Abstract
Background
Deterioration in the National Institutes of Health Stroke Scale (NIHSS) in the early days after stroke is associated with progressive infarction, brain edema and/or hemorrhage, leading to worse outcome.
Aims
We sought to determine whether a stable NIHSS score represents an adverse or favorable course.
Methods
Brain magnetic resonance images (MRI) from a research cohort of acute ischemic stroke patients were analyzed. Using NIHSS scores at baseline and follow-up (day 3-5), patients were categorized into early neurological deterioration (END, ΔNIHSS ≥4), early neurological recovery (ENR, ΔNIHSS, ≥−4) or early neurological stability (ENS, ΔNIHSS between −3 and 3). The association between these categories and the volume of infarct growth, volume of swelling, parenchymal hematoma (PH) and 3 month modified Rankin Scale (mRS) score were evaluated.
Results
Patients with END or ENS were less likely to be independent (mRS 0-2) at 3 months compared to those with ENR (P<0.001). Patients with END or ENS were observed to have significantly greater infarct growth and swelling volumes than those with ENR (P=0.03; P<0.001, respectively). Brain edema was more common than the other imaging markers investigated and was independently associated with a stable or worsening NIHSS score after adjustment for age, baseline stroke volume, infarct growth volume, presence of PH, and reperfusion (P<0.0001).
Conclusions
Stable NIHSS score in the subacute period after ischemic stroke may not be benign, and is associated with tissue injury including infarct growth and brain edema. Early improvement is considerably more likely to occur in the absence of these factors.
Keywords: outcome, secondary neurological injury, ischemic stroke, MRI, edema, deterioration
INTRODUCTION
In patients suffering acute stroke, the degree of neurological deficit frequently changes after initial presentation (1). The evolution of neurological impairment in the first few days after stroke onset influences long-term clinical outcome (2). Accordingly, the subacute National Institutes of Health Stroke Scale (NIHSS) score in the days following stroke is a stronger predictor of long-term global disability than the baseline score (3).
Prior studies have focused on the clinical importance of dramatic worsening after stroke (4–6), termed early neurological deterioration (END), and commonly defined as an increase of ≥4 in the NIHSS (1,7,8). Up to one-third of stroke patients experience END (3,6), although the frequency varies with the precise definition used (9). The relationship of END with poor long-term outcome is well established (5,6,10–12). In contrast, less is known about patients with early neurological stability (ENS), who exhibit a stable neurological exam in the subacute period.
Understanding the factors that predict subacute deficit changes can advance prognostication and may identify putative therapeutic targets to improve long-term outcome. Several factors have been postulated to contribute to secondary neurological injury such as brain edema (6,13), infarct growth (2,12,14,15), parenchymal hemorrhage (PH) (4,10), revascularization status (16,17), and metabolic factors including hyperglycemia (5,10,11,18,19). Recent MRI-based methods can distinguish and quantify several of these processes in a wide array of stroke severity (20).
AIMS AND HYPOTHESIS
In this study, we sought to determine whether subacute stability in neurological deficit, ENS, was a sign of uneventful recovery or unrecognized injury. We also sought to characterize the relative contributions of brain edema, infarct growth, parenchymal hemorrhage, and revascularization to early neurological course after stroke. We hypothesized that ENS and END are both adverse clinical manifestations of secondary tissue injury.
METHODS
Patient Characteristics
Patients enrolled in the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET, NCT00238537) were analyzed. The details of the cohort have been previously described (21). In brief, the EPITHET study enrolled acute hemispheric ischemic stroke patients who presented 3–6 hr after symptom onset, with a NIHSS score of more than 4. MRI, magnetic resonance angiography (MRA), and perfusion-weighted imaging (PWI) studies and NIHSS assessments were performed at baseline and at day 3-5. Long-term outcome was measured with 90-day modified Rankin Scale (mRS) score, with good outcome defined as mRS of 0-2 and poor outcome as mRS of 3-6 (21).
EPITHET patients lacking day 3-5 MRI or PWI, or patients with DWI of insufficient quality were excluded from the present analysis. The Institutional Review Board approved this study, and all patients or their legally authorized representative originally provided informed consent.
Early neurological deterioration, stability and recovery
The change in NIHSS (ΔNIHSS) was derived for each subject by subtracting follow-up NIHSS from the baseline score. In accordance with prior literature, we defined early neurological deterioration (END) as an increase of ≥4 points and early neurological recovery (ENR) as a decrease of ≥4 points (1,4,8,11). Patients with ΔNIHSS values between −3 and 3 were defined as having early neurological stability (ENS). Since ENS and END both were hypothesized to represent adverse outcomes, we combined these categories in some of our analyses.
