in the United States. For instance, volunteer facilities may have higher initial data quality or interest in data accuracy, and if so, we may be underestimating the potential contribution of 2D barcode scanning. Second, although we controlled for a number of factors when examining the association of 2D barcode scanning with lot number and expiration date accuracy, other variables such as user characteristics, workflow practices, traditional method of data entry, and EMR type may play a role in data accuracy. Another limitation is the possibility of misclassification in the subset of data where the team matched EMRs with a separate text file to identify if a record was scanned or entered by traditional methods. Misclassification might occur if a scanned lot number failed to populate into an otherwise complete vaccination record. In this case, lot numbers in the scan log would not be matched to the EMR and records could not be identified as scanned. The reference file protocol was designed to ensure the data were comprehensive and from the most reliable source, but the file is limited by the data available (e.g., data were not always complete and not obtained directly from all vaccine manufacturers). If a lot number was accurately recorded but not included in the reference file, the record would be misclassified as inaccurate.
The improvement in data accuracy of scanned vaccine administration records observed in this evaluation has the ability to make a significant impact on vaccine safety given the number of records that could be affected by this change. Each year in the United States, approximately 10 million vaccines are administered to children younger than one year of age.[24] If the accuracy of all lot numbers recorded for this age group increased by 1.8%, this could equate to an additional 180,000 vaccine administration records with the correct lot number. For the same age group, an 11% increase in accurate expiration dates would mean 1.1 million additional vaccination records would contain accurate expiration dates. The number of records with more accurate data could grow considerably when we consider the millions of additional vaccinations given to individuals over one year of age.
Conclusions
Our evaluation demonstrates that vaccine lot number and expiration date are more accurately captured via 2D barcode scanning than when entered via traditional methods. These findings suggest that increased availability and use of 2D barcodes on vaccines and widespread adoption of 2D barcode scanning will improve vaccination data accuracy. Improving interoperability between EMRs and 2D barcode scanners so that all data elements encoded in 2D barcodes can automatically populate would likely increase adoption of 2D barcode scanning technology. Future research should explore reasons for variations in differences in data quality by month of year, vaccine manufacturer, and vaccine type. A similar study should be conducted to examine the accuracy of recorded manufacturer when entered via 2D barcode scanning once EMRs can populate this required data field.
Acknowledgments
David Friedman, Andrew Wiesenthal, Katharine Benedict, Elizabeth Sobzyck, Robbie Locklar, Marshall Gaddis, Nikos Papageorgiou, and Ana Boltik
Funding: All phases of this evaluation were supported by the Centers for Disease Control and Prevention, Atlanta, GA.
Abbreviations
- 2D
Two-Dimensional
- EMR
Electronic medical record
- FDA
U.S. Food and Drug Administration
- IIS
Immunization Information System
- NDC
National Drug Code/product identifier
- VAERS
Vaccine Adverse Event Reporting System
- VFC
Vaccines for Children
Footnotes
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this manuscript to disclose.
Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Where facilities had existing EMRs capable of identifying which records were entered using a 2D scanner, we referred to those facilities as having native scan logs. At installed scan log facilities, the team had to install a text document to record vaccine information populated through 2D scanning and then match the text file records to the EMR data.
References
- 1. (Pub. L. No. 99-660, 42 U.S.C. 300aa-1 to 300aa-34).National Childhood Vaccine Injury Act of 1986. 1986 http://www.hrsa.gov/vaccinecompensation/authorizinglegislation.pdf. Accessed September 25, 2015.
- 2.American Academy of Pediatrics. American Academy of Pediatrics & GS1 healthcare US guideline for suppliers. 2012 http://www2.aap.org/immunization/pediatricians/pdf/barcoding_guidance_manufacturers_022212.pdf. Accessed September 18, 2016.
