Abstract
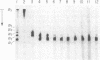
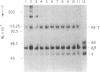
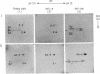
In addition to generating polymeric products from human fibrinogen, human erythrocyte transglutaminase (protein-glutamine:amine gamma-glutamyltransferase, EC 2.3.2.13) was shown to catalyze the intramolecular reaction of crosslinking two of the constituent chains within monomeric fibrinogen itself. This internally fused protein derivative contains appreciable amounts of the N epsilon-(gamma-glutamyl)lysine bridge peptide and displays the A alpha.gamma hybrid chain pattern of crosslinking, characteristic for the actions of tissue transglutaminases on fibrinogen. Diagnostic analysis in pathological situations, where such enzymes might have escaped from cells into the plasma environment, should include a search for the internally crosslinked soluble fibrinogen monomer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner S. C., Wold F. Human erythrocyte transglutaminase. Purification and properties. Biochim Biophys Acta. 1978 Jan 12;522(1):74–83. doi: 10.1016/0005-2744(78)90323-6. [DOI] [PubMed] [Google Scholar]
- Cariello L., Wilson J., Lorand L. Activation of transglutaminase during embryonic development. Biochemistry. 1984 Dec 18;23(26):6843–6850. doi: 10.1021/bi00321a087. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. The structure and evolution of vertebrate fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:13–27. doi: 10.1111/j.1749-6632.1983.tb23231.x. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Fowler W. E. Electron microscopy of fibrinogen, its plasmic fragments and small polymers. Ann N Y Acad Sci. 1983 Jun 27;408:146–163. doi: 10.1111/j.1749-6632.1983.tb23242.x. [DOI] [PubMed] [Google Scholar]
- Fink M. L., Chung S. I., Folk J. E. gamma-Glutamylamine cyclotransferase: specificity toward epsilon-(L-gamma-glutamyl)-L-lysine and related compounds. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4564–4568. doi: 10.1073/pnas.77.8.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M., Price S. J., Palmer T. A rapid and sensitive procedure for the quantitative determination of plasma amino acids. Clin Chim Acta. 1982 Oct 13;125(1):89–95. doi: 10.1016/0009-8981(82)90049-3. [DOI] [PubMed] [Google Scholar]
- Henschen A., Lottspeich F., Kehl M., Southan C. Covalent structure of fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:28–43. doi: 10.1111/j.1749-6632.1983.tb23232.x. [DOI] [PubMed] [Google Scholar]
- Lorand L., Murthy S. N., Velasco P. T., Karush F. Identification of transglutaminase substrates in inside-out vesicles from human erythrocytes: immunoblotting with anti-dansyl antibody. Biochem Biophys Res Commun. 1986 Jan 29;134(2):685–689. doi: 10.1016/s0006-291x(86)80474-0. [DOI] [PubMed] [Google Scholar]
- Lorand L. New approaches to old problems in the clotting of fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:226–232. doi: 10.1111/j.1749-6632.1983.tb23247.x. [DOI] [PubMed] [Google Scholar]
- Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968 Jan;7(1):208–223. doi: 10.1021/bi00841a026. [DOI] [PubMed] [Google Scholar]
- Moroi M., Inoue N., Yamasaki M. Analysis of the fibrin-polymerizing reaction using sodium dodecylsulfate-agarose gel electrophoresis. Biochim Biophys Acta. 1975 Jan 30;379(1):217–226. doi: 10.1016/0005-2795(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Murthy S. N., Lorand L. Cross-linked A alpha.gamma chain hybrids serve as unique markers for fibrinogen polymerized by tissue transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9679–9682. doi: 10.1073/pnas.87.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pucci P., Malorni A., Marino G., Metafora S., Esposito C., Porta R. Beta-endorphin modification by transglutaminase in vitro: identification by FAB/MS of glutamine-11 and lysine-29 as acyl donor and acceptor sites. Biochem Biophys Res Commun. 1988 Jul 29;154(2):735–740. doi: 10.1016/0006-291x(88)90201-x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Shainoff J. R., Urbanic D. A., DiBello P. M. Immunoelectrophoretic characterizations of the cross-linking of fibrinogen and fibrin by factor XIIIa and tissue transglutaminase. Identification of a rapid mode of hybrid alpha-/gamma-chain cross-linking that is promoted by the gamma-chain cross-linking. J Biol Chem. 1991 Apr 5;266(10):6429–6437. [PubMed] [Google Scholar]
- Slayter H. S. Electron microscopic studies of fibrinogen structure: historical perspectives and recent experiments. Ann N Y Acad Sci. 1983 Jun 27;408:131–145. doi: 10.1111/j.1749-6632.1983.tb23241.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J., Hainfeld J., Haschemeyer R. H., Mosesson M. W. Analysis of human fibrinogen by scanning transmission electron microscopy. Ann N Y Acad Sci. 1983 Jun 27;408:164–179. doi: 10.1111/j.1749-6632.1983.tb23243.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams R. C. Morphology of fibrinogen monomers and of fibrin protofibrils. Ann N Y Acad Sci. 1983 Jun 27;408:180–193. doi: 10.1111/j.1749-6632.1983.tb23244.x. [DOI] [PubMed] [Google Scholar]