Imaging Analysis
Region-of-interest (ROI) analysis was conducted using a semi-automated method in Analyze 11.0 (Biomedical Imaging Resource, Rochester, MN), as previously described (20,22). Briefly, the baseline and follow-up stroke ROIs were initially outlined on DWI. Lesion volumes were determined and the total change in lesion volume (ΔDWI) from baseline to follow-up was calculated. The component volumes attributable to brain edema, quantified by measuring the lesional swelling volume (20), infarct growth, and PH were determined for each subject by comparing baseline and follow-up scans (see Supplementary Figure 1 for an example of this imaging analysis approach).
New neuroanatomic areas of infarction that were not present on the baseline DWI were first identified on the follow-up DWI in the axial, sagittal, and coronal planes and then outlined. The presence of PH (defined as PH1 and PH2) was based on the designation from the original EPITHET report (21) and the volume was quantified in Analyze 11.0. Swelling volumes were calculated based on the relationship: swelling volume = ΔDWI volume − infarct growth volume − PH volume. Each variable was furthermore dichotomized and analyzed regarding its association with poor outcome. Swelling volume was dichotomized at >11mL and infarct growth at ΔASPECTS score >2 based on thresholds previously demonstrated to be predictive of clinical outcome (20). HT was dichotomized based on the presence or absence of PH (23).
Revascularization was assessed by both reperfusion and recanalization measures (24,25). Reperfusion was defined as >90% reduction in the volume of the perfusion-weighted imaging deficit between baseline and day 3-5, as previously reported (21). To assess recanalization, we evaluated vessel occlusion status between baseline and day 3-5 MRA. We defined persistent occlusion by the continued presence of occlusion at the same site between baseline and follow-up angiographic study. Partial recanalization was defined as an improvement in the degree of obstruction but without complete resolution. Complete recanalization was defined as an occluded baseline MRA that was normal at follow-up. Finally, a normal study had a patent MRA at baseline and follow-up.
Statistical Analysis
Differences between ENR, ENS and END groups for binary variables were analyzed using the Fisher’s exact or chi-squared test. Continuous variables were compared between ΔNIHSS groups using ANOVA or Kruskal-Wallis testing, as appropriate. Univariate regression was performed to investigate the association between imaging variables and ΔNIHSS. Multivariate linear regression modeling was then performed to assess the independent effects of swelling, infarct growth, HT, and reperfusion status on continuous ΔNIHSS score. All tests were two-sided and performed with the threshold for significance set at P<0.05 using JMP Pro 11.0 (SAS Institute, Cary, NC).
RESULTS
Study Population
Of 101 subjects originally enrolled in the EPITHET study, 75 were included in the present analysis. Thirteen patients were excluded because of insufficient DWI quality, 11 had no follow-up MRI, and 2 had no follow-up NIHSS assessment. Of patients included in this investigation, 31 had ENR (41%), 36 had ENS (48%) and 8 had END (11%). The clinical characteristics of each group are reported in Table 1. The groups were similar in age, comorbidities, and admission NIHSS. Patients exhibiting ENR had smaller baseline stroke lesions on DWI (P=0.02) and were more likely to have experienced reperfusion (P=0.0002).
Table 1.