- 3.Immunization Information Systems (IIS): recommended core data elements. Centers for Disease Control and Prevention Web Site; http://www.cdc.gov/vaccines/programs/iis/core-data-elements.html. Published December 18, 2012. Updated May 5, 2015. Accessed September 21, 2016. [Google Scholar]
- 4.CDC. Progress in Immunization Information Systems—United States, 2011. MMWR. 2013;62:48–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy DG, Shore AD, Morlock LL, Miller MR. Pediatric vaccination errors: Application of the “5 rights” framework to a national error reporting database. Vaccine. 2009;27:3890–6. doi: 10.1016/j.vaccine.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Mullooly J, Drew L, DeStefano F, et al. Quality of HMO vaccination databases used to monitor childhood vaccine safety. Am J Epidemiol. 1999;149:186–194. doi: 10.1093/oxfordjournals.aje.a009785. [DOI] [PubMed] [Google Scholar]
- 7.Samuels RC, Appel L, Reddy SI, Tilson RS. Improving accuracy in a computerized immunization registry. Ambul Pediatr. 2002;2:187–192. doi: 10.1367/1539-4409(2002)002<0187:iaiaci>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Varricchio F, Reed J, VAERS working group Follow-up study of medication errors reported to the Vaccine Adverse Event Reporting System (VAERS) South Med J. 2006;99(5):486–89. doi: 10.1097/01.smj.0000216513.73448.af. [DOI] [PubMed] [Google Scholar]
- 9.Wilton R, Pennisi A. Evaluating the accuracy of transcribed computer-stored immunization data. Pediatrics. 1994;94:902–6. [PubMed] [Google Scholar]
- 10.Pereira JA, Quach S, Hamid JS, et al. Exploring the feasibility of integrating barcode scanning technology into vaccine inventory recording in seasonal influenza vaccination clinics. Vaccine. 2012;30:794–802. doi: 10.1016/j.vaccine.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. Bar code label requirement for human drug products and biological products, final rule. 2004. (21 C.F.R. Parts 201, 606, and 610 [Docket No. 2002N-0204]). [PubMed] [Google Scholar]
- 12.National Drug Code Directory. U. S. Food and Drug Administration Web Site; http://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm. Updated September 25, 2015. Accessed September 26, 2015. [Google Scholar]
- 13.U.S. Food and Drug Administration. Guidance for industry: bar code label requirements—questions and answers. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/UCM267392.pdf. Published August 2011. Accessed September 3, 2015.
- 14.Immunization Information Systems (IIS): Two-Dimensional (2D) Barcodes. Centers for Disease Control and Prevention Web site; http://www.cdc.gov/vaccines/programs/iis/2d-vaccine-barcodes/index.html#shipping. Published July 27, 2016. Accessed September 19, 2016. [Google Scholar]
- 15.O’Connor AC, Kennedy ED, Loomis RJ, et al. Prospective cost-benefit analysis of a two-dimensional barcode for vaccine production, clinical documentation, and public health reporting and tracking. Vaccine. 2013;31:3179–86. doi: 10.1016/j.vaccine.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 16.Pereira JA, Quach S, Hamid JS, et al. The integration of barcode scanning technology into Canadian public health immunization settings. Vaccine. 2014;32:2748–2755. doi: 10.1016/j.vaccine.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Fierro L, Gaddis M, Kinney M, et al. Implementation pilot for two-dimensional (2D) vaccine barcode utilization: summary report. http://www.cdc.gov/vaccines/programs/iis/2d-vaccine-barcodes/downloads/pilot-summary.pdf. Published November 2014. Accessed September 8, 2016.
- 18.VAERS Data – Database. Vaccine Adverse Event Reporting System (VAERS) Web Site; https//vaers.hhs.gov/data/data. Updated October 12, 2015. Accessed October 31, 2015. [Google Scholar]
- 19.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Influenza vaccine (flu): flu vaccination coverage, United States, 2014–15 influenza season. Centers for Disease Control and Prevention Web Site; http://www.cdc.gov/flu/fluvaxview/coverage-1415estimates.htm. Updated September 17, 2016. Accessed October 5, 2015. [Google Scholar]
- 21.Cullen KA, Stokley S, Markowitz LE. Uptake of human papillomavirus vaccine among adolescent males and females: Immunization Information System sentinel sites, 2009–2012. Academic Pediatr. 2014;14:497–504. doi: 10.1016/j.acap.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GS1 Healthcare GTIN allocation rules: GTIN allocation rules for the healthcare sector. GS1 Web Site; http://www.gs1.org/docs/gsmp/healthcare/GS1_Healthcare_GTIN_Allocation_Rules.pdf. Published July 2015. Accessed September 28, 2016. [Google Scholar]
- 23.Kirkwood B, Robinson P, David S, et al. EHR-IIS 2D barcode functional capabilities report: version 1.1. http://www.cdc.gov/vaccines/programs/iis/2d-vaccine-barcodes/downloads/barcode-functional-capabilities.pdf. Published October 2014. Accessed September 28, 2016.
- 24.VAERS Data. Vaccine Adverse Event Reporting System (VAERS) Web Site; https://vaers.hhs.gov/data/index. Accessed September 3, 2015. [Google Scholar]


