Abstract
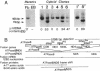
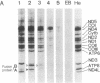
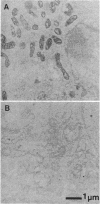
Mutant mitochondrial DNA with large-scale deletions (delta-mtDNA) has been frequently observed in patients with chronic progressive external ophthalmoplegia (CPEO), a subgroup of the mitochondrial encephalomyopathies. To exclude involvement of the nuclear genome in expression of the mitochondrial dysfunction characteristic of CPEO, we introduced the mtDNA of a CPEO patient into clonal mtDNA-less HeLa cells and isolated cybrid clones. Quantitation of delta-mtDNA in the cybrids revealed that delta-mtDNA was selectively propagated with higher levels of delta-mtDNA correlating with slower cellular growth rate. In these cybrid clones, translational complementation of the missing tRNAs occurred only when delta-mtDNA was less than 60% of the total mtDNA, whereas accumulation of delta-mtDNA to greater than 60% resulted in progressive inhibition of overall mitochondrial translation as well as reduction of cytochrome c oxidase activity throughout the organelle population. Because these cybrids shared the same nuclear background as HeLa cells, these results suggest that large-scale deletion mutations of mtDNA alone are sufficient for the mitochondrial dysfunction characteristic of CPEO.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- Goto Y., Koga Y., Horai S., Nonaka I. Chronic progressive external ophthalmoplegia: a correlative study of mitochondrial DNA deletions and their phenotypic expression in muscle biopsies. J Neurol Sci. 1990 Dec;100(1-2):63–69. doi: 10.1016/0022-510x(90)90014-e. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hayashi J. I., Yonekawa H., Tagashira Y. Nuclear but not mitochondrial genome involvement in 3-methylcholanthrene-induced expression of tumorigenicity in mouse somatic cells. Cancer Res. 1989 Sep 1;49(17):4715–4720. [PubMed] [Google Scholar]
- Hayashi J., Werbin H., Shay J. W. Effects of normal human fibroblast mitochondrial DNA on segregation of HeLaTG Mitochondrial DNA and on tumorigenicity of HeLaTG cells. Cancer Res. 1986 Aug;46(8):4001–4006. [PubMed] [Google Scholar]
- Hayashi J., Yonekawa H., Murakami J., Tagashira Y., Pereira-Smith O. M., Shay J. W. Mitochondrial genomes in intraspecies mammalian cell hybrids display codominant or dominant/recessive behavior. Exp Cell Res. 1987 Sep;172(1):218–227. doi: 10.1016/0014-4827(87)90108-x. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Shimoizumi H., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet. 1991 Sep;49(3):590–599. [PMC free article] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Riley M., Cottrell B., Doolittle R. F., Attardi G. Identification of the polypeptides encoded in the unassigned reading frames 2, 4, 4L, and 5 of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1563–1567. doi: 10.1073/pnas.83.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane M. A., Hammans S. R., Sweeney M., Holt I. J., Beattie T. J., Brett E. M., Harding A. E. Pearson syndrome and mitochondrial encephalomyopathy in a patient with a deletion of mtDNA. Am J Hum Genet. 1991 Jan;48(1):39–42. [PMC free article] [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Schon E. A., DiMauro S., Miranda A. F. Heteroplasmy of mitochondrial genomes in clonal cultures from patients with Kearns-Sayre syndrome. Biochem Biophys Res Commun. 1989 Apr 28;160(2):765–771. doi: 10.1016/0006-291x(89)92499-6. [DOI] [PubMed] [Google Scholar]
- Nakase H., Moraes C. T., Rizzuto R., Lombes A., DiMauro S., Schon E. A. Transcription and translation of deleted mitochondrial genomes in Kearns-Sayre syndrome: implications for pathogenesis. Am J Hum Genet. 1990 Mar;46(3):418–427. [PMC free article] [PubMed] [Google Scholar]
- Orii Y., Okunuki K. Studies on cytochrome a. XV. Cytochrome oxidase activity of the Okunuki preparation and its activation by heat, alkali and detergent treatments. J Biochem. 1965 Dec;58(6):561–568. doi: 10.1093/oxfordjournals.jbchem.a128243. [DOI] [PubMed] [Google Scholar]
- Rotig A., Colonna M., Bonnefont J. P., Blanche S., Fischer A., Saudubray J. M., Munnich A. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet. 1989 Apr 22;1(8643):902–903. doi: 10.1016/s0140-6736(89)92897-3. [DOI] [PubMed] [Google Scholar]
- Rötig A., Cormier V., Blanche S., Bonnefont J. P., Ledeist F., Romero N., Schmitz J., Rustin P., Fischer A., Saudubray J. M. Pearson's marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J Clin Invest. 1990 Nov;86(5):1601–1608. doi: 10.1172/JCI114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]