Clinical and Imaging Characteristics of the EPITHET cohort
|
ENR
(n=31) |
ENS
(n=36) |
END
(n=8) |
P value | ||
|---|---|---|---|---|---|
| Age, y, mean +/− SD | 71 ± 13 | 73 ± 14 | 75 ± 8 | 0.69 | |
| Sex, male, n (%) | 16 (52) | 20 (56) | 5 (63) | 0.85 | |
| Admission MAP (mmHg), mean +/− SD | 99 ± 12 | 100 ± 12 | 106 ± 14 | 0.55 | |
| Admission glucose (mmol/L), median [IQR] | 7 [6-8] | 7 [6-8] | 8 [7-12.5] | 0.09 | |
| Smoking history, yes, n (%) | 11 (35) | 15 (42) | 3 (38) | 0.87 | |
| Comorbidities, n (%) | |||||
| Diabetes mellitus | 6 (19) | 8 (22) | 3 (38) | 0.55 | |
| Hypertension | 21 (68) | 23 (64) | 8 (100) | 0.13 | |
| Hyperlipidemia | 17 (55) | 12 (33) | 4 (50) | 0.20 | |
| Atrial fibrillation | 12 (39) | 16 (44) | 2 (25) | 0.59 | |
| IV tPA, n (%) | 19 (61) | 12 (33) | 4 (50) | 0.07 | |
| Time to IV tPA treatment, min, mean +/− SD | 293 ± 45 | 293 ± 50 | 292 ± 44 | 0.99 | |
| Admission NIHSS, median [IQR] | 11 [10-16] | 14 [7-18] | 12 [10-17] | 0.71 | |
| Follow-up NIHSS, median [IQR] | 3 [2-8] | 13 [8-19] | 21 [17-24] | <0.0001*** | |
| Admission DWI volume, mL, median [IQR] | 11 [7-31] | 31 [12-69] | 23 [10-116] | 0.02* | |
| Admission PWI volume, mL, median [IQR] | 142 [94-214] | 198 [83-257] | 208 [101-369] | 0.30 | |
| Swelling volume, mL, mean ± SD | 7 ± 4.2 | 32 ± 3.9 | 48 ± 8.2 | <0.0001*** | |
| Infarct growth volume, mL, median [IQR] | 0.9 [0-6] | 10 [0-32] | 9 [1-40] | 0.03* | |
| Parenchymal Hemorrhage, n (%) | 1 (3) | 7 (19) | 2 (25) | 0.04* | |
| Reperfusion, n (%) | 20 (69) | 7 (22) | 1 (13) | 0.0002*** | |
DWI indicates diffusion-weighted imaging; IV tPA, intravenous tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; MAP, mean arterial pressure; PWI, perfusion-weighted imaging; reperfusion, >90% reduction in perfusion-weighted imaging deficit volume between baseline and day 3-5.
p<0.05;
p<0.01;
p<0.001
ENS and Functional Outcome
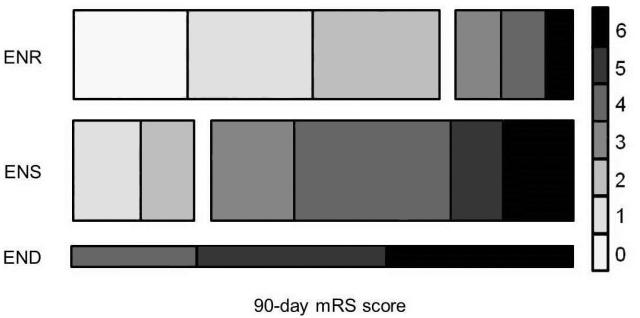
We found that ΔNIHSS between baseline and day 3-5 was independently associated with 90-day mRS score after adjustment for age, sex, baseline NIHSS, baseline stroke volume, and admission blood glucose levels (P<0.001; see Supplemental Table 1). Poor functional outcome was common in patients with ENS (75%) and END (100%) as compared to those with ENR (25%) (Figure 1, P<0.001).
Figure 1.
Distribution of 90-day modified Rankin Scale (mRS) scores for patients with early neurological recovery (ENR), stability (ENS), and deterioration (END). The right-hand key represents each category of mRS as labeled. The height of each bar represents the proportion of the cohort exhibiting each course of neurological deficit evolution (ENR: 41%, ENS: 48%, END: 11%).
Imaging Markers of Secondary Neurological Injury are associated with ENS
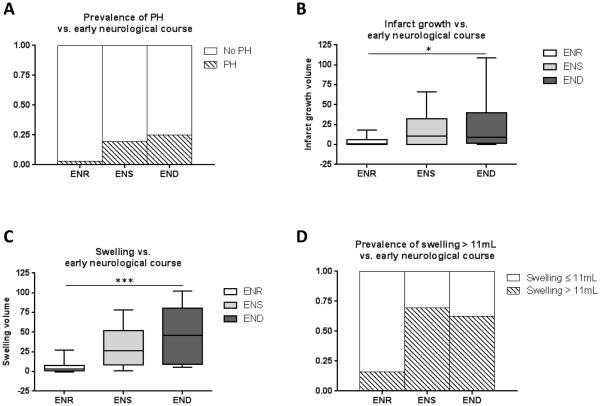
Patients with and without PH had differing ΔNIHSS, (2±7 versus −3±5, respectively, P=0.006). Accordingly, patients exhibiting early recovery had a lower incidence of PH (3%, P=0.04) versus ENS (19%) and END (25%) (see Figure 2A).
Figure 2.
Association of early neurological course with markers of secondary neurological injury. A, There is a trend towards higher incidence of parenchymal hemorrhage (PH) in patients with early neurological stability (ENS) and deterioration (END) than those with early neurological recovery (ENR; P=0.04). B, There was a significant difference in infarct growth in patients among the three categories (*, P=0.03). C, Swelling volume demonstrates a stepwise association with ENR, ENS, and END (ANOVA; ***, P<0.0001). D, Using a threshold for swelling of >11mL, subjects with swelling were significantly more likely to have ENS or END than ENR (P<0.0001).
Larger volume of infarct growth was associated with ΔNIHSS (ρ=0.34, P<0.005). In accord, Figure 2B demonstrates that median infarct growth volume was smaller in the early recovery group relative to ENS and END (Kruskal-Wallis test, P=0.03).
Figure 2C demonstrates a stepwise increase in swelling volume in patients with ENR (7±4mL), compared to those with ENS (32±4mL) and those with END (48±8mL; ANOVA, P<0.0001). When dichotomized at the threshold of >11ml, swelling was evident in only 16% of patients with ENR, but in 69% of those with ENS and 63% of those with END (P<0.0001, Figure 2D).
Supplementary Figure 2 depicts the relative frequency of each type of secondary injury in patients with ENS and END combined. Swelling was evident in 68% of patients, whereas PH and infarct growth were evident in 20% and 25%, respectively. Swelling alone, without co-association of PH or infarct growth, was observed in 32% of patients. Infarct growth alone and PH alone were each observed in 2% of patients. Only 27% of patients who experienced ENS or END did not have PH, infarct growth or swelling.
To compare with the aforementioned imaging markers of secondary injury, we also evaluated revascularization (e.g., both reperfusion and recanalization), which is consistently associated with good outcome (26–29). Reperfusion was observed frequently with ENR (69%), less frequently with ENS (22%) and rarely with END (13%) (P<0.001). Similarly, recanalization was exhibited by 36% of patients with ENR, by 9% with ENS and 0% with END (P<0.001).
Independent Predictors of Early Neurological Course
We next developed a multivariate model to investigate independent predictors of early change in NIHSS as a continuous outcome variable. We incorporated the imaging markers of secondary injury in addition to previously reported predictors of ΔNIHSS, including admission glucose, baseline DWI lesion volume and reperfusion status (5,6,10,12,30). Of these, only swelling volume and reperfusion independently predicted ΔNIHSS (Table 2, model 1; see Supplementary Tables 2 and 3 for additional model diagnostics). These results were unchanged when substituting recanalization status for reperfusion (Supplementary Table 4).
Table 2.
Univariate and Multivariate Predictors of Change in NIHSS from Baseline to Day 3-5
| Univariate Analyses | Multivariate Model 1 | Multivariate Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔNIHSS |
ΔNIHSS
R2 =0.44 |
ENR vs. ENS+END
R2 = 0. 38 |
|||||||||
| β | 95% CI | P value | adjusted β | 95% CI | P value | adjusted OR | 95% CI | P value | |||
| Age | 0.027 | −0.078 - 0.13 | 0.61 | ||||||||
| Admission Glucose | 5.96 | 2.07 - 9.85 | 0.055 | 3.54 | −0.57 - 7.66 | 0.09 | |||||
| Admission DWI | 3.04 | 0.40 - 5.67 | 0.0043** | −0.83 | −4.31 - 2.65 | 0.66 | |||||
| Infarct Growth | 2.53 | 0.76 - 4.30 | 0.01* | 0.02 | −0.02 - 0.05 | 0.33 | 0.93 | 0.26 - 3.6 | 0.91 | ||
| Swelling | 0.11 | 0.071 - 0.15 | <0.0001*** | 0.10 | 0.02 - 0.17 | 0.007** | 0.94 | 0.88 - 0.98 | 0.014* | ||
| PH | −2.71 | −4.6 - -0.82 | 0.0055** | −1.26 | −2.98 - 0.46 | 0.15 | 0.26 | 0.012 - 2.3 | 0.27 | ||
| Reperfusion | 2.6 | 1.3 - 4.0 | 0.0001*** | 1.37 | 0.05 - 2.69 | 0.043* | 4.1 | 1.2 - 17 | 0.036* | ||
Admission glucose and admission DWI volume were log transformed before inclusion in Multivariate Model 1. Data are from the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET) cohort. ΔNIHSS indicates the change in NIHSS score from baseline to day 3-5; CI, confidence interval; DWI, diffusion-weighted imaging; ENR, early neurological recovery; ENS, early neurological stability; END, early neurological deterioration; PH, presence of parenchymal hemorrhage 1 or 2; reperfusion, >90% reduction in perfusion-weighted imaging deficit volume between baseline and day 3-5.
p<0.05;
p<0.01;
p<0.001
Because our results suggest that both ENS and END represent a similar adverse neurological course, we dichotomized ΔNIHSS into ENR versus ENS and END. In multivariate logistic regression, the presence of swelling volume and absence of reperfusion were independent predictors of ENS and END (Table 2, model 2). Although logistic regression limited the number of dependent variables included in the model (31), the addition of the other listed variables did not alter the independent association of brain edema.
DISCUSSION
In this study, we report that a stable neurological exam in the first few days after stroke is an adverse prognostic sign for recovery. This association is present irrespective of the initial NIHSS score, suggesting that factors associated with early change in NIHSS have an independent relationship with outcome. Furthermore, in this cohort of moderate-to-severe stroke, almost half of all patients exhibited ENS. These data underscore the clinical importance of early neurological stability and of studying the factors that may be associated with its incidence.
It is widely accepted that neurological deterioration portends poor outcome (5,6,10,12), and is associated with secondary injury from brain edema (6,13), infarct growth (2,12,14,15), parenchymal hemorrhage (PH)(4,10), lack of reperfusion (16,17), and/or metabolic factors (5,10,11,18,19). Here we report that patients with ENS are also at high risk for poor long-term outcome, and that ongoing tissue injury in the form of brain edema and infarct growth might mediate this association.
Although PH was associated with END and ENS, it was the least common form of secondary neurological injury. Although we hypothesized that PH may lead to ENS or END independently, its frequent co-association with edema made it difficult to study as a separate entity in this cohort.
Although infarct growth was associated with END and ENS in univariate analysis, unexpectedly, it was not an independent predictor of the change in NIHSS. While prior studies that have shown that large infarct expansion causes neurodeterioration and poor long-term outcome (32,33), we found that it occurred infrequently in this study cohort. We hypothesize that the effect of infarct growth on outcome was collinear with reperfusion status. Therefore, the association between infarct growth and outcome may be mediated by the stronger effect leveraged by reperfusion.
Reperfusion, defined as >90% reduction in PWI deficit volume between baseline and day 3-5, was associated with ENR. Moreover, it was observed less frequently in patients with ENS and almost absent in END. Reperfusion was also an independent predictor of improving NIHSS score in multivariate analyses. These findings are in accord with prior studies demonstrating the robust clinical benefit of reperfusion (29,34). That said, it was not the only independent predictor of ΔNIHSS, suggesting that reperfusion status accounts for some, but not all of the variability in early neurological course. Our analyses reveal that brain edema may be another possible contributor.
Brain edema was the most common form of secondary neurological injury, and it occurred in isolation in about one-third of patients. This, in conjunction with our finding that swelling volume independently predicted worsening NIHSS score, suggests that moderate swelling may be a more common form of secondary injury than previously appreciated. In accord, moderate swelling has been shown to predict poor three month disability (20). Validation in additional cohorts and prospective study would be necessary to establish whether there is any causal link between markers of brain edema (e.g., swelling volume) and poor functional outcome.
Unexplained ENS-END was less common in our study relative to prior reports (18,35). Specifically, our study accounted for about three quarters of cases. Although this may be explained by increased detection via our imaging methods, alternatives such as differences in cohort severity and/or treatment rates with IV tPA are also possible. The unaccounted for sources of ENS-END may include metabolic effects (11), systemic post-stroke complications such as infection (36), disruptions in local perfusion from thrombus extension (8,18), or other unknown sources.
Our study has limitations. First, this was a retrospective analysis, performed in a cohort which included moderate to severe infarction. Our results may not be generalizable to small and/or mild strokes. Second, our sample size was relatively small, particularly with respect to the END subgroup. We used a change of ≥4 NIHSS points to assign individuals to ENR, ENS, and END, based on accepted definitions (5,7), and because of inter-rater reliability that may be encountered with smaller differences (37). The uneven sample sizes of ENR, ENS and END subgroups may have skewed our results about early neurological course and long term outcome. However, our analysis of ΔNIHSS as a continuous variable avoided these limitations and supports the effect of swelling on early outcome. Finally, even though the characteristics of the END subgroup are similar to prior reports (5,6), the exclusion of 11 patients that lacked day 3-5 MRI may have introduced bias into our analyses. These patients may have been more likely to have neurological deterioration preventing the imaging from being performed. While this may underestimate the prevalence for each factor on END, it does not affect our main finding that neurological stability represents an equally adverse neurological course in the days after stroke. Finally, our method for measuring edema is based upon morphometric analysis, and future methods that directly measure water content may provide additional insight into the role of brain edema after stroke.
CONCLUSIONS
Our study identifies early neurological stability as an adverse prognostic sign for recovery after stroke. Ongoing tissue injury, including infarct growth, hemorrhage and brain edema may manifest as a persistence in the severity of neurological deficit, preventing early neurological recovery. In accord, early neurological recovery was considerably more likely to occur in the absence of these processes. Identifying therapeutic strategies to limit the clinical impact of each of these factors may thereby promote both early and long-term recovery.
Supplementary Material
Acknowledgments
FUNDING
The original funding for the EPITHET trial was from the National Health and Medical Research Council, Australia for the EPITHET trial (S.M.D., G.A.D.). The analysis performed for this study was funded in part by the NIH/NINDS K23NS076597 (W.T.K.).
Footnotes
CONFLICTS OF INTERST
The authors declare no competing interests.
REFERENCES
- 1.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25(2):362–5. doi: 10.1161/01.str.25.2.362. [DOI] [PubMed] [Google Scholar]
- 2.Alawneh JA, Moustafa RR, Baron JC. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke. 2009;40(6):e443–50. doi: 10.1161/STROKEAHA.108.532465. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke. 2012;43(6):1537–41. doi: 10.1161/STROKEAHA.111.636928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinkstok SM, Beenen LF, Majoie CB, Marquering HA, de Haan RJ, Roos YB. Early deterioration after thrombolysis plus aspirin in acute stroke: a post hoc analysis of the Antiplatelet Therapy in Combination with Recombinant t-PA Thrombolysis in Ischemic Stroke trial. Stroke. 2014;45(10):3080–2. doi: 10.1161/STROKEAHA.114.006268. [DOI] [PubMed] [Google Scholar]
- 5.Davalos A, Cendra E, Teruel J, Martinez M, Genis D. Deteriorating ischemic stroke: risk factors and prognosis. Neurology. 1990;40(12):1865–9. doi: 10.1212/wnl.40.12.1865. [DOI] [PubMed] [Google Scholar]
- 6.Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999;30(12):2631–6. doi: 10.1161/01.str.30.12.2631. [DOI] [PubMed] [Google Scholar]
- 7.Brott TG, Haley EC, Jr., Levy DE, Barsan W, Broderick J, Sheppard GL, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23(5):632–40. doi: 10.1161/01.str.23.5.632. [DOI] [PubMed] [Google Scholar]
- 8.Arenillas JF, Rovira A, Molina CA, Grive E, Montaner J, Alvarez-Sabin J. Prediction of early neurological deterioration using diffusion- and perfusion-weighted imaging in hyperacute middle cerebral artery ischemic stroke. Stroke. 2002;33(9):2197–203. doi: 10.1161/01.str.0000027861.75884.df. [DOI] [PubMed] [Google Scholar]
- 9.Siegler JE, Martin-Schild S. Early Neurological Deterioration (END) after stroke: the END depends on the definition. Int J Stroke. 2011;6(3):211–2. doi: 10.1111/j.1747-4949.2011.00596.x. [DOI] [PubMed] [Google Scholar]
- 10.Grotta JC, Welch KM, Fagan SC, Lu M, Frankel MR, Brott T, et al. Clinical deterioration following improvement in the NINDS rt-PA Stroke Trial. Stroke. 2001;32(3):661–8. doi: 10.1161/01.str.32.3.661. [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Naganuma M, Okada Y, Hasegawa Y, Shiokawa Y, Nakagawara J, et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the Stroke Acute Management with Urgent Risk Factor Assessment and Improvement rt-PA Registry. Cerebrovasc Dis. 2012;34(2):140–6. doi: 10.1159/000339759. [DOI] [PubMed] [Google Scholar]
- 12.Georgiadis D, Engelter S, Tettenborn B, Hungerbuhler H, Luethy R, Muller F, et al. Early recurrent ischemic stroke in stroke patients undergoing intravenous thrombolysis. Circulation. 2006;114(3):237–41. doi: 10.1161/CIRCULATIONAHA.105.597435. [DOI] [PubMed] [Google Scholar]
- 13.Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87–94. doi: 10.1136/jnnp-2014-308327. [DOI] [PubMed] [Google Scholar]
- 14.Saqqur M, Molina CA, Salam A, Siddiqui M, Ribo M, Uchino K, et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38(1):69–74. doi: 10.1161/01.STR.0000251800.01964.f6. [DOI] [PubMed] [Google Scholar]
- 15.Awadh M, MacDougall N, Santosh C, Teasdale E, Baird T, Muir KW. Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: incidence and association with atrial fibrillation. Stroke. 2010;41(9):1990–5. doi: 10.1161/STROKEAHA.109.569459. [DOI] [PubMed] [Google Scholar]
- 16.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31(8):1812–6. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 17.Felberg R a, Okon NJ, El-Mitwalli A, Burgin WS, Grotta JC, Alexandrov AV. Early dramatic recovery during intravenous tissue plasminogen activator infusion: Clinical pattern and outcome in acute middle cerebral artery stroke. Stroke. 2002;33(5):1301–7. doi: 10.1161/01.str.0000015556.48283.74. [DOI] [PubMed] [Google Scholar]
- 18.Seners P, Turc G, Tisserand M, Legrand L, Labeyrie MA, Calvet D, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke. 2014;45(7):2004–9. doi: 10.1161/STROKEAHA.114.005426. [DOI] [PubMed] [Google Scholar]
- 19.Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Beasley TM, et al. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(7):e207–13. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45(12):3643–8. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 22.Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20(2):193–201. doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39(8):2249–56. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 24.Cho T-H, Nighoghossian N, Mikkelsen IK, Derex L, Hermier M, Pedraza S, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke; a journal of cerebral circulation. 2015;46(6):1582–9. doi: 10.1161/STROKEAHA.114.007964. [DOI] [PubMed] [Google Scholar]
- 25.Tsai JP, Albers GW. Reperfusion versus recanalization: The winner is… Stroke. 2015;46:1433–4. doi: 10.1161/STROKEAHA.115.009268. [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Koziol JA. Recanalization and stroke outcome. Circulation. 2007;115(20):2602–5. doi: 10.1161/CIRCULATIONAHA.107.698225. [DOI] [PubMed] [Google Scholar]
- 27.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 28.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 29.Cho TH, Nighoghossian N, Mikkelsen IK, Derex L, Hermier M, Pedraza S, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke. 2015;46(6):1582–9. doi: 10.1161/STROKEAHA.114.007964. [DOI] [PubMed] [Google Scholar]
- 30.Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial doppler monitoring. Stroke. 2000;31(3):610–4. doi: 10.1161/01.str.31.3.610. [DOI] [PubMed] [Google Scholar]
- 31.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Rajajee V, Kidwell C, Starkman S, Ovbiagele B, Alger JR, Villablanca P, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67(6):980–4. doi: 10.1212/01.wnl.0000237520.88777.71. [DOI] [PubMed] [Google Scholar]
- 33.Liebeskind DS, Jahan R, Nogueira RG, Jovin TG, Lutsep HL, Saver JL, et al. Serial Alberta Stroke Program early CT score from baseline to 24 hours in Solitaire Flow Restoration with the Intention for Thrombectomy study: a novel surrogate end point for revascularization in acute stroke. Stroke. 2014;45(3):723–7. doi: 10.1161/STROKEAHA.113.003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36(11):2400–3. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 35.Siegler JE, Boehme AK, Albright KC, George AJ, Monlezun DJ, Beasley TM, et al. A proposal for the classification of etiologies of neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(8):e549–56. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karepov VG, Gur AY, Bova I, Aronovich BD, Bornstein NM. Stroke-in-evolution: infarct-inherent mechanisms versus systemic causes. Cerebrovascular diseases. 2006;21:42–6. doi: 10.1159/000089593. [DOI] [PubMed] [Google Scholar]
- 37.Josephson SA, Hills NK, Johnston SC. NIH Stroke Scale reliability in ratings from a large sample of clinicians. Cerebrovasc Dis. 2006;22(5-6):389–95. doi: 10.1159/000094857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.